Impact of an Omega-3-Enriched Sheep Diet on the Microbiota and Chemical Composition of Kefalograviera Cheese
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.1.1. Animals and Dietary Treatments
2.1.2. Milk Collection
2.1.3. Kefalograviera Cheese Production
2.2. Microbiological Analysis of Kefalograviera Cheese-Cultured Methodology
Identification of Microbiota by MALDI-TOF MS
2.3. Chemical Analysis
2.3.1. Determination of the Kefalograviera Cheese Fatty Acids Composition
- AI = (C12:0 + (4 × C14:0) + C16:0)/(Σn-3 PUFA + Σn-6 PUFA + Σ MUFA).
- TI = (C14:0 + C16:0 + C18:0)/((0.5 × C18:1) + (0.5 × other MUFA) + (0.5 × Σn-6 PUFA) + (3 × Σn-3 PUFA) + Σn-3 PUFA/Σn-6PUFA).
- DFA = UFA + C18:0;
- OFA = C12:0 + C14:0 + C16:0;
- H/H index = DFA/OFA.
2.3.2. Determination of Kefalograviera Cheese TBARs
2.4. Statistical Analysis
3. Results
4. Discussion
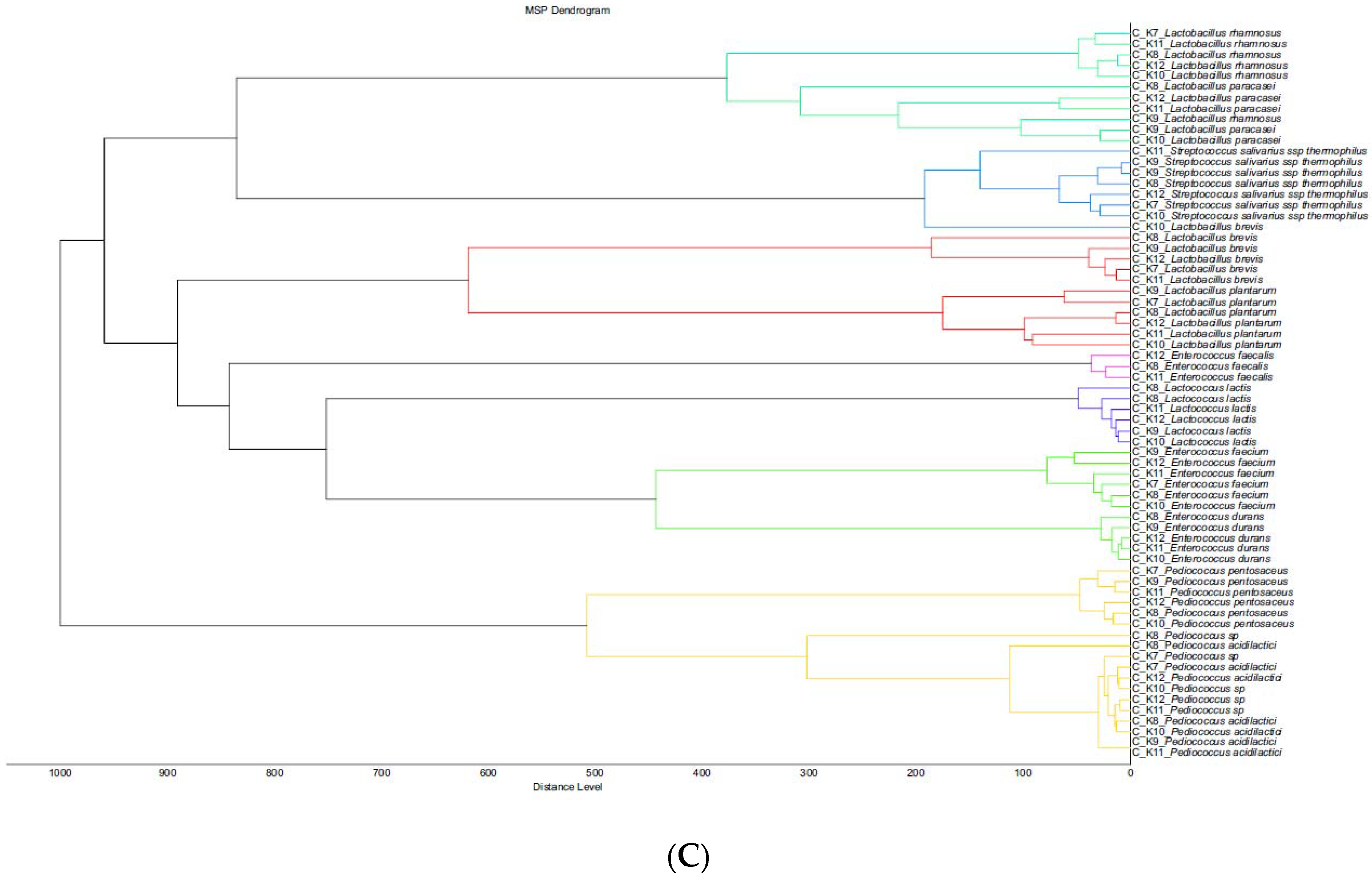
4.1. Microbial Dynamics in Kefalograviera Samples
4.2. Chemical Composition of the Cheeses—TBARs Values
4.3. Fatty Acid Content of the Cheeses
4.4. Health Indexes
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- EU Kefalograviera Profile. Available online: https://ec.europa.eu/info/food-farming-fisheries/food-safety-and-quality/certification/quality-labels/geographical-indications-register/ (accessed on 12 December 2021).
