Mineral Content and Volatile Profiling of Prunus avium L. (Sweet Cherry) By-Products from Fundão Region (Portugal)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Standards and Reagents
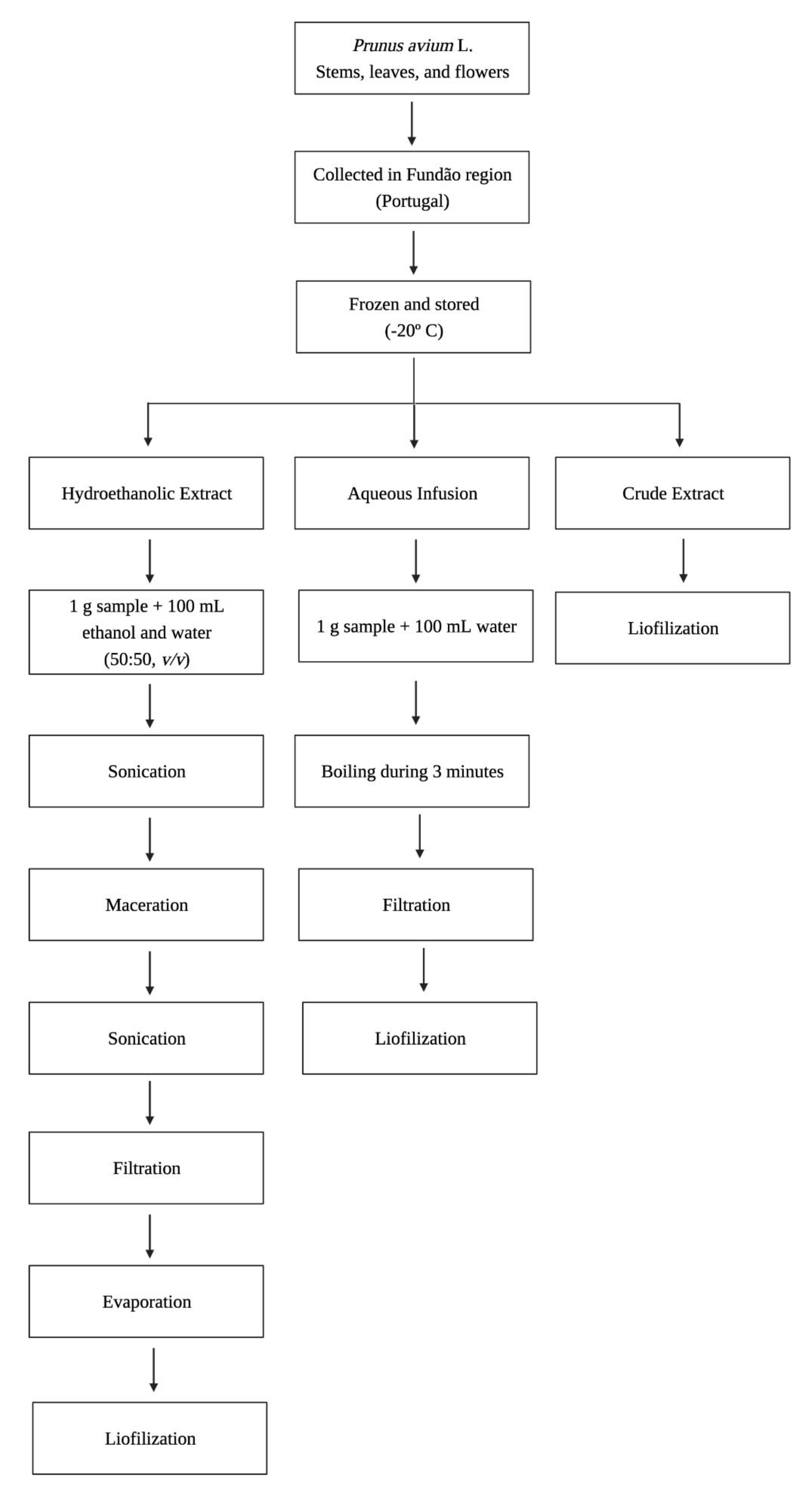
2.2. Plant Material and Extract Preparation
2.3. Mineral Analysis
2.3.1. Sample Digestion
2.3.2. ICP-MS Analysis
2.4. Volatile Organic Compound (VOC) Analysis
2.4.1. Volatile Extraction by Headspace Solid-Phase Microextraction (HS-SPME)
2.4.2. Gas Chromatography-Mass Spectrometry (GS-MS) Analysis
2.5. Statistical Analysis
3. Results and Discussion
3.1. Mineral Content
3.1.1. Essential Elements
3.1.2. Non-Essential Elements
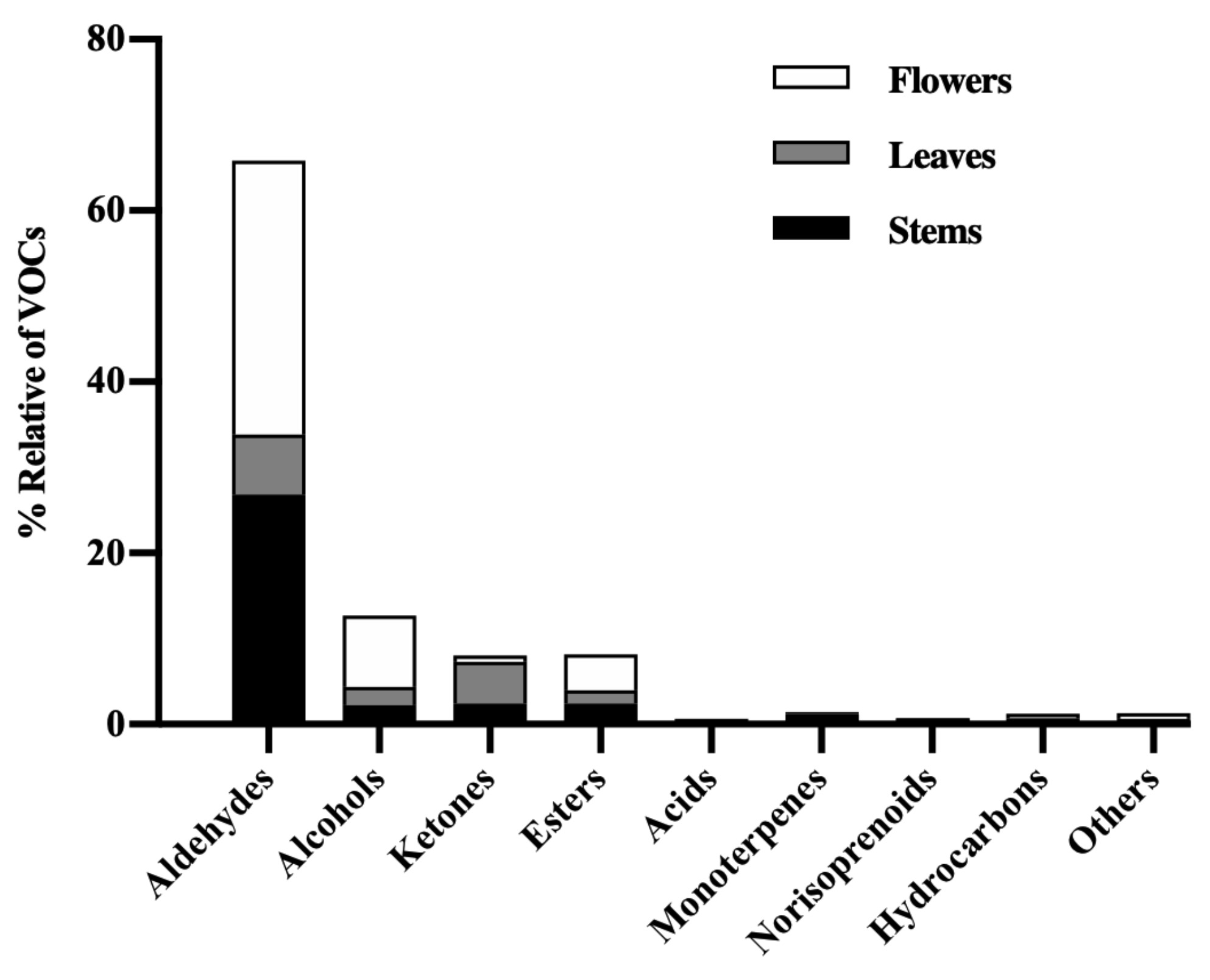
3.2. VOC Profiles
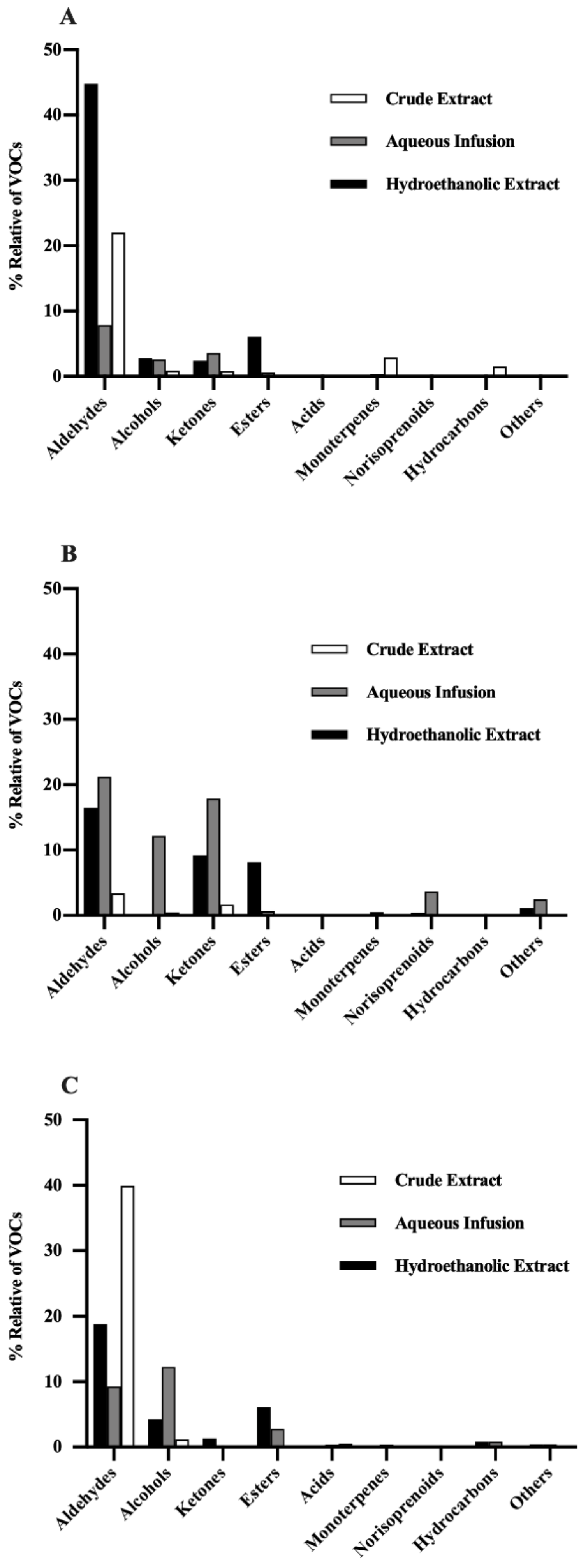
3.2.1. Aldehydes
3.2.2. Alcohols
3.2.3. Ketones
3.2.4. Esters
3.2.5. Acids, Monoterpenes, Norisoprenoids, and Hydrocarbons
3.2.6. Heterocyclics
3.2.7. Lactones
3.2.8. Phenols
3.2.9. Phenylpropenes
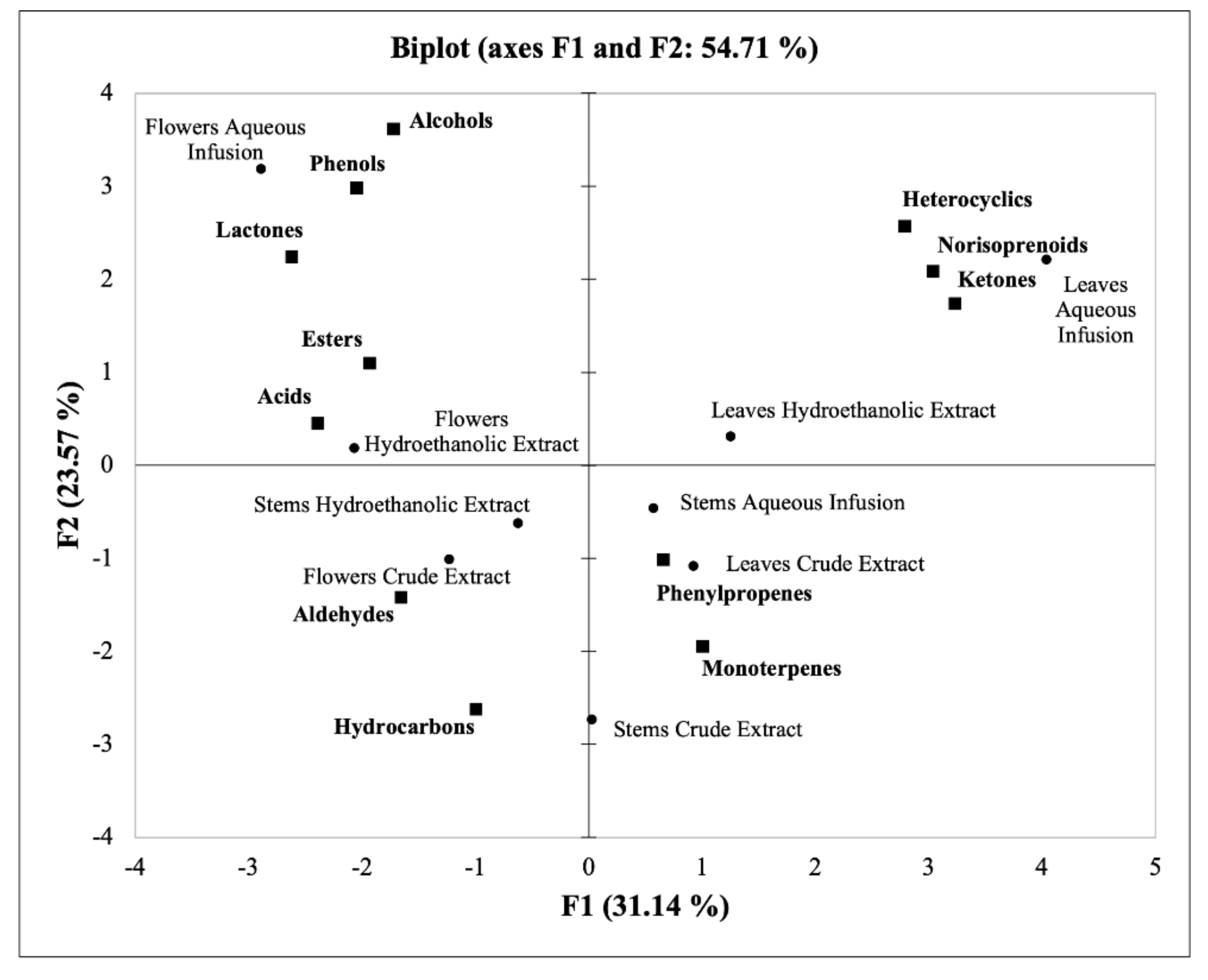
3.3. Principal Component Analysis (PCA)
| A (SD) c | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Stems | Leaves | Flowers | ||||||||||||||
| Compounds | RT | Most Abundant Ions (m/z) | RI a (Reported) | RI b (Calculated) | Rmatch | Hydroethanolic Extract | Aqueous Infusion | Crude Extract | Hydroethanolic Extract | Aqueous Infusion | Crude Extract | Hydroethanolic Extract | Aqueous Infusion | Crude Extract | Sensorial Description | |
| Aldehydes | ||||||||||||||||
| 1 | Crotonaldehyde L2 | 2.51 | 41/70 | 629 | - | 933 | nd | nd | 1.73 × 10+08 (1.54 × 10+08) | 1.33 × 10+09 (5.35 × 10+07) | nd | nd | nd | nd | nd | |
| 2 | 3-Methyl-butanal L2 | 2.56 | 44/58 | 652 | - | 653 | nd | nd | 3.04 × 10+08 (6.76 × 10+07) | nd | 5.47 × 10+08 (1.01 × 10+08) | nd | 1.60 × 10+10 (8.26 × 10+07) | 3.16 × 10+09 (8.56 × 10+08) | 7.59 × 10+08 (2.10 × 10+07) | Fatty, sour, and peach |
| 3 | 2-Methyl-butanal L2 | 2.65 | 57 | 662 | - | 841 | 3.61 × 10+08 (5.54 × 10+07) | nd | 4.88 × 10+08 (1.27 × 10+08) | 7.07 × 10+08 (2.99 × 10+08) | 1.27 × 10+09 (1.05 × 10+08) | 5.93 × 10+07 (7.72 × 10+06) | 2.62 × 10+09 (1.63 × 10+08) | 3.43 × 10+09 (2.07 × 10+09) | 1.21 × 10+09 (6.70 × 10+07) | Green, grass, and fruity |
| 4 | Pentanal L2 | 3.00 | 44/58 | 669 | - | 882 | nd | nd | 9.90 × 10+08 (9.38 × 10+08) | nd | nd | nd | 6.76 × 10+08 (8.50 × 10+08) | nd | 1.02 × 10+09 (5.00 × 10+08) | Almond, bitter, malt, oil, and pungent |
| 5 | 3-Methyl-2-butenal L2 | 4.41 | 55/84 | 782 | - | 949 | nd | nd | nd | nd | nd | 3.90 × 10+07 (1.30 × 10+07) | nd | nd | nd | Almond and roasted |
| 6 | Hexanal L2 | 4.74 | 44/56/72 | 800 | 804 | 954 | 1.35 × 10+10 (9.21 × 10+08) | 1.26 × 10+10 (9.48 × 10+08) | 3.11 × 10+10 (5.59 × 10+09) | 1.99 × 10+10 (1.90 × 10+09) | 3.52 × 10+10 (3.28 × 10+09) | 4.33 × 10+09 (3.40 × 10+08) | nd | 6.58 × 10+09 (3.98 × 10+09) | 2.34 × 10+10 (3.10 × 10+09) | Fatty, green, and grassy |
| 7 | 2-Methyl-2-pentenal L2 | 5.42 | 55/69/98 | 837 | 931 | 977 | nd | nd | nd | nd | 3.54 × 10+09 (4.16 × 10+08) | nd | nd | nd | nd | Fruity |
| 8 | (E)-2-Hexenal L2 | 5.98 | 55/69/83 | 854 | 850 | 941 | 1.03 × 10+09 (5.05 × 10+07) | 1.42 × 10+09 (3.70 × 10+07) | 6.20 × 10+09 (5.38 × 10+09) | 6.06 × 10+10 (3.03 × 10+09) | 3.21 × 10+10 (6.03 × 10+09) | 1.31 × 10+10 (1.40 × 10+09) | nd | nd | 1.75 × 10+09 (1.50 × 10+09) | Fresh, fruity, green |
| 9 | (Z)-2-Hexen-1-ol L2 | 6.30 | 57/67/82 | 868 | 866 | 909 | 7.08 × 10+08 (1.43 × 10+08) | nd | 1.27 × 10+10 (4.94 × 10+09) | nd | nd | nd | nd | nd | nd | Fruity, green, and wine |
| 10 | (E)-4-Hepten-1-al L2 | 7.19 | 55/67/84 | 900 | 900 | 936 | nd | nd | nd | nd | 1.91 × 10+09 (1.76 × 10+09) | nd | nd | nd | nd | Dairy |
| 11 | Heptanal L2 | 7.25 | 55/70/81 | 901 | 902 | 887 | 6.79 × 10+09 (6.19 × 10+09) | 1.48 × 10+09 (1.44 × 10+09) | nd | nd | 7.66 × 10+09 (1.04 × 10+10) | 2.15 × 10+08 (2.40 × 10+08) | nd | 9.45 × 10+09 (9.77 × 10+09) | 7.36 × 10+08 (1.30 × 10+09) | Fresh, green, and citrus |
| 12 | (E,E)-2,4-Hexadienal L2 | 7.48 | 81 | 911 | 910 | 905 | nd | nd | nd | nd | nd | 9.29 × 10+07 (6,72 × 10+06) | nd | nd | nd | |
| 13 | (Z)-2-Heptenal L2 | 8.84 | 55/83 | 958 | 956 | 938 | 1.82 × 10+10 (3.50 × 10+09) | 4.90 × 10+09 (9.00 × 10+08) | 1.24 × 10+10 (4.47 × 10+09) | 3.34 × 10+09 (2.44 × 10+09) | nd | 2.21 × 10+08 (3.00 × 10+07) | 2.00 × 10+10 (5.94 × 10+09) | nd | 1.67 × 10+09 (2.90 × 10+09) | Almond |
| 14 | Benzaldehyde L1 | 8.98 | 51/77/105 | 962 | 961 | 968 | 1.65 × 10+11 (1.65 × 10+11) | 5.33 × 10+10 (6.94 × 10+09) | 1.95 × 10+11 (9.61 × 10+10) | 3.29 × 10+09 (9.94 × 10+08) | 1.82 × 10+10 (1.04 × 10+10) | 9.19 × 10+08 (1.00 × 10+08) | 2.48 × 10+11 (2.90 × 10+10) | 4.28 × 10+09 (2.59 × 10+09) | 5.37 × 10+11 (2.30 × 10+11) | Almond and cherry |
| 15 | Octanal L1 | 10.22 | 57/69 | 1003 | 1003 | 946 | 4.20 × 10+09 (1.09 × 10+09) | 2.00 × 10+09 (2.35 × 10+08) | 2.54 × 10+09 (5.52 × 10+08) | nd | 1.06 × 10+09 (6.07 × 10+08) | 9.09 × 10+07 (3.70 × 10+07) | 1.56 × 10+09 (1.36 × 10+09) | 1.76 × 10+09 (1.41 × 10+09) | 2.06 × 10+09 (2.30 × 10+08) | Citrus, fatty, fruit, green, lemon, and honey |
| 16 | Phenylacetaldehyde L1 | 11.39 | 65/91 | 1045 | 1042 | 964 | nd | nd | nd | nd | nd | nd | nd | 1.56 × 10+09 (9.54 × 10+08) | nd | Berry, geranium, honey, nut, and pungent |
| 17 | Benzeneacetaldehyde | 11.40 | 65/91 | 1045 | 1042 | 940 | nd | nd | nd | nd | 2.57 × 10+09 (4.94 × 10+08) | nd | nd | nd | nd | Berry, geranium, honey, nut, and pungent |
| 18 | 4-Methyl-benzaldehyde L2 | 12.58 | 91/119 | 1079 | 1081 | 940 | 3.34 × 10+11 (5.62 × 10+11) | nd | nd | nd | nd | nd | nd | nd | nd | Flavoring agents |
