Extract from the Macroalgae Ulva rigida Induces Table Grapes Resistance to Botrytis cinerea
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Algal Extracts
2.1.1. On-Shore Biomass Cultivation and Processing of Ulva rigida
2.1.2. Thermochemical Extraction
2.1.3. Organic Solvents Extraction
2.1.4. Characterization and Chemical Composition Analysis
2.2. In Vitro Effects of Ulva rigida Extracts on B. cinerea Fungus
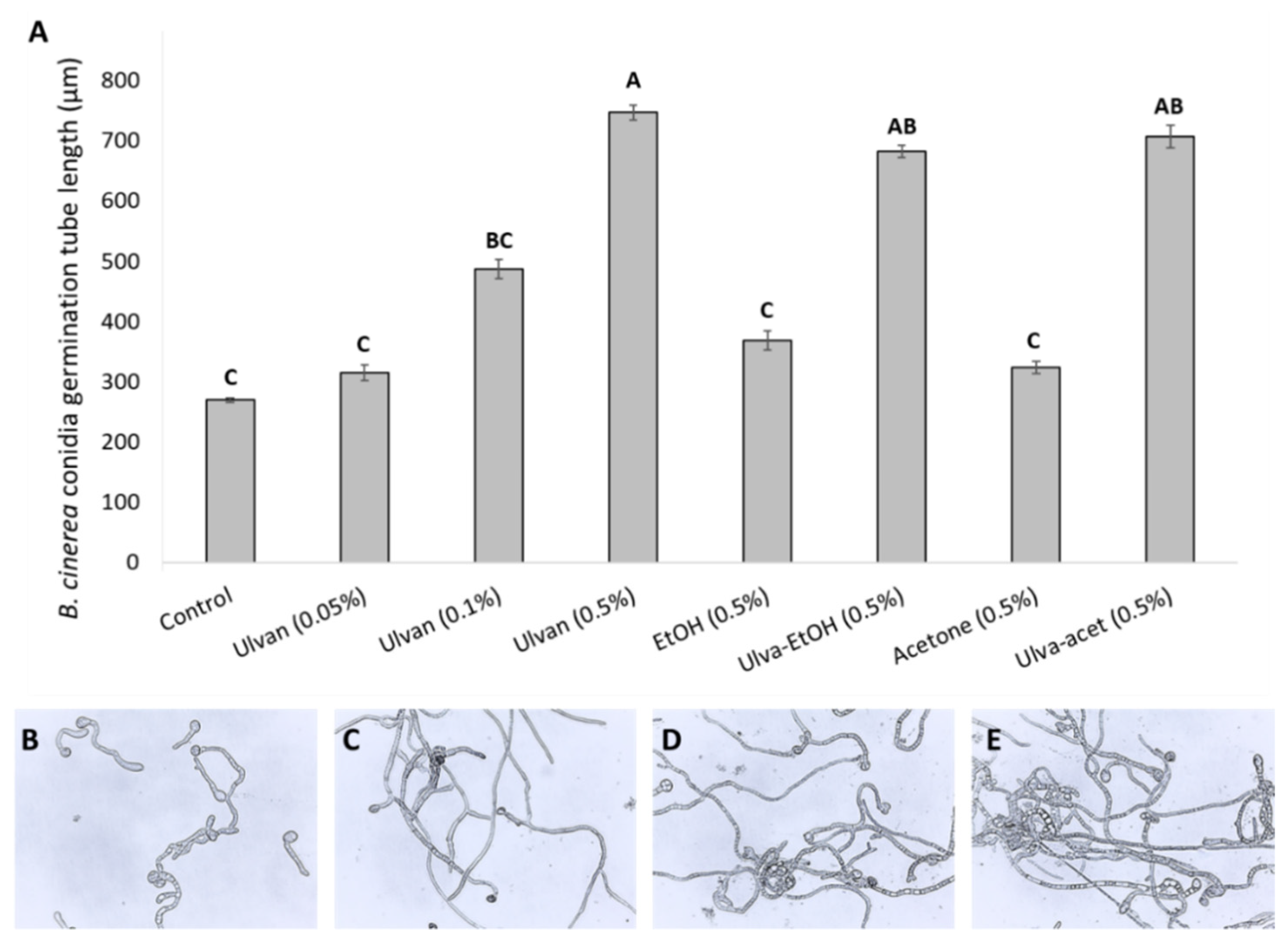
2.2.1. In Vitro Effect of Extracts on Conidia Germination
2.2.2. In Vitro Effect on Mycelium Grown on Agar Plate
2.2.3. In Vitro Effect on Fungal Growth Kinetics
2.3. Measuring Fruit Response to Ulva rigida Extracts
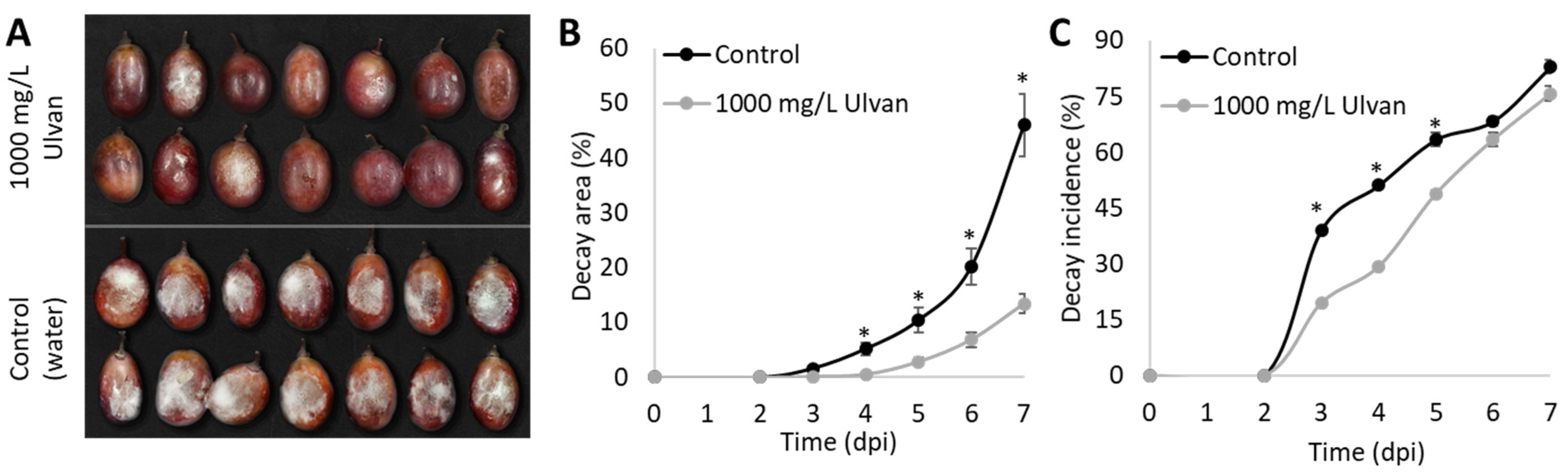
2.3.1. In Vivo Test on Grapes Inoculated with B. cinerea
2.3.2. Evaluation of Fruit Quality Parameters
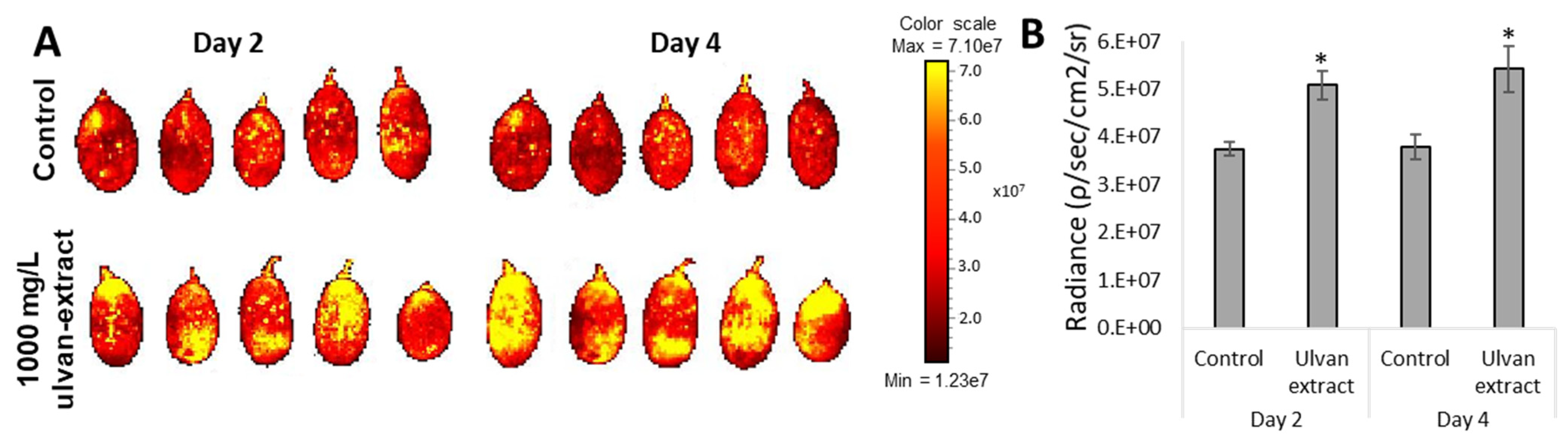
2.3.3. Reactive Oxygen Species Levels
2.3.4. Free Radical Scavenging Activity
2.3.5. Enzymatic Activity and Protein Assays
2.4. Statistical Analysis
3. Results
3.1. In Vitro Effects of Ulva rigida Extracts on B. cinerea Growth
3.2. In Vivo Effects of Ulva rigida Extract on B. cinerea Growth
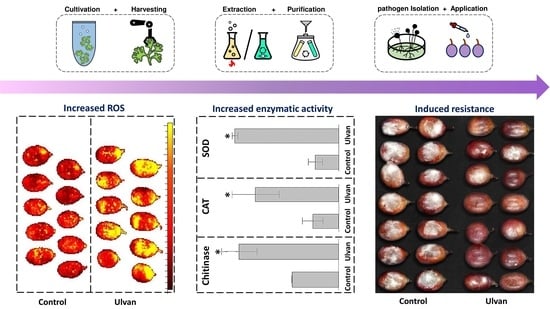
3.3. Induction of Fruit Defense Response
3.4. Characterization and Chemical Composition of Crude Extract of Ulva rigida Sulfated Polysaccharides (Infrared Spectroscopy and Elemental Analysis)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dean, R.; Van Kan, J.A.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant. Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbey, J.A.; Percival, D.; Abbey, L.; Asiedu, S.K.; Prithiviraj, B.; Schilder, A. Biofungicides as alternative to synthetic fungicide control of grey mould (Botrytis cinerea)—Prospects and challenges. Biocontrol Sci. Technol. 2019, 29, 207–228. [Google Scholar] [CrossRef]
- Zhang, W. Global pesticide use: Profile, trend, cost/benefit and more. Proc. Int. Acad. Ecol. Environ. Sci. 2018, 8, 1–27. [Google Scholar]
- Zhang, H.; Adwoa Serwah Boateng, N.; Legrand Ngolong Ngea, G.; Shi, Y.; Lin, H.; Yang, Q.; Wang, K.; Zhang, X.; Zhao, L.; Droby, S. Unravelling the fruit microbiome: The key for developing effective biological control strategies for postharvest diseases. Compr. Rev. Food Sci. 2021, 20, 4906–4930. [Google Scholar] [CrossRef] [PubMed]
