Antifreeze Peptides Preparation from Tilapia Skin and Evaluation of Its Cryoprotective Effect on Lacticaseibacillus rhamnosus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Determination of Antifreeze Activity
2.3. Screening of Proteases
2.4. Optimization of Enzymatic Hydrolysis Conditions
2.5. Determination of Molecular Weight (Mw) Distribution
2.6. Analysis of Amino Acid Composition
2.7. Identification of Peptide Sequences
2.8. Determination of β-GAL and LDH Activities
2.9. Assay of Extracellular Protein
2.10. Scanning Electron Microscopy
2.11. Statistical Analysis
3. Results and Discussion
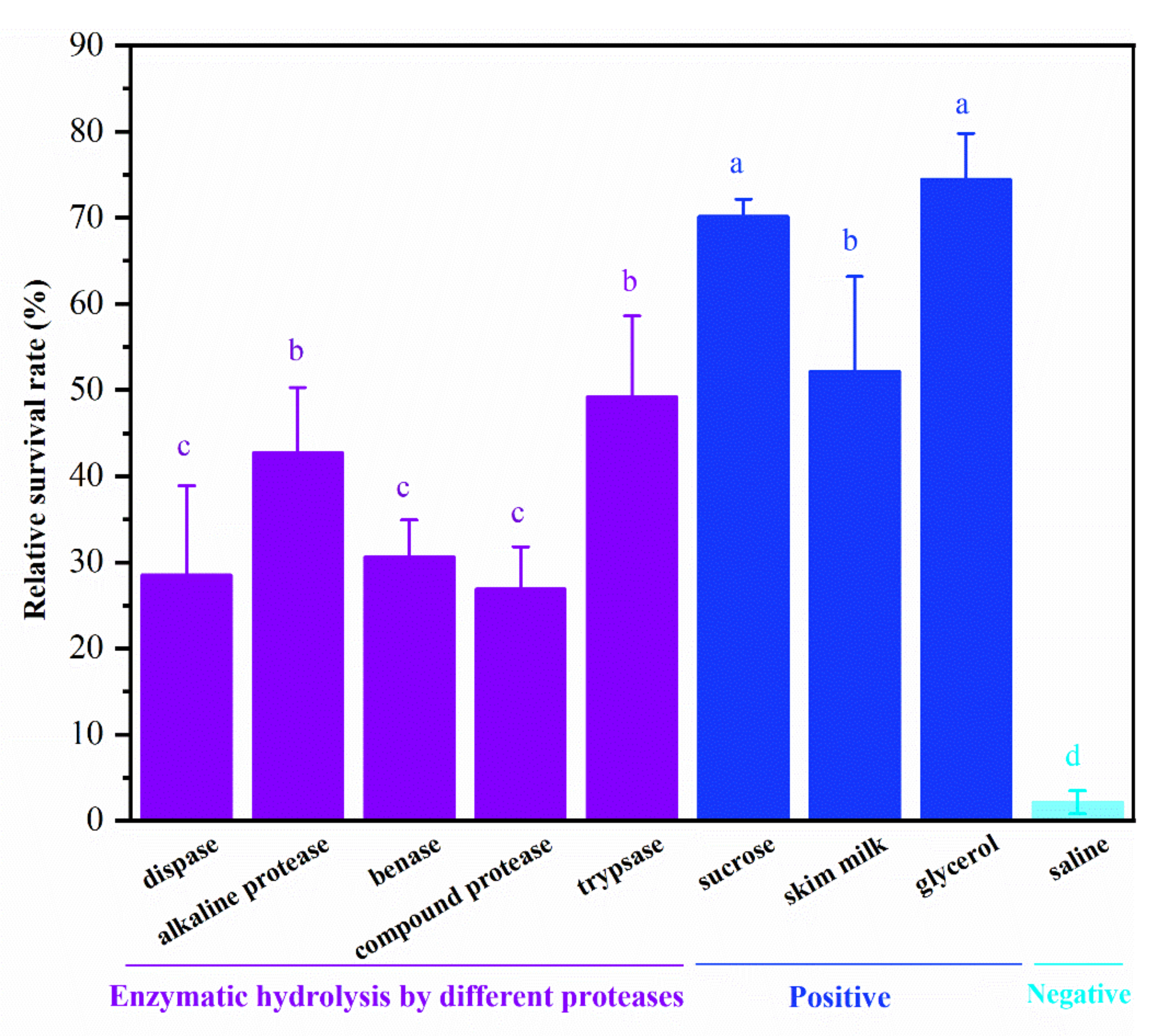
3.1. Selection of Protease
3.2. Optimization of Enzymatic Hydrolysis Conditions
3.2.1. Single-Factor Experiments
3.2.2. Establishing the Fitted Model
3.2.3. Response Surface Analysis
3.2.4. Optimal Conditions and Verification
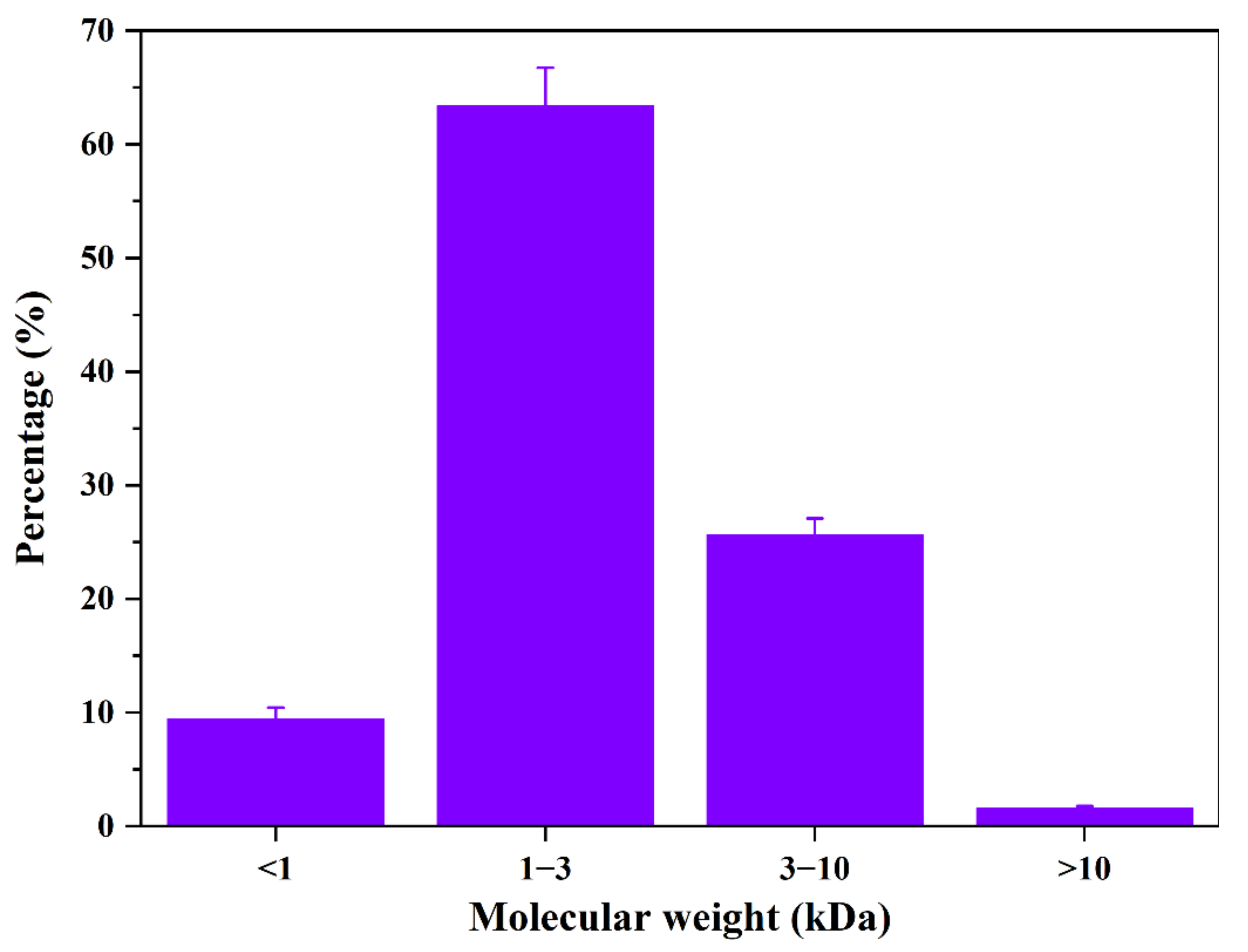
3.3. Mw Distribution and Amino Acid Composition of APT
3.4. Peptide Sequences of APT
3.5. Effect of APT on Cell Metabolic Activity
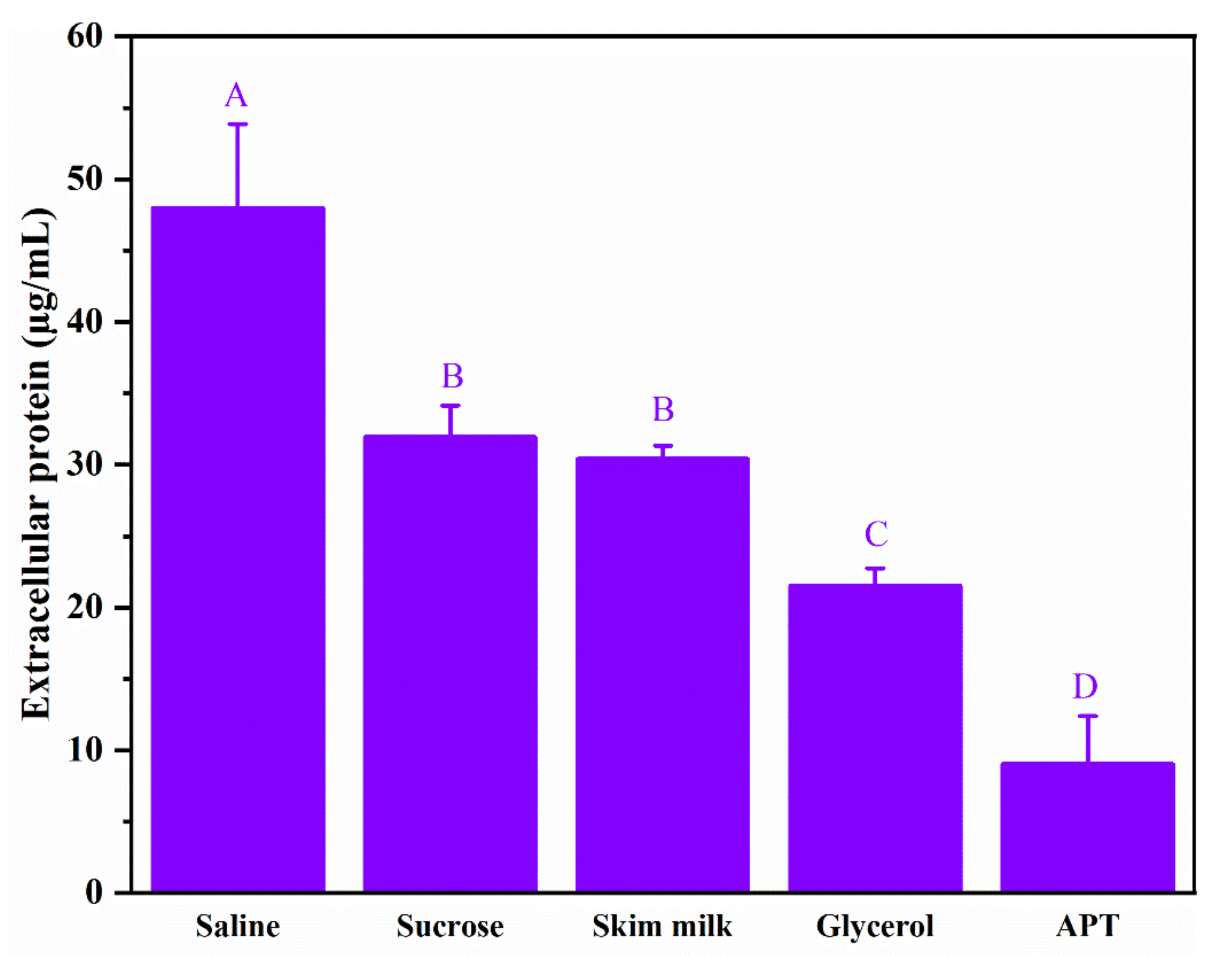
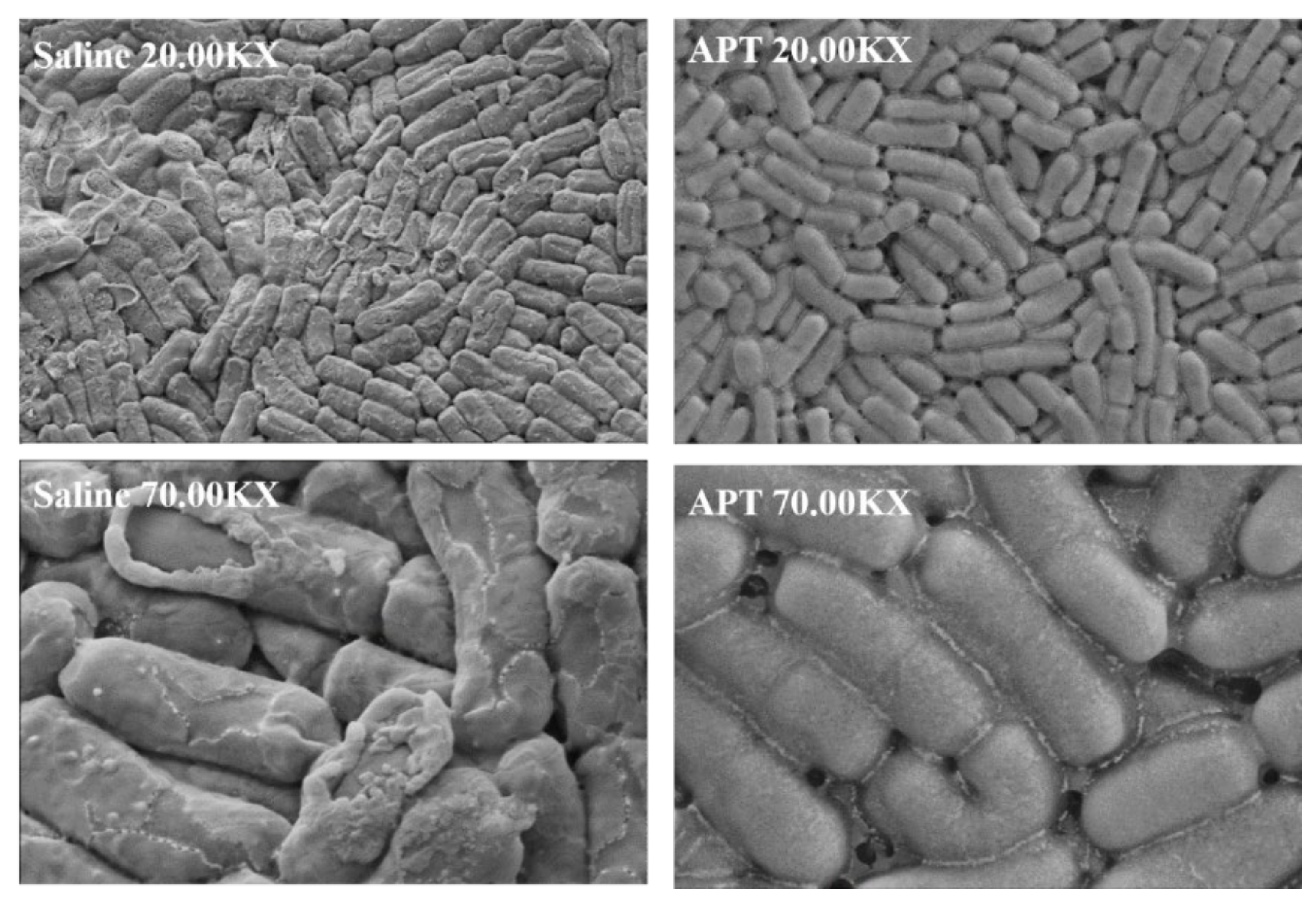
3.6. Effect of APT on Cell Membrane Permeability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chaiyasut, C.; Sivamaruthi, B.S.; Lailerd, N.; Sirilun, S.; Khongtan, S.; Fukngoen, P.; Peerajan, S.; Saelee, M.; Chaiyasut, K.; Kesika, P.; et al. Probiotics Supplementation Improves Intestinal Permeability, Obesity Index and Metabolic Biomarkers in Elderly Thai Subjects: A Randomized Controlled Trial. Foods 2022, 11, 268. [Google Scholar] [CrossRef]
- Harahap, I.A.; Suliburska, J. Probiotics and Isoflavones as a Promising Therapeutic for Calcium Status and Bone Health: A Narrative Review. Foods 2021, 10, 2685. [Google Scholar] [CrossRef] [PubMed]
