Storage Conditions and Adsorption Thermodynamic Properties for Purple Corn
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Construction of Adsorption Isotherms
2.3. Determination of Equilibrium Moisture
2.4. Adjustment of Adsorption Isotherms
2.5. Thermodynamic Parameters
3. Results and Discussion
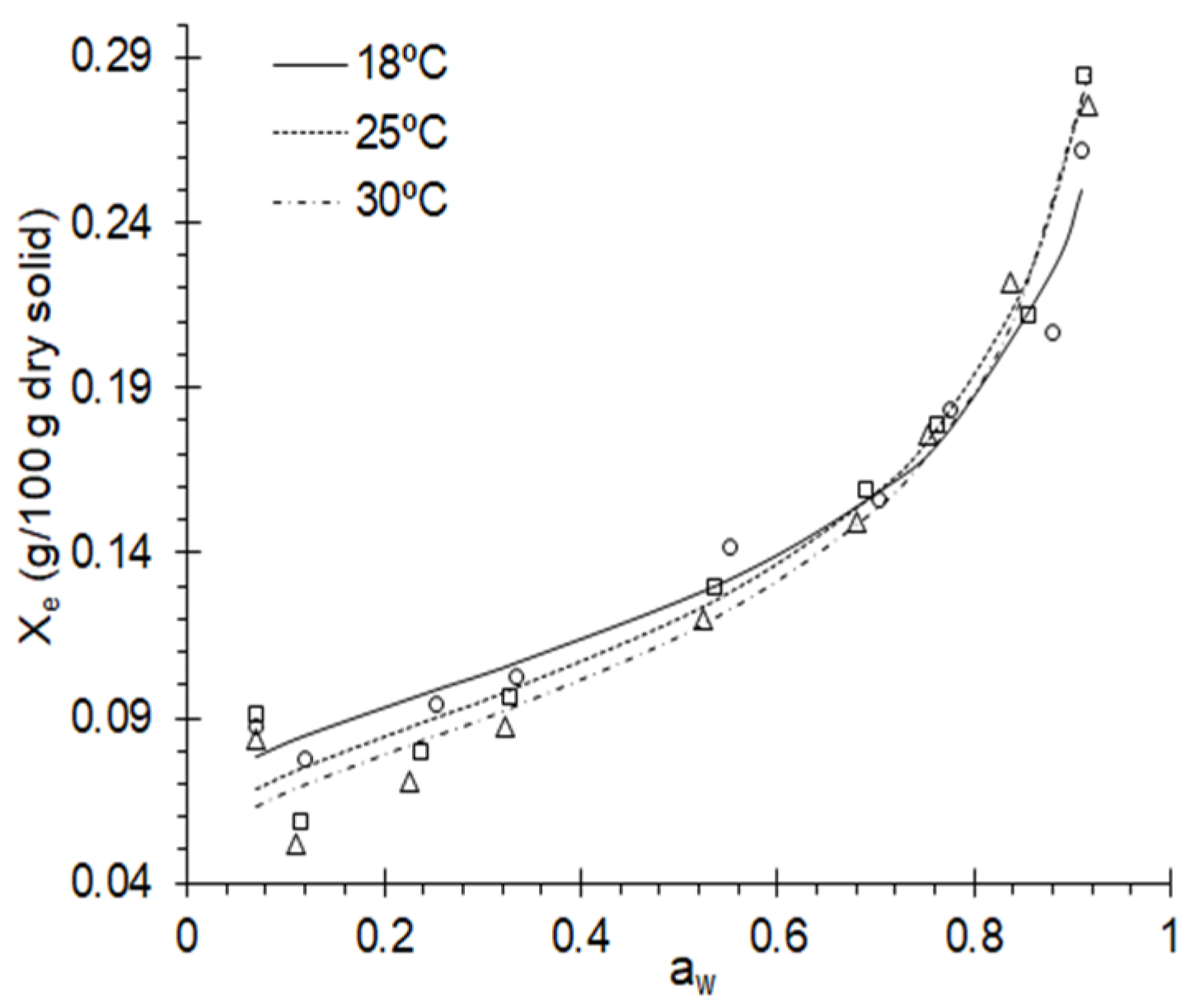
3.1. Adsorption Isotherms
3.2. Adjustment of Adsorption Isotherms
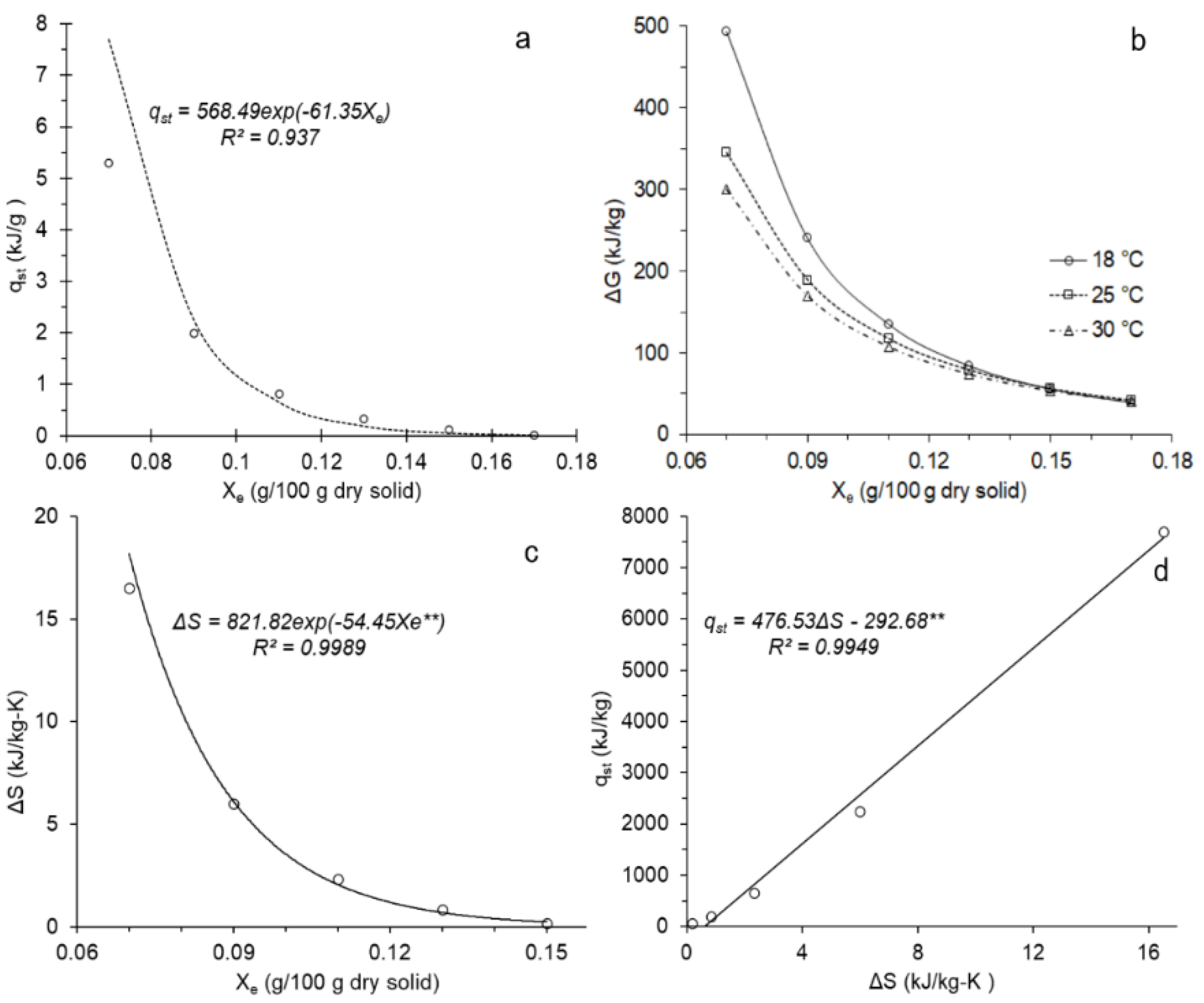
3.3. Thermodynamic Parameters
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lao, F.; Sigurdson, G.T.; Giusti, M.M. Health Benefits of Purple Corn (Zea mays L.) Phenolic Compounds. Compr. Rev. Food Sci. Food Saf. 2017, 16, 234–246. [Google Scholar] [CrossRef]
- Del Pozo-Insfran, D.; Brenes, C.H.; Serna, S.O.; Talcott, S.T. Polyphenolic and antioxidant content of white and blue corn (Zea mays L.) products. Food Res. Int. 2006, 39, 696–703. [Google Scholar] [CrossRef]
- Loarca-Piña, G.; Neri, M.; Figueroa, J.D.; Castaño, E.; Ramos, M.; Reynoso, R.; Mendoza, S. Chemical characterization, antioxidant and antimutagenic evaluations of pigmented corn. J. Food Sci. Technol. 2019, 56, 3177–3184. [Google Scholar] [CrossRef]
- Lao, F.; Giusti, M.M. The effect of pigment matrix, temperature and amount of carrier on the yield and final color properties of spray dried purple corn (Zea mays L.) cob anthocyanin powders. Food Chem. 2017, 227, 376–382. [Google Scholar] [CrossRef]
- Jing, P.; Giusti, M. Characterization of anthocyanin-rich waste from purple corncobs (Zea mays L.) and its application to color milk. J. Agric. Food Chem. 2005, 53, 8775–8781. [Google Scholar] [CrossRef] [PubMed]
- De Pascual-Teresa, S.; Santos-Buelga, C.; Rivas-Gonzalo, J.C. LCMS analysis of anthocyanins from purple corn cob. J. Sci. Food Agric. 2002, 82, 1003–1006. [Google Scholar] [CrossRef]
- Colombo, F.; Di Lorenzo, C.; Petroni, K.; Silano, M.; Pilu, R.; Falletta, E.; Biella, S.; Restani, P. Pigmented Corn Varieties as Functional Ingredients for Gluten-Free Products. Foods 2021, 10, 1770. [Google Scholar] [CrossRef] [PubMed]
- Bacchetti, T.; Masciangelo, S.; Micheletti, A.; Ferretti, G. Carotenoids, Phenolic Compounds and Antioxidant Capacity of Five Local Italian Corn (Zea Mays L.) Kernels. J. Nutr. Food Sci. 2013, 3, 237. [Google Scholar] [CrossRef]
- Gullón, P.; Eibes, G.; Lorenzo, J.M.; Pérez-Rodríguez, N.; Lú-Chau, T.A.; Gullón, B. Green sustainable process to revalorize purple corn cobs within a biorefinery frame: Co-production of bioactive extracts. Sci. Total Environ. 2020, 709, 136236. [Google Scholar] [CrossRef] [PubMed]