| 19 | NonanalL1 | 13.26 | 55/77 | 1104 | 1104 | 913 | 1.28 × 10+10 (5.36 × 10+09) | 9.28 × 10+09 (9.45 × 10+08) | 1.16 × 10+10 (1.27 × 10+09) | 8.29 × 10+09 (1.40 × 10+09) | 1.16 × 10+10 (6.68 × 10+09) | 7.13 × 10+08 (3.20 × 10+08) | nd | 1.08 × 10+10 (8.65 × 10+09) | nd | Apple, citrus, fruity, grape, green, orange, and rose |
| 20 | Lilac aldehyde (isomer A) L2 | 14.32 | 43/55/93 | 1145 | 1140 | 955 | nd | nd | nd | nd | nd | nd | 2.04 × 10+09 (3.04 × 10+09) | 2.72 × 10+10 (2.12 × 10+10) | 4.82 × 10+10 (5.20 × 10+09) | |
| 21 | Lilac aldehyde (isomer B) L2 | 14.56 | 43/55/67/93 | 1154 | 1148 | 946 | 1.66 × 10+09 (2.28 × 10+08) | nd | nd | nd | nd | nd | 1.35 × 10+10 (8.63 × 10+09) | 6.06 × 10+10 (3.77 × 10+10) | 3.96 × 10+10 (3.80 × 10+10) | |
| 22 | Cucumber aldehyde L2 | 14.64 | 70 | 1155 | 1151 | 894 | nd | nd | nd | nd | nd | 4.27 × 10+07 (7.02 × 10+06) | nd | nd | nd | Cucumber, green, and wax |
| 23 | (E,Z)-2,6-nonadienal L2 | 14.65 | 70 | 1155 | 1153 | 905 | 3.00 × 10+08 (3.60 × 10+07) | nd | nd | nd | nd | nd | nd | nd | nd | Cucumber, green, and wax |
| 24 | (E)-2-Nonenal L2 | 14.87 | 55/70/83 | 1162 | 1159 | 893 | 2.60 × 10+09 (5.25 × 10+08) | nd | nd | 8.84 × 10+08 (1.32 × 10+08) | nd | nd | nd | nd | nd | Cucumber and green |
| 25 | Lilac aldehyde (isomer C) L2 | 14.99 | 55/93 | 1169 | 1163 | 945 | nd | nd | nd | nd | nd | nd | 7.66 × 10+09 (1.43 × 10+09) | 2.47 × 10+10 (1.53 × 10+10) | 2.12 × 10+10 (3.50 × 10+09) | |
| 26 | Safranal L2 | 16.01 | 91/107/121 | 1201 | 1198 | 933 | nd | nd | nd | nd | 7.84 × 10+09 (5.31 × 10+08) | nd | nd | nd | nd | Herb |
| 27 | Decanal L1 | 16.21 | 47/67 | 1206 | 1205 | 875 | 3.91 × 10+09 (6.50 × 10+08) | 9.22 × 10+09 (5.89 × 10+08) | 3.42 × 10+09 (3.13 × 10+08) | 4.42 × 10+09 (7.10 × 10+08) | nd | 1.47 × 10+08 (8.10 × 10+07) | nd | nd | 3.33 × 10+09 (4.00 × 10+08) | Floral, fried, orange peel, penetrating, and tallow |
| 28 | 2,5-Dimethylbenzaldehyde L2 | 16.47 | 77/105/133 | 1208 | 1214 | 928 | 8.24 × 10+08 (1.28 × 10+08) | 3.90 × 10+09 (2.44 × 10+08) | nd | nd | nd | nd | nd | nd | nd | |
| 29 | (Z)-2-Decenal L2 | 17.78 | 55/70/83 | 1252 | 1261 | 943 | 2.29 × 10+09 (5.55 × 10+08) | 9.45 × 10+08 (7.20 × 10+07) | nd | nd | nd | nd | nd | nd | nd | Orange and tallow |
| 30 | Citral L2 | 17.93 | 69 | 1276 | 1166 | 933 | nd | nd | nd | nd | 1.11 × 10+09 (3.45 × 10+08) | nd | nd | nd | nd | Lemon |
| 31 | p-Propylbenzaldehyde L2 | 18.12 | 91/119/148 | - | 1273 | 921 | 5.69 × 10+08 (1.75 × 10+08) | 1.39 × 10+09 (2.88 × 10+08) | 2.87 × 10+09 (1.78 × 10+08) | nd | 5.22 × 10+09 (1.16 × 10+09) | 2.43 × 10+08 (3.70 × 10+07) | nd | nd | 2.00 × 10+09 (2.00 × 10+08) | |
| Total aldehydes | 5.69 × 10+11 | 1.00 × 10+11 | 2.80 × 10+11 | 9.94 × 10+10 | 1.28 × 10+11 | 2.02 × 10+10 | 3.12 × 10+11 | 1.54 × 10+11 | 6.63 × 10+11 | |||||||
| Alcohols | ||||||||||||||||
| 32 | 1-Penten-3-ol L2 | 2.83 | 57 | 684 | - | 882 | nd | nd | nd | nd | 1.62 × 10+09 (1.67 × 10+08) | nd | nd | nd | nd | Butter, fish, green, oxidized, and wet earth |
| 33 | 3-Methyl-1-butanol L1 | 3.55 | 55/70 | 736 | - | 923 | nd | nd | nd | nd | nd | nd | 8.92 × 10+07 (1.29 × 10+08) | 1.87 × 10+09 (1.17 × 10+09) | nd | Burnt, cocoa, floral, and malt |
| 34 | 2-Methyl-1-butanol L2 | 3.61 | 43/73 | - | - | 941 | nd | nd | nd | nd | nd | nd | nd | 2.89 × 10+08 (1.87 × 10+08) | nd | Fish oil, green, malt, onion, and wine |
| 35 | (Z)-2-Penten-1-ol L2 | 4.14 | 57/68 | 767 | - | 917 | nd | nd | nd | nd | 7.20 × 10+08 (1.41 × 10+08) | 3.84 × 10+07 (9.49 × 10+06) | nd | nd | nd | Jasmin, green, plastic, and rubber |
| 36 | 3-Hexen-1-ol L1 | 6.03 | 55/67/82 | 857 | 855 | 926 | nd | 1.55 × 10+09 (1.72 × 10+09) | nd | nd | 2.41 × 10+09 (9.33 × 10+08) | nd | nd | nd | nd | Green |
| 37 | (Z)-2-Hexen-1-ol L2 | 6.30 | 57/67 | 868 | 866 | 863 | nd | nd | nd | nd | 1.18 × 10+09 (6.78 × 10+07) | nd | nd | nd | nd | |
| 38 | 2-Hexen-1-ol L2 | 6.31 | 57/67 | 862 | 866 | 963 | nd | 8.37 × 10+09 (6.53 × 10+09) | nd | nd | nd | nd | nd | nd | nd | Green |
| 39 | 1-Hexanol L1 | 6.40 | 56/69 | 868 | 870 | 884 | nd | 1.30 × 10+10 (1.60 × 10+09) | nd | nd | 1.85 × 10+10 (3.77 × 10+09) | 1.40 × 10+09 (1.20 × 10+09) | 2.88 × 10+09 (3.92 × 10+08) | nd | nd | Banana, flower, grass, and herb |
| 40 | 1-Octen-3-ol L2 | 9.54 | 43/55/57 | 980 | 980 | 882 | 7.02 × 10+09 (1.42 × 10+09) | 1.40 × 10+10 (2.60 × 10+09) | 9.36 × 10+09 (9.26 × 10+08) | nd | 2.87 × 10+09 (1.85 × 10+08) | 1.07 × 10+08 (9.30 × 10+07) | 7.89 × 10+09 (2.10 × 10+09) | 1.51 × 10+09 (9.34 × 10+08) | 7.34 × 10+09 (1.20 × 10+09) | Fruity, woody, green, mushroom, and nut |
| 41 | 2-Ethyl-1-hexanol L2 | 10.99 | 55/57 | 1030 | 1029 | 916 | 1.48 × 10+09 (1.29 × 10+09) | 1.60 × 10+10 (1.67 × 10+09) | nd | nd | 4.60 × 10+10 (5.27 × 10+10) | 9.96 × 10+08 (8.70 × 10+07) | 7.97 × 10+09 (3.05 × 10+08) | 4.35 × 10+09 (2.70 × 10+09 | 8.67 × 10+09 (1.10 × 10+09) | Oily, rose, and sweet |
| 42 | Benzyl alcohol L2 | 11.11 | 51/79/108 | 1036 | 1033 | 908 | 3.17 × 10+09 (5.57 × 10+08) | 2.37 × 10+09 (2.08 × 10+09) | 9.07 × 10+08 (1.27 × 10+09) | nd | nd | nd | nd | nd | nd | Berry, citrus, cherry, floral, and grape |
| 43 | Phenylethyl alcohol L1 | 13.45 | 91 | 1116 | 1112 | 906 | 7.19 × 10+08 (1.17 × 10+08) | 7.21 × 10+08 (6.27 × 10+08) | 5.52 × 10+08 (4.97 × 10+08) | nd | nd | 8.44 × 10+06 (1.50 × 10+07) | 1.13 × 10+10 (3.84 × 10+08) | 5.53 × 10+09 (3.47 × 10+09) | 3.70 × 10+09 (6.00 × 10+08) | Fruit, honey, lilac, rose, and wine |
| 44 | Lilac alcohol (isomer D) L2 | 16.04 | 55/93/111 | 1232 | 1199 | 950 | nd | nd | nd | nd | nd | nd | nd | nd | nd | |
