Curcumin Suppresses Lead-Induced Inflammation and Memory Loss in Mouse Model and In Silico Molecular Docking
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagent
2.2. Animals Designing and Treatment
- Gr. i: Untreated control NaAc (1%, w/v) + normal saline
- Gr. ii: Pb (1%, w/v) PbAc (1%, w/v) + normal saline
- Gr. iii: Pb + Cur 100 mg/kgBW PbAc (1%, w/v) + Cur 100 mg/kgBW in normal saline
- Gr. iv: Pb + Cur 200 mg/kgBW PbAc (1%, w/v) + Cur 200 mg/kgBW in normal saline
- Gr. v: Cur 200 mg/kgBW NaAc (1%, w/v) + Cur 200 mg/kgBW in normal saline
2.3. Water Maze Swimming Test
2.4. Forced Swimming Test
2.5. Blood Sampling and Tissue Harvesting
2.6. Measurement of Lipid Peroxidation in Plasma, Red Blood Cell (RBC), and Brain Tissue
2.7. Measurement of Acetylcholinesterase Activity in Plasma, RBC, and Brain Tissue
2.8. Enzyme-Linked Immunosorbent Assay (ELISA)
2.9. Western Blot Assay
2.10. Immunohistochemistry Study
2.11. In Silico Study
2.11.1. Molecular Docking Study
2.11.2. The Chemical Absorption, Distribution, Metabolism, Excretion, and Toxicity (ADMET) Analysis
2.12. Statistical Analysis
3. Results
3.1. Effect of Curcumin on Lead-Induced Mice Body Weight Changes
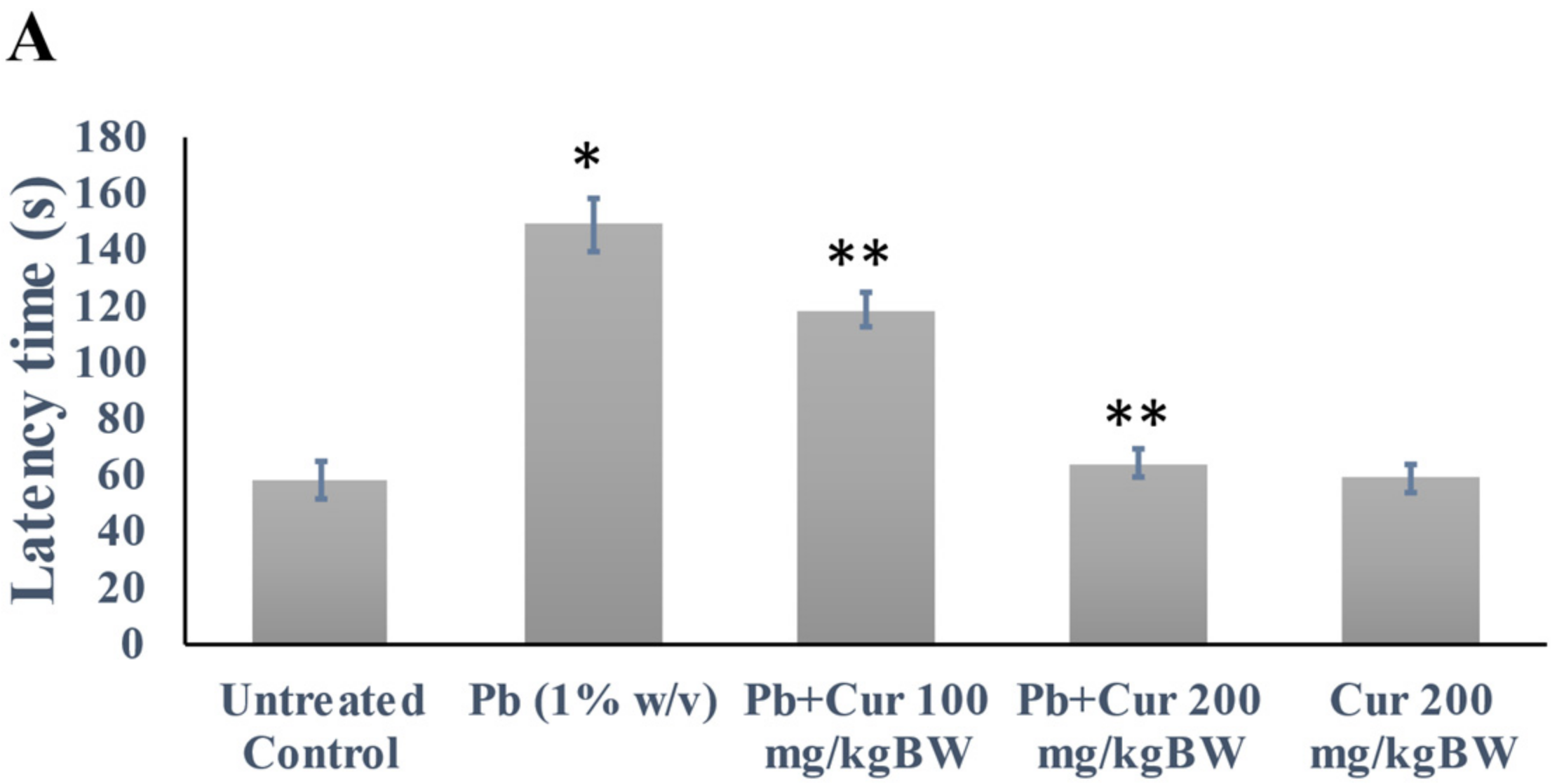
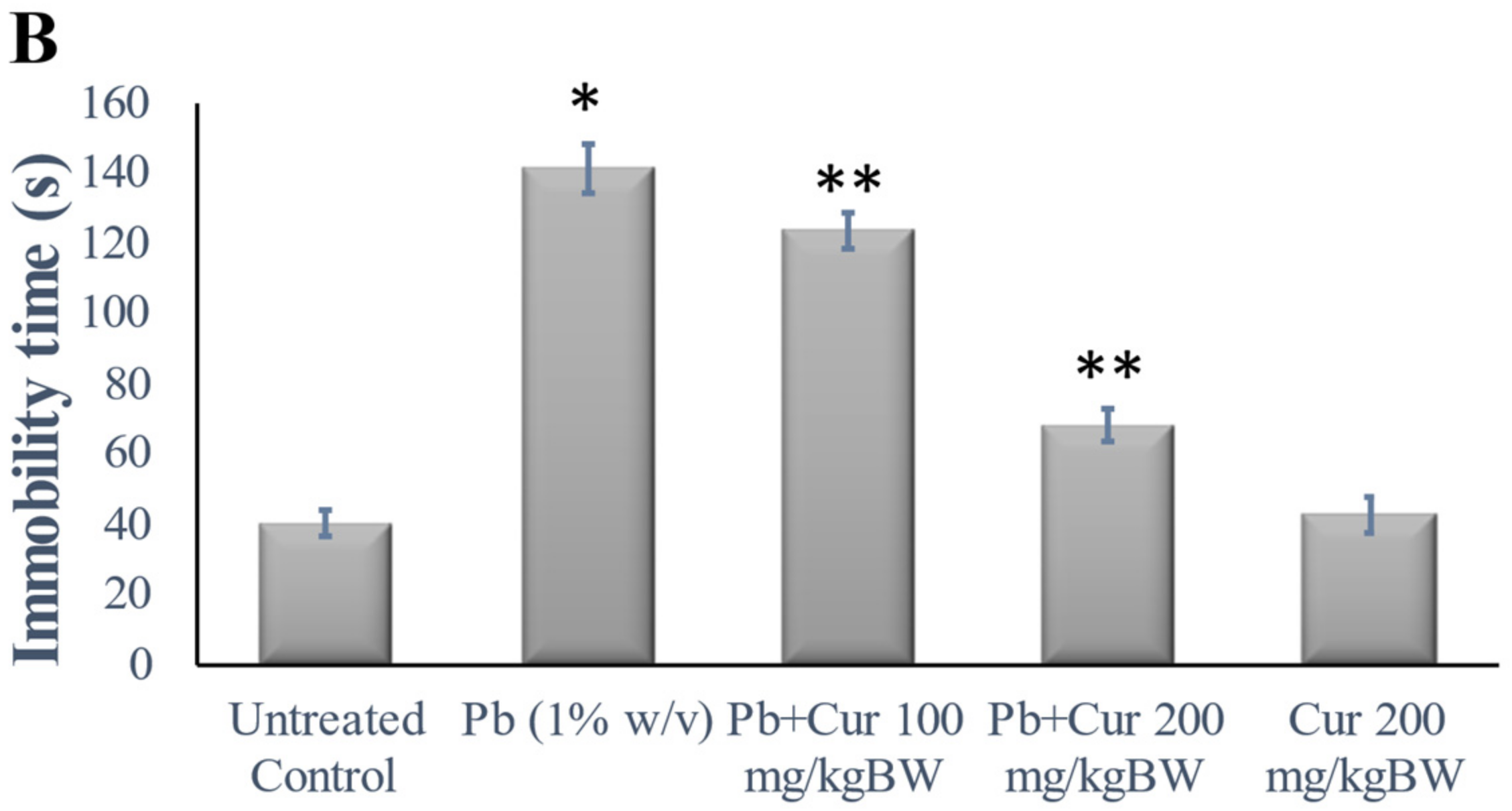
3.2. Validation of Curcumin’s Effect against Lead-Induced Cognitive Dysfunction
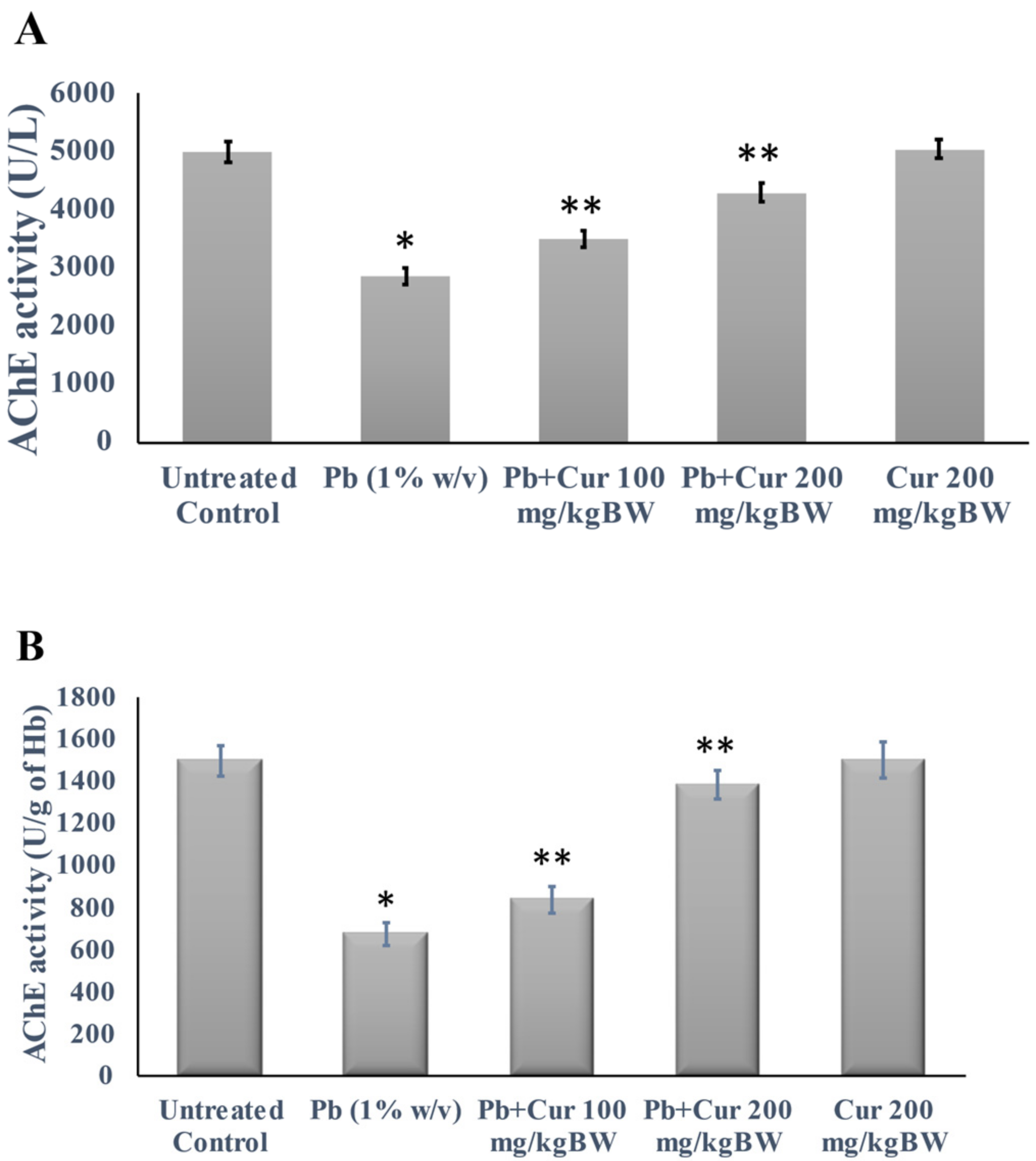
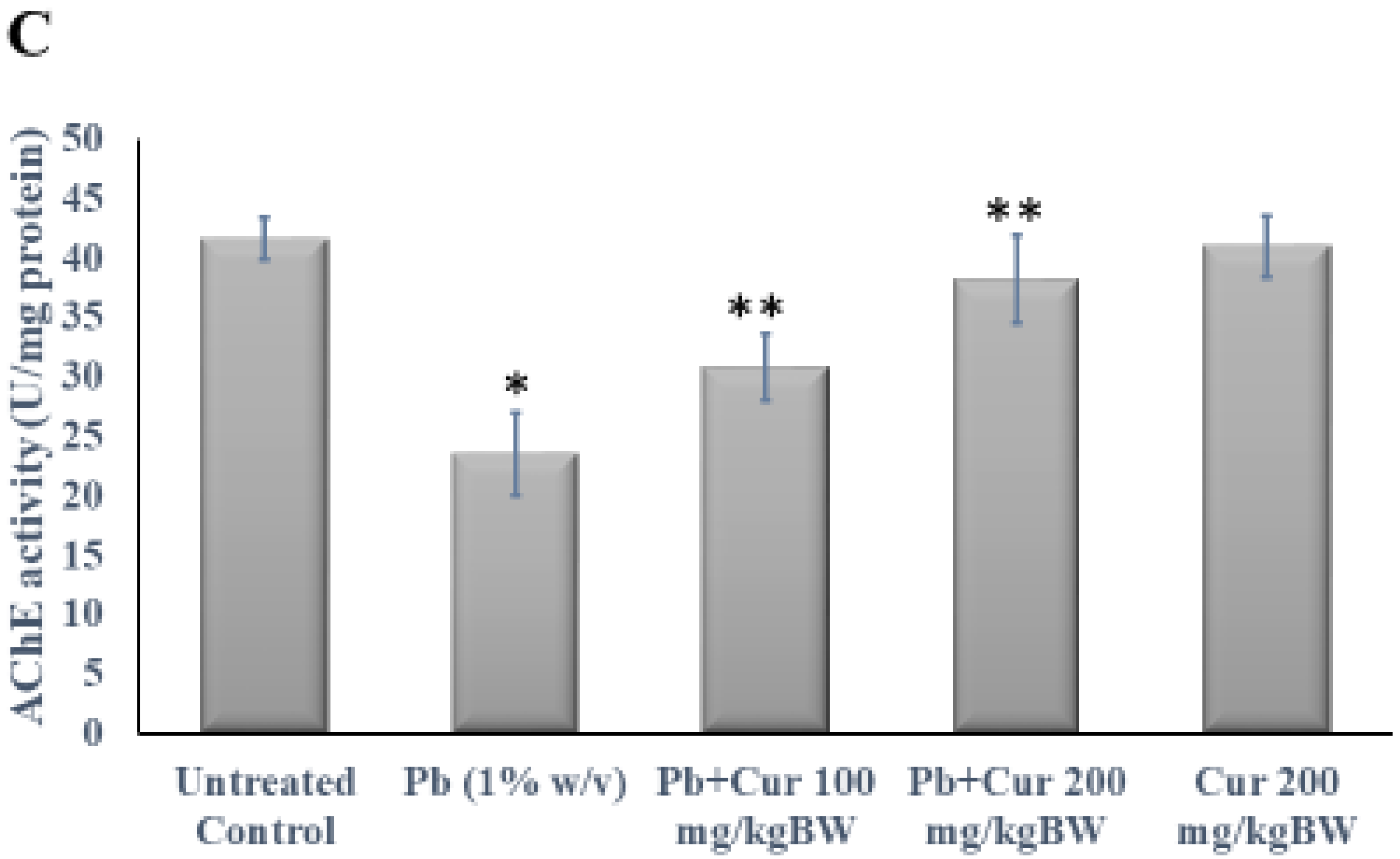
3.3. Protective Effect of Curcumin on Cholinergic Dysfunction
3.4. Protective Effect of Curcumin on Oxidative Stress Parameters
3.5. Protective Effect of Curcumin on Inflammatory Profile
3.6. Molecular Docking In Silico
3.6.1. Binding Mechanism of Curcumin with Acetylcholinesterase (AChE)
3.6.2. ADMET Analysis and Drug-Likeliness Properties of Curcumin
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rana, M.N.; Tangpong, J.; Rahman, M.M. Toxicodynamics of Lead, Cadmium, Mercury and Arsenic-induced kidney toxicity and treatment strategy: A mini-review. Toxicol. Rep. 2018, 5, 704–713. [Google Scholar] [CrossRef]