- Lago, C.; Cassani, E.; Zanzi, C.; Landoni, M.; Trovato, R.; Pilu, R. Development and study of a maize cultivar rich in anthocyanins: Coloured polenta, a new functional food. Plant Breed. 2014, 133, 201–217. [Google Scholar] [CrossRef]
- Escribano-Bailón, M.T.; Santos-Buelga, C.; Rivas-Gonzalo, J.C. Anthocyanins in cereals. J. Chromatogr. A 2004, 1054, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Hernández, A.D.; Salinas-Moreno, Y.; Ramírez-Díaz, J.L.; Alemán-De la Torre, I.; Bautista-Ramírez, E.; Flores-López, H.E. Anthocyanins and color in grain and cob of peruvian purple corn grown in Jalisco, Mexico. Rev. Mex. Cienc. Agrícolas 2019, 10, 1071–1082. [Google Scholar] [CrossRef]
- Cuevas-Montilla, E.; Hillebrand, S.; Antezana, A.; Winterhalter, P. Soluble and bound phenolic compounds in different Bolivian purple corn (Zea mays L.) Cultivars. J. Agric. Food Chem. 2011, 59, 7068–7074. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhai, W. Identification and antioxidant activity of anthocyanins extracted from the seed and cob of purple corn (Zea mays L.). Innovative Food Sci. Emerg. Technol. 2010, 11, 169–176. [Google Scholar] [CrossRef]
- Chen, C.; Weng, Y. Moisture Sorption isotherms of Oolong tea. Food Bioprocess Technol. 2010, 3, 226–233. [Google Scholar] [CrossRef]
- Aghazadeh, N.; Esmaili, M.; Mohtarami, F. Prediction of Equilibrium Moisture Contents of Black Grape Seeds (Siah Sardasht cultivar) at Various Temperatures and Relative Humidity: Shelf-Life Criteria. Nutr. Food Sci. Res. 2021, 8, 45–52. [Google Scholar]
- Fleurat-Lessard, F. Integrated management of the risks of stored grain spoilage by seed borne fungi and contamination by storage mould mycotoxins—An update. J. Stored Prod. Res. 2017, 71, 22–40. [Google Scholar] [CrossRef]
- Udomkun, P.; Argyropoulos, D.; Nagle, M.; Mahayothee, B.; Müller, J. Sorption behaviour of papayas as affected by compositional and structural alterations from osmotic pretreatment and drying. J. Food Eng. 2015, 157, 14–23. [Google Scholar] [CrossRef]
- Anwar, F.; Naseer, R.; Bhanger, M.I.; Ashraf, S.; Talpur, F.N.; Aladedunye, F.A. Physico-chemical characteristics of citrus seeds and seed oils from Pakistan. J. Am. Oil. Chem. Soc. 2008, 85, 321–330. [Google Scholar] [CrossRef]
- Pumacahua-Ramos, A.; Gómez, J.A.; Telis-Romero, J.; Villa-Vélez, H.A.; Lopes, J.F. Isotherms and isosteric heat of sorption of two varieties of Peruvian quinoa. Sci. Agropecu. 2016, 7, 409–417. [Google Scholar] [CrossRef][Green Version]
- De Oliveira, G.H.H.; Aragão, D.M.S.; De Oliveira, A.P.L.; Silva, M.G.; Gusmão, A.C.A. Modelagem e propriedades termodinâmicas na secagem de morangos. Braz. J. Food Technol. 2015, 18, 314–321. [Google Scholar] [CrossRef]