- ELSTAT. Livestock and Livestock Products. 2019. Available online: https://www.statistics.gr/el/statistics/-/publication/SPK33/- (accessed on 1 February 2022).
- Greek Codex Alimentarius. Official Journal of the Republic of Greece; National Printing Office: Athens, Greece, 2003; Volume B. [Google Scholar]
- Fox, P.F.; Guinee, T.P.; Cogan, T.M.; McSweeney, P.L.H. (Eds.) Biochemistry of Cheese Ripening. In Fundamentals of Cheese Science; Springer: New York, NY, USA, 2017; pp. 391–442. [Google Scholar]
- Martini, M.; Liponi, G.; Salari, F. Effect of forage: Concentrate ratio on the quality of ewe’s milk, especially on milk fat globules characteristics and fatty acids composition. J. Dairy Res. 2010, 77, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Bennato, F.; Ianni, A.; Innosa, D.; Grotta, L.; D’Onofrio, A.; Martino, G. Chemical-nutritional characteristics and aromatic profile of milk and related dairy products obtained from goats fed with extruded linseed. Asian-Australas. J. Anim. Sci. 2019, 33, 148–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pulina, G.; Nudda, A.; Battacone, G.; Cannas, A. Effects of nutrition on the contents of fat, protein, somatic cells, aromatic compounds, and undesirable substances in sheep milk. Anim. Feed Sci. Technol. 2006, 131, 255–291. [Google Scholar] [CrossRef]
- Simopoulos, A. An increase in the Omega-6/Omega-3 fatty acid ratio increases the risk for obesity. Nutrients 2016, 8, 128. [Google Scholar] [CrossRef] [Green Version]
- Ponnampalam, E.N.; Hopkins, D.L.; Jacobs, J.L. Increasing omega-3 levels in meat from ruminants under pasture-based systems: An invited review. Sci. Tech. Rev. Off. Int. Epizoot. 2018, 37, 57–70. [Google Scholar] [CrossRef]
- Renes, E.; Gómez-Cortés, P.; de la Fuente, M.A.; Fernández, D.; Tornadijo, M.E.; Fresno, J.M. Effect of forage type in the ovine diet on the nutritional profile of sheep milk cheese fat. J. Dairy Sci. 2020, 103, 63–71. [Google Scholar] [CrossRef] [Green Version]
- Nudda, A.; Battacone, G.; Boaventura Neto, O.; Cannas, A.; Francesconi, A.H.D.; Atzori, A.S.; Pulina, G. Feeding strategies to design the fatty acid profile of sheep milk and cheese. R. Bras. Zootec. 2014, 43, 445–456. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Cortés, P.; Juárez, M.; de la Fuente, M.A. Milk fatty acids and potential health benefits: An updated vision. Trends Food Sci. Technol. 2018, 81, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Field, C.J.; Blewett, H.H.; Proctor, S.; Vine, D. Human health benefits of vaccenic acid. Appl. Physiol. Nutr. Metab. 2009, 34, 979–991. [Google Scholar] [CrossRef]
- Sales-Campos, H.; Souza, P.R.; Peghini, B.C.; da Silva, J.S.; Cardoso, C.R. An overview of the modulatory effects of oleic acid in health and disease. Mini Rev. Med. Chem. 2013, 13, 201–210. [Google Scholar]
- Djordjevic, J.; Ledina, T.; Baltic, M.Z.; Trbovic, D.; Babic, M.; Bulajic, S. Fatty Acid Profile of Milk. In IOP Conference Series: Earth and Environmental Science. Proceedings of the 60th International Meat Industry Conference MEATCON2019; Kopaonik, Serbia, 22–25 September 2019, IOP Publishing: Bristol, UK, 2019; Volume 333, p. 012057. [Google Scholar] [CrossRef]
- Kannan, N.; Rao, A.; Nair, A. Microbial production of omega-3 fatty acids: An overview. J. Appl. Microbiol. 2021, 131, 2114–2130. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, A.C.; Olazábal, L.; Torre, A.; Loperena, L. Antarctic microorganisms as source of the omega-3 polyunsaturated fatty acids. World J. Microbiol. Biotechnol. 2014, 30, 1869–1878. [Google Scholar] [CrossRef] [PubMed]
- Grygier, A.; Myszka, K.; Juzwa, W.; Białas, W.; Rudzińska, M. Galactomyces geotrichum mold isolated from a traditional fried cottage cheese produced omega-3 fatty acids. Int. J. Food Microbiol. 2020, 319, 108503. [Google Scholar] [CrossRef] [PubMed]
- Alessandria, V.; Ferrocino, I.; De Filippis, F.; Fontana, M.; Rantsiou, K.; Ercolini, D.; Cocolin, L. Microbiota of an Italian Grana-like cheese during manufacture and ripening, unraveled by 16S rRNA-based approaches. Appl. Environ. Microbiol. 2016, 82, 3988–3995. [Google Scholar] [CrossRef] [Green Version]
- Tzora, A.; Nelli, A.; Voidarou, C.; Fthenakis, G.; Rozos, G.; Theodorides, G.; Bonos, E.; Skoufos, I. Microbiota “Fingerprint” of Greek Feta Cheese through Ripening. Appl. Sci. 2021, 11, 5631. [Google Scholar] [CrossRef]
- Tsigkrimani, M.; Bakogianni, M.; Paramithiotis, S.; Bosnea, L.; Pappa, E.; Drosinos, E.H.; Skandamis, P.N.; Mataragas, M. Microbial Ecology of Artisanal Feta and Kefalograviera Cheeses, Part I: Bacterial Community and Its Functional Characteristics with Focus on Lactic Acid Bacteria as Determined by Culture-Dependent Methods and Phenotype Microarrays. Microorganisms 2022, 10, 161. [Google Scholar] [CrossRef]
- Renes, E.; Gómez-Cortés, P.; de la Fuente, M.A.; Linares, D.M.; Tornadijo, M.E.; Fresno, J.M. CLA-producing adjunct cultures improve the nutritional value of sheep cheese fat. Food Res. Int. 2019, 116, 819–826. [Google Scholar] [CrossRef] [Green Version]
- Premier Nutrition. Premier Atlas 2014. Ingredients Matrix; Premier Nutrition: Brereton, UK, 2014; Available online: https://www.premiernutrition.co.uk/news-insight/new-global-animal-feed-ingredients-matrix-launched (accessed on 1 February 2022).