- Liu, C.M.; Zheng, G.H.; Ming, Q.L.; Sun, J.M.; Cheng, C. Protective effect of puerarin on lead-induced mouse cognitive im-pairment via altering activities of acetylcholinesterase, monoamine oxidase and nitric oxide synthase. Environ. Toxicol. Phar-macol. 2013, 35, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, B.; Zeng, H.; Cai, C.; Hu, Q.; Cai, S.; Xu, L.; Meng, X.; Zou, F. Role of the mitochondrial Ca2+ uniporter in Pb2+-induced oxidative stress in human neuroblastoma cells. Brain Res. 2014, 1575, 12–21. [Google Scholar] [CrossRef]
- Indo, H.P.; Yen, H.C.; Nakanishi, I.; Matsumoto, K.; Tamura, M.; Nagano, Y.; Matsui, H.; Gusev, O.; Cornette, R.; Okuda, T.; et al. A mitochondrial super-oxide theory for oxidative stress diseases and aging. J. Clin. Biochem. Nutr. 2015, 56, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, L.; Liu, J.Y.; Dong, J.X.; Xiao, Q.; Zhao, J.; Jiang, F.L. Toxicity of Pb2+ on rat liver mitochondria induced by oxidative stress and mitochondrial permeability transition. Toxicol. Res. 2017, 6, 822–830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gleichmann, M.; Mattson, M.P. Neuronal calcium homeostasis and dysregulation. Antioxid. Redox Signal. 2011, 14, 1261–1273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tangpong, J.; Satarug, S. Alleviation of lead poisoning in the brain with aqueous leaf extract of the Thunbergia laurifolia (Linn.). Toxicol. Lett. 2010, 198, 83–88. [Google Scholar] [CrossRef]
- Indo, H.P.; Hawkins, C.L.; Nakanishi, I.; Matsumoto, K.; Matsui, H.; Suenaga, S.; Davies, M.J.; St Clair, D.K.; Ozawa, T.; Majima, H.J. Role of mitochondrial reactive oxygen species in the activation of cellular signals, molecules and function. Pharmacol. Mitochondria 2017, 240, 439–456. [Google Scholar] [CrossRef]