| 45 | Lilac alcohol (isomer C) L2 | 16.42 | 43/55 | 1219 | 1212 | 953 | nd | nd | nd | nd | nd | nd | 2.03 × 10+10 (1.76E09) | 1.90 × 10+11 (1.32 × 10+11) | nd | |
| Total alcohols | 3.53 × 10+10 | 3.31 × 10+10 | 1.08 × 10+10 | 0.00 × 10+00 | 7.33 × 10+10 | 2.55 × 10+09 | 7.07 × 10+10 | 2.04 × 10+11 | 1.97 × 10+10 | |||||||
| Ketones | ||||||||||||||||
| 46 | 1-Penten-3-one L2 | 2.85 | 55 | 681 | - | 822 | nd | nd | nd | nd | nd | 2.79 × 10+08 (2.00 × 10+07) | 2.51 × 10+08 (2.18 × 10+08) | nd | nd | Fish, green, mustard, and pungent |
| 47 | 2-Pentanone L2 | 2.88 | 43/86 | 685 | - | 881 | nd | nd | nd | nd | nd | nd | nd | 1.94 × 10+08 (1.25 × 10+08) | nd | Fruit and pungent |
| 48 | 3-Penten-2-one L2 | 3.59 | 69/84 | 733 | - | 930 | nd | nd | 6.38 × 10+07 (1.11 × 10+08) | nd | nd | nd | nd | nd | nd | |
| 49 | 2-Heptanone L1 | 6.91 | 43/58 | 891 | 890 | 887 | nd | 1.66 × 10+09 (2.70 × 10+08) | 3.67 × 10+08 (3.19 × 10+08) | nd | 9.37 × 10+08 (1.04 × 10+08) | 6.05 × 10+07 (1.80 × 10+06) | 3.99 × 10+08 (8.94 × 10+07) | 8.71 × 10+08 (5.42 × 10+08) | 7.35 × 10+08 (9.70 × 10+07) | Blue cheese, fruit, green, nut, and spice |
| 50 | 4-Methyl-2-heptanone L2 | 8.25 | 43/58/85 | 943 | 936 | 923 | 2.20 × 10+08 (3.01 × 10+07) | nd | nd | nd | nd | nd | 4.99 × 10+07 (8.64 × 10+07) | 9.67 × 10+07 (1.19 × 10+08) | nd | |
| 51 | 6-Methyl-2-heptanone L2 | 8.74 | 43/58 | 956 | 953 | 804 | nd | nd | nd | nd | nd | 6.22 × 10+07 (2.34 × 10+06) | nd | 2.98 × 10+08 (1.81 × 10+08) | nd | |
| 52 | 1-Octen-3-one L2 | 9.43 | 43/55/70 | 979 | 976 | 905 | 2.97 × 10+10 (4.05 × 10+10) | nd | 4.62 × 10+09 (4.59 × 10+09) | nd | nd | nd | 2.09 × 10+10 (4.68 × 10+09) | nd | nd | Mushroom |
| 53 | 6-Methyl-5-heptene-2-one L2 | 9.66 | 43/58 | 986 | 984 | 912 | nd | 8.72 × 10+09 (1.40 × 10+10) | nd | 5.01 × 10+10 (4.81 × 10+09) | 9.20 × 10+10 (7.45 × 10+09) | 9.50 × 10+09 (1.20 × 10+09) | nd | nd | nd | Citrus, mushroom, pepper, rubber, and strawberry |
| 54 | 1,1,3-Trimethyl-2-cyclohexanone L2 | 11.15 | 51/55/82 | 1036 | 1034 | 851 | nd | nd | nd | 1.85 × 10+09 (5.48 × 10+07) | 1.48 × 10+09 (2.58 × 10+08) | nd | nd | nd | nd | |
| 55 | (E)-3-Octen-2-one L2 | 11.24 | 43/55/111 | 1033 | 1035 | 927 | nd | 3.56 × 10+09 (4.88 × 10+08) | 3.40 × 10+09 (2.95 × 10+09) | nd | nd | nd | nd | nd | 2.18 × 10+09 (2.40 × 10+08) | Dull, green, nut, and rose |
| 56 | 3,5,5-Trimethylcyclohex-2-en-1-one L2 | 11.87 | 54/82 | 1124 | 1058 | 869 | nd | nd | nd | nd | 1.81 × 10+09 (3.14 × 10+09) | nd | nd | nd | 1.34 × 10+09 (1.20 × 10+09) | Cedarwood and spice |
| 57 | Acetophenone L2 | 12.05 | 77/105 | 1065 | 1062 | 946 | 3.30 × 10+08 (4.53 × 10+07) | 3.01 × 10+10 (3.59 × 10+09) | 1.53 × 10+09 (4.17 × 10+08) | nd | nd | nd | nd | 1.29 × 10+09 (8.87 × 10+08) | nd | Almonds, flower, meat and must |
| 58 | 2-Nonanone L1 | 12.83 | 71 | - | 1092 | 1090 | nd | nd | nd | nd | nd | nd | nd | 2.89 × 10+08 (1.77 × 10+08) | nd | |
| 59 | 6-Methyl-3,5-heptadiene-2-one L2 | 13.18 | 43/79/109 | 1107 | 1101 | 933 | nd | nd | nd | nd | 1.43 × 10+09 (2.80 × 10+08) | nd | nd | nd | nd | Spice |
| 60 | Jasmone L2 | 21.28 | 43/79/91/104 | 1394 | 1391 | 798 | nd | 1.03 × 10+09 (8.93 × 10+08) | nd | nd | nd | nd | nd | nd | nd | |
| 61 | Nerylacetone L2 | 22.66 | 43/69 | 1453 | 1446 | 915 | nd | nd | 3.06 × 10+08 (5.30 × 10+08) | 3.48 × 10+09 (3.20 × 10+09) | 1.17 × 10+10 (1.81 × 10+09) | nd | nd | nd | nd | Fruit |
| Total ketones | 3.03 × 10+10 | 4.51 × 10+10 | 1.03 × 10+10 | 5.54 × 10+10 | 1.08 × 10+11 | 9.90 × 10+09 | 2.16 × 10+10 | 3.04 × 10+09 | 7.35 × 10+08 | |||||||
| Esters | ||||||||||||||||
| 62 | Ethyl acetate L2 | 2.20 | 43/45/61/70 | 612 | - | 933 | 8.57 × 10+09 (3.63 × 10+09) | 1.59 × 10+09 (1.92 × 10+09) | nd | 1.53 × 10+09 (3.22 × 10+08) | nd | nd | 1.74 × 10+10 (2.94 × 10+09) | nd | nd | Fruity, pineapple, and pleasant |
| 63 | Ethyl propanoate L2 | 3.17 | 57/77 | 709 | - | 828 | nd | nd | nd | 6.02 × 10+09 (9.80 × 10+09) | nd | nd | 4.16 × 10+09 (3.40 × 10+09) | nd | nd | Apple, pineapple, rum, and strawberry |
| 64 | Ethyl isobutyrate L1 | 3.93 | 43/71 | 755 | - | 924 | nd | nd | nd | nd | nd | nd | 1.43 × 10+09 (1.77 × 10+08) | nd | nd | |
| 65 | (Z)-Ethyl crotonate L2 | 5.77 | 69/99 | 830 | 845 | 871 | nd | nd | nd | nd | nd | nd | 7.32 × 10+08 (5.37 × 10+07) | nd | nd | Tropical fruit |
| 66 | Ethyl-2-methylbutanoate L1 | 5.89 | 57/102 | 849 | 850 | 968 | nd | nd | nd | nd | nd | nd | 4.72 × 10+09 (4.52 × 10+08) | nd | nd | |
| 67 | Ethyl isovalerate L1 | 6.00 | 57/88 | 854 | 854 | 892 | nd | nd | nd | nd | nd | nd | 8.28 × 10+09 (4.71 × 10+08) | nd | nd | Apple, fruit, pineapple, and sour |
| 68 | Isoamyl acetate L1 | 6.59 | 43/55/70 | 876 | 838 | 880 | nd | nd | nd | nd | nd | nd | nd | 1.91 × 10+08 (2.34 × 10+08) | nd | Apple, banana, glue, and pear |
| 69 | 2-Methylbutyl acetate L2 | 6.65 | 43/70 | 880 | 879 | 903 | nd | nd | nd | nd | nd | nd | 3.55 × 10+08 (2.15 × 10+08) | nd | Ester, fresh, fruit, and pineapple | |
| 70 | Ethyl pentanoate L2 | 7.24 | 57/85/133 | 900 | 902 | 897 | nd | nd | nd | nd | nd | nd | 1.32 × 10+10 (1.10 × 10+10) | nd | nd | Apple, dry fish, herb, nut, and yeast |
| 71 | Amyl acetate L2 | 7.60 | 43/55/70 | 828 | 914 | 828 | nd | nd | nd | nd | nd | nd | 2.76 × 10+08 (9.83 × 10+07) | nd | nd | Banana |
| 72 | Methyl hexanoate L2 | 7.89 | 43/74/87 | 925 | 924 | 846 | nd | nd | 1.88 × 10+08 (1.65 × 10+08) | nd | nd | 2.93 × 10+07 (2.80 × 10+07) | nd | 5.62 × 10+08 (3.40 × 10+08) | 8.27 × 10+08 (7.11 × 10+06) | Ester, fresh, fruit, and pineapple |