- Tilocca, B.; Costanzo, N.; Morittu, V.M.; Spina, A.A.; Soggiu, A.; Britti, D.; Roncada, P.; Piras, C. Milk microbiota: Characterization methods and role in cheese production. J. Proteom. 2020, 210, 103534. [Google Scholar] [CrossRef]
- Rau, J.; Eisenberg, T.; Männig, A.; Wind, C.; Lasch, P.; Sting, R. MALDI-UP—An Internet Platform for the Exchange of MALDI-TOF Mass Spectra. Asp. Food Control. Anim. Health 2016, 1, 1–17. Available online: https://ejournal.cvuas.de/issue201601.asp (accessed on 1 February 2022).
- Currò, S.; Manuelian, C.L.; Penasa, M.; Cassandro, M.; De Marchi, M. Technical note: Feasibility of near infrared transmittance spectroscopy to predict cheese ripeness. J. Dairy Sci. 2017, 100, 8759–8763. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification, Canadian. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [Green Version]
- Standard 15884; Milk Fat. Preparation of Fatty Acid Methyl Esters. ISO (International Organization for Standardization): Geneva, Switzerland, 2002.
- Papaloukas, L.; Sinapis, E.; Arsenos, G.; Kyriakou, G.; Basdagianni, Z. Effect of season on fatty acid and terpene profiles of milk from Greek sheep raised under a semi-extensive production system. J. Dairy Res. 2016, 83, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Ulbricht, T.L.V.; Southgate, D.A.T. Coronary heart disease: Seven dietary factors. Lancet 1991, 338, 982–992. [Google Scholar] [CrossRef]
- Paszczyk, B.; Łuczyńska, J. The Comparison of Fatty Acid Composition and Lipid Quality Indices in Hard Cow, Sheep, and Goat Cheeses. Foods 2020, 9, 1667. [Google Scholar] [CrossRef]
- Osmari, E.K.; Cecato, U.; Macedo, F.A.F.; Souza, N.E. Nutritional quality indices of milk fat from goats on diets supplemented with different roughages. Small Rumin. Res. 2011, 98, 128–132. [Google Scholar] [CrossRef]
- Papastergiadis, A.; Mubiru, E.; van Langenhove, H.; De Meulenaer, B. Malondialdehyde measurement in oxidized foods: Evaluation of the spectrophotometric thiobarbituric acid reactive substances (TBARS) test in various foods. J. Agric. Food Chem. 2012, 60, 9589–9594. [Google Scholar] [CrossRef]
- Sinanoglou, V.J.; Koutsouli, P.; Fotakis, C.; Sotiropoulou, G.; Cavouras, D.; Bizelis, I. Assessment of lactation stage and breed effect on sheep milk fatty acid profile and lipid quality indices. Dairy Sci. Technol. 2015, 95, 509–531. [Google Scholar] [CrossRef] [Green Version]
- Simopoulos, A.P. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp. Biol. Med. 2008, 233, 674–688. [Google Scholar] [CrossRef]
- Zeppa, G.; Giordano, M.; Gerbi, V.; Arlorio, M. Fatty acid composition of Piedmont “Ossolano” cheese. Dairy Sci. Technol. 2003, 83, 167–173. [Google Scholar] [CrossRef] [Green Version]
- Formaggioni, P.; Malacarne, M.; Franceschi, P.; Zucchelli, V.; Faccia, M.; Battelli, G.; Brasca, M.; Summer, A. Characterization of Formaggella della Valle di Scalve Cheese Produced from Cows Reared in Valley Floor Stall or in Mountain Pasture: Fatty Acids Profile and Sensory Properties. Foods 2020, 9, 383. [Google Scholar] [CrossRef] [Green Version]
- Fragkou, I.A.; Skoufos, J.; Cripps, P.J.; Kyriazakis, I.; Papaioannou, N.; Boscos, C.M.; Tzora, A.; Fthenakis, G.C. Differences in susceptibility to Mannheimia haemolytica-associated mastitis between two breeds of dairy sheep. J. Dairy Res. 2007, 74, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Mayo, B.; Rodríguez, J.; Vázquez, L.; Flórez, A.B. Microbial Interactions within the Cheese Ecosystem and Their Application to Improve Quality and Safety. Foods 2021, 10, 602. [Google Scholar] [CrossRef] [PubMed]
- Bojanic Rasovic, M.; Mayrhofer, S.; Martinovic, A.; Dürr, K.; Domig, K.J. Lactococci of local origin as potential starter cultures for traditional Montenegrin cheese production. Food Technol. Biotechnol. 2017, 55, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Santiago-López, L.; Aguilar-Toalá, J.E.; Hernández-Mendoza, A.; Vallejo-Cordoba, B.; Liceaga, A.M.; González-Córdova, A.F. Invited review: Bioactive compounds produced during cheese ripening and health effects associated with aged cheese consumption. J. Dairy Sci. 2018, 101, 3742–3757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.; Wang, C.L.; Sun, Y.; Li, A.L.; Liu, F.; Meng, X.C. Microencapsulation of Lactobacillus rhamnosus GG by Transglutaminase Cross-Linked Soy Protein Isolate to Improve Survival in Simulated Gastrointestinal Conditions and Yoghurt. J. Food Sci. 2016, 81, M1726–M1734. [Google Scholar] [CrossRef]
