Effects of Enzymatic Konjac Glucomannan Hydrolysates on Textural Properties, Microstructure, and Water Distribution of Grass Carp Surimi Gels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of KGM Enzymatic Hydrolysates
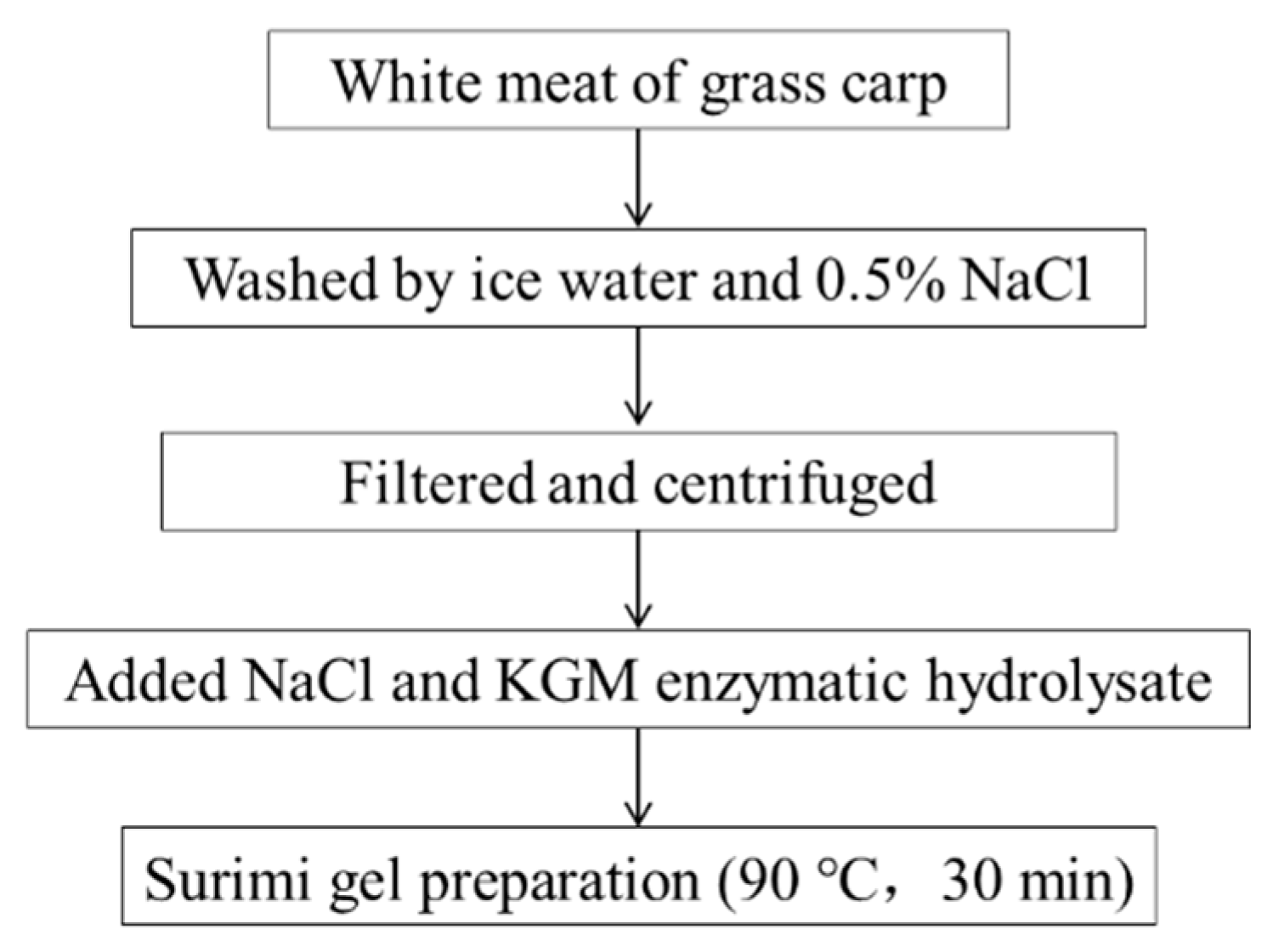
2.3. Preparation of Surimi with KGM Enzymatic Hydrolysates
2.4. Molecular Weight of KGM Enzymatic Hydrolysates
2.5. Intrinsic Viscosity
2.6. Light Microcopy Analysis
2.7. Scanning Electron Microscope
2.8. Fourier Transform Infrared Reflection (FT-IR) Spectrum
2.9. Texture Analysis Profile (TPA)
2.10. Gel Strength
2.11. Whiteness
2.12. Water Holding Capacity (WHC)
2.13. Low Field Nuclear Magnetic Resonance (LF-NMR)
2.14. Magnetic Resonance Imaging (MRI)
2.15. Statistical Analysis
3. Results and Discussion
3.1. Molecular Characteristics
3.2. Microstructure
3.3. Secondary Structure Analysis
3.4. Textural Properties and Whiteness
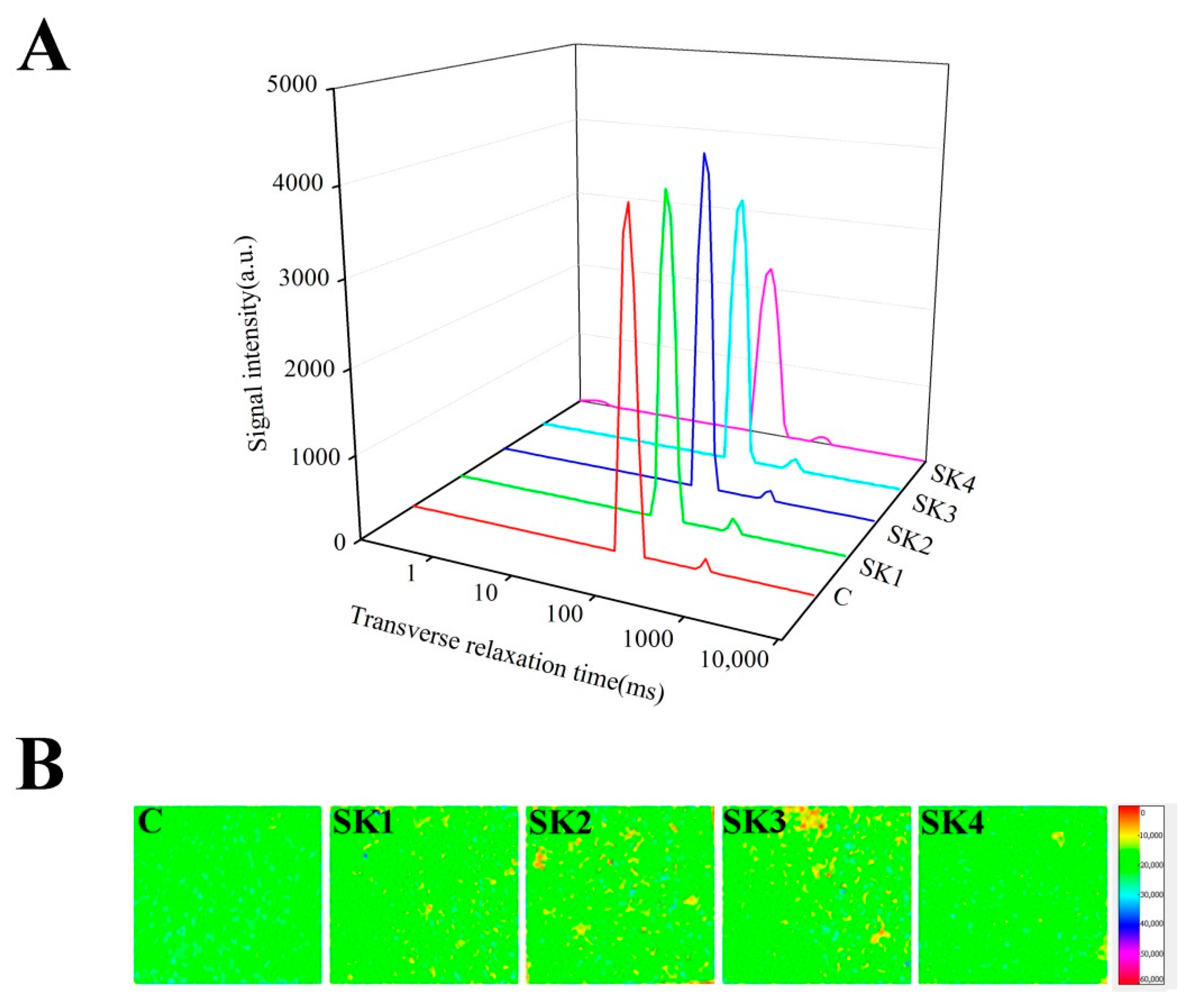
3.5. WHC and Water Molecules Distribution
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jia, R.; Katano, T.; Yoshimoto, Y.; Gao, Y.; Watanabe, Y.; Nakazawa, N.; Osako, K.; Okazaki, E. Sweet potato starch with low pasting temperature to improve the gelling quality of surimi gels after freezing. Food Hydrocoll. 2018, 81, 467–473. [Google Scholar] [CrossRef]
- Zhang, B.; Hao, G.-J.; Cao, H.-J.; Tang, H.; Zhang, Y.-Y.; Deng, S.-G. The cryoprotectant effect of xylooligosaccharides on denaturation of peeled shrimp (Litopenaeus vannamei) protein during frozen storage. Food Hydrocoll. 2018, 77, 228–237. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, X.-L.; Shen, C.-L.; Deng, S.-G. Understanding the influence of carrageenan oligosaccharides and xy-looligosaccharides on ice-crystal growth in peeled shrimp (Litopenaeus vannamei) during frozen storage. Food Funct. 2018, 9, 4394–4403. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Cao, H.-J.; Lin, H.-M.; Deng, S.-G.; Wu, H. Insights into ice-growth inhibition by trehalose and alginate oligosac-charides in peeled Pacific white shrimp (Litopenaeus vannamei) during frozen storage. Food Chem. 2019, 278, 482–490. [Google Scholar] [CrossRef]
- Zhang, H.; Xiong, Y.; Bakry, A.M.; Xiong, S.; Yin, T.; Zhang, B.; Huang, J.; Liu, Z.; Huang, Q. Effect of yeast β-glucan on gel properties, spatial structure and sensory characteristics of silver carp surimi. Food Hydrocoll. 2019, 88, 256–264. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, W.; Xu, M.; Fang, C.; Liu, J. Characterization of silver carp myosin glycated with phosphorylated konjac oligo-lucomannan. J. Sci Food Agr. 2021, 101, 6117–6124. [Google Scholar] [CrossRef]