- Phyu, M.P.; Tangpong, J. Neuroprotective effects of xanthone derivative of Garcinia mangostana against lead-induced acetyl-cholinesterase dysfunction and cognitive impairment. Food Chem. Toxicol. 2014, 70, 151–156. [Google Scholar] [CrossRef]
- Basha, D.C.; Rani, M.U.; Devi, C.B.; Kumar, M.R.; Reddy, G.R. Perinatal lead exposure alters postnatal cholinergic and aminergic system in rat brain: Reversal effect of calcium co-administration. Int. J. Dev. Neurosci. 2012, 30, 343–350. [Google Scholar] [CrossRef] [PubMed]
- de Souza Tavares, W.; Akhtar, Y.; Gonçalves, G.L.P.; Zanuncio, J.C.; Isman, M.B. Turmeric powder and its derivatives from Curcuma longa rhizomes: Insecticidal effects on cabbage looper and the role of synergists. Sci. Rep. 2016, 6, 34093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esatbeyoglu, T.; Huebbe, P.; Ernst, I.M.; Chin, D.; Wagner, A.E.; Rimbach, G. Curcumin-from molecule to biological function. Angew. Chem. Int. Ed. Engl. 2012, 51, 5308–5332. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.C.; Patchva, S.; Koh, W.; Aggarwal, B.B. Discovery of Curcumin, a component of golden spice, and its miraculous biological activities. CEPP 2012, 39, 283–299. [Google Scholar] [CrossRef]
- Ng, T.P.; Chiam, P.C.; Lee, T.; Chua, H.C.; Lim, L.; Kua, E.H. Curry consumption and cognitive function in the elderly. Am. J. Epidemiol. 2006, 164, 898–906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cox, K.H.; Pipingas, A.; Scholey, A.B. Investigation of the effects of solid lipid curcumin on cognition and mood in a healthy older population. J. Psychopharmacol. 2015, 29, 642–651. [Google Scholar] [CrossRef]
- Abu-Taweel, G.M. Neurobehavioral protective properties of Curcumin against the mercury chloride treated mice offspring. Saudi J. Biol. Sci. 2019, 26, 736–743. [Google Scholar] [CrossRef] [PubMed]
- Akinyemi, A.J.; Oboh, G.; Fadaka, A.O.; Olatunji, B.P.; Akomolafe, S. Curcumin administration suppress acetylcholinesterase gene expression in cadmium treated rats. Neurotoxicology 2017, 62, 75–79. [Google Scholar] [CrossRef]