- Grujović, M.Ž.; Mladenović, K.G.; Semedo-Lemsaddek, T.; Laranjo, M.; Stefanović, O.D.; Kocić-Tanackov, S.D. Advantages and Disadvantages of Non-Starter Lactic Acid Bacteria from Traditional Fermented Foods: Potential Use as Starters or Probiotics. Compr. Rev. Food Sci. Food Safty 2022. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wu, S.; He, J.; He, C.; Wang, P.; Zeng, Q.; Yang, F. Salt-Induced Osmotic Stress Stimulates Selenium Biotransformation in Lactobacillus rhamnosus ATCC 53103. LWT 2020, 131, 109763. [Google Scholar] [CrossRef]
- Rascón, M.P.; Huerta-Vera, K.; Pascual-Pineda, L.A.; Contreras-Oliva, A.; Flores-Andrade, E.; Castillo-Morales, M.; Bonilla, E.; González-Morales, I. Osmotic Dehydration Assisted Impregnation of Lactobacillus rhamnosus in Banana and Effect of Water Activity on the Storage Stability of Probiotic in the Freeze-Dried Product. LWT 2018, 92, 490–496. [Google Scholar] [CrossRef]
- Shori, A.B. The Potential Applications of Probiotics on Dairy and Non-Dairy Foods Focusing on Viability during Storage. Biocatal. Agric. Biotechnol. 2015, 4, 423–431. [Google Scholar] [CrossRef]
- Andrade, R.; Santos, E.; Azoubel, P.; Ribeiro, E. Increased Survival of Lactobacillus rhamnosus ATCC 7469 in Guava Juices with Simulated Gastrointestinal Conditions during Refrigerated Storage. Food Biosci. 2019, 32, 100470. [Google Scholar] [CrossRef]
- Yang, H.; He, M.; Wu, C. Cross Protection of Lactic Acid Bacteria during Environmental Stresses: Stress Responses and Underlying Mechanisms. LWT 2021, 144, 111203. [Google Scholar] [CrossRef]
- Chen, X.; Wu, J.; Li, L.; Wang, S.Y. Cryoprotective Activity and Action Mechanism of Antifreeze Peptides Obtained from Tilapia Scales on Streptococcus thermophilus during Cold Stress. J. Agric. Food Chem. 2019, 67, 1918–1926. [Google Scholar] [CrossRef]
- Chen, X.; Wu, J.; Cai, X.; Wang, S. Production, Structure–Function Relationships, Mechanisms, and Applications of Antifreeze Peptides. Compr. Rev. Food Sci. Food Safty 2020, 20, 542–562. [Google Scholar] [CrossRef]