- Lu, Y.; Xiong, G.; Li, X.; Wu, W.; Qiao, Y.; Ding, A.; Liao, L.; Wang, J.; Wang, L. Cryoprotective Effect and Mechanism of Dextran with Different Molecular Weights on Denaturation of Myofibrillar Protein from Grass Carp (Ctenopharyngodon idella). Food Sci. 2016, 37, 289–294. [Google Scholar]
- Somjit, K.; Ruttanapornwareesakul, Y.; Hara, K.; Nozaki, Y. The cryoprotectant effect of shrimp chitin and shrimp chitin hydrolysate on denaturation and unfrozen water of lizardfish surimi during frozen storage. Food Res. Int. 2005, 38, 345–355. [Google Scholar] [CrossRef]
- Behera, S.S.; Ray, R.C. Konjac glucomannan, a promising polysaccharide of Amorphophalluskonjac K. Koch in health care. Int. J. Biol. Macromol. 2016, 92, 942–956. [Google Scholar] [CrossRef]
- Du, X.; Li, J.; Chen, J.; Li, B. Effect of degree of deacetylation on physicochemical and gelation properties of konjac glucomannan. Food Res. Int. 2012, 46, 270–278. [Google Scholar] [CrossRef]
- Ji, L.; Xue, Y.; Zhang, T.; Li, Z.; Xue, C. The effects of microwave processing on the structure and various quality parameters of Alaska pollock surimi protein-polysaccharide gels. Food Hydrocoll. 2017, 63, 77–84. [Google Scholar] [CrossRef]
- Liu, J.; Xu, Q.; Zhang, J.; Zhao, P.; Ding, Y. Characterization of silver carp (Hypophthalmichthys molitrix) myosin protein glycated with konjac oligo-glucomannan. Food Hydrocoll. 2016, 57, 114–121. [Google Scholar] [CrossRef]
- Zhang, T.; Li, Z.; Wang, Y.; Xue, Y.; Xue, C. Effects of konjac glucomannan on heat-induced changes of physicochemical and structural properties of surimi gels. Food Res. Int. 2016, 83, 152–161. [Google Scholar] [CrossRef]
- Iglesias-Otero, M.A.; Borderías, J.; Tovar, C.A. Use of Konjac glucomannan as additive to reinforce the gels from low-quality squid surimi. J. Food Eng. 2010, 101, 281–288. [Google Scholar] [CrossRef]
- Liu, J.; Wang, X.; Ding, Y. Optimization of adding konjac glucomannan to improve gel properties of low-quality surimi. Carbohydr. Polym. 2013, 92, 484–489. [Google Scholar] [CrossRef]