- Chen, C. Validation of the Component Model for Prediction of Moisture Sorption Isotherms of Two Herbs and other Products. Foods 2019, 8, 191. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Miranda, M.; Cruz y Victoria, M.T.; Vizcarra-Mendoza, M.G.; Anaya-Sosa, I. Determination of moisture sorption isotherms and their thermodynamics properties of nixtamalized maize flour. Rev. Mex. Ing. Química 2014, 13, 165–178. [Google Scholar]
- Isquierdo, E.P.; Caldeira, D.S.A.; Siqueira, V.C.; Martins, E.A.S.; Quequeto, W. Fittings of adsorption isotherm models and thermodynamic properties of urunday seeds. Eng. Agríc. 2020, 40, 374–380. [Google Scholar] [CrossRef]
- Goneli, A.L.D.; Corrêa, P.C.; Oliveira, G.H.H.; Afonso Júnior, P.C. Water sorption properties of coffee fruits, pulped and green coffee. LWT—Food Sci. Technol. 2013, 50, 386–391. [Google Scholar] [CrossRef]
- Saberi, B.; Vuong, Q.V.; Chockchaisawasdee, S.; Golding, J.B.; Scarlett, C.J.; Stathopoulos, C.E. Water Sorption Isotherm of Pea Starch Edible Films and Prediction Models. Foods 2015, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Talla, A. Predicting sorption isotherms and net isosteric heats of sorption of maize grains at different temperaturas. Int. J. Food Eng. 2014, 10, 393–401. [Google Scholar] [CrossRef]
- Moussaoui, H.; Bahammou, Y.; Idlimam, A.; Lamharrar, A.; Abdenouri, N. Investigation of hygroscopic equilibrium and modeling sorption isotherms of the argan products: A comparative study of leaves, pulps, and fruits. Food Bioprod. Process. 2019, 114, 12–22. [Google Scholar] [CrossRef]
- Saleh, R.M.; Karim, N.A.; Hensel, O.; Sturm, B. Mathematical modelling of adsorption isotherms of Malaysian variety of purple flesh sweet potato at different temperatures. Therm. Sci. Eng. Prog. 2018, 7, 326–330. [Google Scholar] [CrossRef]
- Fonseca, N.N.; Resende, O.; Ferreira, J.W.N.; Silva, L.C.D.M.; Andrade, E.G.; Oliveira, L.P. Desorption isotherms of graniferous sorghum grains. Res. Soc. Dev. 2020, 9, 1–16. [Google Scholar] [CrossRef]
- Resende, O.; Oliveira, D.E.C.; Costa, L.M.; Ferreira, W.N. Thermodynamic properties of baru fruits (Dipteryx alata Vogel). Eng. Agríc. 2017, 37, 739–749. [Google Scholar] [CrossRef]
- Staudt, P.B.; Kechinski, C.P.; Tessaro, I.C.; Marczak, L.D.F.; Soares, R.D.P.; Cardozo, N.S.M. A new method for predicting sorption isotherms at different temperatures using the BET model. J. Food Eng. 2013, 114, 139–145. [Google Scholar] [CrossRef]
- Zeymer, J.S.; Corrêa, P.C.; Oliveira, G.H.; Baptestini, F.M.; Campos, R.C. Mathematical modeling and hysteresis of sorption isotherms for paddy rice grains. Eng. Agríc. 2019, 39, 524–532. [Google Scholar] [CrossRef]