- Dwiastuti, M.E.; Soesanto, L.; Aji, T.G.; Devy, N.F.; Hardiyanto. Biological control strategy for postharvest diseases of citrus, apples, grapes and strawberries fruits and application in Indonesia. Egypt J. Biol. Pest. Control 2021, 31, 141. [Google Scholar] [CrossRef]
- Kusstatscher, P.; Cernava, T.; Abdelfattah, A.; Gokul, J.; Korsten, L.; Berg, G. Microbiome approaches provide the key to biologically control postharvest pathogens and storability of fruits and vegetables. FEMS Microbiol. Ecol. 2020, 96, fiaa119. [Google Scholar] [CrossRef]
- Romanazzi, G.; Sanzani, S.M.; Bi, Y.; Tian, S.; Martínez, P.G.; Alkan, N. Induced resistance to control postharvest decay of fruit and vegetables. Postharvest. Biol. Technol. 2016, 122, 82–94. [Google Scholar] [CrossRef]
- Krishnamoorthi, R.; Sivakumar, S. Antifungal activity of seaweed Ulva lactuca extracted crude protein against pathogenic fungi. Asian J. Pharm. Clin. Res. 2019, 12, 393–396. [Google Scholar] [CrossRef]
- Raj, G.A.; Chandrasekaran, M.; Jegan, S.; Venkatesalu, V. Phytochemical analysis and antifungal activity of Ulva Species from the kanniyakumari gulf of mannar, South Coast India. Nat. Prod. Ejbps 2017, 4, 9. [Google Scholar] [CrossRef] [Green Version]
- Paulert, R.; Talamini, V.; Cassolato, J.E.F.; Duarte, M.E.R.; Noseda, M.D.; Paulert, R.; Stadnik, M.J. Effects of sulfated polysaccharide and alcoholic extracts from green seaweed Ulva fasciata on anthracnose severity and growth of common bean (Phaseolus vulgaris L.). J. Plant. Dis. Prot. 2009, 116, 263–270. [Google Scholar] [CrossRef]
- Kidgell, J.T.; Magnusson, M.; de Nys, R.; Glasson, C.R.K. Ulvan: A systematic review of extraction, composition and function. Algal Res. 2019, 39, 101422. [Google Scholar] [CrossRef]
- Beckers, G.J.M.; Spoel, S.H. Fine-tuning plant defence signalling: Salicylate versus jasmonate. Plant. Biol. 2006, 8, 1–10. [Google Scholar] [CrossRef]
- Cluzet, S.; Torregrosa, C.; Jacquet, C.; Lafitte, C.; Fournier, J.; Mercier, L.; Salamagne, S.; Briand, X.; Esquerré-Tugayé, M.T.; Dumas, B. Gene expression profiling and protection of Medicago truncatula against a fungal infection in response to an elicitor from green algae Ulva spp. Plant. Cell. Environ. 2004, 27, 917–928. [Google Scholar] [CrossRef]
