Frankfurter-Type Sausage Enriched with Buckwheat By-Product as a Source of Bioactive Compounds
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Sausages Samples with Buckwheat Husk
2.2. pH Value
2.3. Weight Losses and Yield
2.4. Instrumental Texture Profile Analysis
2.5. Instrumental Color Measurement
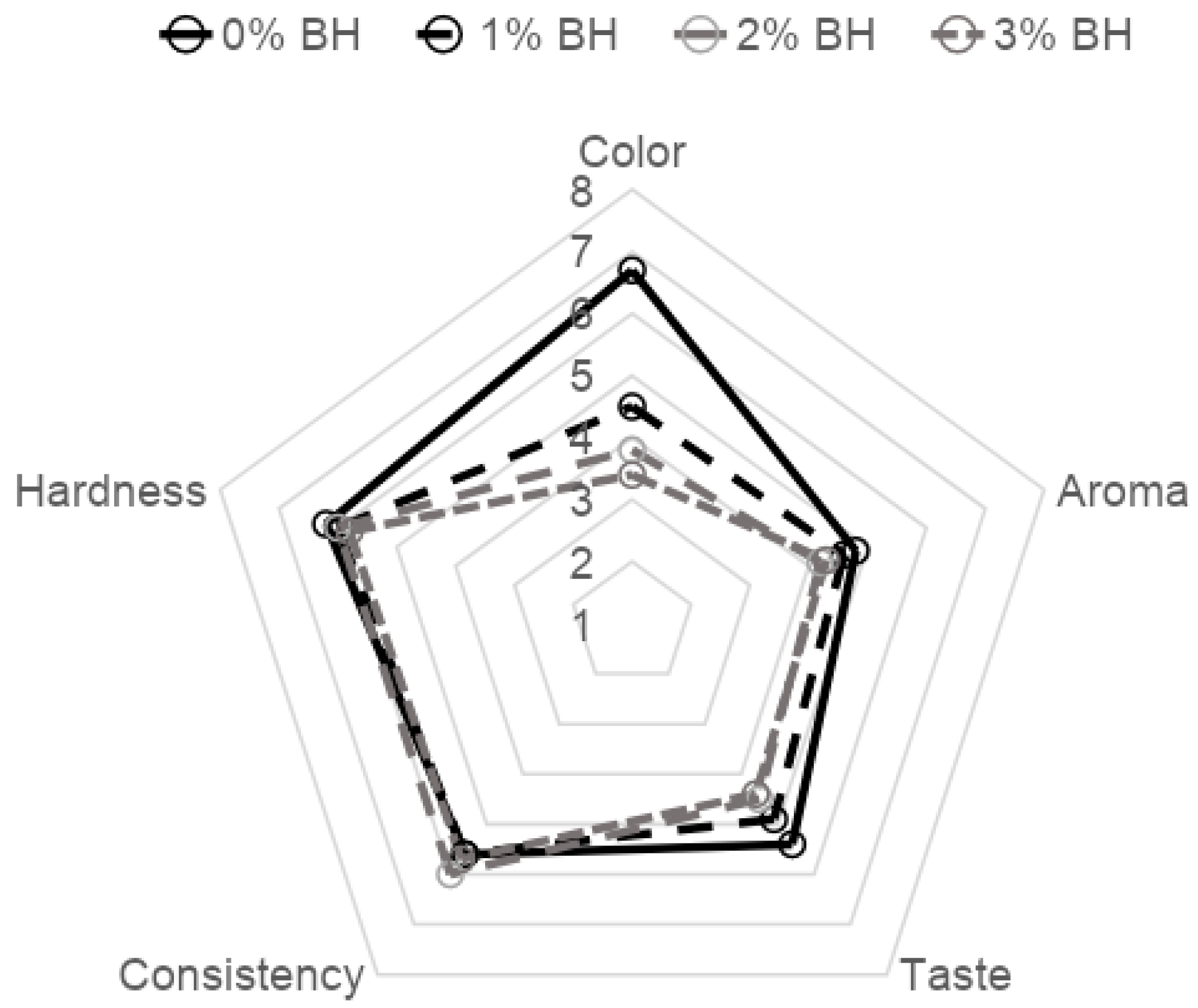
2.6. Organoleptic Evaluation
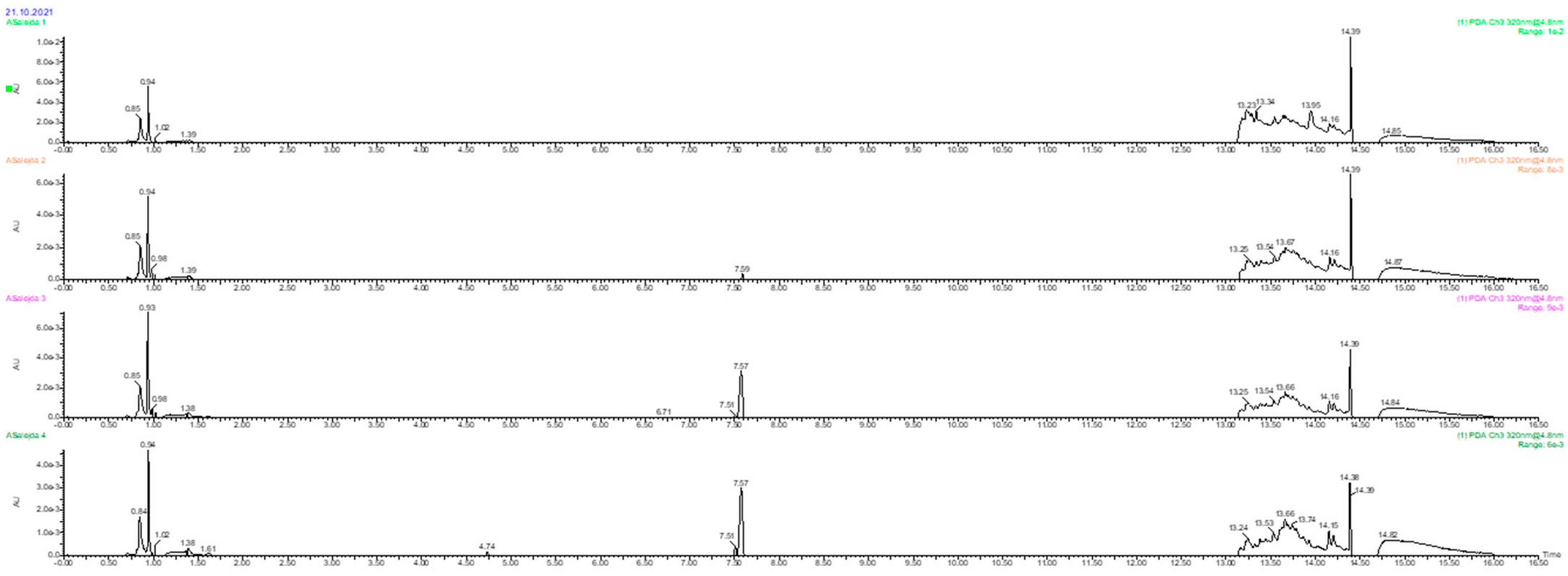
2.7. Identification of Polyphenols
2.8. Amino Acid Analysis
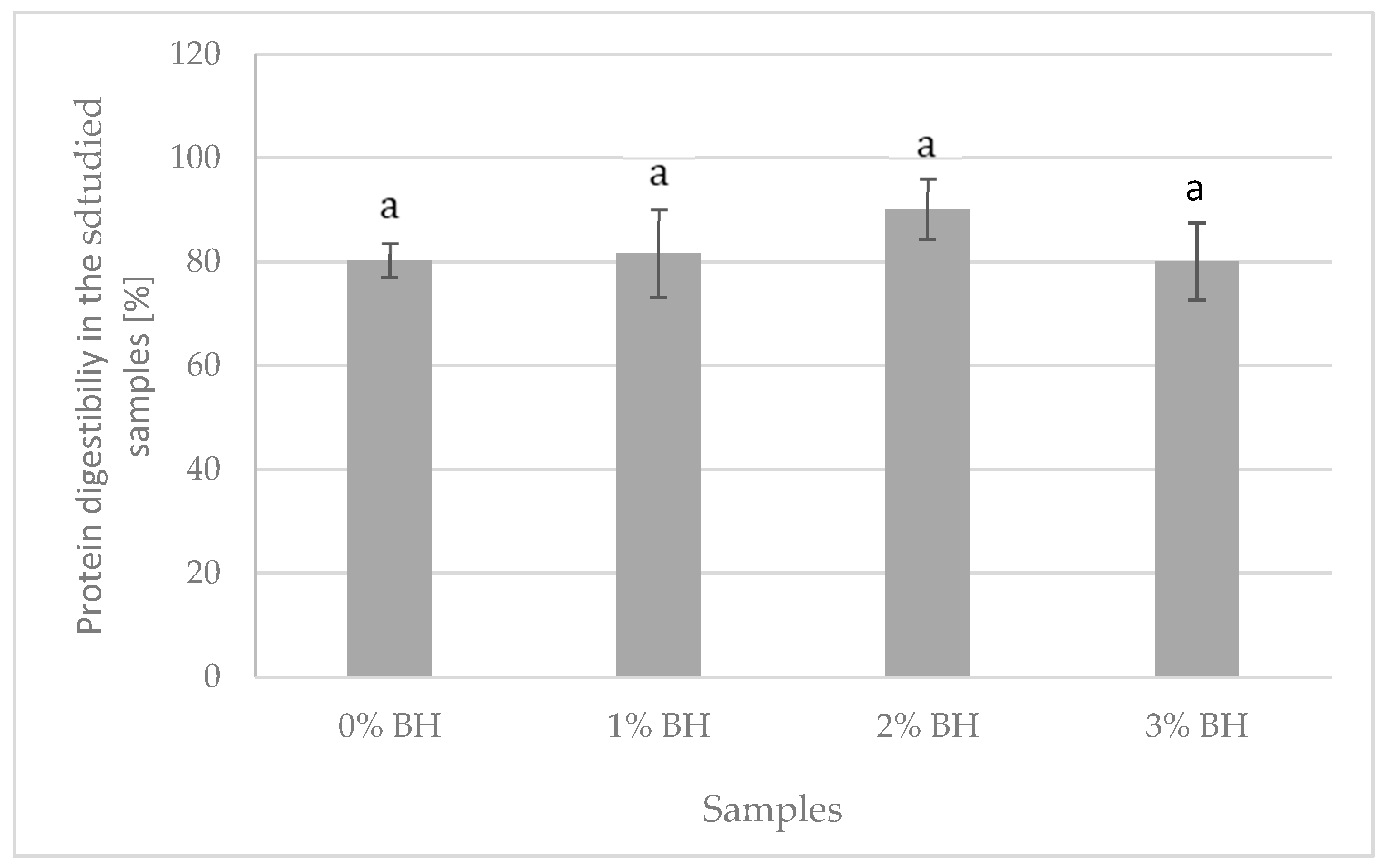
2.9. In Vitro Protein Digestibility Determination
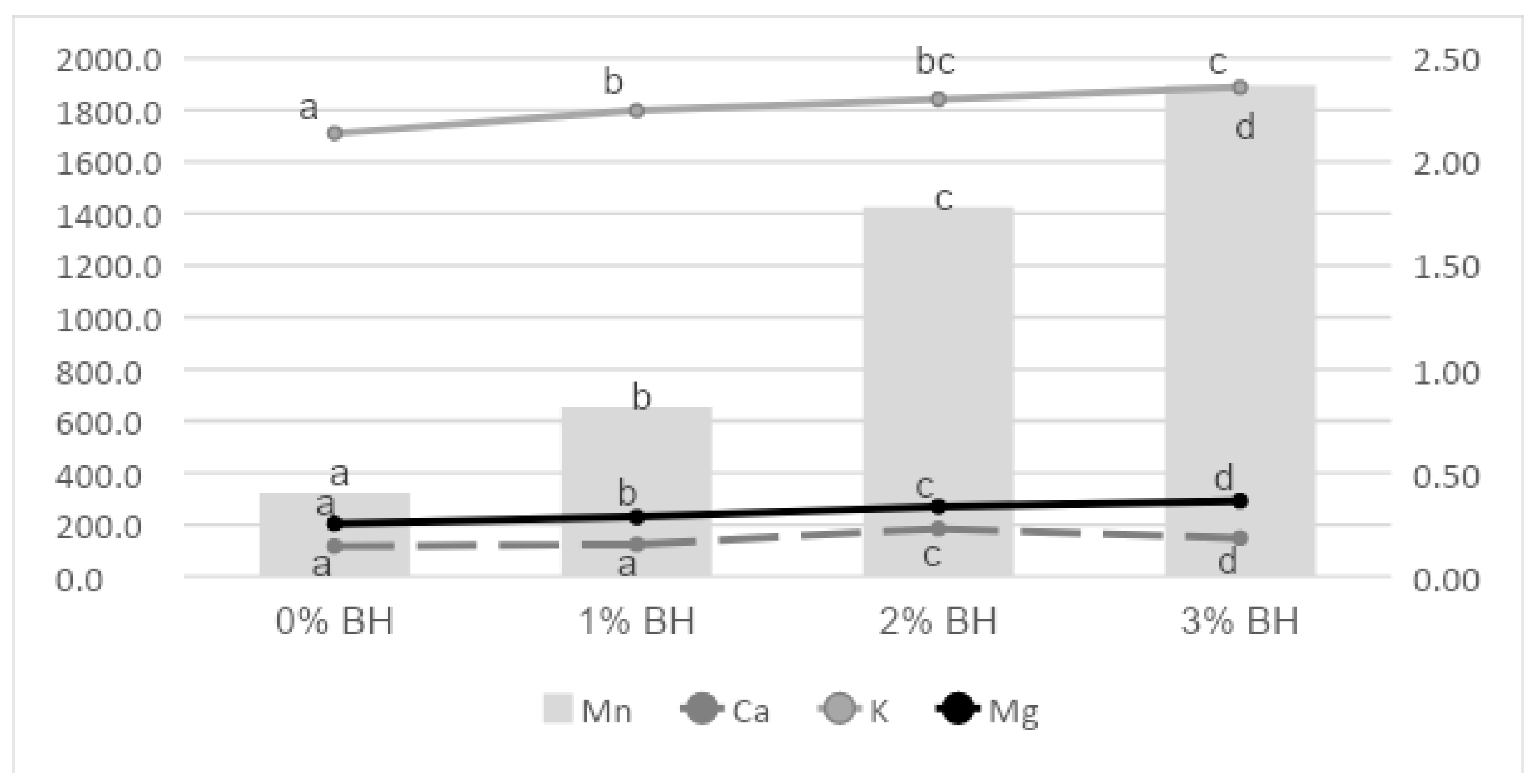
2.10. Determination of Trace Elements
2.11. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Serrano, A.; Librelotto, J.; Cofrades, S.; Sánchez-Muniz, F.J.; Jiménez-Colmenero, F. Composition and physicochemical characteristics of restructured beef steaks containing walnuts as affected by cooking method. Meat Sci. 2007, 77, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Hygreeva, D.; Pandey, M.C.; Radhakrishna, K. Potential applications of plant based derivatives as fat replacers, antioxidants and antimicrobials in fresh and processed meat products. Meat Sci. 2014, 98, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Kanner, J. Oxidative processes in meat and meat products: Quality implications. Meat Sci. 1994, 36, 169–189. [Google Scholar] [CrossRef]
- Fung, D.Y. Microbial hazards in food: Food-borne infections and intoxications. In Handbook of Meat Processing; Blackwell Publishing: Hoboken, NJ, USA, 2010; pp. 481–500. [Google Scholar]