- Sgarbi, E.; Lazzi, C.; Tabanelli, G.; Gatti, M.; Neviani, E.; Gardini, F. Nonstarter lactic acid bacteria volatilomes produced using cheese components. J. Dairy Sci. 2013, 96, 4223–4234. [Google Scholar] [CrossRef] [Green Version]
- Hayaloglu, A.A. Cheese: Microbiology of Cheese, Reference Module. In Food Science; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar] [CrossRef]
- Papadopoulou, O.S.; Argyri, A.A.; Varzakis, E.E.; Tassou, C.C.; Chorianopoulos, N.G. Greek functional Feta cheese: Enhancing quality and safety using a Lactobacillus plantarum strain with probiotic potential. Food Microbiol. 2018, 74, 21–33. [Google Scholar] [CrossRef]
- Praagman, J.; Vissers, L.E.T.; Mulligan, A.A.; Laursen, A.S.D.; Beulens, J.W.J.; van der Schouw, Y.T.; Wareham, N.J.; Hansen, C.P.; Khaw, K.T.; Jakobsen, M.U.; et al. Consumption of individual saturated fatty acids and the risk of myocardial infarction in a UK and a Danish cohort. Int. J. Cardiol. 2019, 279, 18–26. [Google Scholar] [CrossRef] [Green Version]
- Caroprese, M.; Albenzio, M.; Bruno, A.; Fedele, V.; Santillo, A.; Sevi, A. Effect of solar radiation and flaxseed supplementation on milk production and fatty acid profile of lactating ewes under high ambient temperature. J. Dairy Sci. 2011, 94, 3856–3867. [Google Scholar] [CrossRef] [Green Version]
- Parapouli, M.; Delbès-Paus, C.; Kakouri, A.; Koukkou, A.I.; Montel, M.C.; Samelis, J. Characterization of a wild, novel nisin a-producing Lactococcus strain with an L. lactis subsp. cremoris genotype and an L. lactis subsp. lactis phenotype, isolated from Greek raw milk. Appl. Environ. Microbiol. 2013, 79, 3476–3484. [Google Scholar] [CrossRef] [Green Version]
- Pappa, E.; Kondyli, E.; Bosnea, L.; Mataragas, M.; Giannouli, A.; Tsiraki, M. Semi-Industrial Production of Kashkaval of Pindos Cheese Using Sheep or a Mixture of Sheep-Goat Milk and the Utilization of the Whey for Manufacturing Urda Cheese. Foods 2020, 9, 736. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, M.; Corsetti, A.; Tosti, N.; Rossi, J.; Corbo, M.R.; Gobbetti, M. Characterization of non-starter lactic acid bacteria from Italian ewe cheeses based on phenotypic, genotypic, and cell wall protein analyses. Appl. Environ. Microbiol. 2001, 67, 2011–2020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Bryan, C.A.; Koo, O.K.; Sostrin, M.L.; Ricke, S.C.; Crandall, P.G.; Johnson, M.G. Chapter 15—Characteristics of Bacteriocins and Use as Food Antimicrobials in the United States. In Food and Feed Safety Systems and Analysis; Ricke, S.C., Atungulu, G.G., Rainwater, C., Park, S.H., Eds.; Academic Press: Hong Kong, China, 2018; pp. 273–286. [Google Scholar] [CrossRef]
- Papagianni, M. Ribosomally synthesized peptides with antimicrobial properties: Biosynthesis, structure, function, and applications. Biotechnol. Adv. 2003, 21, 465–499. [Google Scholar] [CrossRef]
- Voidarou, C.; Tzora, A.; Skoufos, I.; Vassos, D.; Gallogiannis, K.; Alexopoulos, A.; Berzitzoglou, E. Experimental Effect of Ozone upon Some Indicator Bacteria for Preservation of an Ecologically Protected Watery System. Water Air Soil Pollut. 2007, 181, 161–171. [Google Scholar] [CrossRef]
- Foulquie Moreno, M.R.; Sarantinopoulos, P.; Tsakalidou, E.; De Vuyst, L. The role and application of enterococci in food and health. Int. J. Food Microbiol. 2006, 106, 1–24. [Google Scholar] [CrossRef]
- Suzzi, G.; Caruso, M.; Gardini, F.; Lombardi, A.; Vannini, L.; Guerzoni, M.; Andrighetto, C.; Lanorte, M.T. A survey of the enterococci isolated from an artisanal Italian goat’s cheese (semicotto caprino). J. Appl. Microbiol. 2000, 89, 267–274. [Google Scholar] [CrossRef] [Green Version]
- Jafari, B.; Rezaie, A.; Alizadeh, S. Isolation and identification of potentially probiotic bacteria from traditional dairy products of Ardabil region in Iran. Ann. Biol. Res. 2011, 2, 311–317. Available online: https://www.scholarsresearchlibrary.com/articles/isolation-and-identification-of-potentially-probiotic-bacteria-fromtraditional-dairy-products-of-ardabil-region-in-iran.pdf (accessed on 1 February 2022).