| 73 | Methyl hexanoate L2 | 7.90 | 43/59/74 | 925 | 924 | 874 | nd | 3.12 × 10+08 (3.20 × 10+08) | nd | nd | nd | nd | nd | nd | nd | Ester, fresh, fruit, and pineapple |
| 74 | Ethyl (E)-2-pentenoate L2 | 8.59 | 55/83/99 | - | 948 | 908 | nd | nd | nd | nd | nd | nd | 3.45 × 10+08 (5.98 × 10+08) | nd | nd | |
| 75 | Ethyl hexanoate L1 | 10.10 | 43/70/88/99 | 1000 | 999 | 911 | 3.34 × 10+10 (8.90 × 10+08) | nd | nd | 3.54 × 10+10 (3.33 × 10+09) | nd | 1.19 × 10+08 (3.30 × 10+07) | 4.13 × 10+10 (1.70 × 10+08) | nd | nd | Apple peel, brandy, fruit gum, overripe fruit, and pineapple |
| 76 | Ethyl 2-hexenoate L2 | 11.43 | 55/97 | 1037 | 1043 | 875 | nd | nd | nd | nd | nd | nd | 4.13 × 10+09 (1.70 × 10+08) | nd | nd | |
| 77 | Methyl benzoate L2 | 12.91 | 51/77/105/136 | 1094 | 1092 | 886 | nd | 5.76 × 10+09 (2.80 × 10+08) | nd | nd | nd | nd | nd | nd | nd | Herb, lettuce, prune, and violet |
| 78 | Ethyl heptanoate L1 | 13.04 | 43/88 | 1097 | 1097 | 921 | 2.56 × 10+09 (2.31 × 10+08) | nd | nd | 3.38 × 10+09 (4.04 × 10+09) | nd | nd | 1.10 × 10+09 (5.18 × 10+08) | nd | nd | Brandy, fruit, and wine |
| 79 | Ethyl benzoate L2 | 15.17 | 77/105 | 1171 | 1169 | 933 | 1.30 × 10+10(9.88 × 10+09) | nd | 1.32 × 10+09 (9.99 × 10+07) | nd | nd | nd | nd | 2.73 × 10+10 (3.55 × 10+09) | nd | Chamomile, celery, fat, flower, and fruit |
| 80 | Diethyl succinate L1 | 15.41 | 101/129 | 1182 | 1177 | 885 | nd | nd | nd | nd | nd | nd | nd | 5.93 × 10+08 (1.03 × 10+09) | nd | Cotton, fabric, floral, fruit, and wine |
| 81 | Methyl salicylate L2 | 15.81 | 92/120/152 | 1192 | 1191 | 910 | nd | nd | nd | nd | 3.30 × 10+09 (8.01 × 10+08) | 1.32 × 10+08 (1.70 × 10+07) | 3.30 × 10+09 (8.01 × 10+08) | nd | nd | |
| 82 | Ethyl octanoate L1 | 15.97 | 47/88/101 | 1196 | 1197 | 919 | 1.51 × 10+10 (1.30 × 10+10) | nd | nd | 1.54 × 10+09 (1.78 × 10+08) | nd | 2.61 × 10+07 (9.27 × 10+06) | nd | 5.58 × 10+09 (3.38 × 10+09) | nd | Apricot, brandy, fat, floral, and pineapple |
| 83 | Ethyl phenylacetate L1 | 17.21 | 65/91/164 | 1246 | 1241 | 907 | nd | nd | nd | nd | nd | nd | nd | 9.67 × 10+09 (7.77 × 10+08) | nd | Floral, fruit, honey, and rose |
| 84 | Ethyl (E)-2-octenoate L2 | 17.35 | 55/73/125 | 1249 | 1246 | 903 | nd | nd | nd | nd | nd | nd | nd | 2.43 × 10+09 (1.22 × 10+08) | nd | Fruit |
| 85 | Ethyl nonanoate L1 | 18.70 | 88/101 | 1296 | 1294 | 903 | 4.41 × 10+09 (5.27 × 10+08) | nd | nd | 1.13 × 10+09 (2.85 × 10+08) | nd | nd | nd | nd | nd | Floral |
| 86 | Ethyl phenylpropanoate L2 | 20.10 | 91/104/178 | 1353 | 1347 | 799 | nd | nd | nd | nd | nd | nd | 8.73 × 10+07 (1.51 × 10+08) | nd | nd | Flower and honey |
| 87 | Ethyl undecanoate L2 | 23.8 | 43/88/101 | 1494 | 1492 | 881 | nd | nd | nd | nd | nd | nd | 3.62 × 10+08 (1.49 × 10+08) | nd | nd | Coconut and cognac |
| 88 | Methyl dihydrojasmonate L2 | 27.42 | 83 | 1649 | 1646 | 836 | 1.34 × 10+08 (6.74 × 10+07) | nd | nd | nd | 2.10 × 10+08 (4.74 × 10+06) | nd | nd | nd | nd | Floral and jasmine |
| 89 | Ethyl myristate L2 | 30.60 | 88/101 | 1794 | 1793 | 882 | nd | nd | nd | nd | nd | nd | 8.04 × 10+08 (2.12 × 10+08) | nd | nd | Wax |
| 90 | Isopropyl myristate L2 | 31.21 | 43/60/228 | 1827 | 1822 | 873 | 1.73 × 10+08 (5.13 × 10+07) | 2.49 × 10+08 (7.47 × 10+06) | 1.32 × 10+08 (2.67 × 10+07) | 1.20 × 10+08 (4.77 × 10+07) | 2.96 × 10+08 (1.14 × 10+08) | 1.22 × 10+07 (1.18 × 10+06) | 1.15 × 10+08 (2.24 × 10+07) | 1.09 × 10+08 (6.73 × 10+07) | 1.28 × 10+08 (6.60 × 10+07) | Dairy |
| 91 | Ethyl palmitate L2 | 34.56 | 88/101 | 1993 | 1993 | 906 | nd | nd | nd | nd | nd | nd | 4.19 × 10+09 (1.18 × 10+09) | nd | nd | Wax |
| Total esters | 7.73 × 10+10 | 7.91 × 10+09 | 1.64 × 10+09 | 4.91 × 10+10 | 3.81 × 10+09 | 3.19 × 10+08 | 1.01 × 10+11 | 4.68 × 10+10 | 9.55 × 10+08 | |||||||
| Acids | ||||||||||||||||
| 92 | Acetic acid L2 | 3.14 | 43/45/60 | 610 | - | 926 | nd | 8.95 × 10+08 (7.76 × 10+08) | 1.39 × 10+09 (4.33 × 10+08) | nd | 3.19 × 10+08 (2.80 × 10+08) | nd | nd | 1.33 × 10+09 (8.56 × 10+08) | 4.88 × 10+09 (1.90 × 10+09) | Acid, fruit, pungent, sour, and vinegar |
| 93 | Octanoic acid L2 | 15.30 | 43/55/60/73 | 1180 | 1174 | 869 | nd | nd | nd | nd | nd | nd | 2.53 × 10+09 (1.98 × 10+09) | nd | nd | Cheese, fat, grass, and oil |
| 94 | Nonanoic acid L2 | 17.97 | 69/73/92/120 | 1273 | 1268 | 913 | 8.91 × 10+08 (1.48 × 10+09) | 6.33 × 10+08 (6.58 × 10+08) | 3.81 × 10+08 (4.74 × 10+09) | 1.06 × 10+09 (5.34 × 10+08) | nd | 9.53 × 10+07 (9.90 × 10+07) | 4.03 × 10+09 (1.10 × 10+09) | Fat, green, and sour | ||
| 95 | Decanoic acid L2 | 30.55 | 60/73/129 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 4.29 × 10+09 (5.26 × 10+09) | nd | Dust, fat, and grass |
| Total acids | 8.91 × 10+08 | 1.53 × 10+09 | 1.77 × 10+09 | 1.06 × 10+09 | 3.19 × 10+08 | 9.53 × 10+07 | 2.53 × 10+09 | 5.62 × 10+09 | 8.91 × 10+09 | |||||||
| Monoterpenes | ||||||||||||||||
| 96 | Limonene L1 | 11.01 | 67/79/93 | 1030 | 1029 | 904 | nd | nd | 3.49 × 10+10 (3.23 × 10+09) | nd | nd | nd | nd | nd | nd | Coriander, floral, lavender, lemon, and rose |
| 97 | Terpinolene L2 | 12.71 | 79/93/121/136 | 1088 | 1086 | 953 | nd | nd | 1.87 × 10+09 (1.70 × 10+09) | nd | nd | nd | nd | nd | nd | Pine |
| 98 | Linalool L1 | 13.12 | 55/71/93 | 1099 | 1099 | 855 | nd | nd | nd | nd | 2.98 × 10+09 (4.99 × 10+08) | nd | nd | 5.58 × 10+09 (3.49 × 10+09) | nd | |
| 99 | α-Terpineol L2 | 15.91 | 59/93/121 | 1189 | 1194 | 926 | nd | 4.49 × 10+09 (2.56 × 10+08) | nd | nd | nd | nd | nd | nd | nd | Anise, fresh, mint, and oil |