- Jaulneau, V.; Lafitte, C.; Corio-Costet, M.F.; Stadnik, M.J.; Salamagne, S.; Briand, X.; Esquerré-Tugayé, M.T.; Dumas, B. An Ulva armoricana extract protects plants against three powdery mildew pathogens. Eur. J. Plant. Pathol. 2011, 131, 393–401. [Google Scholar] [CrossRef]
- Abouraïcha, E.; El Alaoui-Talibi, Z.; El Boutachfaiti, R.; Petit, E.; Courtois, B.; Courtois, J.; El Modafar, C. Induction of natural defense and protection against Penicillium expansum and Botrytis cinerea in apple fruit in response to bioelicitors isolated from green algae. Sci. Hort. 2015, 181, 121–128. [Google Scholar] [CrossRef]
- Chemodanov, A.; Robin, A.; Goldberg, A. Design of marine macroalgae photobioreactor integrated into building to support seagriculture for biorefinery and bioeconomy. Bioresour. Technol. 2017, 241, 1084–1093. [Google Scholar] [CrossRef]
- Duanis-Assaf, D.; Galsurker, O.; Davydov, O.; Feygenberg, O.; Sagi, M.; Poverenov, E.; Fluhr, R.; Alkan, N. Double-stranded RNA targeting fungal ergosterol biosynthesis pathway controls Botrytis cinerea and postharvest grey mold. Plant. Biotechnol. J. 2021, 20, 226–237. [Google Scholar] [CrossRef]
- Solairaj, D.; Ngea Guillaume Legrand, N.; Yang, Q.; Zhang, H.M. Isolation of pathogenic fungi causing postharvest decay in table grapes and in vivo biocontrol activity of selected yeasts against them. Physiol. Mol. Plant Pathol. 2020, 110, 101478. [Google Scholar] [CrossRef]
- Galsurker, O.; Diskin, S.; Duanis-Assaf, D.; Doron-Faigenboim, A.; Maurer, D.; Feygenberg, O.; Alkan, N. Harvesting Mango Fruit with a Short Stem-End Altered Endophytic Microbiome and Reduce Stem-End Rot. Microorganisms 2020, 8, 558. [Google Scholar] [CrossRef]
- Cheung, L.M.; Cheung, P.C.K.; Ooi, V.E.C. Antioxidant activity and total phenolics of edible mushroom extracts. Food Chem. 2003, 81, 249–255. [Google Scholar] [CrossRef]
- Sudheeran, P.K.; Feygenberg, O.; Maurer, D.; Alkan, N. Improved cold tolerance of mango fruit with enhanced anthocyanin and flavonoid contents. Molecules 2018, 23, 1832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Assis, J.S.; Maldonado, R.; Muñoz, T.; Escribano, M.I.; Merodio, C. Effect of high carbon dioxide concentration on PAL activity and phenolic contents in ripening cherimoya fruit. Postharvest Biol. Technol. 2001, 23, 33–39. [Google Scholar] [CrossRef] [Green Version]