- Terzić-Vidojević, A.; Veljović, K.; Begović, J.; Filipić, B.; Popović, D.; Tolinački, M.; Miljković, M.; Kojić, M.; Golić, N. Diversity and antibiotic susceptibility of autochthonous dairy enterococci isolates: Are they safe candidates for autochthonous starter cultures? Front. Microbiol. 2015, 6, 954. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.B.; Ebersole, J.L. A novel bioactivity of omega-3 polyunsaturated fatty acids and their ester derivatives. Mol. Oral Microbiol. 2010, 25, 75–80. [Google Scholar] [CrossRef]
- Chanda, W.; Joseph, T.P.; Guo, X.F.; Wang, W.D.; Liu, M.; Vuai, M.S.; Padhiar, A.A.; Zhong, M.T. Effectiveness of omega-3 polyunsaturated fatty acids against microbial pathogens. J. Zhejiang Univ. Sci. B 2018, 19, 253–262. [Google Scholar] [CrossRef]
- Pikhtirova, A.; Bujok, J.; Pecka-Kiełb, E.; Zachwieja, A.; Vasil, M.; Elečko, J.; Zigo, F. Fatty acid profile of ewe’s milk infected with Staphylococcus spp. Iran. J. Vet. Res. 2020, 21, 216–220. [Google Scholar] [PubMed]
- Tonamo, A.; Komlósi, I.; Varga, L.; Kačániová, M.; Peles, F. Identification of ovine-associated staphylococci by MALDI-TOF mass spectrometry. Acta Aliment. 2021, 50, 210–218. Available online: https://akjournals.com/view/journals/066/50/2/article-p210.xml (accessed on 1 February 2022). [CrossRef]
- Vasileiou, N.G.C.; Chatzopoulos, D.C.; Cripps, P.J.; Ioannidi, K.S.; Gougoulis, D.A.; Chouzouris, T.M.; Lianou, D.T.; Gonzalez-Valerio, T.C.; Vallverdu, R.G.; Argyros, S.; et al. Evaluation of efficacy of a biofilm-embedded bacteria-based vaccine against staphylococcal mastitis in sheep-A randomized, placebo-controlled field study. J. Dairy Sci. 2019, 102, 9328–9344. [Google Scholar] [CrossRef] [PubMed]
- Branciari, R.; Galarini, R.; Miraglia, D.; Ranucci, D.; Valiani, A.; Giusepponi, D.; Servili, M.; Acuti, G.; Pauselli, M.; Trabalza-Marinucci, M. Dietary Supplementation with Olive Mill Wastewater in Dairy Sheep: Evaluation of Cheese Characteristics and Presence of Bioactive Molecules. Animals 2020, 10, 1941. [Google Scholar] [CrossRef]
- Branciari, R.; Mughetti, L.; Ranucci, D.; Miraglia, D.; Valiani, A.; Acuti, G.; Selvaggini, R.; Trabalza-Marinucci, M. Influence of manufacturing procedure on the compositional and sensory properties of n-3 fatty acid-enriched pecorino cheese. J. Dairy Res. 2014, 81, 455–461. [Google Scholar] [CrossRef]
- Zamora, R.; Hidalgo, F.J. Coordinate contribution of lipid oxidation and Maillard reaction to the nonenzymatic food browning. Crit. Rev. Food Sci. Nutr. 2005, 45, 49–59. [Google Scholar] [CrossRef]
- Fusaro, I.; Giammarco, M.; Odintsov Vaintrub, M.; Chincarini, M.; Manetta, A.C.; Mammi, L.; Palmonari, A.; Formigoni, A.; Vignola, G. Effects of three different diets on the fatty acid profile and sensory properties of fresh Pecorino cheese “Primo Sale”. Asian-Australas. J. Anim. Sci. 2020, 33, 1991–1998. [Google Scholar] [CrossRef] [Green Version]
- De La Fuente, L.F.; Barbosa, E.; Carriedo, J.A.; Gonzalo, C.; Arenas, R.; Fresno, J.M.; San Primitivo, F. Factors influencing variation of fatty acid content in ovine milk. J. Dairy Sci. 2009, 92, 3791–3799. [Google Scholar] [CrossRef] [Green Version]