- Akinyemi, A.J.; Oboh, G.; Ogunsuyi, O.; Abolaji, A.O.; Udofia, A. Curcumin-supplemented diets improve antioxidant enzymes and alter acetylcholinesterase genes expression level in Drosophila melanogaster model. Metab. Brain Dis. 2018, 33, 369–375. [Google Scholar] [CrossRef]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS). Scientific Opinion on the re-evaluation of curcu-min (E 100) as a food additive. EFSA J. 2010, 8, 1679. [Google Scholar] [CrossRef]
- Rafiee, Z.; Nejatian, M.; Daeihamed, M.; Jafari, S.M. Application of curcumin-loaded nanocarriers for food, drug and cos-metic purposes. Trends Food Sci. Technol. 2019, 88, 445–458. [Google Scholar] [CrossRef]
- Rana, M.N.; Tangpong, J.; Rahman, M.A. Xanthones protects lead-induced chronic kidney disease (CKD) via activating Nrf-2 and modulating NF-kB, MAPK pathway. Biochem. Biophys. Rep. 2020, 21, 100718. [Google Scholar] [CrossRef] [PubMed]
- Meagher, E.A.; FitzGerald, G.A. Indices of lipid peroxidation in vivo: Strengths and limitations. Free Radic. Biol. Med. 2000, 28, 1745–1750. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Feather-stone, R.M. A new and rapid colorimetric determination of acetylcholin-esterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Mason, L.H.; Harp, J.P.; Han, D.Y. Pb neurotoxicity: Neuropsychological effects of lead toxicity. Biomed. Res. Int. 2014, 2014, 840547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reddy, G.R.; Devi, B.C.; Chetty, C.S. Developmental lead neurotoxicity: Alterations in brain cholinergic system. Neurotoxicology 2007, 28, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Toscano, C.D.; Guilarte, T.R. Lead neurotoxicity: From exposure to molecular effects. Brain Res. Rev. 2005, 49, 529–554. [Google Scholar] [CrossRef]