- Bustos-Vanegas, J.D.; Corrêa, P.C.; Zeymer, J.S.; Baptestini, F.M.; Campos, R.C. Moisture sorption isotherms of quinoa seeds: Thermodynamic analysis. Eng. Agríc. 2018, 38, 941–950. [Google Scholar] [CrossRef]
- Bessa, J.F.V.; Resende, O.; De Oliveira, D.E.C.; De Lima, R.R.; Quequeto, W.D.; Siqueira, V.C. Adsorption isotherms and thermodynamic properties of Carthamus tinctorius L. seeds. Rev. Bras. Eng. Agríc. E Ambient. 2021, 25, 696–702. [Google Scholar] [CrossRef]
- Hassini, L.; Bettaieb, E.; Desmorieux, H.; Torres, S.S.; Touil, A. Desorption isotherms and thermodynamic properties of prickly pear seeds. Ind. Crops Prod. 2015, 67, 457–465. [Google Scholar] [CrossRef]
- Botelho, F.M.; Neto, N.J.B.; Botelho, S.C.C.; De Oliveira, G.H.H.; Hauth, M.R. Sorption isotherms of Brazil nuts. Rev. Bras. Eng. Agríc. E Ambient. 2019, 23, 776–781. [Google Scholar] [CrossRef]
- Ade, A.R.; Ajav, E.A.; Raji, A.O.; Adetayo, S.A.; Arowora, K.A. Moisture sorption isotherms of Mesquite seed (Prosopis africana). Agric. Eng. Int. CIGR J. 2016, 18, 273–281. [Google Scholar]
- Oliveira, G.H.H.; Corrêa, P.C.; Araújo, E.F.; Valente, D.S.M.; Botelho, F.M. Desorption isotherms and thermodynamic properties of sweet corn cultivars (Zea Mays L.). Int. J. Food Sci. Technol. 2010, 45, 546–554. [Google Scholar] [CrossRef]
- Choque-Quispe, D.; Ramos-Pacheco, B.S.; Solano-Reynoso, A.M.; Ligarda-Samanez, C.A.; Choque-Quispe, Y.; Peralta-Guevara, D.E.; Quispe-Quispe, Y. Drying and color in punamuña leaves (Satureja boliviana). DYNA 2021, 88, 31–37. [Google Scholar] [CrossRef]
- Zeymer, J.S.; Corrêa, P.C.; De Oliveira, G.H.H.; Baptestini, F.M. Thermodynamic properties of water desorption in lettuce seeds. Semin. Cienc. Agrar. 2018, 39, 921–932. [Google Scholar] [CrossRef]
- Moreira, R.; Chenlo, F.; Torres, M.D.; Vallejo, N. Thermodynamic analysis of experimental sorption isotherms of loquat and quince fruits. J. Food Eng. 2008, 88, 514–521. [Google Scholar] [CrossRef]
- Campos, R.C.; Corrêa, P.C.; Zaidan, I.R.; Zaidan, Ú.R.; Leite, R.A. Moisture sorption isotherms of sunflower seeds: Thermodynamic analysis. Ciênc. E Agrotecnol. 2019, 43, 2. [Google Scholar] [CrossRef]
- Wang, P.; Fu, N.; Li, D.; Wang, L. Predicting Storage Conditions for Rice Seed with Thermodynamic Analysis. Int. J. Food Eng. 2017, 13, 20170129. [Google Scholar] [CrossRef]
- Barati, M.; Zare, D.; Zomorodian, A. Moisture sorption isotherms and thermodynamic properties of safflower seed using empirical and neural network models. Food Meas. 2016, 10, 236–246. [Google Scholar] [CrossRef]
- Labuza, T.P. Moisture Sorption: Practical Aspects of Isotherm Measurement and Use; American Association of Cereal Chemists: St. Paul, MN, USA, 1984; ISBN 0913250341. [Google Scholar]