- Vlaeminck, B.; Fievez, V.; Cabrita, A.; Fonseca, A.; Dewhurst, R. Factors affecting odd- and branched-chain fatty acids in milk: A review. Anim. Feed Sci. Technol. 2006, 131, 389–417. [Google Scholar] [CrossRef]
- Skoufos, I.; Tzora, A.; Giannenas, I.; Karamoutsios, A.; Tsangaris, G.; Fthenakis, G.C. Milk quality characteristics of Boutsiko, Frisarta and Karagouniko sheep breeds reared in the mountainous and semimountainous areas of Western and Central Greece. Int. J. Dairy Technol. 2017, 70, 345–353. [Google Scholar] [CrossRef]
- Park, Y.W.; Juárez, M.; Ramos, M.; Haenlein, G.F.W. Physico-chemical characteristics of goat and sheep milk. Small Rumin. Res. 2007, 68, 88–113. [Google Scholar] [CrossRef] [Green Version]
- Akbaș, A.; Tașҫi, F.; Elmaz, Ỏ.; Saatci, M. Evaluation of Milk Yield and Milk Composition of Honamlı Goats. J. Hell. Vet. Med. Soc. 2021, 72, 2747–2754. [Google Scholar] [CrossRef]
- Balthazar, C.; Pimentel, T.; Ferrão, L.; Almada, C.; Santillo, A.; Albenzio, M.; Mollakhalili, N.; Mortazavian, A.; Nascimento, J.; Silva, M.; et al. Sheep Milk: Physicochemical Characteristics and Relevance for Functional Food Development. Compr. Rev. Food Sci. Food Saf. 2017, 16, 247–262. [Google Scholar] [CrossRef] [PubMed]
- Haenlein, G.F.; Wendorff, W.L. Sheep Milk. In Handbook of Milk of Non-Bovine Mammals; Park, Y.W., Haenlein, G.F., Eds.; Blackwell Publishing: Oxford, UK, 2006. [Google Scholar] [CrossRef]
- Zhang, R.H.; Mustafa, A.F.; Zhao, X. Effects of feeding oilseeds rich in linoleic and linolenic fatty acids to lactating ewes on cheese yield and on fatty acid composition of milk and cheese. Anim. Feed Sci. Technol. 2006, 127, 220–233. [Google Scholar] [CrossRef]
- Mollica, M.P.; Trinchese, G.; Cimmino, F.; Penna, E.; Cavaliere, G.; Tudisco, R.; Musco, N.; Manca, C.; Catapano, A.; Monda, M.; et al. Milk Fatty Acid Profiles in Different Animal Species: Focus on the Potential Effect of Selected PUFAs on Metabolism and Brain Functions. Nutrients 2021, 13, 1111. [Google Scholar] [CrossRef]
- Santin Junior, I.A.; Silva, K.C.C.; Cucco, D.C. Milk Fatty Acids Profile and the Impact on Human Health. Dairy Vet. Sci. J. 2019, 10, 555779. [Google Scholar] [CrossRef]



| Ingredients (on Fresh Weight Basis) | Control Diet (g/Day/Ewe) | Experimental Diet (g/Day/Ewe) |
|---|---|---|
| Lucerne hay | 1.200 | 1.200 |
| Barley straw | 300 | 300 |
| Corn grain | 540 | 590 |
| Barley grain | 330 | 150 |
| Wheat bran | 180 | 110 |
| Sunflower seed meal (36% crude protein) | 30 | 180 |
| Soyabean meal (47% crude protein) | 300 | 150 |
| Cotton seed | 30 | 80 |
| Flaxseed | - | 75 |
| Lupin seed | - | 75 |
| Molasses | 20 | 20 |
| Premix 1 with vitamins and inorganic minerals | 60 | 60 |
| Total dry matter intake/day | 2630 | 2640 |
| Chemical Analysis (%) | ||
| Dry matter | 87.4 | 87.5 |
| Crude protein (N × 6.25) | 16.1 | 16.1 |
| Ether extract | 2.82 | 4.01 |
| Neutral detergent fibre | 33.8 | 35.1 |
| Acid detergent fibre | 19.5 | 19.9 |
| Acid detergent lignin | 4.51 | 4.98 |
| Ash | 6.55 | 6.52 |
| Starch | 18 | 16.1 |
| Calculated analysis | ||
| Calcium | 11.5 | 11.6 |
| Phosphorus (total) | 3.82 | 3.98 |
| PDI g/kg DM | 89.4 | 87.5 |
| PDIA g/kg DM | 44.3 | 42.7 |
| UFL | 0.71 | 0.71 |
| Isolated Bacteria | Group A Day 30 | Group B Day 60 | Group C Day 90 | ||||
|---|---|---|---|---|---|---|---|