- Canfield, R.L.; Henderson, C.R.J.; Cory-Slechta, D.A.; Cox, C.; Jusko, T.A.; Lanphear, B.P. Intellectual impairment in children with blood lead concentrations below 10 μg per deciliter. N. Engl. J. 2003, 348, 1517–1526. [Google Scholar] [CrossRef] [Green Version]
- Vorhees, C.V.; Williams, M.T. Morris water maze: Procedures for assessing spatial and related forms of learning and memory. Nat. Protoc. 2006, 1, 848–858. [Google Scholar] [CrossRef] [Green Version]
- Tian, H.; Ding, N.; Guo, M.; Wang, S.; Wang, Z.; Liu, H.; Yang, J.; Li, Y.; Ren, J.; Jiang, J.; et al. Analysis of Learning and Memory Ability in an Alzheimer’s Disease Mouse Model using the Morris Water Maze. J. Vis. Exp. 2019, 29, e60055. [Google Scholar] [CrossRef]
- Hasselmo, M.E. The role of acetylcholine in learning and memory. Curr. Opin. Neurobiol. 2006, 16, 710–715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newman, E.L.; Gupta, K.; Climer, J.R.; Monaghan, C.K.; Hasselmo, M.E. Cholinergic modulation of cognitive processing: In-sights drawn from computational models. Front. Behav. Neurosci. 2012, 6, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.H.; Lee, C.J.; Chen, L.C.; Lee, T.L.; Hsieh, Y.Y.; Han, C.H.; Yang, C.H.; Huang, W.J.; Hou, W.C. Acetylcholinesterase inhibitory activity and neuroprotection: In vitro, molecular docking, and improved learning and memory functions of deme-thylcurcumin in scopolamine-induced amnesia ICR mice. Food Funct. 2020, 11, 2328–2338. [Google Scholar] [CrossRef] [PubMed]
- Oz, M.; Atalik, K.E.N.; Yerlikaya, F.H.; Demir, E.A. Curcumin alleviates cisplatin-induced learning and memory impairments. Neurobiol. Learn. Mem. 2015, 123, 43–49. [Google Scholar] [CrossRef]
- Sarlak, Z.; Oryan, S.; Moghaddasi, M. Interaction between the antioxidant activity of curcumin and cholinergic system on memory retention in adult male Wistar rats. Iran. J. Basic Med. Sci. 2015, 18, 398–403. [Google Scholar]
- Mary, C.P.V.; Vijayakumar, S.; Shankar, R. Metal chelating ability and antioxidant properties of Curcumin-metal complexes—A DFT approach. J. Mol. Graph. Model. 2018, 79, 1–14. [Google Scholar] [CrossRef]