- Calvo, M.M.; García, M.L.; Selgas, M.D. Dry fermented sausages enriched with lycopene from tomato peel. Meat Sci. 2008, 80, 1672010172. [Google Scholar] [CrossRef] [PubMed]
- Skiepko, N.; Chwastowska-Siwiecka, I.; Kondratowicz, J. Properties of lycopene and utilizing it to produce functional foods. Zywnosc-Nauka Technol. Jakosc 2015, 103, 20–32. [Google Scholar]
- Perales-Jasso, Y.J.; Gamez-Noyola, S.A.; Aranda-Ruiz, J.; Hernandez-Martinez, C.A.; Gutierrez-Soto, G.; Luna-Maldonado, A.I.; Silva-Vazquez, R.; Hume, M.E.; Mendez-Zamora, G. Oregano powder substitution and shelf life in pork chorizo using Mexican oregano essential oil. Food Sci. Nutr. 2018, 6, 1254–1260. [Google Scholar] [CrossRef]
- Shan, B.; Cai, Y.; Brooks, J.D.; Corke, H. Antibacterial and antioxidant effects of five spice and herb extracts as natural preservatives of raw pork. J. Sci. Food Agric. 2009, 89, 1879–1885. [Google Scholar] [CrossRef]
- Fasseas, M.K.; Mountzouris, K.C.; Tarantilis, P.A.; Polissiou, M.; Zervas, G. Antioxidant activity in meat treated with oregano and sage essential oils. Food Chem. 2008, 106, 1188–1194. [Google Scholar] [CrossRef]
- De la Cruz-Lapa, P. An integral and rational utility of tara (Caesalpinia spinosa). Rev. Inst. Investig. FIGMMG 2004, 7, 64–73. [Google Scholar]
- Skowyra, M.; Janiewicz, U.; Salejda, A.M.; Krasnowska, G.; Almajano, M.P. Effect of Tara (Caesalpinia spinosa) Pod Powder on the Oxidation and Colour Stability of Pork Meat Batter During Chilled Storage. Food Technol. Biotechnol. 2015, 53, 419–427. [Google Scholar] [CrossRef]
