The Solubility and Structures of Porcine Myofibrillar Proteins under Low-Salt Processing Conditions as Affected by the Presence of L-Lysine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. MFP Extraction
2.3. Preparation of MFP Suspensions with Assorted Levels of Ionic Strength and Lys
2.4. MFP Solubility
2.5. MFP Turbidity
2.6. Measurement of the Particle Size
2.7. Measurement of Circular Dichroism (CD) Spectra
2.8. Measurement of Intrinsic Tryptophan Fluorescence
2.9. Statistical Analysis
3. Results
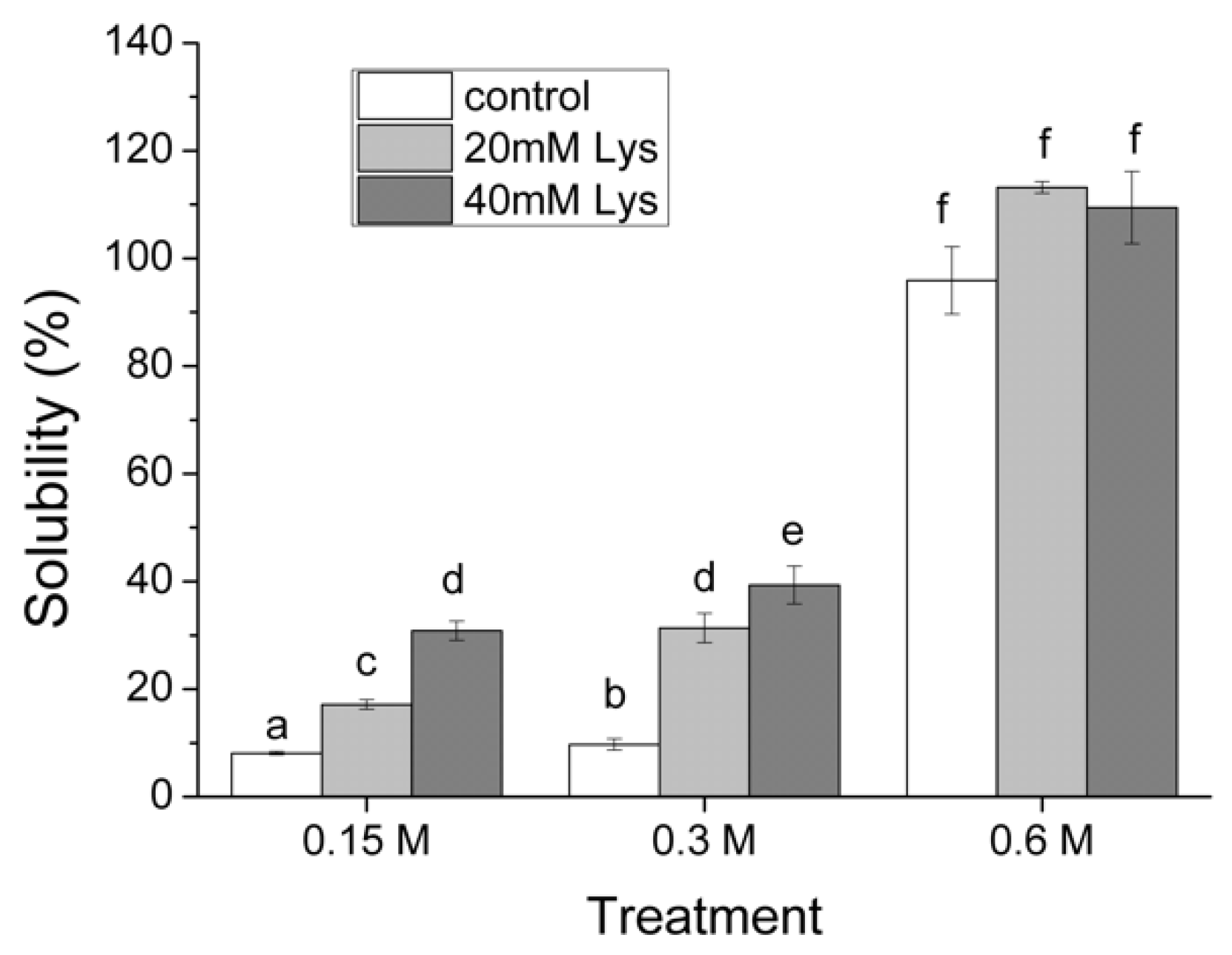
3.1. Solubility
3.2. Turbidity
3.3. Particle Size Distribution
3.4. Protein Secondary Structure
3.5. Intrinsic Tryptophan of Fluorescence
4. Discussion
4.1. Solubilization of MFP under Different Ionic Strengths in the Presence of Lys
4.2. Structural Changes of MFPs under Different Ionic Strengths in the Presence of Lys
4.2.1. Swelling of Myofibrils
4.2.2. Dissociation of Myosin Filaments and Uncoiling of α-Helix
4.2.3. Unfolding of the Tertiary Structure
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lawrie, R.A.; Ledward, D.A. Chemical and biochemical constitution of muscle. In Lawrie’s Meat Science; Lawrie, R.A., Ledward, D.A., Eds.; Woodhead Publishing Limited: Abington, UK, 2006; pp. 75–76. [Google Scholar]
- Xiong, Y.L. Structure function relationships of muscle proteins. In Food Proteins and Their Applications; Damodaran, S., Paraf, A., Eds.; Marcel Dekker, Inc.: New York, NY, USA, 1997; pp. 341–392. [Google Scholar]
- Sun, X.D.; Holley, R.A. Factors Influencing Gel Formation by Myofibrillar Proteins in Muscle Foods. Compr. Rev. Food Sci. Food Saf. 2011, 10, 33–51. [Google Scholar] [CrossRef]
- Ishioroshi, M.; Jima, K.; Yasui, T. Heat-induced gelation of myosin: Factors of pH and salt concentrations. J. Food Sci. 1979, 44, 1280–1284. [Google Scholar] [CrossRef]
- Inguglia, E.S.; Zhang, Z.; Tiwari, B.K.; Kerry, J.P.; Burgess, C.M. Salt reduction strategies in processed meat products—A review. Trends Food Sci. Technol. 2017, 59, 70–78. [Google Scholar] [CrossRef]
- Guo, X.; Tao, S.; Pan, J.; Lin, X.; Ji, C.; Liang, H.; Dong, X.; Li, S. Effects of L-Lysine on the physiochemical properties and sensory characteristics of salt-reduced reconstructed ham. Meat Sci. 2020, 166, 108133. [Google Scholar] [CrossRef]
- Guo, X.Y.; Peng, Z.Q.; Zhang, Y.W.; Liu, B.; Cui, Y.Q. The solubility and conformational characteristics of porcine myosin as affected by the presence of L-Lysine and L-histidine. Food Chem. 2015, 170, 212–217. [Google Scholar]
- Takai, E.; Yoshizawa, S.; Ejima, D.; Arakawa, T.; Shiraki, K. Synergistic solubilization of porcine myosin in physiological salt solution by arginine. Int. J. Biol. Macromol. 2013, 62, 647–651. [Google Scholar] [CrossRef] [Green Version]