- Tsai, S.; Chong, G.; Meng, P.J.; Lin, C. Sugars as Supplemental Cryoprotectants for Marine Organisms. Rev. Aquac. 2018, 10, 703–715. [Google Scholar] [CrossRef]
- Chen, X.; Wu, J.; Li, X.; Yang, F.; Huang, D.; Huang, J.; Wang, S.; Guyonnet, V. Snow Flea Antifreeze Peptide for Cryopreservation of Lactic Acid Bacteria. NPJ Sci. Food 2022, 6, 10. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.; Fayter, A.E.R.; Gibson, M.I. Ice Recrystallization Inhibiting Polymers Enable Glycerol-Free Cryopreservation of Microorganisms. Biomacromolecules 2018, 19, 3371–3376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Wang, S. Cryoprotective Effect of Antifreeze Glycopeptide Analogues Obtained by Nonenzymatic Glycation on Streptococcus thermophilus and Its Possible Action Mechanism. Food Chem. 2019, 288, 239–247. [Google Scholar] [CrossRef]
- Wang, W.; Chen, M.; Wu, J.; Wang, S. Hypothermia Protection Effect of Antifreeze Peptides from Pigskin Collagen on Freeze-Dried Streptococcus thermophiles and Its Possible Action Mechanism. LWT Food Sci. Technol. 2015, 63, 878–885. [Google Scholar] [CrossRef]
- Wang, S.; Agyare, K.; Damodaran, S. Optimisation of Hydrolysis Conditions and Fractionation of Peptide Cryoprotectants from Gelatin Hydrolysate. Food Chem. 2009, 115, 620–630. [Google Scholar] [CrossRef]
- Zhang, J.; Du, G.C.; Zhang, Y.; Liao, X.Y.; Wang, M.; Li, Y.; Chen, J. Glutathione Protects Lactobacillus sanfranciscensis against Freeze-Thawing, Freeze-Drying, and Cold Treatment. Appl. Environ. Microbiol. 2010, 76, 2989–2996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Wu, J.H.; Li, L.; Wang, S.Y. The Cryoprotective Effects of Antifreeze Peptides from Pigskin Collagen on Texture Properties and Water Mobility of Frozen Dough Subjected to Freeze–Thaw Cycles. Eur. Food Res. Technol. 2017, 243, 1149–1156. [Google Scholar] [CrossRef]