- Yang, J.; Vittori, N.; Wang, W.; Shi, Y.-C.; Hoeflinger, J.L.; Miller, M.J.; Pan, Y. Molecular weight distribution and fermentation of mechanically pre-treated konjac enzymatic hydrolysates. Carbohydr. Polym. 2017, 159, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Fang, C.; Luo, Y.; Ding, Y.; Liu, S. Effects of konjac oligo-glucomannan on the physicochemical properties of frozen surimi from red gurnard (Aspitrigla cuculus). Food Hydrocoll. 2019, 89, 668–673. [Google Scholar] [CrossRef]
- Wang, L.; Xiong, G.; Peng, Y.-B.; Wu, W.; Li, X.; Wang, J.; Qiao, Y.; Liao, L.; Ding, A. The Cryoprotective Effect of Different Konjac Glucomannan (KGM) Hydrolysates on the Glass Carp (Ctenopharyngodon idella) Myofibrillar During Frozen Storage. Food Bioprocess Technol. 2014, 7, 3398–3406. [Google Scholar] [CrossRef]
- Li, B.; Xie, B. Study on molecular chain morphology and chain parameters of konjac glucomannan. Acta Pharm. Sin. 2003, 38, 838–842. [Google Scholar]
- Chiang, P.Y.; Li, J.Y.; Chen, M.L. Rheological characteristics and morphology of dialdehyde starch/meat composites during heating. J. Food Sci. 2009, 74, E112–E119. [Google Scholar] [CrossRef]
- Hoon Moon, J.; Byong Yoon, W.; Park, J.W. Assessing the textural properties of Pacific whiting and Alaska pollock surimi gels prepared with carrot under various heating rates. Food Biosci. 2017, 20, 12–18. [Google Scholar] [CrossRef]
- Xie, M.X.; Yuan, L. Studies on Amide III Infrared Bands for the Secondary Structure Determination of Proteins. Chem. J. Chin. Univ. 2003, 24, 226–231. [Google Scholar]
- Guo, X.; Shi, L.; Xiong, S.; Hu, Y.; You, J.; Huang, Q.; Yin, T. Gelling properties of vacuum-freeze dried surimi powder as influenced by heating method and microbial transglutaminase. LWT 2019, 99, 105–111. [Google Scholar] [CrossRef]
- Ullah, I.; Hu, Y.; You, J.; Yin, T.; Xiong, S.; Din, Z.-U.; Huang, Q.; Liu, R. Influence of okara dietary fiber with varying particle sizes on gelling properties, water state and microstructure of tofu gel. Food Hydrocoll. 2019, 89, 512–522. [Google Scholar] [CrossRef]
- Picout, D.R.; Ross-Murphy, S.B. On the Mark–Houwink parameters for galactomannans. Carbohydr. Polym. 2007, 70, 145–148. [Google Scholar] [CrossRef]
- Vinod, V.T.P.; Sashidhar, R.B. Solution and conformational properties of gum kondagogu (Cochlospermum gossypium)—A natural product with immense potential as a food additive. Food Chem. 2009, 116, 686–692. [Google Scholar] [CrossRef]
- Mao, C.-F.; Chen, C.-H. A kinetic model of the gelation of konjac glucomannan induced by deacetylation. Carbohydr. Polym. 2017, 165, 368–375. [Google Scholar] [CrossRef]