| Total monoterpenes | 0.00 × 10+00 | 4.49 × 10+09 | 3.68 × 10+10 | 0.00 × 10+00 | 2.98 × 10+09 | 0.00 × 10+00 | 0.00 × 10+00 | 5.58 × 10+09 | 0.00 × 10+00 | |||||||
| Norisoprenoids | ||||||||||||||||
| 100 | β-Cyclocitral L1 | 16.59 | 67/81/109/137/152 | 1220 | 1218 | 940 | nd | nd | nd | 1.90 × 10+09 (2.76 × 10+08) | 1.45 × 10+10 (1.13 × 10+09) | 5.81 × 10+08 (5.40 × 10+07) | nd | nd | nd | |
| 101 | α-Ionone L1 | 22.04 | 43/93 | 1426 | 1421 | 795 | nd | 2.49 × 10+08 (7.47+06) | nd | 5.00 × 10+08 (1.26 × 10+08) | 2.03 × 10+09 (1.32 × 10+08) | 5.60 × 10+07 (2.81+06) | nd | nd | nd | Floral and violet |
| 102 | β-Ionone L2 | 23.46 | 43/93/177 | 1491 | 1478 | 878 | nd | nd | nd | nd | 5.53 × 10+09 (2.57 × 10+08) | 2.61 × 10+08 (1.40 × 10+07) | nd | nd | nd | Floral and violet |
| Total norisoprenoids | 0.00 × 10+00 | 2.49 × 10+08 | 0.00 × 10+00 | 2.40 × 10+09 | 2.21 × 10+10 | 8.98 × 10+08 | 0.00 × 10+00 | 0.00 × 10+00 | 0.00 × 10+00 | |||||||
| Hydrocarbons | ||||||||||||||||
| 103 | 2,4-Dimethyl-1-heptene L2 | 5.66 | 43/55/70 | 836 | 841 | 965 | nd | nd | 2.62 × 10+09 (3.45 × 10+08) | nd | nd | 8.68 × 10+08 (1.90 × 10+08) | 3.82 × 10+09 (2.96 × 10+08) | nd | 3.36 × 10+09(5.60 × 10+08) | |
| 104 | 1,3-Dimethylheptane L2 | 6.26 | 43/71/85 | 863 | 864 | 943 | nd | nd | nd | nd | nd | nd | 1.27 × 10+09 (3.75 × 10+07) | nd | nd | |
| 105 | Styrene L2 | 6.98 | 51/78/104 | 893 | 892 | 937 | 2.80 × 10+09 (3.59 × 10+08) | 2.47 × 10+09 (2.95 × 10+08) | 4.25 × 10+09 (5.50 × 10+08) | nd | nd | 4.56 × 10+07 (4.30 × 10+07) | 8.77 × 10+09 (6.85 × 10+09) | nd | nd | |
| 106 | o-Cymene L2 | 10.87 | 91/119 | 1022 | 1025 | 955 | nd | nd | 1.26 × 10+10 (7.35 × 10+09) | nd | nd | nd | nd | nd | nd | |
| Total hydrocarbons | 2.80 × 10+09 | 2.47 × 10+09 | 1.95 × 10+10 | 0.00 × 10+00 | 0.00 × 10+00 | 9.14 × 10+08 | 1.39 × 10+10 | 0.00 × 10+00 | 3.36 × 10+09 | |||||||
| Heterocyclics | ||||||||||||||||
| 107 | 2-Methylfuran L2 | 2.19 | 43/53/82 | 606 | - | 826 | nd | nd | nd | 1.00 × 10+09 (1.68 × 10+08) | 4.18 × 10+08 (3.60 × 10+07) | nd | nd | nd | nd | |
| 108 | Methyltetrahydrofuran L2 | 2.70 | 43/71 | 674 | - | 876 | nd | nd | nd | 2.86 × 10+08 (5.87 × 10+07) | nd | nd | nd | nd | ||
| 109 | 2-Ethylfuran L2 | 3.03 | 53/81 | 703 | - | 960 | nd | nd | nd | 3.94 × 10+09 (4.06 × 10+09) | 1.42 × 10+10 (3.72 × 10+09) | 1.27 × 10+09 (1.30 × 10+09) | nd | nd | nd | Butter and caramel |
| 110 | 2,4-Dimethylfuran L2 | 3.22 | 53/67/96 | 729 | - | 850 | nd | nd | nd | nd | nd | nd | nd | 2.77 × 10+08 (1.85 × 10+08) | nd | |
| 110 | 2,4-Dimethylfuran L2 | 3.22 | 53/67/96 | 729 | - | 850 | nd | nd | nd | nd | nd | nd | nd | 2.77 × 10+08 (1.85 × 10+08) | nd | |
| 111 | Furfural L1 | 5.44 | 95 | 833 | 832 | 878 | nd | nd | nd | nd | nd | nd | nd | 2.30 × 10+09 (1.39 × 10+09) | 2.50 × 10+09 (7.00 × 10+07) | Almond, baked potatoes, bread, burnt, and spice |
| 112 | 2,3-Dimethylpyrazine L2 | 7.66 | 67/108 | 925 | 916 | 905 | nd | nd | nd | nd | nd | nd | nd | 3.80 × 10+08 (2.49 × 10+08) | nd | Caramel, cocoa, hazelnut, peanut butter, and roasted |
| 113 | 2-Penthylfuran L2 | 9.84 | 81 | 993 | 990 | 924 | nd | nd | nd | 1.70 × 10+09 (1.48 × 10+09) | nd | nd | nd | nd | nd | Butter, floral, fruit, and green bean |
| Total heterocyclics | 0.00 × 10+00 | 0.00 × 10+00 | 0.00 × 10+00 | 6.64 × 10+09 | 1.49 × 10+10 | 1.27 × 10+09 | 0.00 × 10+00 | 2.96 × 10+09 | 2.50 × 10+09 | |||||||
| Lactones | ||||||||||||||||
| 114 | 4-Methyl-4-vinylbutyrolactone L2 | 11.17 | 55/67/111 | 1043 | 1035 | 923 | nd | nd | nd | nd | nd | nd | 6.54 × 10+09 (7.67 × 10+08) | 5.74 × 10+09 (3.60 × 10+09) | nd | |
| Total lactones | 0.00 × 10+00 | 0.00 × 10+00 | 0.00 × 10+00 | 0.00 × 10+00 | 0.00 × 10+00 | 0.00 × 10+00 | 6.54 × 10+09 | 5.74 × 10+09 | 0.00 × 10+00 | |||||||
| Phenols | ||||||||||||||||
| 115 | 4-Ethylphenol L2 | 15.16 | 107/122 | 1169 | 1169 | 921 | nd | nd | nd | nd | nd | nd | nd | 4.43 × 10+09 (3.09 × 10+09) | nd | Leather, phenol, spice, and stable |
| Total phenols | 0.00 × 10+00 | 0.00 × 10+00 | 0.00 × 10+00 | 0.00 × 10+00 | 0.00 × 10+00 | 0.00 × 10+00 | 0.00 × 10+00 | 4.43 × 10+09 | 0.00 × 10+00 | |||||||
| Phenylpropenes | ||||||||||||||||
| 116 | Estragole L2 | 15.99 | 51/77/91/121/133/148 | 1196 | 1197 | 883 | nd | nd | nd | nd | nd | 6.45 × 10+07 (5.70 × 10+07) | nd | nd | nd | Anise and licorice |
| 117 | Apioline L2 | 27.99 | 149/222 | 1682 | 1672 | 865 | nd | nd | nd | nd | nd | 5.12 × 10+06 (8.88 × 10+06) | nd | nd | nd | |
| Total phenylpropenes | 0.00 × 10+00 | 0.00 × 10+00 | 0.00 × 10+00 | 0.00 × 10+00 | 0.00 × 10+00 | 6.96 × 10+07 | 0.00 × 10+00 | 0.00 × 10+00 | 0.00 × 10+00 | |||||||
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dias, T.R.; Bernardino, R.L.; Meneses, M.J.; Sousa, M.; Sá, R.; Alves, M.G.; Silva, B.M.; Oliveira, P.F. Emerging Potential of Natural Products as an Alternative Strategy to Pharmacological Agents Used Against Metabolic Disorders. Curr. Drug Metab. 2016, 17, 582–597. [Google Scholar] [CrossRef] [PubMed]
- Nunes, A.R.; Gonçalves, A.C.; Falcão, A.; Alves, G.; Silva, L.R. Prunus avium L. (Sweet Cherry) By-Products: A Source of Phenolic Compounds with Antioxidant and Anti-Hyperglycemic Properties—A Review. Appl. Sci. 2021, 11, 8516. [Google Scholar] [CrossRef]
- Gonçalves, A.C.A.; Bento, C.; Silva, B.; Simões, M.; Silva, L. Nutrients, Bioactive Compounds and Bioactivity: The Health Benefits of Sweet Cherries (Prunus avium L.). Curr. Nutr. Food Sci. 2019, 15, 208–227. [Google Scholar] [CrossRef]
- Maatallah, S.; Dabbou, S.; Castagna, A.; Guizani, M.; Hajlaoui, H.; Ranieri, A.M.; Flamini, G. Prunus persica by-products: A source of minerals, phenols and volatile compounds. Sci. Hortic. 2019, 261, 109016. [Google Scholar] [CrossRef]