- Weisburger, J.H.; Veliath, E.; Larios, E.; Pittman, B.; Zang, E.; Hara, Y. Tea polyphenols inhibit the formation of mutagens during the cooking of meat. Mutat. Res. Genet. Toxicol. Environ. Mutagenesis 2002, 516, 19–22. [Google Scholar] [CrossRef]
- Salejda, A.M.; Krasnowska, G.; Tril, U. Attempt to utilize antioxidant properties of green tea extract in the production of model meat products. Zywnosc-Nauka Technol. Jakosc 2011, 18, 107–118. [Google Scholar] [CrossRef]
- Lee, M.; Woo, S.; Oh, S.; Kwon, T. Changes in contents and composition of insoluble dietary fiber during buckwheat germination. Korean J. Food Nutr. 1995, 8, 23–31. [Google Scholar]
- Gordon, D. The importance of dietary fiber in human nutrition and health. Korean J. Nutr. 1992, 25, 37–41. [Google Scholar]
- Baumgertel, A.; Grimm, R.; Eisenbeiss, W.; Kreis, W. Purification and characterization of a flavonol 3-O-betaheterodisaccharidase from the dried herb of Fagopyrum esculentum. Phytochemistry 2003, 64, 411–418. [Google Scholar] [CrossRef]
- Dziedzic, K.; Górecka, D.; Kobus-Cisowska, J.; Jeszka, M. Możliwość wykorzystania gryki w produkcji żywności funkcjonalnej. Nauka Przyr. Technol. 2010, 4, 28. [Google Scholar]
- Choy, A.L.; Morrison, D.P.; Hughes, G.J.; Marriott, J.P.; Small, M.D. Quality and antioxidant properties of instant noodles enhanced with common buckwheat flour. J. Cereal Sci. 2013, 57, 281–287. [Google Scholar] [CrossRef]
- Trzebska-Jeske, I.; Nadolna, I.; Rutkowska, U.; Secomska, B. Wpływ obróbki mechanicznej na wartość odżywczą kasz produkowanych w kraju. Rocznik PZH 1973, 24, 717–724. [Google Scholar]