| Control Diet | Experimental Diet (1st Month) | Experimental Diet (2nd Month) | p | ||||
| Total viable counts | 6 | 7.440 ± 0.594 | 6 | 7.181 ± 0.620 | 6 | 7.767 ± 1.355 | 0.560 |
| Lactococcus lactis | 6 | 6.999 ± 0.569 | 6 | 7.522 ± 0.315 | 6 | 6.977 ± 0.999 | 0.327 |
| Lactobacillus rhamnosus | 3 | 7.352 ± 0.453 b | 6 | 6.145 ± 0.224 a | 6 | 7.488 ± 0.274 b | <0.001 |
| Lactobacillus plantarum | 3 | 6.310 ± 0.084 | 6 | 6.805 ± 1.537 | 6 | 7.421 ± 0.352 | 0.310 |
| Lactobacillus brevis | 3 | 6.060 ± 0.283 | 0 | - | 6 | 7.036 ± 0.869 | |
| Lactobacillus fermentum | 1 | 3.699 ± 0.000 | 0 | - | 0 | - | |
| Lactobacillus paracasei | 3 | 7.201 ± 1.154 ab | 6 | 6.320 ± 1.104 a | 6 | 8.415 ± 0.662 b | 0.009 |
| Enterococcus durans | 0 | - | 0 | - | 6 | 5.351 ± 0.189 | |
| Enterococcus faecium | 4 | 6.128 ± 1.309 | 4 | 6.033 ± 0.229 | 6 | 5.665 ± 1.254 | 0.780 |
| Enterococcus faecalis | 1 | 6.301 ± 0.000 | 4 | 6.070 ± 0.267 | 4 | 5.382 ± 0.846 | |
| Staphylococcus caprae | 1 | 4.477 ± 0.000 | 0 | - | 0 | - | |
| Staphylococcus haemolyticus | 1 | 4.699 ± 0.000 | 0 | - | 0 | - | |
| Staphylococcus haulius | 1 | 3.477 ± 0.000 | 0 | - | 0 | - | |
| Streptococcus sal. sp. thermophilus | 4 | 7.979 ± 0.783 | 4 | 8.089 ± 0.182 | 6 | 8.431 ± 0.350 | 0.333 |
| Pediococcus spp. | 0 | - | 0 | - | 6 | 5.003 ± 0.446 | |
| Pediococcus pentosaceus | 0 | - | 4 | 6.064 ± 0.136 | 6 | 6.685 ± 0.777 | |
| Pediococcus acidilactici | 0 | - | 2 | 4.278 ± 0.032 | 6 | 4.422 ± 0.481 | |
| Fatty Acids | Group A Day 30 | Group B Day 60 | Group C Day 90 | |
|---|---|---|---|---|
| Control Diet | Experimental Diet (1st Month) | Experimental Diet (2nd Month) | p | |
| C6:0 | 0.71 ± 0.028 | 1.11 ± 0.325 | 1.32 ± 0.113 | 0.115 |
| C8:0 | 0.62 ± 0.106 | 1.18 ± 0.856 | 1.75 ± 0.092 | 0.226 |
| C10:0 | 6.61 ± 0.035 b | 5.68 ± 0.240 a | 5.83 ± 0.071 a | 0.015 |
| C11:0 | 0.11 ± 0.014 a | 0.16 ± 0.028 ab | 0.32 ± 0.064 b | 0.031 |
| C12:0 | 2.13 ± 0.028 | 3.4 ± 1.782 | 4.89 ± 0.304 | 0.164 |
| C13:0 | 0.25 ± 0.057 | 0.25 ± 0.064 | 0.45 ± 0.049 | 0.066 |
| C14:0 | 8.41 ± 0.021 | 9.93 ± 2.249 | 11.62 ± 0.078 | 0.188 |
| C14:1n5 | 0.87 ± 0.021 | 0.6 ± 0.382 | 0.34 ± 0.028 | 0.198 |
| C15:0 | 0.91 ± 0.007 | 0.93 ± 0.071 | 1.08 ± 0.049 | 0.067 |
| C15:1n5 | 0.34 ± 0.014 | 0.33 ± 0.035 | 0.25 ± 0.035 | 0.093 |
| C16:0 | 28.37 ± 0.573 | 25.96 ± 1.605 | 27.41 ± 0.325 | 0.197 |
| C16:1n7 | 2.24 ± 0.127 | 1.58 ± 0.898 | 0.93 ± 0.014 | 0.184 |
| C16:1n9 | 0.43 ± 0.049 | 0.39 ± 0.071 | 0.38 ± 0.028 | 0.698 |
| C17:1n7 | 0.46 ± 0.035 | 0.34 ± 0.177 | 0.27 ± 0.007 | 0.297 |
| C18:0 | 12.72 ± 0.071 | 11.06 ± 2.404 | 9.24 ± 0.177 | 0.185 |
| C18:1n9c | 30.49 ± 0.601 | 29.72 ± 5.982 | 24.26 ± 1.011 | 0.299 |
| C18:1n7c | 0.28 ± 0.028 | 0.72 ± 0.636 | 1.19 ± 0.177 | 0.205 |
| C18:2n6t | 0.48 ± 0.001 | 0.82 ± 0.389 | 1.02 ± 0.017 | 0.196 |
| C18:2n6c | 2.25 ± 0.007 | 3.08 ± 1.117 | 3.88 ± 0.233 | 0.189 |
| C18:3n6 | 0.49 ± 0.064 | 0.93 ± 0.714 | 1.26 ± 0.17 | 0.325 |
| C18:3n3 | 0.16 ± 0.014 a | 0.72 ± 0.064 b | 1.23 ± 0.071 c | 0.001 |
| C20:0 | 0.26 ± 0.035 | 0.31 ± 0.085 | 0.26 ± 0.028 | 0.653 |
| C20:4n6 | 0.25 ± 0.014 | 0.21 ± 0.035 | 0.19 ± 0.001 | 0.152 |
| Totals | 99.84 ± 0.099 | 99.41 ± 0.113 | 99.37 ± 0.057 | |