- Li, B.; Xie, B.J. Single molecular chain geometry of konjac glucomannan as a high quality dietary fiber in East Asia. Food Res. Int. 2006, 39, 127–132. [Google Scholar] [CrossRef]
- Yin, T.; Yao, R.; Ullah, I.; Xiong, S.; Huang, Q.; You, J.; Hu, Y.; Shi, L. Effects of nanosized okara dietary fiber on gelation properties of silver carp surimi. LWT 2019, 111, 111–116. [Google Scholar] [CrossRef]
- Jian, W.; Wu, H.; Wu, L.; Wu, Y.; Jia, L.; Pang, J.; Sun, Y.M. Effect of molecular characteristics of Konjac glucomannan on gelling and rheological properties of Tilapia myofibrillar protein. Carbohydr. Polym. 2016, 150, 21–31. [Google Scholar] [CrossRef]
- Luo, X.; He, P.; Lin, X. The mechanism of sodium hydroxide solution promoting the gelation of Konjac glucomannan (KGM). Food Hydrocoll. 2013, 30, 92–99. [Google Scholar] [CrossRef]
- Zou, Y.; Ma, G. A new criterion to evaluate water vapor interference in protein secondary structural analysis by FTIR spectroscopy. Int. J. Mol. Sci. 2014, 15, 10018–10033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stani, C.; Vaccari, L.; Mitri, E.; Birarda, G. FTIR investigation of the secondary structure of type I collagen: New insight into the amide III band. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 229, 118006. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Mayer, S.G.; Park, J.W. FT-IR and Raman spectroscopies determine structural changes of tilapia fish protein isolate and surimi under different comminution conditions. Food Chem. 2017, 226, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Gonzalez, I.; Carmona, P.; Moreno, P.; Borderias, J.; Sanchez-Alonso, I.; Rodriguez-Casado, A.; Careche, M. Protein and water structural changes in fish surimi during gelation as revealed by isotopic H/D exchange and Raman spectroscopy. Food Chem. 2008, 106, 56–64. [Google Scholar] [CrossRef]
- Xu, J.; Mukherjee, D.; Chang, S.K.C. Physicochemical properties and storage stability of soybean protein nanoemulsions prepared by ultra-high pressure homogenization. Food Chem. 2017, 240, 1005–1013. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, F.; Wang, X. Changes of protein secondary structures of pollock surimi gels under high-temperature (100 °C and 120 °C) treatment. J. Food Eng. 2016, 171, 159–163. [Google Scholar] [CrossRef]
- Tao, Z.; Yong, X.; Li, Z.; Wang, Y.; Xue, C. Effects of deacetylation of konjac glucomannan on Alaska Pollock surimi gels subjected to high-temperature (120 °C) treatment. Food Hydrocoll. 2015, 43, 125–131. [Google Scholar]