- Zalesskaja, E.W.; Mielnikow, E.M. Izmienije mineralnogo sostawa jadricy pri gidrotermiczeskoj obrabotke. Technology 1978, 7, 43–44. [Google Scholar]
- Amarowicz, R.; Fornal, Ł. Characteristics of buckwheat grain mineral components and dietary fiber. Fagopyrum 1987, 7, 3–6. [Google Scholar]
- Ikedai, S.; Yamashita, Y.; Kreft, I. Mineral composition of buckwheat by-products and its processing characteristics to konjak preparation. Fagopyrum 1999, 16, 89–94. [Google Scholar]
- Sedej, I.; Sakac, M.; Mandic, A.; Misan, A.; Tumbas, V.; Canadanovic-Brunet, J. Buckwheat (Fagopyrum esculentum Moench) grain and fractions: Antioxidant compounds and activities. J. Food Sci. 2012, 77, 954–959. [Google Scholar] [CrossRef] [PubMed]
- Hęś, M.; Gorecka, D.; Dziedzic, K. Antioxidant properties of extracts from buckwheat by-products. Acta Sci. Pol. Technol. Aliment. 2012, 11, 167–174. [Google Scholar] [PubMed]
- Dziadek, K.; Kopeć, A.; Pastucha, E.; Piątkowska, E.; Leszczyńska, T.; Pisulewska, E.; Witkowicz, R.; Francik, R. Basic chemical composition and bioactive compounds content in selected cultivars of buckwheat whole seeds, dehulled seeds and hulls. J. Cereal Sci. 2016, 69, 1–8. [Google Scholar] [CrossRef]
- Borkowska, B.; Robaszewska, A. Use of buckwheat grain in various industry branches. Zesz. Nauk. Akad. Mor. Gdyni 2012, 73, 43–55. [Google Scholar]
- Zarzecka, K.; Gugała, M.; Mystkowska, I. Wartość odżywcza i możliwości wykorzystania gryki. Postępy Fitoter. 2014, 1, 28–31. [Google Scholar]
- Ecovative. Available online: https://ecovativedesign.com/mycocomposite (accessed on 1 February 2022).