- Hayakawa, T.; Ito, T.; Wakamatsu, J.; Nishimura, T.; Hattori, A. Myosin filament depolymerizes in a low ionic strength solution containing L-histidine. Meat Sci. 2010, 84, 742–746. [Google Scholar] [CrossRef]
- Hayakawa, T.; Ito, T.; Wakamatsu, J.; Nishimura, T.; Hattori, A. Myosin is solubilized in a neutral and low ionic strength solution containing L-histidine. Meat Sci. 2009, 82, 151–154. [Google Scholar] [CrossRef]
- Chen, X.; Zou, Y.; Han, M.; Pan, L.; Xing, T.; Xu, X.; Zhou, G. Solubilisation of myosin in a solution of low ionic strength L-histidine: Significance of the imidazole ring. Food Chem. 2016, 196, 42–49. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, X.; Peng, Z.; Jamali, M.A. A review of recent progress in reducing NaCl content in meat and fish products using basic amino acids. Trends Food Sci. Technol. 2022, 119, 215–226. [Google Scholar] [CrossRef]
- Huff-Lonergan, E.; Mitsuhashi, T.; Beekman, D.D.; Parrish, F.C.; Olson, D.G.; Robson, R.M. Proteolysis of specific muscle structural proteins by µ-calpain at low pH and temperature is similar to degradation in postmortem bovine muscle. J. Anim. Sci. 1996, 74, 993–1008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.; Xiong, Y.L.; Chen, J. Protein Oxidation at Different Salt Concentrations Affects the Cross-Linking and Gelation of Pork Myofibrillar Protein Catalyzed by Microbial Transglutaminase. J. Food Sci. 2013, 78, C823–C831. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Lian, H.; Jia, H.; Li, S.; Hao, R.; Wang, Y. Ultrasound treatment modified the functional mode of gallic acid on properties of fish myofibrillar protein. Food Chem. 2020, 320, 126637. [Google Scholar] [CrossRef] [PubMed]
- Greenfield, N.J. Using circular dichroism spectra to estimate protein secondary structure. Nat. Protoc. 2007, 1, 2876–2890. [Google Scholar] [CrossRef]
- Kristinsson, H.G.; Hultin, H.O. Changes in conformation and subunit assembly of cod myosin at low and high pH and after subsequent refolding. J. Agric. Food Chem. 2003, 51, 7187–7196. [Google Scholar] [CrossRef]
- Offer, G.; Trinick, J. On the mechanism of water holding in meat: The swelling and shrinking of myofibrils. Meat Sci. 1983, 8, 245–281. [Google Scholar] [CrossRef]
- Li, S.; Li, L.; Zhu, X.; Ning, C.; Cai, K.; Zhou, C. Conformational and charge changes induced by L-Arginine and L-Lysine increase the solubility of chicken myosin. Food Hydrocoll. 2019, 89, 330–336. [Google Scholar] [CrossRef]
- Xiong, Y.L.; Lou, X.; Wang, C.; Moody, W.G.; Harmon, R.J. Protein extraction from chicken myofibrils irrigated with various polyphosphate and NaCl solutions. J. Food Sci. 2000, 65, 96–100. [Google Scholar] [CrossRef]
- Li, S.; Zheng, Y.; Xu, P.; Zhu, X.; Zhou, C. L-Lysine and L-arginine inhibit myosin aggregation and interact with acidic amino acid residues of myosin: The role in increasing myosin solubility. Food Chem. 2018, 242, 22–28. [Google Scholar] [CrossRef]