- Corrêa, P.C.; Baptestini, F.M.; Vanegas, J.D.B.; Leite, R.; Botelho, F.M.; De Oliveira, G.H.H. Kinetics of water sorption of damaged bean grains: Thermodynamic properties. Rev. Bras. Eng. Agríc. E Ambient. 2017, 21, 556–561. [Google Scholar] [CrossRef][Green Version]
- Silva, H.W.; Costa, L.M.; Resende, O.; Oliveira, D.E.; Soares, R.S.; Vale, L.S. Thermodynamic properties of pepper seeds—Variety “Cabacinha”. Científica 2016, 44, 14–22. [Google Scholar] [CrossRef][Green Version]
- Soleimani, M.; Tabil, L.; Shahedi, M.; Emani, S. Sorption isotherm of hybrid seed corn. In Proceedings of the Canadian Society for Engineering in Agricultural, Food, Environmental, and Biological Systems—CSBE, Edmonton, AB, Canada, 16–19 July 2006. [Google Scholar] [CrossRef]
- Karunanithy, C.; Muthukumarappan, K.; Donepudi, A. Moisture Sorption Characteristics of Corn Stover and Big Bluestem. J. Renew. Energy 2013, 2013, 4. [Google Scholar] [CrossRef]
- Resende, O.; Corrêa, C.P.; Gonell, A.L.D.; Ribeiro, D.M. Isotermas e Calor Isostérico de Sorção do Feijão. Ciênc. Tecnol. Aliment. 2006, 26, 626–631. [Google Scholar] [CrossRef][Green Version]
- Tsami, E.; Maroulis, Z.B.; Marinos-Kouris, D.; Saravacos, G.D. Heat sorption of water in dried fruits. Int. J. Food Sci. Technol. 1990, 25, 350–363. [Google Scholar] [CrossRef]
- Rizvi, S.S. Thermodynamic properties of foods in dehydration. In Engineering Properties of Foods; Taylor & Francis: Boca Ratón, FL, USA, 2005; pp. 259–346. [Google Scholar] [CrossRef]
- McMinn, W.A.M.; Al-Muhtaseb, A.H.; Magee, T.R.A. Enthalpy-entropy compensation in sorption phenomena of starch materials. Food Res. Int. 2005, 38, 505–510. [Google Scholar] [CrossRef]
- Gabas, A.L.; Menegalli, F.C.; Telis-Romero, J. Water sorption enthalpy-entropy compensation based on isotherms of plum skin and pulp. J. Food Sci. 2000, 65, 680. [Google Scholar] [CrossRef]
- Beristain, C.I.; Garcia, H.S.; Azuara, E. Enthalpyentropy compensation in food vapor adsorption. J. Food Eng. 1996, 30, 405–415. [Google Scholar] [CrossRef]
- Madamba, P.S.; Driscoll, R.H.; Buckle, K.A. Enthalpy-entropy compensation models for sorption and browning of garlic. J. Food Eng. 1996, 28, 109–119. [Google Scholar] [CrossRef]
- Tsami, E. Net isosteric heat of sorption in dried fruits. J. Food Eng. 1991, 14, 327–335. [Google Scholar] [CrossRef]
- Krug, R.R.; Hunter, W.G.; Grieger, R.A. Enthalpy-entropy compensation. 1. Some fundamental statistical problems associated with the analysis of van’t Hoff and Arrhenius data. J. Phys. Chem. 1976, 80, 2335–2341. [Google Scholar] [CrossRef]