- Liu, S.; Zhao, P.; Zhang, J.; Xu, Q.; Ding, Y.; Liu, J. Physicochemical and functional properties of silver carp (Hypophthalmichthys molitrix) myofibrillar protein glycated with konjac oligo-glucomannan. Food Hydrocoll. 2017, 67, 216–223. [Google Scholar] [CrossRef]
- Liu, J.; Luo, Y.; Gu, S.; Xu, Q.; Zhang, J.; Zhao, P.; Ding, Y. Physicochemical, conformational and functional properties of silver carp myosin glycated with konjac oligo-glucomannan: Implications for structure-function relationships. Food Hydrocoll. 2017, 72, 136–144. [Google Scholar] [CrossRef]
- Tadpitchayangkoon, P.; Park, J.W.; Yongsawatdigul, J. Gelation characteristics of tropical surimi under water bath and ohmic heating. LWT 2012, 46, 97–103. [Google Scholar] [CrossRef] [Green Version]
- Balange, A.K.; Benjakul, S. Effect of oxidised tannic acid on the gel properties of mackerel (Rastrelliger kanagurta) mince and surimi prepared by different washing processes. Food Hydrocoll. 2009, 23, 1693–1701. [Google Scholar] [CrossRef]
- Ma, S.; Zhu, P.; Wang, M.; Wang, F.; Wang, N. Effect of konjac glucomannan with different molecular weights on physicochemical properties of corn starch. Food Hydrocoll. 2019, 96, 663–670. [Google Scholar] [CrossRef]
- Andrés-Bello, A.; Iborra-Bernad, C.; García-Segovia, P.; Martínez-Monzó, J. Effect of Konjac Glucomannan (KGM) and Carboxymethylcellulose (CMC) on some Physico-Chemical and Mechanical Properties of Restructured Gilthead Sea Bream (Sparus aurata) Products. Food Bioprocess Technol. 2013, 6, 133–145. [Google Scholar] [CrossRef]
- Li, T.; Rui, X.; Li, W.; Chen, X.; Jiang, M.; Dong, M. Water distribution in tofu and application of T2 relaxation measurements in determination of Tofu’s water holding capacity. J. Agric. Food Chem. 2014, 62, 8594–8601. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Wang, J.Z.; Zhang, C.H.; Sun, H.M.; Wang, C.Q.; Xie, X.L. Effects of oxidation on water distribution and physicochemical properties of porcine myofibrillar protein gel. Food Biophys. 2014, 9, 169–178. [Google Scholar] [CrossRef]
- Marín, D.; Alemán, A.; Montero, P.; Gómez-Guillén, M.C. Protein aggregation, water binding and thermal gelation of saltground hake muscle in the presence of wet and dried soy phosphatidylcholine liposomes. Food Hydrocoll. 2018, 82, 466–477. [Google Scholar] [CrossRef] [Green Version]