| Saturated fatty acids—SFA | 61.10 ± 0.537 | 59.98 ± 4.773 | 64.18 ± 0.941 | 0.418 |
| Unsaturated fatty acids—UFA | 38.74 ± 0.636 | 39.44 ± 4.660 | 35.20 ± 0.997 | 0.383 |
| Mono unsaturated fatty acids—MUFA | 35.11 ± 0.707 | 33.68 ± 6.908 | 27.61 ± 1.117 | 0.290 |
| Poly unsaturated fatty acids—PUFA | 3.63 ± 0.071 | 5.76 ± 2.249 | 7.59 ± 0.120 | 0.121 |
| PUFA n3 (omega-3) | 0.16 ± 0.014 a | 0.72 ± 0.064 b | 1.23 ± 0.071 c | 0.001 |
| PUFA n6 (omega-6) | 2.99 ± 0.021 | 4.11 ± 1.471 | 5.10 ± 0.219 | 0.191 |
| Ratio n6/n3 (omega-6/omega-3) | 18.66 ± 1.789 b | 5.75 ± 1.556 a | 4.14 ± 0.064 a | 0.003 |
| Index of atherogenicity (AI): (C12:0 + 4 × C14:0 + C16:0)/UFA | 1.66 ± 0.035 | 1.75 ± 0.524 | 2.24 ± 0.085 | 0.278 |
| Index of Thrombogenicity (TI): (C14:0 + C16:0 + C18:0)/(0.5 × MUFA + 0.5 × PUFAn6 + 3 × PUFAn3 + n3/n6) | 2.53 ± 0.085 | 2.21 ± 0.339 | 2.38 ± 0.042 | 0.442 |
| Hypocholesterolaemic fatty acids (DFA) (UFA + C18:0) | 51.46 ± 0.566 | 50.50 ± 7.064 | 44.44 ± 1.174 | 0.323 |
| Hypercholesterolaemic fatty acids (OFA) (C12:0 + C14:0 + C16:0) | 38.91 ± 0.523 | 39.30 ± 5.636 | 43.93 ± 0.707 | 0.365 |
| H/H index (Hypocholesterolaemic/Hypocholesterolaemic fatty acids) | 1.323 ± 0.033 | 1.285 ± 0.368 | 1.012 ± 0.042 | 0.383 |
| Group A Day 30 | Group B Day 60 | Group C Day 90 | ||
|---|---|---|---|---|
| Control Diet | Experimental Diet (1st Month) | Experimental Diet (2nd Month) | p | |
| Moisture (%) | 39.17 ± 1.80 | 40.95 ± 1.21 | 39.70 ± 1.58 | 0.157 |
| Total solids (%) | 60.85 ± 1.81 b | 58.33 ± 1.46 a | 60.30 ± 1.58 ab | 0.041 |
| Fat (%) | 28.03 ± 3.41 | 26.23 ± 1.24 | 26.87 ± 1.38 | 0.431 |
| Protein (%) | 27.88 ± 2.71 | 27.17 ± 1.11 | 28.82 ± 1.06 | 0.309 |
| Salt (%) | 2.16 ± 0.06 b | 2.08 ± 0.02 a | 2.08 ± 0.02 a | 0.016 |
| pH | 4.84 ± 0.09 | 4.86 ± 0.07 | 4.79 ± 0.16 | 0.757 |
| TBARS (mg/kg) | 4.2 | 3.4 | 3.6 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tzora, A.; Nelli, A.; Voidarou, C.; Fotou, K.; Bonos, E.; Rozos, G.; Grigoriadou, K.; Papadopoulos, P.; Basdagianni, Z.; Giannenas, I.; et al. Impact of an Omega-3-Enriched Sheep Diet on the Microbiota and Chemical Composition of Kefalograviera Cheese. Foods 2022, 11, 843. https://doi.org/10.3390/foods11060843
Tzora A, Nelli A, Voidarou C, Fotou K, Bonos E, Rozos G, Grigoriadou K, Papadopoulos P, Basdagianni Z, Giannenas I, et al. Impact of an Omega-3-Enriched Sheep Diet on the Microbiota and Chemical Composition of Kefalograviera Cheese. Foods. 2022; 11(6):843. https://doi.org/10.3390/foods11060843
Chicago/Turabian StyleTzora, Athina, Aikaterini Nelli, Chrysoula (Chrysa) Voidarou, Konstantina Fotou, Eleftherios Bonos, Georgios Rozos, Katerina Grigoriadou, Panagiotis Papadopoulos, Zoitsa Basdagianni, Ilias Giannenas, and et al. 2022. "Impact of an Omega-3-Enriched Sheep Diet on the Microbiota and Chemical Composition of Kefalograviera Cheese" Foods 11, no. 6: 843. https://doi.org/10.3390/foods11060843
APA StyleTzora, A., Nelli, A., Voidarou, C., Fotou, K., Bonos, E., Rozos, G., Grigoriadou, K., Papadopoulos, P., Basdagianni, Z., Giannenas, I., & Skoufos, I. (2022). Impact of an Omega-3-Enriched Sheep Diet on the Microbiota and Chemical Composition of Kefalograviera Cheese. Foods, 11(6), 843. https://doi.org/10.3390/foods11060843