- Abeles, F.B.; Bosshart, R.P.; Forrence, L.E.; Habig, W.H. Preparation and purification of glucanase and chitinase from bean leaves. Plant Physiol. 1971, 47, 129–134. [Google Scholar] [CrossRef] [Green Version]
- Beers, R.F.; Sizer, I.W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J. Biol. Chem. 1952, 195, 133–140. [Google Scholar] [CrossRef]
- Giannopolitis, C.; Ries, S. Superoxide dismutases: II. Purification and quantitative relationship with water-soluble protein in seedlings. Plant Physiol. 1977, 59, 315–318. [Google Scholar] [CrossRef] [Green Version]
- Costa, C.; Alves, A.; Pinto, P.R.; Sousa, R.A.; Borges da Silva, A.; Rui, L.R.; Rodrigues, A.E. Characterization of ulvan extracts to assess the effect of different steps in the extraction procedure. Carbohydr. Polym. 2012, 88, 537–546. [Google Scholar] [CrossRef]
- Del Rocío Quezada-Rodríguez, P.; Fajer-Ávila, J.E. The dietary effect of ulvan from Ulva clathrata on hematological-immunological parameters and growth of tilapia (Oreochromis niloticus). J. Appl. Phycol. 2017, 29, 423–431. [Google Scholar] [CrossRef]
- Lahaye, M.; Robic, A. Structure and functional properties of ulvan, a polysaccharide from green seaweeds. Biomacromolecules 2007, 8, 1765–1774. [Google Scholar] [CrossRef]
- Kitinoja, L.; Cantwell, M. Identification of appropriate postharvest technologies for improving market access and incomes for small horticultural farmers in Sub-Saharan Africa and South Asia. Acta Hort. 2010, 934, 31–40. [Google Scholar] [CrossRef] [Green Version]
- Robin, A.; Chavel, P.; Chemodanov, A.; Israel, A.; Golberg, A. Diversity of monosaccharides in marine macroalgae from the Eastern Mediterranean Sea. Algal Res. 2017, 28, 118–127. [Google Scholar] [CrossRef]
- Chiquito-Contreras, R.G.; Murillo-Amador, B.; Carmona-Hernandez, S.; Chiquito-Contreras, C.J.; Hernandez-Montiel, L.G. Effect of marine bacteria and ulvan on the activity of antioxidant defense enzymes and the bio-protection of papaya fruit against Colletotrichum gloeosporioides. Antioxidants 2019, 8, 580. [Google Scholar] [CrossRef] [Green Version]
- Stadnik, M.J. Ulvan effect on conidial germination and appressoria formation of Colletotrichum gloeosporioides. Phytoparasitica 2014, 42, 631–640. [Google Scholar] [CrossRef]