- Siauciunas, R.; Valanciene, V. Influence of buckwheat hulls on the mineral composition and strength development of easily fusible clay body. Appl. Clay Sci. 2020, 197, 105794. [Google Scholar] [CrossRef]
- Cucina, M.; Pezzolla, D.; Tacconi, C.; Gigliotti, G. Pretreatments for enhanced biomethane production from buckwheat hull: Effects on organic matter degradation and process sustainability. J. Environ. Manag. 2021, 285, 112098. [Google Scholar] [CrossRef]
- Wronkowska, M.; Zieliński, H.; Szmatowicz, B.; Ostaszyk, A.; Lamparski, G.; Majkowska, A. Effect of roasted buckwheat flour and hull enrichment on the sensory qualities, acceptance and safety of innovative mixed rye/wheat and wheat bakery products. J. Food Process. Preserv. 2019, 43, 8. [Google Scholar] [CrossRef]
- Hęś, M.; Szwengiel, A.; Dziedzic, K.; Le Thanh-Blicharz, J.; Kmiecik, D.; Górecka, D. The Effect of Buckwheat Hull Extract on Lipid Oxidation in Frozen-Stored Meat Products. J. Food Sci. 2017, 82, 882–889. [Google Scholar] [CrossRef]
- Püssa, T.; Pällin, R.; Raudsepp, P.; Soidla, R.; Rei, M. Inhibition of lipid oxidation and dynamics of polyphenol content in me-chanically deboned meat supplemented with sea buckthorn (Hippophae rhamnoides) berry residues. Food Chem. 2008, 107, 714–721. [Google Scholar] [CrossRef]
- Mazur, M.; Salejda, A.M.; Pilarska, K.M.; Krasnowska, G.; Nawirska-Olszańska, A.; Kolniak-Ostek, J.; Bąbelewski, P. The Influence of Viburnum opulus Fruits Addition on Some Quality Properties of Homogenized Meat Products. Appl. Sci. 2021, 11, 3141. [Google Scholar] [CrossRef]
- Kolniak-Ostek, J. Chemical composition and antioxidant capacity of different anatomical parts of pear (Pyrus communis L.). Food Chem. 2016, 203, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Simpson, R.J.; Neuberger, M.R.; Lin, T.Y. Complete amino acid analysis of proteins from a single hydrolysate. J. Biol. Chem. 1976, 251, 1936–1940. [Google Scholar] [CrossRef]
- Spackman, D.H.; Stein, W.H.; Moore, S. Automatic recording apparatus for use in the chromatography amino acid. Anal. Chem. 1958, 30, 1190–1206. [Google Scholar] [CrossRef]
- Kowalczewski, P.Ł.; Olejnik, A.; Białas, W.; Rybicka, I.; Zielińska-Dawidziak, M.; Siger, A.; Kubiak, P.; Lewandowicz, G. The nutritional value and biological activity of concentrated protein fraction of potato juice. Nutrients 2019, 11, 1523. [Google Scholar] [CrossRef] [PubMed]
- Zielińska-Dawidziak, M.; Tomczak, A.; Burzyńska, M.; Rokosik, E.; Dwiecki, K.; Piasecka-Kwiatkowska, D. Comparison of Lupinus angustifolius protein digestibility in dependence on protein, amino acids, trypsin inhibitors and polyphenolic compounds content. Int. J. Food Sci. Technol. 2020, 55, 2029–2040. [Google Scholar] [CrossRef]
- ISO 1871:2009; Food and Feed Products–General Guidelines for the Determination of Nitrogen by the Kjeldahl Method. ISO: Geneva, Switzerland, 2009.
- PN-EN 13805:2003; Foodstuffs–Determination of Trace Elements–Pressure Digestion. PKN: Warszawa, Poland, 2003.
- PN-EN 1134:1999; Fruit and Vegetable Juices–Determination of Sodium, Potassium, Calcium and Magnesium Content by Atomic Absorption Spectrometry (AAS). PKN: Warszawa, Poland, 2013.
- PN-EN 14084:2004; Foodstuffs–Determination of Trace Elements–Determination of Lead, Cadmium, Zinc, Copper and Iron by Atomic Absorption Spectrometry (AAS) after Microwave Digestion. PKN: Warszawa, Poland, 2004.
- Sol-Hee, L.; Gye-Woong, K.; Juhui, C.; Hack-Youn, K. Effect of Buckwheat (Fagopyrum esculentum) Powder on the Physicochemical and Sensory Properties of Emulsion-type Sausage. Korean J. Food Sci. Anim. Resour. 2018, 38, 927–935. [Google Scholar]
- Lee, M.S.; Shon, K.H. Content comparison on dietary fiber and rutin of Korean buckwheat according to growing district and classification. Korean J. Soc. Food Sci. 1994, 10, 249–253. [Google Scholar]
- Campagnol, P.C.B.; Dos Santos, B.A.; Wagner, R.; Terra, N.N.; Pollonio, M.A.R. The Effect of Soy Fiber Addition on the Quality of Fermented Sausages at Low-Fat Content. J. Food Qual. 2013, 36, 41–50. [Google Scholar] [CrossRef]
- Fang, Z.; Lin, P.; Ha, M.; Warner, R.D. Effects of incorporation of sugarcane fibre on the physicochemical and sensory properties of chicken sausage. Int. J. Food Sci. Technol. 2019, 54, 1036–1044. [Google Scholar] [CrossRef]
- Ferjančič, B.; Kugler, S.; Korošec, M.; Polak, T.; Bertoncelj, J. Development of low-fat chicken bologna sausages enriched with inulin, oat fibre or psyllium. Int. J. Food Sci. Technol. 2021, 56, 1818–1828. [Google Scholar] [CrossRef]
- Kim, C.J.; Kim, H.W.; Hwang, K.E.; Song, D.H.; Ham, Y.K.; Choi, J.H.; Kim, Y.B.; Choi, Y.S. Effects of dietary fiber extracted from pumpkin (Cucurbita maxima duch.) on the physic-chemical and sensory characteristics of reduced-fat frankfurters. Korean J. Food Sci. Anim. Resour. 2016, 36, 309–318. [Google Scholar] [CrossRef]