- Gonçalves, A.C.; Campos, G.; Pinto, E.; Oliveira, A.S.; Almeida, A.; de Pinho, P.G.; Alves, G.; Silva, L.R. Essential and non-essential elements, and volatile organic compounds for the discrimination of twenty-three sweet cherry cultivars from Fundão, Portugal. Food Chem. 2021, 367, 130503. [Google Scholar] [CrossRef] [PubMed]
- Pinto, E.; Almeida, A.; Ferreira, I.M. Essential and non-essential/toxic elements in rice available in the Portuguese and Spanish markets. J. Food Compos. Anal. 2016, 48, 81–87. [Google Scholar] [CrossRef]
- Kuppusamy, S.; Thavamani, P.; Megharaj, M.; Nirola, R.; Lee, Y.B.; Naidu, R. Assessment of antioxidant activity, minerals, phenols and flavonoid contents of common plant/tree waste extracts. Ind. Crops Prod. 2016, 83, 630–634. [Google Scholar] [CrossRef]
- Zhang, X.; Ahuja, J.K.; Burton-Freeman, B.M. Characterization of the nutrient profile of processed red raspberries for use in nutrition labeling and promoting healthy food choices. Nutr. Health Aging 2019, 5, 225–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Aguilar, L.; Rojas-Molina, A.; Ibarra-Alvarado, C.; Rojas-Molina, J.I.; Vázquez-Landaverde, P.A.; Luna-Vázquez, F.J.; Zavala-Sánchez, M.A. Nutritional Value and Volatile Compounds of Black Cherry (Prunus serotina) Seeds. Molecules 2015, 20, 3479–3495. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, A.; Silva, L.; de Pinho, P.G.; Gil-Izquierdo, A.; Valentão, P.; Silva, B.M.; Pereira, J.A.; Andrade, P.B. Volatile profiling of Ficus carica varieties by HS-SPME and GC–IT-MS. Food Chem. 2010, 123, 548–557. [Google Scholar] [CrossRef]
- de Pinho, P.G.; Ribeiro, B.; Gonçalves, R.F.; Baptista, P.; Valentão, P.; Seabra, R.M.; Andrade, P.B. Correlation between the Pattern Volatiles and the Overall Aroma of Wild Edible Mushrooms. J. Agric. Food Chem. 2008, 56, 1704–1712. [Google Scholar] [CrossRef] [PubMed]
- Lanciotti, R.; Gianotti, A.; Patrignani, F.; Belletti, N.; Guerzoni, M.; Gardini, F. Use of natural aroma compounds to improve shelf-life and safety of minimally processed fruits. Trends Food Sci. Technol. 2004, 15, 201–208. [Google Scholar] [CrossRef]
- Tylewicz, U.; Inchingolo, R.; Rodriguez-Estrada, M.T. Food aroma compounds. In Nutraceutical and Functional Food Components—Effects of Innovative Processing Techniques, 1st ed.; Galanakis, C., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 295–332. [Google Scholar]
- Desbois, A.P.; Smith, V.J. Antibacterial free fatty acids: Activities, mechanisms of action and biotechnological potential. Appl. Microbiol. Biotechnol. 2009, 85, 1629–1642. [Google Scholar] [CrossRef] [Green Version]
- Shyr, T.W.; Shie, J.W.; Jiang, C.H.; Li, J.J. A Textile-Based Wearable Sensing Device Designed for Monitoring the Flexion Angle of Elbow and Knee Movements. Sensors 2014, 14, 4050–4059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ludwig, I.A.; Sánchez, L.; De Peña, M.; Cid, C. Contribution of volatile compounds to the antioxidant capacity of coffee. Food Res. Int. 2014, 61, 67–74. [Google Scholar] [CrossRef]
- García-Oliveira, P.; Fraga-Corral, M.; Pereira, A.G.; Prieto, M.A.; Simal-Gandara, J. Solutions for the sustainability of the food production and consumption system. Crit. Rev. Food Sci. Nutr. 2020, 62, 1–17. [Google Scholar] [CrossRef]
- Krivokapić, S.; Vlaović, M.; Vratnica, B.D.; Perović, A.; Perović, S. Biowaste as a Potential Source of Bioactive Compounds—A Case Study of Raspberry Fruit Pomace. Foods 2021, 10, 706. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.C.; Rodrigues, M.; Santos, A.O.; Alves, G.; Silva, L.R. Antioxidant Status, Antidiabetic Properties and Effects on Caco-2 Cells of Colored and Non-Colored Enriched Extracts of Sweet Cherry Fruits. Nutrients 2018, 10, 1688. [Google Scholar] [CrossRef] [Green Version]
- U.S.D. of Agriculture, Noncitrus Fruits and Nuts 2020 Summary. 2020. Available online: https://downloads.usda.library.cornell.edu/usda-esmis/files/zs25x846c/sf269213r/6t054c23t/ncit0521.pdf (accessed on 14 November 2021).
- Gonçalves, A.C.A.; Campos, G.; Alves, G.; Garcia-Viguera, C.; Moreno, D.A.; Silva, L.R. Physical and phytochemical composition of 23 Portuguese sweet cherries as conditioned by variety (or genotype). Food Chem. 2020, 335, 127637. [Google Scholar] [CrossRef]
- Nunes, A.; Gonçalves, A.; Alves, G.; Falcão, A.; Garcia-Viguera, C.; Moreno, D.A.; Silva, L. Valorisation of Prunus avium L. By-Products: Phenolic Composition and Effect on Caco-2 Cells Viability. Foods 2021, 10, 1185. [Google Scholar] [CrossRef]
- Amaro, F.; Pinto, J.; Rocha, S.; Araújo, A.M.; Miranda-Gonçalves, V.; Jerónimo, C.; Henrique, R.; de Lourdes Bastos, M.; Carvalho, M.; de Pinho, P.G. Volatilomics Reveals Potential Biomarkers for Identification of Renal Cell Carcinoma: An In Vitro Approach. Metabolites 2020, 10, 174. [Google Scholar] [CrossRef] [PubMed]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- De Pinho, P.G.; Gonçalves, R.; Valentão, P.; Pereira, D.; Seabra, R.M.; Andrade, P.B.; Sottomayor, M. Volatile composition of Catharanthus roseus (L.) G. Don using solid-phase microextraction and gas chromatography/mass spectrometry. J. Pharm. Biomed. Anal. 2009, 49, 674–685. [Google Scholar] [CrossRef] [PubMed]
- Tiburski, J.H.; Rosenthal, A.; Deliza, R.; Godoy, R.L.D.O.; Pacheco, S. Nutritional properties of yellow mombin (Spondias mombin L.) pulp. Food Res. Int. 2011, 44, 2326–2331. [Google Scholar] [CrossRef] [Green Version]