- Nakasawa, T.; Takahashi, M.; Matsuzawa, F.; Aikawa, S.; Togashi, Y.; Saitoh, T.; Yamagishi, A.; Yazawa, M. Critical Regions for Assembly of Vertebrate Nonmuscle Myosin II. Biochemistry 2004, 44, 174–183. [Google Scholar] [CrossRef]
- Puolanne, E.; Halonen, M. Theoretical aspects of water-holding in meat. Meat Sci. 2010, 86, 151–165. [Google Scholar] [CrossRef] [PubMed]
- Saleem, R.; Ahmad, R. Effect of ultrasonication on secondary structure and heat induced gelation of chicken myofibrils. J. Food Sci. Technol. 2016, 53, 3340–3348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, T.; Yuan, L.; Mu, J.; Gao, R. The effect of Arginine, Lysine and Histidine in the myosin secondary structure by circular dichroism and Raman spectroscopy. CyTA-J. Food 2019, 17, 656–660. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Zhang, H.; Liu, Q.; Chen, Q.; Kong, B. Solubilization and stable dispersion of myofibrillar proteins in water through the destruction and inhibition of the assembly of filaments using high-intensity ultrasound. Ultrason. Sonochem. 2020, 67, 105160. [Google Scholar] [CrossRef] [PubMed]
- King, L.; Lehrer, S.S. Thermal Unfolding of Myosin Rod and Light Meromyosin: Circular Dichroism and Tryptophan Fluorescence Studies. Biochemistry 1989, 28, 3498–3502. [Google Scholar] [CrossRef]
- Chang, Y.; Ludescher, R.D. Quenching of tryptophan fluorescence in skeletal myosin rod. Proc. SPIE 1992, 1640, 159–166. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Ludescher, R.D. Tryptophan photophysics in rabbit skeletal myosin rod. Biophys. Chem. 1994, 49, 113–126. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Wang, W.; Wang, S.; Shen, Y.; Pan, J.; Dong, X.; Li, S. The Solubility and Structures of Porcine Myofibrillar Proteins under Low-Salt Processing Conditions as Affected by the Presence of L-Lysine. Foods 2022, 11, 855. https://doi.org/10.3390/foods11060855
Li X, Wang W, Wang S, Shen Y, Pan J, Dong X, Li S. The Solubility and Structures of Porcine Myofibrillar Proteins under Low-Salt Processing Conditions as Affected by the Presence of L-Lysine. Foods. 2022; 11(6):855. https://doi.org/10.3390/foods11060855
Chicago/Turabian StyleLi, Xiuping, Wenhui Wang, Shouyin Wang, Yuqing Shen, Jinfeng Pan, Xiuping Dong, and Shengjie Li. 2022. "The Solubility and Structures of Porcine Myofibrillar Proteins under Low-Salt Processing Conditions as Affected by the Presence of L-Lysine" Foods 11, no. 6: 855. https://doi.org/10.3390/foods11060855
APA StyleLi, X., Wang, W., Wang, S., Shen, Y., Pan, J., Dong, X., & Li, S. (2022). The Solubility and Structures of Porcine Myofibrillar Proteins under Low-Salt Processing Conditions as Affected by the Presence of L-Lysine. Foods, 11(6), 855. https://doi.org/10.3390/foods11060855