- Yadav, S.; Pathera, A.K.; Islam, R.U.; Malik, A.K.; Sharma, D.P. Effect of wheat bran and dried carrot pomace addition on quality characteristics of chicken sausage. Asian-Australas. J. Anim. Sci. 2018, 31, 729–737. [Google Scholar] [CrossRef]
- Park, K.S.; Choi, Y.S.; Kim, H.Y.; Kim, H.W.; Song, D.H.; Hwang, K.E.; Choi, S.G.; Kim, C.J. Quality characteristics of chicken emulsion sausages with different levels of makgeolli lees fiber. Korean J. Food Sci. Anim. Resour. 2012, 32, 54–61. [Google Scholar] [CrossRef]
- Bejosano, F.P.; Corke, H. Amaranthus and buckwheat protein concentrate effects on an emulsion-type meat product. Meat Sci. 1998, 50, 343–353. [Google Scholar] [CrossRef]
- Salejda, A.M.; Janiewicz, U.; Korzeniowska, M.; Kolniak-Ostek, J.; Krasnowska, G. Effect of walnut green husk addition on some quality properties of cooked sausages. LWT-Food Sci. Technol. 2016, 65, 751–757. [Google Scholar] [CrossRef]
- Kim, H.W.; Hwang, K.E.; Song, D.H.; Kim, Y.J.; Ham, Y.K.; Jeong, T.J.; Choi, Y.S.; Kim, C.J. Germinated barley as a functional ingredient in chicken sausages: Effect on physicochemical and technological properties at different levels. J. Food Sci. Technol. 2016, 53, 872–879. [Google Scholar] [CrossRef][Green Version]
- Shin, J.H.; Kang, M.J.; Kim, R.J.; Sung, N.J. The Quality Characteristics of Sausage with Added Black Garlic Extracts. Korean J. Food Cook. Sci. 2011, 27, 701–711. [Google Scholar] [CrossRef]
- Seo, J.K.; Parvin, R.; Yim, D.G.; Zahid, M.A.; Yang, H.S. Effects on quality properties of cooked pork sausages with Caesalpinia sappan L. extract during cold storage. J. Food Sci. Technol. 2019, 56, 4946–4955. [Google Scholar]
- Jin, S.K.; Choi, J.S.; Lee, S.J.; Hur, S.J. Effect of Coptis chinensis Franch Addition on the Quality Characteristics of Sausages During Cold Storage. Food Bioprocess. Technol. 2015, 8, 1045–1053. [Google Scholar] [CrossRef]
- Biel, W.; Maciorowski, R. Evaluation of chemical composition and nutritional quality of buckwheat groat, bran and hull (Fagopyrum esculentum Möench L.). Italian J. Food Sci. 2013, 25, 384–389. [Google Scholar]
- Noriham, A.; Ariffaizuddin, R.; Noorlaila, A. Effects of the Addition of Okara Flour on the Proximate and Amino Acid Compositions of Beef Sausage. In Proceedings of the 2nd International Conference on Food Quality, Safety and Security, Colombo, Sri Lanka, 25–26 October 2018; Volume 1, pp. 26–32. [Google Scholar]
- Jin, S.K.; Park, J.H. Effect of the Addition of Schisandra chinensis Powder on the Physico-chemical Characteristics of Sausage. Asian-Australas. J. Anim. Sci. 2013, 26, 753–761. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Romano, A.; Giosafatto, C.V.; Masi, P.; Mariniello, L. Impact of dehulling on the physico-chemical properties and in vitro protein digestion of common beans (Phaseolus vulgaris L.). Food Funct. 2015, 6, 1345–1351. [Google Scholar] [CrossRef] [PubMed]
- Gilani, G.S.; Xiao, C.W.; Cockell, K.A. Impact of Antinutritional Factors in Food Proteins on the Digestibility of Protein and the Bioavailability of Amino Acids and on Protein Quality. Br. J. Nutr. 2012, 108, 315–332. [Google Scholar] [CrossRef]
- Hur, S.J.; Lim, B.O.; Park, G.B.; Joo, S.T. Effects of Various Fiber Additions on Lipid Digestion during In Vitro Digestion of Beef Patties. J. Food Sci. 2009, 74, 653–657. [Google Scholar] [CrossRef]
- Torres, J.D.; Dueik, V.; Carré, D.; Bouchon, P. Effect of the Addition of Soluble Dietary Fiber and Green Tea Polyphenols on Acrylamide Formation and In Vitro Starch Digestibility in Baked Starchy Matrices. Molecules 2019, 24, 3674. [Google Scholar] [CrossRef]
- Lin, Y.; Chen, K.; Tu, D.; Yu, X.; Dai, Z.; Shen, Q. Characterization of dietary fiber from wheat bran (Triticum aestivum L.) and its effect on the digestion of surimi protein. LWT-Food Sci. Technol. 2019, 102, 106–112. [Google Scholar] [CrossRef]
- Ikeda, S.; Yamashita, Y. Buckwheatasa dietary source of zinc, copper and manganese. Fagopyrum 1994, 14, 29–34. [Google Scholar]
- Zając, M.; Guzik, P.; Kulawik, P.; Tkaczewska, J.; Florkiewicz, A.; Migdał, W. The quality of pork loaves with the addition of hemp seeds, de-hulled hemp seeds, hemp protein and hemp flour. LWT 2019, 105, 190–199. [Google Scholar] [CrossRef]
- Kim, H.W.; Setyabrata, D.; Lee, Y.; Jones, O.G.; Kim, Y.H.B. Effect of House Cricket (Acheta domesticus) Flour Addition on Physicochemical and Textural Properties of Meat Emulsion Under Various Formulations. J. Food Sci. 2017, 82, 2787–2793. [Google Scholar] [CrossRef] [PubMed]
- Wahlsten, A. Phenolic Compounds in Fagopyrum sp. Grains (buckwheat): Profile, Bioactivity and Effect of Processing; Swedish University of Agricultural Sciences: Uppsala, Switzerland, 2019; Available online: https://stud.epsilon.slu.se/15069/ (accessed on 13 January 2022).