- Rivas-Garcia, T.; Murillo-Amador, B.; Nieto-Garibay, A.; Chiquito-Contreras, R.G.; Rincon-Enriquez, G.; Hernandez-Montiel, L.G. Effect of ulvan on the biocontrol activity of Debaryomyces hansenii and Stenotrophomonas rhizophila against fruit rot of Cucumis melo L. Agronomy 2018, 8, 273. [Google Scholar] [CrossRef] [Green Version]
- Paulert, R.; Ebbinghaus, D.; Urlass, C.; Moerschbacher, B.M. Priming of the oxidative burst in rice and wheat cell cultures by ulvan, a polysaccharide from green macroalgae, and enhanced resistance against powdery mildew in wheat and barley plants. Plant. Pathol. 2010, 59, 634–642. [Google Scholar] [CrossRef]
- de Freitas, M.B.; Stadnik, M.J. Ulvan-induced resistance in Arabidopsis thaliana against Alternaria brassicicola requires reactive oxygen species derived from NADPH oxidase. Physiol. Mol. Plant Pathol. 2015, 90, 49–56. [Google Scholar] [CrossRef]
- Dumas, B.; Jaulneau, V.; Lafitte, C.; Jacquet, C.; Fournier, S.; Salamagne, S.; Briand, X.; Esquerré-Tugayé, M.T. Ulvan, a sulfated polysaccharide from green algae, activates plant immunity through the jasmonic acid signaling pathway. J. Biomed. Biotechnol. 2010, 2010, 525291. [Google Scholar] [CrossRef] [Green Version]
- Lacan, D.; Baccou, J.C. High levels of antioxidant enzymes correlate with delayed senescence in nonnetted muskmelon fruits. Planta 1998, 204, 377–382. [Google Scholar] [CrossRef]
- Saunders, J.; O’Neill, N. The characterization of defense responses to fungal infection in alfalfa. BioControl 2004, 49, 715–728. [Google Scholar] [CrossRef]
- Muzzarelli, P.J.S.; Riccardo, A.A. Chitin and Chitinases; Birkhäuser Verlag: Boston, MA, USA, 1999; ISBN 3764358157/9783764358150. [Google Scholar]
- Carver, T.L.W.; Robbins, M.P.; Zeyen, R.J.; Dearne, G.A. Effects of PAL-specific inhibition on suppression of activated defence and quantitative susceptibility of oats to Erysiphe graminis. Physiol. Mol. Plant Pathol. 1992, 41, 149–163. [Google Scholar] [CrossRef]
- De Simone, N.; Pace, B.; Grieco, F.; Chimienti, M.; Tyibilika, V.; Santoro, V.; Capozzi, V.; Colelli, G.; Spano, G.; Russo, P. Botrytis cinerea and table grapes: A review of the main physical, chemical, and bio-based control treatments in post-harvest. Foods 2020, 9, 1138. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, F.; Wang, X.; Liu, X.; Hou, Y.; Zhang, Q. Extraction of the polysaccharides from five algae and their potential antioxidant activity in vitro. Carbohydr. Polym. 2010, 82, 118–121. [Google Scholar] [CrossRef]