- Chen, X.; Li, L.; Yang, F.; Wu, J.; Wang, S. Effects of Gelatin-Based Antifreeze Peptides on Cell Viability and Oxidant Stress of Streptococcus thermophilus during Cold Stage. Food Chem. Toxicol. 2020, 136, 111056. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, J.; Chen, L.; Zhou, Y.; Wu, J. Preparation, Isolation and Hypothermia Protection Activity of Antifreeze Peptides from Shark Skin Collagen. LWT Food Sci. Technol. 2014, 55, 210–217. [Google Scholar] [CrossRef]
- Jiang, J.; Zhou, F.; Xian, C.; Shi, Y.; Wang, X. Effects of Radio Frequency Tempering on the Texture of Frozen Tilapia Fillets. Foods 2021, 10, 2663. [Google Scholar] [CrossRef] [PubMed]
- Nie, Y.; Chen, J.; Xu, J.; Zhang, Y.; Yang, M.; Yang, L.; Wang, X.; Zhong, J. Vacuum Freeze-Drying of Tilapia Skin Affects the Properties of Skin and Extracted Gelatins. Food Chem. 2022, 374, 131784. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Gao, Y.; Chen, J.; Liu, R. Identification and Release Kinetics of Peptides from Tilapia Skin Collagen during Alcalase Hydrolysis. Food Chem. 2022, 378, 132089. [Google Scholar] [CrossRef]
- Chen, J.; Sun, S.; Li, Y.; Liu, R. Proteolysis of Tilapia Skin Collagen: Identification and Release Behavior of ACE-Inhibitory Peptides. LWT 2021, 139, 110502. [Google Scholar] [CrossRef]
- Chen, X.; Shi, X.; Cai, X.; Yang, F.; Li, L.; Wu, J.; Wang, S. Ice-Binding Proteins: A Remarkable Ice Crystal Regulator for Frozen Foods. Crit. Rev. Food Sci. Nutr. 2021, 61, 3436–3449. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.Y.; Hsieh, C.H.; Hung, C.C.; Jao, C.L.; Chen, M.C.; Hsu, K.C. Fish Skin Gelatin Hydrolysates as Dipeptidyl Peptidase IV Inhibitors and Glucagon-like Peptide-1 Stimulators Improve Glycaemic Control in Diabetic Rats: A Comparison between Warm- and Cold-Water Fish. J. Funct. Foods 2015, 19, 330–340. [Google Scholar] [CrossRef]