- Dietrych-Szóstak, D. Flavonoids in hulls of different varieties of buckwheat and their antioxidant activity. In Proceedings of the 9th International Symposium on Buckwheat, Prague, Czech Republic, 18–22 August 2004; pp. 621–625. [Google Scholar]
- Nikfarjam, B.A.; Hajiali, F.; Adineh, M.; Nassiri-Asl, M. Anti-inflammatory Effects of Quercetin and Vitexin on Activated Human Peripheral Blood Neutrophils: The effects of quercetin and vitexin on human neutrophils. J. Pharmacopunct. 2017, 20, 127–131. [Google Scholar] [CrossRef]
- Guimarães, C.C.; Oliveira, D.D.; Valdevite, M.; Fachin Saltoratto, A.L.; Vaz Pereira, S.I.; de Castro França, S.; Soares Pereira, A.M.; Pereira, P.S. The glycosylated flavonoids vitexin, isovitexin, and quercetrin isolated from Serjania erecta Radlk (Sapindaceae) leaves protect PC12 cells against amyloid-β25-35 peptide-induced toxicity. Food Chem. Toxicol. 2015, 86, 88–94. [Google Scholar] [CrossRef] [PubMed]
| Ingredients [g] | Formulation | |||
|---|---|---|---|---|
| 0% BH | 1% BH | 2% BH | 3% BH | |
| Pork | 200 | 200 | 200 | 200 |
| Back fat | 120 | 120 | 120 | 120 |
| Ice | 80 | 80 | 80 | 80 |
| Curing salt | 4.8 | 4.8 | 4.8 | 4.8 |
| Sugar | 4 | 4 | 4 | 4 |
| Seasoning | 2.4 | 2.4 | 2.4 | 2.4 |
| Buckwheat husk (BH) | 0 | 4 | 8 | 12 |
| Formulation | Thermal Losses | Storage Losses | Yield of Production |
|---|---|---|---|
| 0% BH | 14.0 a ± 0.53 | 1.93 d ± 0.01 | 85.8 b ± 0.38 |
| 1% BH | 14.2 a ± 0.67 | 1.69 c ± 0.01 | 85.8 b ± 0.74 |
| 2% BH | 15.5 c ± 0.53 | 1.52 b ± 0.01 | 84.5 a ± 0.53 |
| 3% BH | 14.9 bc ± 0.52 | 1.36 a ± 0.01 | 85.5 b ± 0.89 |
| Storage Time [Weeks] | Formulation | Hardness [N] | Springiness [mm] | Gumminess [N] | Chewiness [Nm] | Cohesiveness [–] |
|---|---|---|---|---|---|---|
| 0 | 0% BH | 52.9 bc ± 1.76 | 0.68 a ± 0.06 | 18.2 b ± 1.02 | 12.2 a ± 1.71 | 0.33 a ± 0.03 |
| 1% BH | 47.6 a ± 1.33 | 0.68 a ± 0.06 | 14.8 a ± 1.20 | 10.1 a ± 1.55 | 0.31 a ± 0.02 | |
| 2% BH | 49.4 ab ± 4.31 | 0.65 a ± 0.03 | 17.3 ab ± 1.34 | 11.4 a ± 0.82 | 0.34 ab ± 0.04 | |
| 3% BH | 52.1 bc ± 1.94 | 0.68 ab ± 0.03 | 16.9 ab ± 1.78 | 11.5 a ± 1.02 | 0.33 a ± 0.04 | |
| 2 | 0% BH | 54.3 cd ± 3.58 | 0.70 bc ± 0.05 | 22.7 c ± 4.31 | 16.0 b ± 4.00 | 0.42 d ± 0.04 |
| 1% BH | 60.0 d ± 2.66 | 0.75 c ± 0.03 | 25.6 d ± 1.96 | 19.1 c ± 2.04 | 0.43 d ± 0.03 | |
| 2% BH | 58.7 d ± 2.16 | 0.75 c ± 0.04 | 23.6 cd ± 3.96 | 17.3 cb ± 1.68 | 0.38 c ± 0.03 | |
| 3% BH | 58.0 d ± 1.67 | 0.73 bc ± 0.04 | 21.2 c ± 1.93 | 15.4 c ± 1.04 | 0.37 bc ± 0.04 |
| Storage Time [Weeks] | Formulation | L* | a* | b* |
|---|---|---|---|---|
| 0 | 0% BH | 74.21 d ± 0.99 | 4.55 b ± 0.47 | 10.40 c ± 0.35 |
| 1% BH | 62.80 c ± 0.92 | 4.10 a ± 0.22 | 7.55 b ± 0.36 | |
| 2% BH | 57.56 b ± 2.51 | 4.42 b ± 0.35 | 7.04 b ± 0.26 | |
| 3% BH | 53.64 a ± 1.39 | 4.60 b ± 0.25 | 6.98 a ± 0.47 | |
| 2 | 0% BH | 74.55 d ± 0.54 | 5.98 c ±0.70 | 8.89 c ± 0.36 |
| 1% BH | 64.67 c ± 1.54 | 3.66 a ± 0.16 | 6.96 b ± 0.33 | |
| 2% BH | 58.21 b ± 1.55 | 3.38 a ± 0.29 | 6.61 b ± 0.30 | |
| 3% BH | 52.87 a ± 1.88 | 4.94 b ± 0.66 | 5.88 a ± 0.47 |
| Amino Acid | 0% BH | 1% BH | 2% BH | 3% BH |
|---|---|---|---|---|
| ASP | 16.4 a ± 0.05 | 20.7 b ± 0.31 | 21.0 b ± 0.01 | 21.5 b ± 0.39 |
| THR | 7.92 a ± 0.02 | 10.0 b ± 0.18 | 11.9 c ± 0.00 | 12.0 c ± 0.02 |
| SER | 6.52 a ± 0.01 | 8.19 b ± 0.15 | 10.6 c ± 0.01 | 10.9 d ± 0.02 |
| GLU | 29.6 a ± 0.14 | 38.5 c ± 0.54 | 32.8 d ± 0.01 | 40.4 c ± 0.22 |
| PRO | 6.29 a ± 0.10 | 5.81 a ± 0.06 | 9.25 c ± 0.15 | 7.90 b ± 0.35 |
| GLY | 10.3 a ± 0.04 | 11.5 b ± 0.15 | 13.1 d ± 0.01 | 12.7 c ± 0.03 |