- Ryde, U.L.F. A fundamental view of enthalpy-entropy compensation. MedChemComm 2014, 5, 1324–1336. [Google Scholar] [CrossRef]
- Leffler, J.E.; Grunwald, E. Rates and Equilibria of Organic Reactions: As Treated by Statistical, Thermodynamic and Extrathermodynamic Methods; Elsevier: Amsterdam, The Netherlands, 1963; ISBN 978-1-62198-643-0. [Google Scholar]
- Vega, A.; Lara, E.; Lemus, R. Isotermas de adsorción en harina de maíz (Zea mays). Food Sci. Technol. 2006, 26, 4. [Google Scholar] [CrossRef]
- Condon, J.B. Surface Area and Porosity Determinations by Physisorption: Measurements and Theory; Elsevier: Amsterdam, The Netherlands, 2006; ISBN 978-0-444-51964-1. [Google Scholar]
- Silva, K.S.; Romero, J.T.; Mauro, M.A. Sorption isotherms and thermodynamic analysis of seed fruits used to obtain vegetable oil. Lat. Am. Appl. Res. 2015, 45, 21–26. [Google Scholar] [CrossRef]
- Miranda, M.; Vega-Gálvez, A.; Sanders, M.; López, J.; Lemus-Mondaca, R.; Martínez, E.; Di Scala, K. Modelling the water sorption isotherms of quinoaseeds (Chenopodium quinoa Willd.) and determination of sorption heats. Food Bioprocess Technol. 2012, 5, 1686–1693. [Google Scholar] [CrossRef]
- Corrêa, P.C.; Botelho, F.M.; Botelho, S.C.C.; Goneli, A.L.D. Isotermas de sorção de água de frutos de Coffea canephora. Rev. Bras. Eng. Agríc. E Ambient. 2014, 18, 1047–1052. [Google Scholar] [CrossRef][Green Version]
- Majd, K.M.; Karparvarfard, S.H.; Farahnaky, A.; Ansari, S. Thermodynamic properties of water sorption isotherms of grape seed. Int. Agrophysics 2014, 28, 63–71. [Google Scholar] [CrossRef]
- Enrione, J.I.; Hill, S.E.; Mitchell, J.R. Sorption behavior of mixtures of glycerol and starch. J. Agric. Food Chem. 2007, 55, 2956–2963. [Google Scholar] [CrossRef] [PubMed]
- Samapundo, S.; Devlieghere, F.; De Meulenaer, B.; Atukwase, A.; Lamboni, Y.; Debevere, J.M. Sorption isotherms and isosteric heats of sorption of whole yellow dent corn. J. Food Eng. 2007, 79, 168–175. [Google Scholar] [CrossRef]
- Choque-Quispe, D.; Ligarda-Samanez, C.A.; Ramos-Pacheco, B.S.; Taipe-Pardo, F.; Peralta-Guevara, D.E.; Solano-Reynoso, A.M. Evaluation of sorption isotherms of grains and flour of amaranth (Amaranthus caudatus). Rev. ION 2018, 31, 67–81. [Google Scholar] [CrossRef]
- Labuza, T.P.; Kaanane, A.; Chen, J.Y. Effect of temperature on the moisture sorption isotherm and water activity shift of two dehydrated foods. J. Food Sci. 1985, 50, 385–391. [Google Scholar] [CrossRef]
- Telis, V.R.N.; Gabas, A.L.; Menegalli, F.C.; Telis-Romero, J. Water sorption thermodynamic properties applied to persimmon skin and pulp. Thermochim. Acta 2000, 343, 49–56. [Google Scholar] [CrossRef]