- Papapetros, S.; Louppis, A.; Kosma, I.; Kontakos, S.; Badeka, A.; Papastephanou, C.; Kontominas, M.G. Physicochemical, Spectroscopic and Chromatographic Analyses in Combination with Chemometrics for the Discrimination of Four Sweet Cherry Cultivars Grown in Northern Greece. Foods 2019, 8, 442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-Ballesta, M.C.; Dominguez-Perles, R.; Moreno, D.A.; Muries, B.; Alcaraz-López, C.; Bastías, E.; García-Viguera, C.; Carvajal, M. Minerals in plant food: Effect of agricultural practices and role in human health. A review. Agron. Sustain. Dev. 2010, 30, 295–309. [Google Scholar] [CrossRef]
- Alejandro, S.; Höller, S.; Meier, B.; Peiter, E. Manganese in Plants: From Acquisition to Subcellular Allocation. Front. Plant Sci. 2020, 11, 300. [Google Scholar] [CrossRef] [Green Version]
- Mahmood, T.; Anwar, F.; Iqbal, T.; Bhatti, I.A.; Ashraf, M. Mineral Composition of Strawberry, Mulberry and Cherry Fruits at Different Ripening Stages as Analyzed by Inductively Coupled Plasma-Optical Emission Spectroscopy. J. Plant Nutr. 2012, 35, 111–122. [Google Scholar] [CrossRef]
- Meunier, P.J.; Roux, C.; Ortolani, S.; Diaz-Curiel, M.; Compston, J.; Marquis, P.; Cormier, C.; Isaia, G.; Badurski, J.; Wark, J.D.; et al. Effects of long-term strontium ranelate treatment on vertebral fracture risk in postmenopausal women with osteoporosis. Osteoporos. Int. 2009, 20, 1663–1673. [Google Scholar] [CrossRef] [Green Version]
- Serradilla, M.J.; Martín, A.; Ruiz-Moyano, S.; Hernández, A.; López-Corrales, M.; De Guía Córdoba, M. Physicochemical and sensorial characterisation of four sweet cherry cultivars grown in Jerte Valley (Spain). Food Chem. 2012, 133, 1551–1559. [Google Scholar] [CrossRef]
- Hayaloglu, A.A.; Demir, N. Phenolic Compounds, Volatiles, and Sensory Characteristics of Twelve Sweet Cherry (Prunus avium L.) Cultivars Grown in Turkey. J. Food Sci. 2015, 81, C7–C18. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.Q.; He, F.; Zhu, B.Q.; Lan, Y.; Pan, Q.H.; Li, C.Y.; Reeves, M.J.; Wang, J. Free and glycosidically bound aroma compounds in cherry (Prunus avium L.). Food Chem. 2014, 152, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yan, H.; Li, Q.; Lin, H.; Wen, X. Identification of VOCs in essential oils extracted using ultrasound- and microwave-assisted methods from sweet cherry flower. Sci. Rep. 2021, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Dionísio, A.P.; Molina, G.; de Carvalho, D.S.; dos Santos, R.; Bicas, J.; Pastore, G. Natural flavourings from biotechnology for foods and beverages. In Natural Food Additives, Ingredients and Flavourings; Elsevier: Amsterdam, The Netherlands, 2012; pp. 231–259. [Google Scholar]
- Verma, R.S.; Padalia, R.C.; Singh, V.R.; Goswami, P.; Chauhan, A.; Bhukya, B. Natural benzaldehyde from Prunus persica (L.) Batsch. Int. J. Food Prop. 2017, 20, 1259–1263. [Google Scholar] [CrossRef]
- Zhang, J.H.; Sun, H.L.; Chen, S.Y.; Zeng, L.; Wang, T.T. Anti-fungal activity, mechanism studies on α-Phellandrene and Nonanal against Penicillium cyclopium. Bot. Stud. 2017, 58, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Patrignani, F.; Iucci, L.; Belletti, N.; Gardini, F.; Guerzoni, M.E.; Lanciotti, R. Effects of sub-lethal concentrations of hexanal and 2-(E)-hexenal on membrane fatty acid composition and volatile compounds of Listeria monocytogenes, Staphylococcus aureus, Salmonella enteritidis and Escherichia coli. Int. J. Food Microbiol. 2008, 123, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Dogra, A.; Kotwal, P.; Gour, A.; Bhatt, S.; Singh, G.; Mukherjee, D.; Nandi, U. Description of Druglike Properties of Safranal and Its Chemistry behind Low Oral Exposure. ACS Omega 2020, 5, 9885–9891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lertnimitphun, P.; Jiang, Y.; Kim, N.; Fu, W.; Zheng, C.; Tan, H.; Zhou, H.; Zhang, X.; Pei, W.; Lu, Y.; et al. Safranal Alleviates Dextran Sulfate Sodium-Induced Colitis and Suppresses Macrophage-Mediated Inflammation. Front. Pharmacol. 2019, 10, 1281. [Google Scholar] [CrossRef]
- Kreck, M.; Püschel, S.; Wüst, A.M.; Mosandl, A. Biogenetic Studies in Syringa vulgaris L.: Synthesis and Bioconversion of Deuterium-Labeled Precursors into Lilac Aldehydes and Lilac Alcohols. J. Agric. Food Chem. 2002, 51, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Prđun, S.; Svečnjak, L.; Valentić, M.; Marijanović, Z.; Jerković, I. Characterization of Bee Pollen: Physico-Chemical Properties, Headspace Composition and FTIR Spectral Profiles. Foods 2021, 10, 2103. [Google Scholar] [CrossRef]
- Cozzolino, R.; Martignetti, A.; Cefola, M.; Pace, B.; Capotorto, I.; De Giulio, B.; Montemurro, N.; Pellicano, M. Volatile metabolites, quality and sensory parameters of “Ferrovia” sweet cherry cold stored in air or packed in high CO2 modified atmospheres. Food Chem. 2019, 286, 659–668. [Google Scholar] [CrossRef]
- Lewinsohn, E.; Sitrit, Y.; Bar, E.; Azulay, Y.; Meir, A.; Zamir, D.; Tadmor, Y. Carotenoid Pigmentation Affects the Volatile Composition of Tomato and Watermelon Fruits, As Revealed by Comparative Genetic Analyses. J. Agric. Food Chem. 2005, 53, 3142–3148. [Google Scholar] [CrossRef] [PubMed]
- Legua, P.; Domenech, A.; Martínez, J.; Sánchez-Rodríguez, L.; Hernández, F.; Carbonell-Barrachina, A.; Melgarejo, P. Bioactive and Volatile Compounds in Sweet Cherry Cultivars. J. Food Nutr. Res. 2017, 5, 844–851. [Google Scholar] [CrossRef] [Green Version]
- Câmara, J.; Lourenço, S.; Silva, C.; Lopes, A.; Andrade, C.; Perestrelo, R. Exploring the potential of wine industry by-products as source of additives to improve the quality of aquafeed. Microchem. J. 2020, 155, 104758. [Google Scholar] [CrossRef]