- Jiao, X.; Cao, H.; Fan, D.; Huang, J.; Zhao, J.; Yan, B.; Zhou, W.; Zhang, W.; Ye, W.; Zhang, H. Effects of fish oil incorporation on the gelling properties of silver carp surimi gel subjected to microwave heating combined with conduction heating treatment. Food Hydrocoll. 2019, 94, 164–173. [Google Scholar] [CrossRef]
- Li, M.; Li, B.; Zhang, W. Rapid and non-invasive detection and imaging of the hydrocolloid-injected prawns with low-field NMR and MRI. Food Chem. 2018, 242, 16–21. [Google Scholar] [CrossRef]
- Xu, L.; Wang, J.; Lv, Y.; Su, Y.; Chang, C.; Gu, L.; Yang, Y.; Li, J. Influence of konjac glucomannan on the emulsion-filled/non-filled chicken gel: Study on intermolecular forces, microstructure and gelling properties. Food Hydrocoll. 2022, 124, 107269. [Google Scholar] [CrossRef]
- Li, Z.; Wang, J.; Zheng, B.; Guo, Z. Effects of high pressure processing on gelation properties and molecular forces of myosin containing deacetylated konjac glucomannan. Food Chem. 2019, 291, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Jin, X.; Zhang, L.; Chen, X.D. Investigation on water status and distribution in broccoli and the effects of drying on water status using NMR and MRI methods. Food Res. Int. 2017, 96, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Yin, T.; Xiong, S.; You, J.; Hu, Y.; Huang, Q. Gelling properties of silver carp surimi incorporated with konjac glucomannan: Effects of deacetylation degree. Int. J. Biol. Macromol. 2021, 191, 925–933. [Google Scholar] [CrossRef] [PubMed]


| Samples | Mw (kDa) | Polydisversity (Mw/Mn) | Root Mean Square Radius (nm) | Intrinsic Viscosity (dL/g) |
|---|---|---|---|---|
| KGM | 894.60 ± 21.20 e | 1.06 | 108.10 ± 1.50 d | 1832.00 ± 2.29 e |
| K1 | 149.03 ± 1.91 d | 1.14 | 45.20 ± 1.17 c | 397.75 ± 9.55 d |
| K2 | 128.70 ± 5.90 c | 1.15 | 42.20 ± 1.29 b | 303.55 ± 5.42 c |
| K3 | 118.75 ± 2.76 b | 1.20 | 42.00 ± 1.13 b | 188.70 ± 8.16 b |
| K4 | 36.48 ± 1.23 a | 1.34 | 31.25 ± 1.12 a | 36.02 ± 0.67 a |
| Samples | Hardness (g) | Springiness | Cohesiveness | Chewiness (g) | Resilience |
|---|---|---|---|---|---|
| C | 3785.94 ± 95.63 c | 0.887 ± 0.024 a | 0.756 ± 0.010 a | 2539.07 ± 81.93 bc | 0.432 ± 0.008 a |
| SK1 | 4028.11 ± 89.38 b | 0.873 ± 0.041 a | 0.754 ± 0.007 a | 2649.88 ± 97.90 b | 0.421 ± 0.008 b |
| SK2 | 4348.46 ± 192.02 a | 0.884 ± 0.025 a | 0.742 ± 0.008 b | 2855.24 ± 174.84 a | 0.413 ± 0.007 b |
| SK3 | 3476.50 ± 218.31 c | 0.900 ± 0.030 a | 0.752 ± 0.005 ab | 2348.26 ± 96.065 c | 0.412 ± 0.005 b |