- De Oliveira, D.E.C.; Resende, O.; Chaves, T.H.; Souza, K.A.; Smaniotto, T.A.D.S. Propriedades termodinâmicas das sementes de pinhão manso. Biosci. J. 2014, 30, 147157. [Google Scholar]
- De Oliveira, D.E.C.; Resende, O.; Smaniotto, T.A.D.S.; De Sousa, K.A.; Campos, R.C. Propriedades termodinâmicas de grãos de milho para diferentes teores de água de equilíbrio. Pesqui. Agropecu. Trop. 2013, 43, 50–56. [Google Scholar] [CrossRef]
- Nkolo, M.Y.N.; Noah, N.J.; Bardet, S. Effect of enthalpy–entropy compensation during sorption of water vapour in tropical woods: The case of Bubinga (Guibourtia Tessmanii J. L¢eonard; G. Pellegriniana J. L.). Thermochim. Acta 2008, 468, 1–5. [Google Scholar] [CrossRef]
- Ayala-Aponte, A.A. Thermodynamic properties of moisture sorption in cassava flour. DYNA 2016, 83, 139–145. [Google Scholar] [CrossRef]
- Toshkov, N.; Lazarov, L.; Popova, V.; Ivanova, T.; Menkov, N. Thermodynamics of moisture sorption in tobacco (Nicotiana tabacum L.) seeds. E3S Web Conf. 2020, 207, 01019. [Google Scholar] [CrossRef]
| Substance | Equation | R2 |
|---|---|---|
| Sodium hydroxide | 0.998 | |
| Lithium chloride | 0.980 | |
| Potassium Acetate | 0.970 | |
| Magnesium chloride | 0.963 | |
| Magnesium Nitrate | 0.990 | |
| Potassium iodide | 1.000 | |
| Sodium chloride | 0.960 | |
| Potassium chloride | 0.970 | |
| Barium chloride | 0.997 |
| Model | ||
|---|---|---|
| Temperature dependent | ||
| BET | (2) | |
| GAB | (3) | |
| Oswin | (4) | |
| Modified Henderson | (5) | |
| Chung y Pfost | (6) | |
| Temperature independent | ||
| Halsey | (7) | |
| Henderson | (8) | |
| Model | Parameters | R2 | SEE | MAE (%) | Residual Distribution | ||
|---|---|---|---|---|---|---|---|
| Temperature dependent | |||||||
| GAB | 18 °C | Xm | 0.076 | 0.967 | 0.013 | 5.149 | Random |
| CGAB | 1,502,959 | ||||||
| K | 0.755 | ||||||
| 25 °C | Xm | 0.068 | 0.973 | 0.014 | 8.795 | Random | |
| CGAB | 4,501,090 | ||||||
| K | 0.825 | ||||||
| 30 °C | Xm | 0.064 | 0.984 | 0.011 | 8.508 | Random | |
| CGAB | 1,812,258 | ||||||
| K | 0.842 | ||||||
| BET | 18 °C | Xm | 0.028 | 0.301 | 0.056 | 33.845 | Trending |
| CBET | −19.315 | ||||||
| 25 °C | Xm | 0.030 | 0.604 | 0.049 | 26.359 | Trending | |
| CBET | −20.218 | ||||||
| 30 °C | Xm | 0.029 | 0.594 | 0.051 | 27.66 | Trending | |
| CBET | −21.015 | ||||||
| Oswin | 18 °C | A | 0.132 | 0.959 | 0.014 | 6.657 | Random |
| B | 0.264 | ||||||
| 25 °C | A | 0.127 | 0.957 | 0.016 | 9.171 | Slightly random | |
| B | 0.323 | ||||||
| 30 °C | A | 0.121 | 0.966 | 0.015 | 9.870 | Slightly random | |
| B | 0.345 | ||||||
| Modified Henderson | 18 °C | k | 0.336 | 0.912 | 0.020 | 10.794 | Trending |
| n | 2.518 | ||||||
| 25 °C | k | 0.130 | 0.903 | 0.024 | 12.346 | Trending | |