- Abubakar, K.; Muhammad Mailafiya, M.; Danmaigoro, A.; Musa Chiroma, S.; Abdul Rahim, E.B.; Abu Bakar Zakaria, M.Z. Curcumin attenuates lead-induced cerebellar toxicity in rats via chelating activity and inhibition of oxidative stress. Biomolecules 2019, 9, 453. [Google Scholar] [CrossRef] [Green Version]
- Vijayraghavan, S.; Major, A.J.; Everling, S. Muscarinic M1 receptor overstimulation disrupts working memory activity for rules in primate prefrontal cortex. Neuron 2018, 98, 1256–1268. [Google Scholar] [CrossRef] [Green Version]
- Spellman, T.; Rigotti, M.; Ahmari, S.E.; Fusi, S.; Gogos, J.A.; Gordon, J.A. Hippocampal–prefrontal input supports spatial en-coding in working memory. Nature 2015, 522, 309–314. [Google Scholar] [CrossRef] [Green Version]
- Baxter, M.G.; Crimins, J.L. Acetylcholine receptor stimulation for cognitive enhancement: Better the devil you know? Neuron 2018, 98, 1064–1066. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Dogra, S.; Prakash, A. Protective effect of Curcumin (Curcuma longa) against aluminum toxicity: Possible behav-ioral and biochemical alterations in rats. Behav. Brain Res. 2009, 205, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Abolaji, A.O.; Fasae, K.D.; Iwezor, C.E.; Aschner, M.; Farombi, O.E. Curcumin attenuates copper-induced oxidative stress and neurotoxicity in Drosophila melanogaster. Toxicol. Rep. 2020, 7, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Galal, M.K.; Elleithy, E.M.M.; Abdrabou, M.I.; Yasin, N.A.E.; Shaheen, Y.M. Modulation of caspase-3 gene expression and pro-tective effects of garlic and spirulina against CNS neurotoxicity induced by lead exposure in male rats. Neurotoxicology 2019, 72, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Hatcher, H.; Planalp, R.; Cho, J.; Torti, F.M.; Torti, S.V. Curcumin: From ancient medicine to current clinical trials. Cell. Mol. Life Sci. 2008, 65, 1631–1652. [Google Scholar] [CrossRef] [PubMed]