- He, Y.; Bu, L.; Xie, H.; Liang, G. Antioxidant Activities and Protective Effects of Duck Embryo Peptides against H2O2-Induced Oxidative Damage in HepG2 Cells. Poult. Sci. 2019, 98, 7118–7128. [Google Scholar] [CrossRef]
- Wiechelman, K.J.; Braun, R.D.; Fitzpatrick, J.D. Investigation of the Bicinchoninic Acid Protein Assay: Identification of the Groups Responsible for Color Formation. Anal. Biochem. 1988, 175, 231–237. [Google Scholar] [CrossRef]
- Wu, J.; Rong, Y.; Wang, Z.; Zhou, Y.; Wang, S.; Zhao, B. Isolation and Characterisation of Sericin Antifreeze Peptides and Molecular Dynamics Modelling of Their Ice-Binding Interaction. Food Chem. 2015, 174, 621–629. [Google Scholar] [CrossRef]
- Halim, N.R.A.; Yusof, H.M.; Sarbon, N.M. Functional and Bioactive Properties of Fish Protein Hydolysates and Peptides: A Comprehensive Review. Trends Food Sci. Technol. 2016, 51, 24–33. [Google Scholar] [CrossRef]
- Li, L.; Wu, J.H.; Zhang, L.; Chen, X.; Wu, Y.; Liu, J.; Geng, X.; Wang, Z.W.; Wang, S.Y. Investigation of the Physiochemical Properties, Cryoprotective Activity and Possible Action Mechanisms of Sericin Peptides Derived from Membrane Separation. LWT 2017, 77, 532–541. [Google Scholar] [CrossRef]
- Kim, J.S.; Damodaran, S.; Yethiraj, A. Retardation of Ice Crystallization by Short Peptides. J. Phys. Chem. A 2009, 113, 4403–4407. [Google Scholar] [CrossRef]
- Damodaran, S.; Wang, S. Ice Crystal Growth Inhibition by Peptides from Fish Gelatin Hydrolysate. Food Hydrocoll. 2017, 70, 46–56. [Google Scholar] [CrossRef]
- Zhang, L.; Jin, Q.; Luo, J.; Wu, J.; Wang, S.; Wang, Z.; Gong, S.; Zhang, W.; Lan, X. Intracellular Expression of Antifreeze Peptides in Food Grade Lactococcus Lactis and Evaluation of Their Cryoprotective Activity. J. Food Sci. 2018, 83, 1311–1320. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Wang, P.; Chen, Y.; Lin, J.; Zheng, B.; Zeng, H.; Zhang, Y. Preparation, Primary Structure and Antifreeze Activity of Antifreeze Peptides from Scomberomorus niphonius Skin. LWT 2019, 101, 670–677. [Google Scholar] [CrossRef]