| n | 2.005 | ||||||
| 30 °C | k | 0.095 | 0.927 | 0.022 | 12.53 | Trending | |
| n | 1.809 | ||||||
| Chun-Pfost | 18 °C | A | −24.266 | 0.948 | 0.015 | 7.443 | Random |
| B | 19.759 | ||||||
| 25 °C | A | −16.300 | 0.929 | 0.021 | 12.428 | Trending | |
| B | 16.826 | ||||||
| 30 °C | A | −13.974 | 0.940 | 0.020 | 13.612 | Trending | |
| B | 16.113 | ||||||
| Temperature independent | |||||||
| Halsey | 18 °C | A | 0.002 | 0.974 | 0.011 | 5.902 | Random |
| B | 2.867 | ||||||
| 25 °C | A | 0.004 | 0.976 | 0.012 | 8.628 | Random | |
| B | 2.387 | ||||||
| 30 °C | A | 0.005 | 0.975 | 0.013 | 10.412 | Random | |
| B | 2.276 | ||||||
| Henderson | 18 °C | k | 97.702 | 0.912 | 0.020 | 10.794 | Trending |
| n | 2.518 | ||||||
| 25 °C | k | 38.724 | 0.903 | 0.024 | 12.346 | Trending | |
| n | 2.005 | ||||||
| 30 °C | k | 28.762 | 0.927 | 0.022 | 12.529 | Trending | |
| n | 1.809 | ||||||
| Parameters | 18 °C | 25 °C | 30 °C | Ea (kJ/mol) |
|---|---|---|---|---|
| Xm | 0.0764 | 0.0677 | 0.0640 | −10.947 |
| CGAB | 1,502,958.98 | 4,501,089.95 | 1,812,257.53 | 18.843 |
| k | 0.7552 | 0.8252 | 0.8418 | 6.820 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choque-Quispe, D.; Ramos-Pacheco, B.S.; Choque-Quispe, Y.; Aguilar-Salazar, R.F.; Mojo-Quisani, A.; Calla-Florez, M.; Solano-Reynoso, A.M.; Zamalloa-Puma, M.M.; Palomino-Malpartida, Y.G.; Alcarraz-Alfaro, T.; et al. Storage Conditions and Adsorption Thermodynamic Properties for Purple Corn. Foods 2022, 11, 828. https://doi.org/10.3390/foods11060828
Choque-Quispe D, Ramos-Pacheco BS, Choque-Quispe Y, Aguilar-Salazar RF, Mojo-Quisani A, Calla-Florez M, Solano-Reynoso AM, Zamalloa-Puma MM, Palomino-Malpartida YG, Alcarraz-Alfaro T, et al. Storage Conditions and Adsorption Thermodynamic Properties for Purple Corn. Foods. 2022; 11(6):828. https://doi.org/10.3390/foods11060828
Chicago/Turabian StyleChoque-Quispe, David, Betsy S. Ramos-Pacheco, Yudith Choque-Quispe, Rolando F. Aguilar-Salazar, Antonieta Mojo-Quisani, Miriam Calla-Florez, Aydeé M. Solano-Reynoso, Miluska M. Zamalloa-Puma, Ybar G. Palomino-Malpartida, Tarcila Alcarraz-Alfaro, and et al. 2022. "Storage Conditions and Adsorption Thermodynamic Properties for Purple Corn" Foods 11, no. 6: 828. https://doi.org/10.3390/foods11060828
APA StyleChoque-Quispe, D., Ramos-Pacheco, B. S., Choque-Quispe, Y., Aguilar-Salazar, R. F., Mojo-Quisani, A., Calla-Florez, M., Solano-Reynoso, A. M., Zamalloa-Puma, M. M., Palomino-Malpartida, Y. G., Alcarraz-Alfaro, T., & Zamalloa-Puma, A. (2022). Storage Conditions and Adsorption Thermodynamic Properties for Purple Corn. Foods, 11(6), 828. https://doi.org/10.3390/foods11060828