| SK4 | 2869.20 ± 182.68 d | 0.892 ± 0.023 a | 0.7528 ± 0.005 a | 1925.33 ± 97.83 d | 0.414 ± 0.007 b |
| Samples | Breaking Force (g) | Deformation (cm) | Gel Strength (g.cm) | Whiteness | WHC |
|---|---|---|---|---|---|
| C | 361.09 ± 16.13 c | 0.83 ± 0.03 a | 301.24 ± 26.30 c | 81.88 ± 0.23 c | 76.36 ± 0.82% a |
| SK1 | 325.73 ± 13.78 bc | 1.08 ± 0.31 a | 342.40 ± 29.83 b | 82.41 ± 0.33 b | 69.54 ± 1.35% bc |
| SK2 | 305.04 ± 59.16 ab | 1.38 ± 0.21 b | 412.12 ± 10.46 a | 82.35 ± 0.19 b | 72.85 ± 2.52% ab |
| SK3 | 332.09 ± 6.42 bc | 0.81 ± 0.03 a | 270.10 ± 6.78 c | 83.02 ± 0.11 a | 73.07 ± 2.22% a |
| SK4 | 276.46 ± 15.21 a | 0.80 ± 0.05 a | 222.01 ± 22.09 d | 83.40 ± 0.14 a | 68.40 ± 2.18% c |
| Samples | T21 (ms) | PT21 (%) | T22 (ms) | PT22 (%) |
|---|---|---|---|---|
| C | 72.71 ± 0.38 a | 96.97 ± 0.06 a | 340.22 ± 23.35 a | 3.03 ± 0.06 d |
| SK1 | 67.65 ± 0.95 c | 95.08 ± 0.46 c | 290.42 ± 10.27 b | 4.92 ± 0.47 b |
| SK2 | 69.22 ± 0.35 b | 95.83 ± 0.39 b | 288.37 ± 10.79 b | 4.17 ± 0.39 c |
| SK3 | 72.44 ± 1.28 a | 94.46 ± 0.54 c | 277.42 ± 18.72 bc | 5.54 ± 0.53 b |
| SK4 | 73.75 ± 0.70 a | 93.63 ± 0.16 d | 250.33 ± 15.15 c | 6.37 ± 0.16 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, W.; Que, F.; Li, X.; Shi, L.; Deng, W.; Fu, X.; Xiong, G.; Sun, J.; Wang, L.; Xiong, S. Effects of Enzymatic Konjac Glucomannan Hydrolysates on Textural Properties, Microstructure, and Water Distribution of Grass Carp Surimi Gels. Foods 2022, 11, 750. https://doi.org/10.3390/foods11050750
Wu W, Que F, Li X, Shi L, Deng W, Fu X, Xiong G, Sun J, Wang L, Xiong S. Effects of Enzymatic Konjac Glucomannan Hydrolysates on Textural Properties, Microstructure, and Water Distribution of Grass Carp Surimi Gels. Foods. 2022; 11(5):750. https://doi.org/10.3390/foods11050750
Chicago/Turabian StyleWu, Wenjin, Feng Que, Xuehong Li, Liu Shi, Wei Deng, Xiaoyan Fu, Guangquan Xiong, Jing Sun, Lan Wang, and Shanbai Xiong. 2022. "Effects of Enzymatic Konjac Glucomannan Hydrolysates on Textural Properties, Microstructure, and Water Distribution of Grass Carp Surimi Gels" Foods 11, no. 5: 750. https://doi.org/10.3390/foods11050750
APA StyleWu, W., Que, F., Li, X., Shi, L., Deng, W., Fu, X., Xiong, G., Sun, J., Wang, L., & Xiong, S. (2022). Effects of Enzymatic Konjac Glucomannan Hydrolysates on Textural Properties, Microstructure, and Water Distribution of Grass Carp Surimi Gels. Foods, 11(5), 750. https://doi.org/10.3390/foods11050750