- Toskas, G.; Hund, R.D.; Laourine, E.; Cherif, C.; Smyrniotopoulos, V.; Roussis, V. Nanofibers based on polysaccharides from the green seaweed Ulva rigida. Carbohydr. Polym. 2011, 84, 1093–1102. [Google Scholar] [CrossRef]
- Thanh, T.T.; Quach, T.M.; Nguyen, T.N.; Luong, D.V.; Bui, M.L.; Van Tran, T.T. Structure and cytotoxic activity of ulvan extracted from green seaweed Ulva lactuca. Int. J. Biol. Macromol. 2016, 93, 695–702. [Google Scholar] [CrossRef]
- Enrique, H.G.; Jose, A.Z.; Isai, P.R. Isolation and chemical characterization of algal polysaccharides from the green seaweed Ulva clathrata (Roth) C. Agardh. J. Appl. Phycol. 2011, 23, 537–542. [Google Scholar] [CrossRef]
- Yaich, H.; Amira, A.B.; Abbes, F.; Bouaziz, M.; Besbes, S.; Richel, A.; Blecker, C.; Attia, H.; Garna, H. Effect of extraction procedures on structural, thermal and antioxidant properties of ulvan from Ulva lactuca collected in Monastir coast. Int. J. Biol. Macromol. 2017, 105, 1430–1439. [Google Scholar] [CrossRef]


| % of DW | C | H | N | S |
|---|---|---|---|---|
| Ulvan extract in the current study # | 22.74 | 4.61 | 0.63 | 7.00 |
| Costa et al., 2012 [27] | 22.80 | 4.30 | 1.00 | 8.40 |
| del Rocío Quezada-Rodríguez and Fajer-Ávila, 2017 [28] | 19.47 | 3.78 | 0.89 | 8.69 |
| Lahaye and Robic, 2007 [29] | 19.35 | 3.88 | 0.98 | 5.62 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shomron, A.; Duanis-Assaf, D.; Galsurker, O.; Golberg, A.; Alkan, N. Extract from the Macroalgae Ulva rigida Induces Table Grapes Resistance to Botrytis cinerea. Foods 2022, 11, 723. https://doi.org/10.3390/foods11050723
Shomron A, Duanis-Assaf D, Galsurker O, Golberg A, Alkan N. Extract from the Macroalgae Ulva rigida Induces Table Grapes Resistance to Botrytis cinerea. Foods. 2022; 11(5):723. https://doi.org/10.3390/foods11050723
Chicago/Turabian StyleShomron, Alon, Danielle Duanis-Assaf, Ortal Galsurker, Alexander Golberg, and Noam Alkan. 2022. "Extract from the Macroalgae Ulva rigida Induces Table Grapes Resistance to Botrytis cinerea" Foods 11, no. 5: 723. https://doi.org/10.3390/foods11050723
APA StyleShomron, A., Duanis-Assaf, D., Galsurker, O., Golberg, A., & Alkan, N. (2022). Extract from the Macroalgae Ulva rigida Induces Table Grapes Resistance to Botrytis cinerea. Foods, 11(5), 723. https://doi.org/10.3390/foods11050723