- Tüzmen, M.N.; Yücel, N.C.; Kalburcu, T.; Demiryas, N. Effects of Curcumin and tannic acid on the aluminum- and lead-induced oxidative neurotoxicity and alterations in NMDA receptors. Toxicol. Mech. Methods 2015, 25, 120–127. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Yue, Y.; Zheng, X.; Zhang, K.; Chen, S.; Du, Z. Curcumin, inflammation, and chronic diseases: How are they linked? Molecules 2015, 20, 9183–9213. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, W.S.; Albasher, G. Moringa oleifera Lam. extract rescues lead-induced oxidative stress, inflammation, and apoptosis in the rat cerebral cortex. J. Food Biochem. 2021, 45, e13579. [Google Scholar] [CrossRef]
- Phyu, M.P.; Tangpong, J. Protective effect of Thunbergia laurifolia (Linn.) on lead induced acetylcholinesterase dysfunction and cognitive impairment in mice. BioMed Res. Int. 2013, 2013, 1–6. [Google Scholar] [CrossRef]
- Khalaf, A.A.; Moselhy, W.A.; Abdel-Hamed, M.I. The protective effect of green tea extract on lead induced oxidative and DNA damage on rat brain. Neurotoxicology 2012, 33, 280–289. [Google Scholar] [CrossRef]

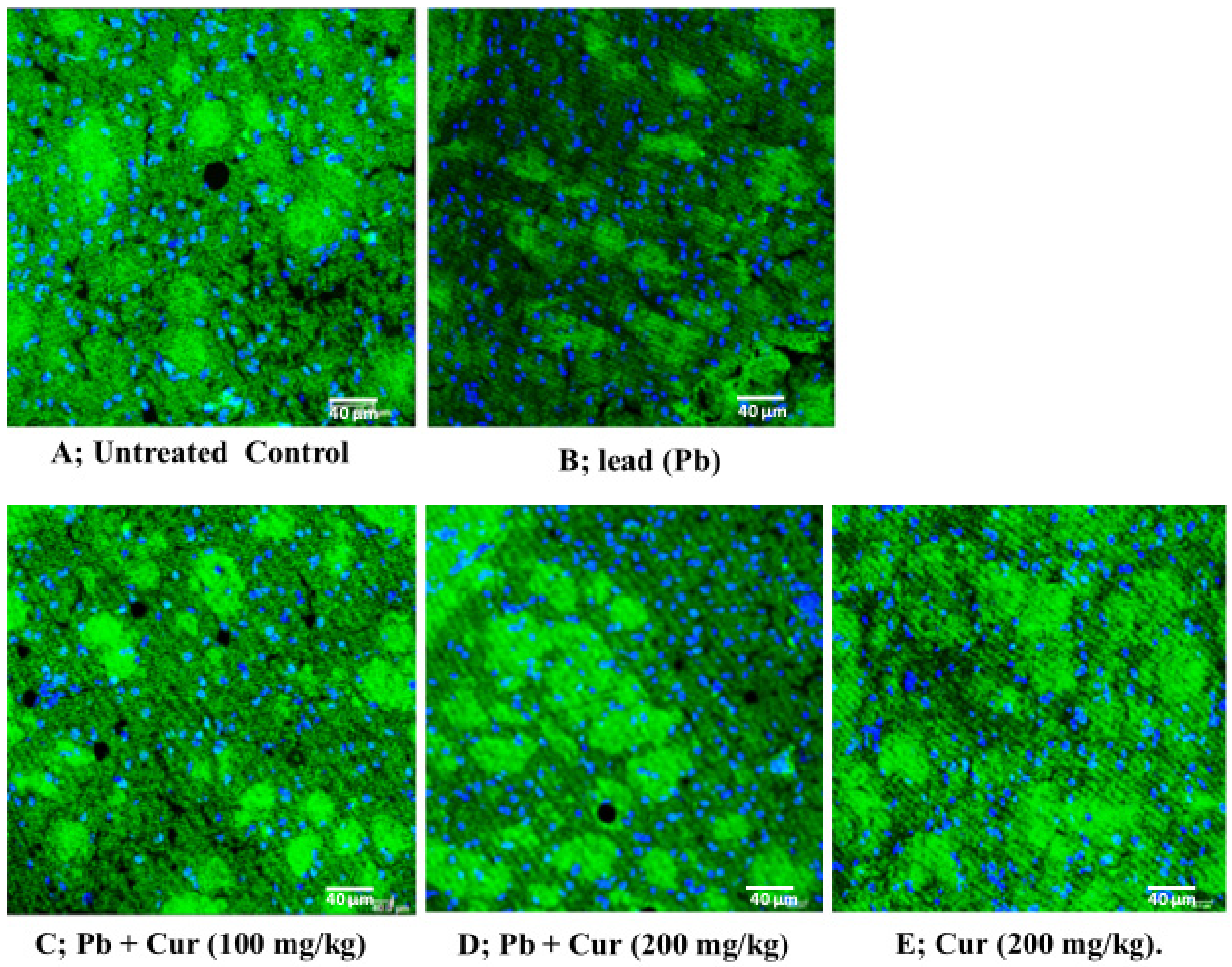
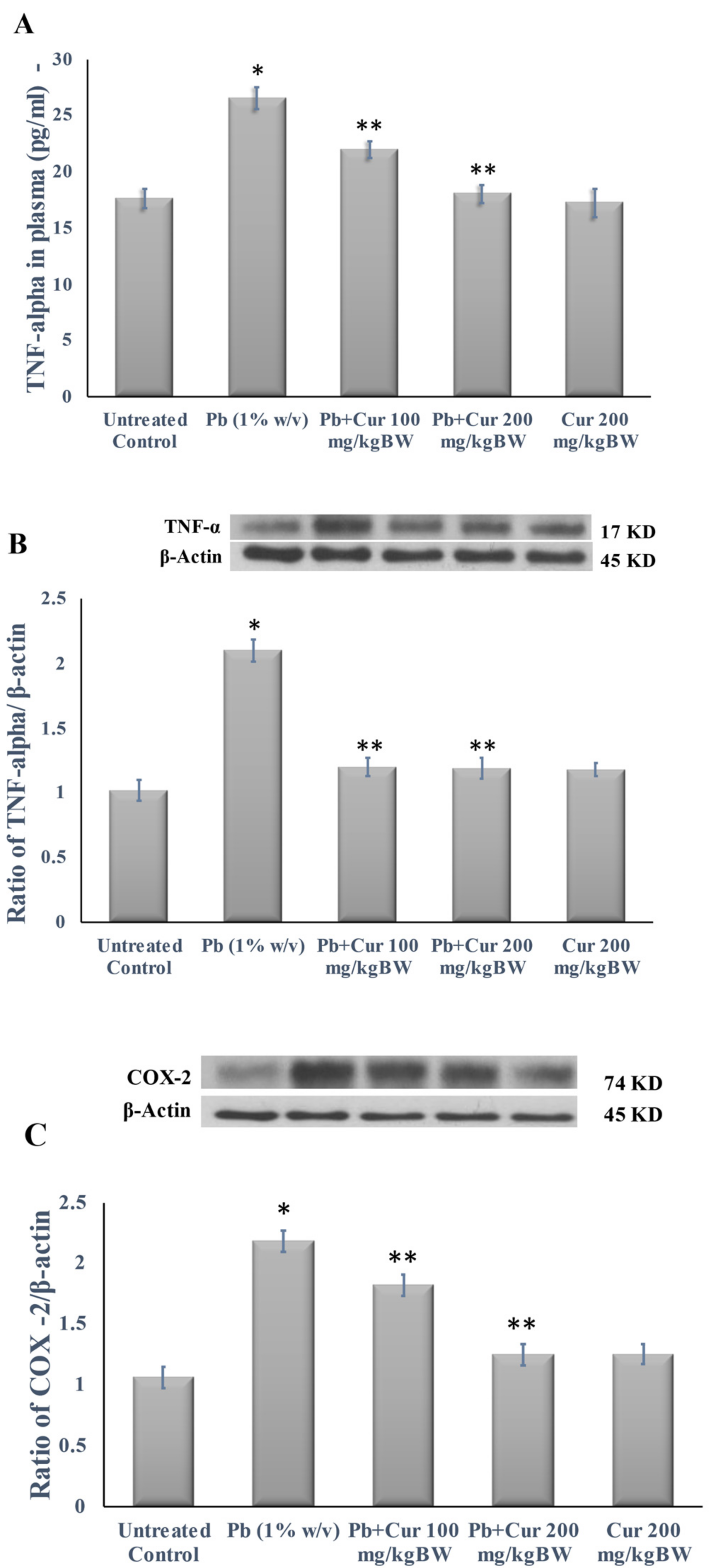
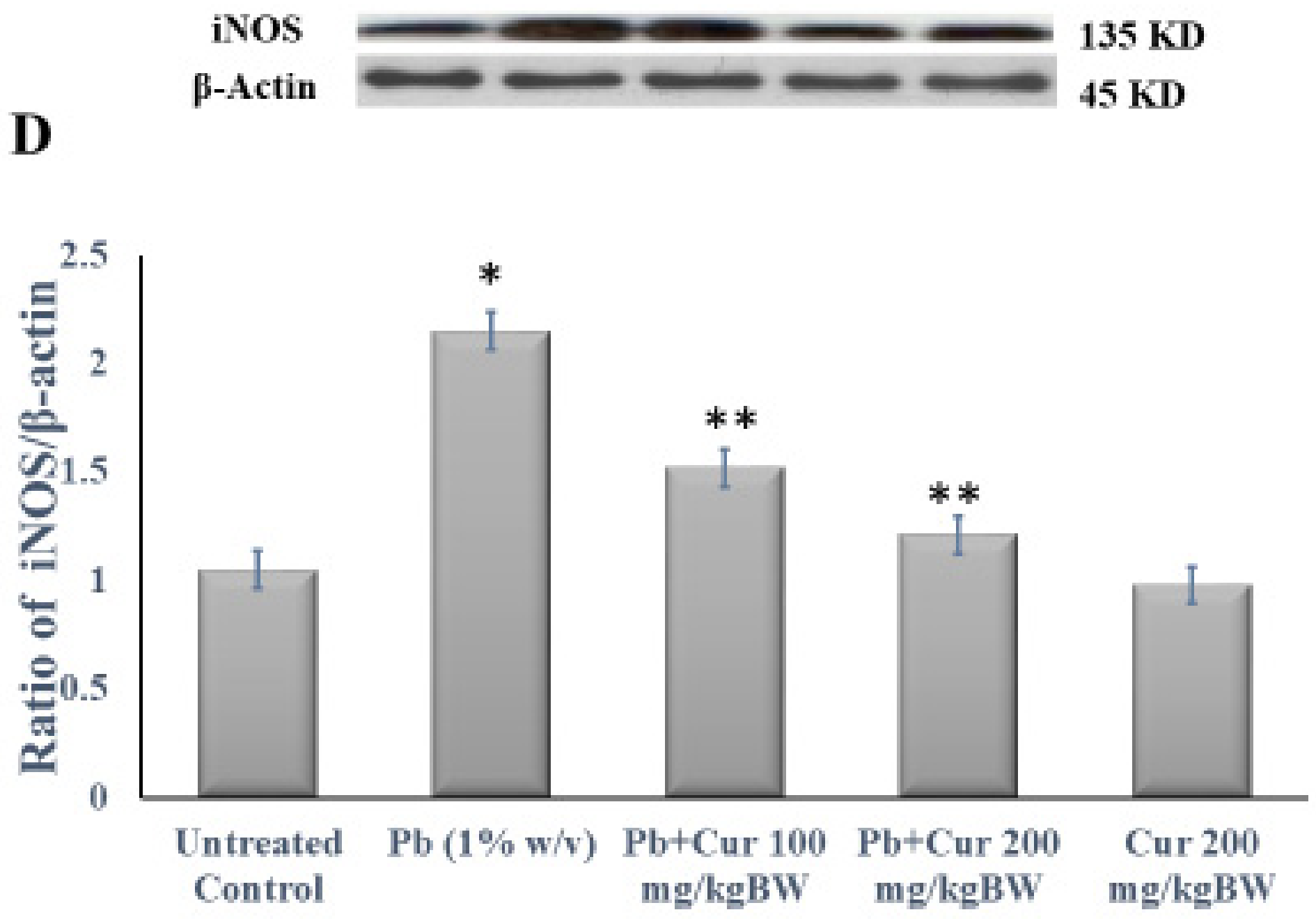

| Group | Initial Body Weight (g) | Final Body Weight (g) |
|---|---|---|
| Untreated control | 29.53 ± 0.35 | 37.16 ± 1.01 |
| Pb (1%, w/v) | 30.01 ± 0.37 | 32.80 ± 0.58 * |
| Pb + Cur 100 mg/kgBW | 30.78 ± 0.37 | 36.50 ± 0.71 ** |
| Pb + Cur 200 mg/kgBW | 30.25 ± 0.46 | 38.69 ± 0.58 ** |
| Cur 200 mg/kgBW | 30.22 ± 0.38 | 37.50 ± 0.80 ** |
| Group | Plasma MDA (nM) | RBC MDA (µM/g Hb) | Brain MDA (nM/g Protein) |
|---|---|---|---|
| Untreated control | 91.43 ± 3.42 | 3.72 ± 0.18 | 37.67 ± 3.43 |
| Pb (1%, w/v) | 178.74 ± 4.54 * | 6.98 ± 0.44 * | 50.80 ± 2.71 * |
| Pb + Cur 100 mg/kgBW | 125.78 ± 3.21 ** | 4.14 ± 0.94 ** | 42.08 ± 0.62 ** |