| ALA | 10.0 a ± 0.00 | 12.2 b ± 0.11 | 18.1 d ± 0.00 | 16.1 c ± 0.01 |
| CYS | 0.37 a ± 0.01 | 0.43 a ± 0.05 | 0.43 a ± 0.01 | 0.56 b ± 0.00 |
| VAL | 8.65 a ± 0.03 | 11.1 b ± 0.19 | 12.8 c ± 0.07 | 11.1 b ± 0.06 |
| MET | 3.03 a ± 0.01 | 3.58 b ± 0.05 | 4.41 d ± 0.00 | 4.17 c ± 0.01 |
| ILE | 7.94 a ± 0.00 | 10.2 b ± 0.17 | 12.1 c ± 0.02 | 12.1 c ± 0.03 |
| LEU | 13.4 a ± 0.04 | 17.1 b ± 0.09 | 16.8 b ± 0.29 | 17.1 b ± 0.11 |
| TYR | 3.71 a ± 0.00 | 5.06 b ± 0.07 | 6.04 c ± 0.00 | 7.38 d ± 0.03 |
| PHE | 7.28 a ± 0.00 | 9.20 b ± 0.16 | 11.5 d ± 0.01 | 10.9 c ± 0.05 |
| HIS | 5.42 a ± 0.03 | 7.39 b ± 0.14 | 5.73 a ± 0.01 | 7.73 c ± 0.07 |
| LYS | 13.8 a ± 0.01 | 17.8 b ± 0.33 | 18.3 b ± 0.10 | 19.8 c ± 0.04 |
| ARG | 11.2 a ± 0.04 | 14.0 b ± 0.15 | 15.4 c ± 0.00 | 15.8 d ± 0.05 |
| Total | 161.8 a | 202.7 b | 220.2 c | 228.0 d |
| PDA RT (min) | TOF1 | TOF2 | [M − H]− (m/z) | Fragment Ions | Compound |
|---|---|---|---|---|---|
| 0.85 | 0.86 | 0.88 | 273.0530 | Either catehin or epicatechin | |
| 0.94 | 1.051 | 1.035 | - | 146.1843; 188.1502 | Quercetin-3-(6-malonyl)-Glucoside |
| 1.018 | 1.035 | 377.1273 | Trehalose dihydrate | ||
| 0.98 | 1.604 | 1.567 | - | 347.0975 | Inosine-5′-monophosphate |
| 1.02 | 2.447 | 2.430 | 267.1691 | 7-Hydroxy-4′-Methoxyisoflavone (Formononetin) | |
| 3.188 | 3.171 | 164.2030 | Sinapic acid | ||
| 3.532 | 3.527 | 271.1116 | Juniperic acid | ||
| 5.325 | 5.317 | 315.1732 | Isorhamnetin-3-Galactoside-6″-Rhamnoside | ||
| 5.581 | 5.560 | - | 257.0191 | Kaempferol-7-O-alpha-l-rhamnoside | |
| 5.857 | 5.874 | 207.0349 | Sinapinaldehyde | ||
| 5.945 | 5.962 | - | 433.9072 | Quercetin derivatives | |
| 6.255 | 6.300 | - | 160.9744; 197.9301 | Rosmarinic acid | |
| 6.821 | 6.852 | 415.1234 | 253.1580 | Flavonoid derivatives | |
| 7.59 | 7.718 | 7.789 | 431.1103 | 311.1306 | Vitexin Apigenin-8-C-glucoside |
| 8.183 | 8.200 | 431.1018 | 269.1367 | Apigenin-7-O-glucoside | |
| 10.474 | 10.522 | - | 253.1515 | Flavonoid derivatives | |
| 10.697 | 10.714 | 269.1435 | - | Apigenin | |
| 13.265 | 13.314 | 595.1830 | Kaempferol-3-Glucoside-3″-Rhamnoside | ||
| 14.471 | 14.522 | - | 311.2462; 293.2622 | Vitexin-2″-O-rhamnoside | |
| 14.539 | 14.589 | - | 253. 259. 265 | Unidentified flavonoid |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salejda, A.M.; Olender, K.; Zielińska-Dawidziak, M.; Mazur, M.; Szperlik, J.; Miedzianka, J.; Zawiślak, I.; Kolniak-Ostek, J.; Szmaja, A. Frankfurter-Type Sausage Enriched with Buckwheat By-Product as a Source of Bioactive Compounds. Foods 2022, 11, 674. https://doi.org/10.3390/foods11050674
Salejda AM, Olender K, Zielińska-Dawidziak M, Mazur M, Szperlik J, Miedzianka J, Zawiślak I, Kolniak-Ostek J, Szmaja A. Frankfurter-Type Sausage Enriched with Buckwheat By-Product as a Source of Bioactive Compounds. Foods. 2022; 11(5):674. https://doi.org/10.3390/foods11050674
Chicago/Turabian StyleSalejda, Anna Marietta, Katarzyna Olender, Magdalena Zielińska-Dawidziak, Monika Mazur, Jakub Szperlik, Joanna Miedzianka, Ireneusz Zawiślak, Joanna Kolniak-Ostek, and Aleksandra Szmaja. 2022. "Frankfurter-Type Sausage Enriched with Buckwheat By-Product as a Source of Bioactive Compounds" Foods 11, no. 5: 674. https://doi.org/10.3390/foods11050674
APA StyleSalejda, A. M., Olender, K., Zielińska-Dawidziak, M., Mazur, M., Szperlik, J., Miedzianka, J., Zawiślak, I., Kolniak-Ostek, J., & Szmaja, A. (2022). Frankfurter-Type Sausage Enriched with Buckwheat By-Product as a Source of Bioactive Compounds. Foods, 11(5), 674. https://doi.org/10.3390/foods11050674