- Damodaran, S. Inhibition of Ice Crystal Growth in Ice Cream Mix by Gelatin Hydrolysate. J. Agric. Food Chem. 2007, 55, 10918–10923. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, Y.; Yu, J.; Wang, F.; Li, X. Identification and Characterization of Cryoprotective Peptides Extracted from Silver Carp (Hypophthalmichthys molitrix) Hydrolysates. Int. J. Food Prop. 2019, 22, 1011–1023. [Google Scholar] [CrossRef] [Green Version]
- Stamov, D.R.; Müller, A.; Wegrowski, Y.; Brezillon, S.; Franz, C.M. Quantitative Analysis of Type I Collagen Fibril Regulation by Lumican and Decorin Using AFM. J. Struct. Biol. 2013, 183, 394–403. [Google Scholar] [CrossRef]
- Ustok, F.I.; Tari, C.; Harsa, S. Biochemical and Thermal Properties of β-Galactosidase Enzymes Produced by Artisanal Yoghurt Cultures. Food Chem. 2010, 119, 1114–1120. [Google Scholar] [CrossRef] [Green Version]
- Heljo, V.P.; Jouppila, K.; Hatanpää, T.; Juppo, A.M. The Use of Disaccharides in Inhibiting Enzymatic Activity Loss and Secondary Structure Changes in Freeze-Dried β-Galactosidase during Storage. Pharm. Res. 2011, 28, 540–552. [Google Scholar] [CrossRef]


| Source | Sum of Squares | df | Mean Square | F Value | p-Value Prob > F | Significant |
|---|---|---|---|---|---|---|
| Model | 863.9864 | 14 | 61.71332 | 4.32713 | 0.0048 | * |
| A | 387.717 | 1 | 387.717 | 27.18541 | 0.0001 | ** |
| B | 2.585408 | 1 | 2.585408 | 0.18128 | 0.6767 | |
| C | 18.97567 | 1 | 18.97567 | 1.33051 | 0.2680 | |
| D | 95.93707 | 1 | 95.93707 | 6.726785 | 0.0212 | * |
| AB | 12.70922 | 1 | 12.70922 | 0.891128 | 0.3612 | |
| AC | 24.6016 | 1 | 24.6016 | 1.724981 | 0.2102 | |
| AD | 6.0516 | 1 | 6.0516 | 0.424318 | 0.5253 | |