| Pb + Cur 200 mg/kgBW | 103.94 ± 5.76 ** | 3.65 ± 0.20 ** | 39.02 ± 1.93 ** |
| Cur 200 mg/kgBW | 87.83 ± 2.12 ** | 3.89 ± 0.11 ** | 38.88 ± 1.46 ** |
| Compound | Protein | PDB ID | Docking Score | Glide e-Model | Glide Energy |
|---|---|---|---|---|---|
| Curcumin | AChE | 4M0E | −9.251 | −75.443 | −52.127 |
| COX-2 | 5KIR | −8.113 | −55.858 | −42.837 | |
| TNF-α | 2AZ5 | −6.857 | −59.754 | −43.063 | |
| IKK | 4KIK | −8.837 | −85.148 | −56.14 | |
| ERK | 1TVO | −5.479 | −62.176 | −46.786 | |
| JNK | 3OY1 | −7.172 | −68.542 | −50.149 | |
| P38 | 5WJJ | −7.739 | −70.85 | −49.057 |
| Compound | Curcumin |
|---|---|
| Star | 1 |
| SASA | 706.80 |
| FOSA | 261.70 |
| FISA | 190.40 |
| QPPCaco | 155.01 |
| QPlogBB | −2.246 |
| QPPMDCK | 65.96 |
| Rule of three | 0 |
| Rule of five | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Changlek, S.; Rana, M.N.; Phyu, M.P.; Karim, N.; Majima, H.J.; Tangpong, J. Curcumin Suppresses Lead-Induced Inflammation and Memory Loss in Mouse Model and In Silico Molecular Docking. Foods 2022, 11, 856. https://doi.org/10.3390/foods11060856
Changlek S, Rana MN, Phyu MP, Karim N, Majima HJ, Tangpong J. Curcumin Suppresses Lead-Induced Inflammation and Memory Loss in Mouse Model and In Silico Molecular Docking. Foods. 2022; 11(6):856. https://doi.org/10.3390/foods11060856
Chicago/Turabian StyleChanglek, Suksan, Mohammad Nasiruddin Rana, Moe Pwint Phyu, Naymul Karim, Hideyuki J. Majima, and Jitbanjong Tangpong. 2022. "Curcumin Suppresses Lead-Induced Inflammation and Memory Loss in Mouse Model and In Silico Molecular Docking" Foods 11, no. 6: 856. https://doi.org/10.3390/foods11060856
APA StyleChanglek, S., Rana, M. N., Phyu, M. P., Karim, N., Majima, H. J., & Tangpong, J. (2022). Curcumin Suppresses Lead-Induced Inflammation and Memory Loss in Mouse Model and In Silico Molecular Docking. Foods, 11(6), 856. https://doi.org/10.3390/foods11060856