- Cardoso, V.M.; Solano, A.G.R.; Prado, M.A.F.; Nunan, E.D.A. Investigation of fatty acid esters to replace isopropyl myristate in the sterility test for ophthalmic ointments. J. Pharm. Biomed. Anal. 2006, 42, 630–634. [Google Scholar] [CrossRef]
- Dickinson, A.J.; Lehner, K.; Mi, J.; Jia, K.-P.; Mijar, M.; Dinneny, J.; Al-Babili, S.; Benfey, P.N. β-Cyclocitral is a conserved root growth regulator. Proc. Natl. Acad. Sci. USA 2019, 116, 10563–10567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murata, M.; Kobayashi, T.; Seo, S. α-Ionone, an Apocarotenoid, Induces Plant Resistance to Western Flower Thrips, Frankliniella occidentalis, Independently of Jasmonic Acid. Molecules 2019, 25, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steele, D.H.; Thornburg, M.J.; Stanley, J.S.; Miller, R.R.; Brooke, R.; Cushman, J.R.; Cruzan, G. Determination of styrene in selected foods. J. Agric. Food Chem. 1994, 42, 1661–1665. [Google Scholar] [CrossRef]
- Srivastava, R.; Bousquières, J.; Cepeda-Vázquez, M.; Roux, S.; Bonazzi, C.; Rega, B. Kinetic study of furan and furfural generation during baking of cake models. Food Chem. 2018, 267, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Premkumar, T.; Govindarajan, S. Antimicrobial study of pyrazine, pyrazole and imidazole carboxylic acids and their hydrazinium salts. World J. Microbiol. Biotechnol. 2005, 21, 479–480. [Google Scholar] [CrossRef]
- Milheiro, J.; Filipe-Ribeiro, L.; Vilela, A.; Cosme, F.; Nunes, F.M. 4-Ethylphenol, 4-ethylguaiacol and 4-ethylcatechol in red wines: Microbial formation, prevention, remediation and overview of analytical approaches. Crit. Rev. Food Sci. Nutr. 2017, 59, 1367–1391. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, R.G. Phenylpropenes: Occurrence, Distribution, and Biosynthesis in Fruit. J. Agric. Food Chem. 2016, 66, 2259–2272. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Shahzad, R.; Lee, I.J. Regulation of flood stress in plants. In Plant Life under Changing Environment; Tripathi, D.K., Chauhan, D.K., Prasad, S.M., Singh, V.P., Chauhan, D.K., Ramawat, N., Sharma, S., Dubey, N.K., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 157–173. [Google Scholar] [CrossRef]
- Cho, H.J.; Do, B.K.; Shim, S.M.; Kwon, H.; Lee, D.H.; Nah, A.H.; Choi, Y.J.; Lee, S.Y. Determination of Cyanogenic Compounds in Edible Plants by Ion Chromatography. Toxicol. Res. 2013, 29, 143–147. [Google Scholar] [CrossRef]
- Arshi, A.; Hosseini, S.M.; Hosseini, F.S.K.; Amiri, Z.Y.; Hosseini, F.S.; Lavasani, M.S.; Kerdarian, H.; Dehkordi, M.S. The anti-cancer effect of amygdalin on human cancer cell lines. Mol. Biol. Rep. 2019, 46, 2059–2066. [Google Scholar] [CrossRef] [PubMed]
- Viant, M.R.; Kurland, I.J.; Jones, M.R.; Dunn, W. How close are we to complete annotation of metabolomes? Curr. Opin. Chem. Biol. 2017, 36, 64–69. [Google Scholar] [CrossRef] [PubMed]
| Element | Cherry Stems | Cherry Leaves | Cherry Flowers |
|---|---|---|---|
| Macrominerals | |||
| Sodium, Na | 79.5 ± 6.0 | <LOQ | <LOQ |
| Phosphorus, P | 1345 ± 54 b | 1058 ± 112 a | 837 ± 10 a,b |
| Trace elements | |||
| Cobalt, Co | <LOQ | 0.057 ± 0.005 | <LOQ |
| Copper, Cu | 24.8 ± 5.5 b,c | 4.22 ± 0.31 a | 6.79 ± 0.34 a |
| Iron, Fe | 32.46 ± 0.92 b | 66.8 ± 4.1 a | 30.9 ± 2.5 b |
| Manganese, Mn | 10.72 ± 0.39 b | 95.0 ± 8.3 a | 18.39 ± 0.35 b |
| Selenium, Se | <LOD | <LOD | <LOD |
| Zinc, Zn | 18.00 ± 1.3 c | 20.9 ± 4.2 c | 35.8 ± 2.8 a,b |
| Element | Cherry Stems | Cherry Leaves | Cherry Flowers |
|---|---|---|---|
| Aluminum, Al | 7.30 ± 0.54 b | 32.2 ± 3.2 a,c | 20.9 ± 1.5 a,b |
| Arsenic, As | <LOD | <LOD | <LOD |
| Barium, Ba | 42.99 ± 0.59 b | 38.9 ± 2.5 a,c | 4.26 ± 0.37 a,b |
| Cadmium, Cd | <LOD | <LOD | <LOD |
| Chromium, Cr | <LOQ | <LOQ | <LOQ |
| Lithium, Li | 0.07 ± 0.01 b | 0.02 ± 0.00 a,c | 0.04 ± 0.00 a,b |
| Nickel, Ni | <LOQ | <LOQ | <LOQ |
| Lead, Pb | 0.35 ± 0.05 b | 0.21 ± 0.02 a,c | 0.04 ± 0.00 a,b |
| Rubidium, Rb | 7.69 ± 0.16 b | 5.34 ± 0.38 a,c | 3.88 ± 0.07 a,b |
| Strontium, Sr | 18.62 ± 0.53 c | 20.3 ± 1.2 c | 2.89 ± 0.17 a,b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nunes, A.R.; Gonçalves, A.C.; Pinto, E.; Amaro, F.; Flores-Félix, J.D.; Almeida, A.; Guedes de Pinho, P.; Falcão, A.; Alves, G.; Silva, L.R. Mineral Content and Volatile Profiling of Prunus avium L. (Sweet Cherry) By-Products from Fundão Region (Portugal). Foods 2022, 11, 751. https://doi.org/10.3390/foods11050751
Nunes AR, Gonçalves AC, Pinto E, Amaro F, Flores-Félix JD, Almeida A, Guedes de Pinho P, Falcão A, Alves G, Silva LR. Mineral Content and Volatile Profiling of Prunus avium L. (Sweet Cherry) By-Products from Fundão Region (Portugal). Foods. 2022; 11(5):751. https://doi.org/10.3390/foods11050751
Chicago/Turabian StyleNunes, Ana R., Ana C. Gonçalves, Edgar Pinto, Filipa Amaro, José D. Flores-Félix, Agostinho Almeida, Paula Guedes de Pinho, Amílcar Falcão, Gilberto Alves, and Luís R. Silva. 2022. "Mineral Content and Volatile Profiling of Prunus avium L. (Sweet Cherry) By-Products from Fundão Region (Portugal)" Foods 11, no. 5: 751. https://doi.org/10.3390/foods11050751
APA StyleNunes, A. R., Gonçalves, A. C., Pinto, E., Amaro, F., Flores-Félix, J. D., Almeida, A., Guedes de Pinho, P., Falcão, A., Alves, G., & Silva, L. R. (2022). Mineral Content and Volatile Profiling of Prunus avium L. (Sweet Cherry) By-Products from Fundão Region (Portugal). Foods, 11(5), 751. https://doi.org/10.3390/foods11050751