| BC | 39.12502 | 1 | 39.12502 | 2.743315 | 0.1199 | |
| BD | 2.839225 | 1 | 2.839225 | 0.199077 | 0.6623 | |
| CD | 4.41 | 1 | 4.41 | 0.309214 | 0.5869 | |
| A2 | 204.7503 | 1 | 204.7503 | 14.3564 | 0.0020 | * |
| B2 | 92.11029 | 1 | 92.11029 | 6.458463 | 0.0235 | * |
| C2 | 57.05622 | 1 | 57.05622 | 4.00059 | 0.0653 | |
| D2 | 16.36413 | 1 | 16.36413 | 1.147397 | 0.3022 | |
| Residual | 199.6673 | 14 | 14.26195 | |||
| Lack of Fit | 181.0135 | 10 | 18.10135 | 3.881537 | 0.1016 | not significant |
| Pure Error | 18.6538 | 4 | 4.66345 | |||
| Cor Total | 1063.654 | 28 |
| Amino Acid | Molar Percentage (%) | Amino Acid | Molar Percentage (%) |
|---|---|---|---|
| Asp | 5.12 | Met | 1.08 |
| Thr | 2.83 | Ile | 1.11 |
| Ser | 3.94 | Leu | 2.69 |
| Glu | 8.64 | Tyr | 0.50 |
| Pro | 12.43 | Phe | 1.50 |
| Gly | 35.45 | Lys | 2.76 |
| Ala | 13.29 | His | 0.62 |
| Cys | 0.05 | Arg | 5.80 |
| Val | 2.20 |
| NO. | Peptide | Length | Mass | m/z | −10lgP | Protein Name |
|---|---|---|---|---|---|---|
| 1 | GPTGEIGATGLAGAR | 15 | 1326.689 | 443.2371 | 45.47 | collagen alpha-2(I) chain precursor |
| 2 | RGPTGEIGATGLAGAR | 16 | 1482.79 | 371.7053 | 49.78 | collagen alpha-2(I) chain precursor |
| 3 | GLSGNIGFPGPK | 12 | 1158.603 | 580.3095 | 24.14 | collagen alpha-2(I) chain precursor |
| 4 | SPAMPVPGPMGPMGPR | 16 | 1577.752 | 526.9243 | 52.54 | collagen alpha-1(I) chain precursor |
| 5 | WLIIDNNQITNAK | 13 | 1541.82 | 514.9476 | 43.46 | lumican |
| 6 | SSGPPVPGPIGPMGPR | 16 | 1501.771 | 501.5981 | 52.57 | collagen, type I, alpha 1b precursor |
| 7 | GLQGFVGLPGSR | 12 | 1202.641 | 602.3278 | 26.16 | collagen alpha-2(I) chain precursor |
| 8 | LVDSGIPAGVF | 11 | 1073.576 | 1074.583 | 32.6 | lumican |
| 9 | GPPGPMGPPGLAGAPGEPGR | 20 | 1783.867 | 892.9423 | 56.6 | collagen alpha-1(I) chain precursor |
| 10 | FMGPLNY | 7 | 840.384 | 841.3916 | 35.48 | lumican |
| 11 | GLTGPIGLPGPAGSPGDKGEPGAQGPVGPSGAR | 33 | 2939.474 | 1470.744 | 58.95 | collagen alpha-1(I) chain precursor |
| 12 | GPTGEIGATGL | 11 | 971.4924 | 972.4996 | 39.71 | collagen alpha-2(I) chain precursor |
| 13 | RGPTGEIGATGL | 12 | 1127.594 | 376.8716 | 30.39 | collagen alpha-2(I) chain precursor |
| 14 | WEVQPVTF | 8 | 1004.497 | 1005.504 | 36.73 | decorin precursor |
| 15 | FPSGLL | 6 | 632.3533 | 633.361 | 22.69 | lumican |
| 16 | GFPGLAGPVGEPGK | 14 | 1297.667 | 649.8418 | 38.04 | collagen alpha-1(I) chain precursor |
| 17 | PGPGPMGLM | 9 | 855.3983 | 856.4062 | 36.06 | collagen alpha-2(I) chain precursor |
| 18 | SPAMPVPGPM | 10 | 982.4616 | 983.4692 | 46.85 | collagen alpha-1(I) chain precursor |
| 19 | GDNGPPGLTGFPGAAGR | 17 | 1571.733 | 786.8741 | 21.81 | collagen alpha-2(I) chain precursor |
| 20 | MVQPQEKAPDPFR | 13 | 1540.734 | 771.3766 | 30.45 | collagen alpha-1(I) chain precursor |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, Y.; Li, W.; Liu, Y.; Jiang, W. Antifreeze Peptides Preparation from Tilapia Skin and Evaluation of Its Cryoprotective Effect on Lacticaseibacillus rhamnosus. Foods 2022, 11, 857. https://doi.org/10.3390/foods11060857
Zeng Y, Li W, Liu Y, Jiang W. Antifreeze Peptides Preparation from Tilapia Skin and Evaluation of Its Cryoprotective Effect on Lacticaseibacillus rhamnosus. Foods. 2022; 11(6):857. https://doi.org/10.3390/foods11060857
Chicago/Turabian StyleZeng, Yan, Weinan Li, Yu Liu, and Wei Jiang. 2022. "Antifreeze Peptides Preparation from Tilapia Skin and Evaluation of Its Cryoprotective Effect on Lacticaseibacillus rhamnosus" Foods 11, no. 6: 857. https://doi.org/10.3390/foods11060857
APA StyleZeng, Y., Li, W., Liu, Y., & Jiang, W. (2022). Antifreeze Peptides Preparation from Tilapia Skin and Evaluation of Its Cryoprotective Effect on Lacticaseibacillus rhamnosus. Foods, 11(6), 857. https://doi.org/10.3390/foods11060857







