Meat Substitution with Oat Protein Can Improve Ground Beef Patty Characteristics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cooking Methods
2.2. Allo-Kramer Shear Force
2.3. Cooking Time and Cooking Yield
2.4. Instrumental Cooked Color
2.5. Instrumental Fresh Color
2.6. Lipid Oxidation
2.7. Moisture Loss
2.8. Experimental Design and Statistical Analysis
3. Results and Discussion
3.1. Allo-Kramer Shear Force
3.2. Cooking Time
3.3. Cooking Yield
3.4. Moisture Loss
3.5. Instrumental Fresh Color
3.6. Thiobarbaurtic Acid Reactive Substances (TBARS)
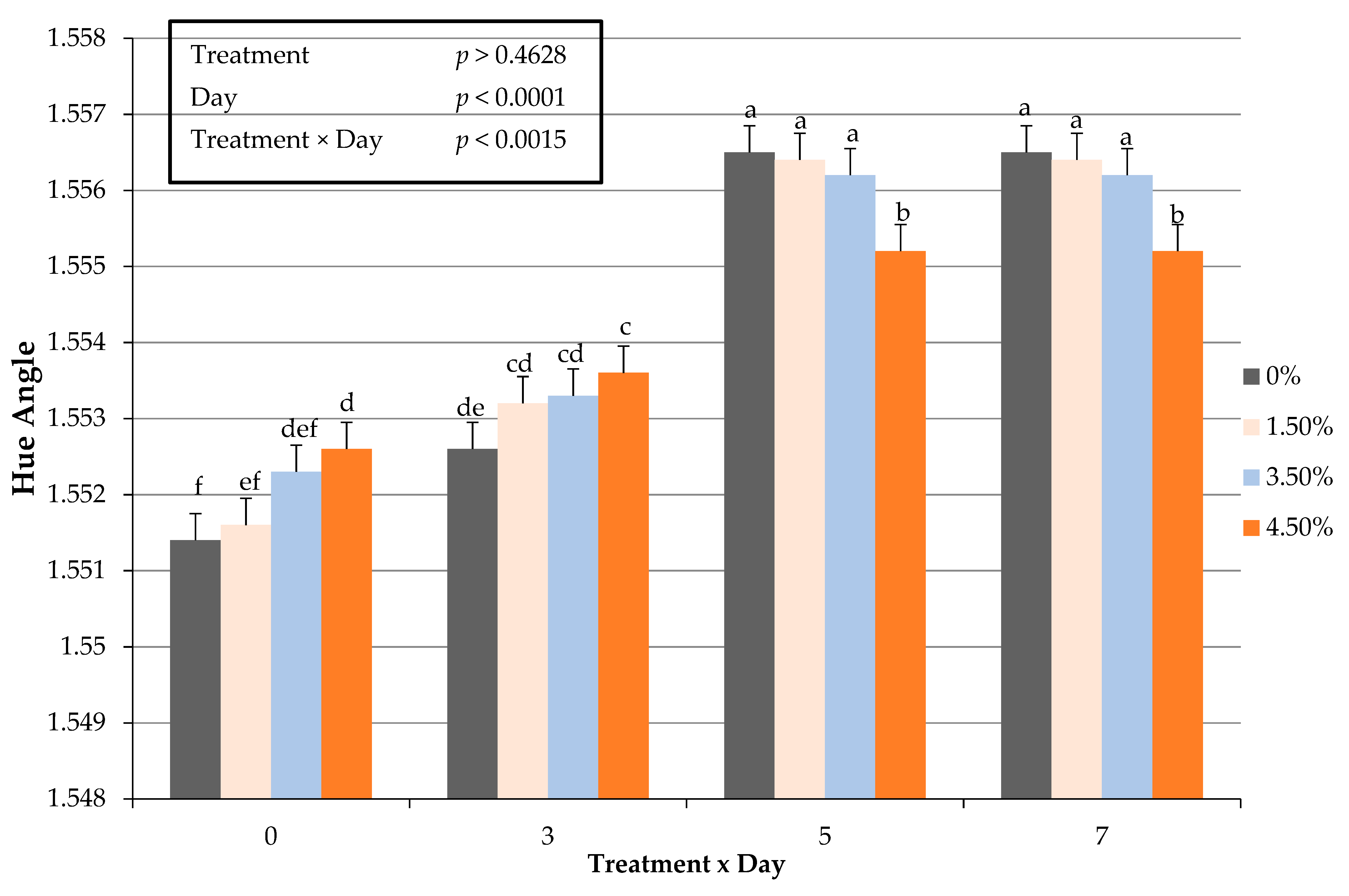
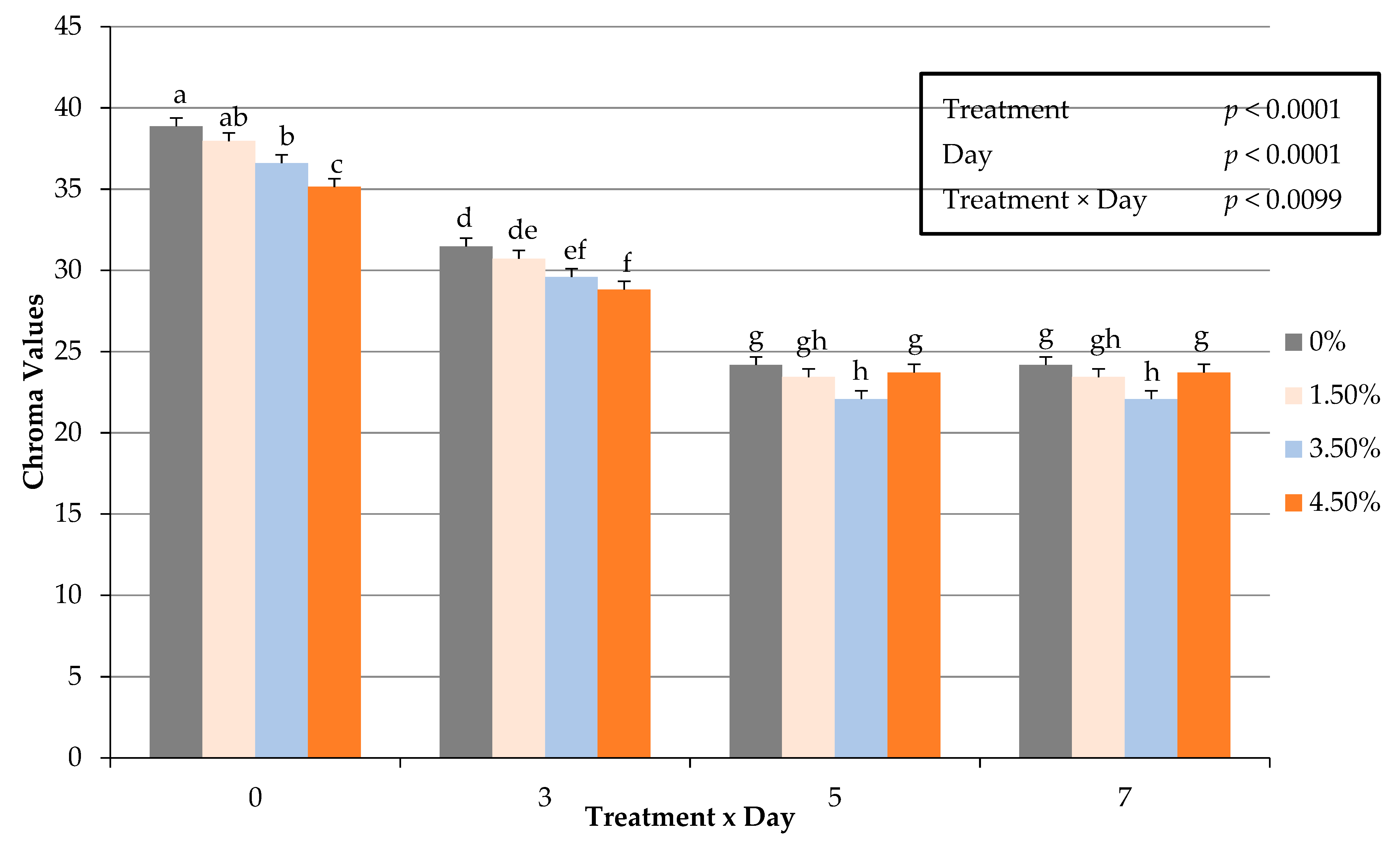
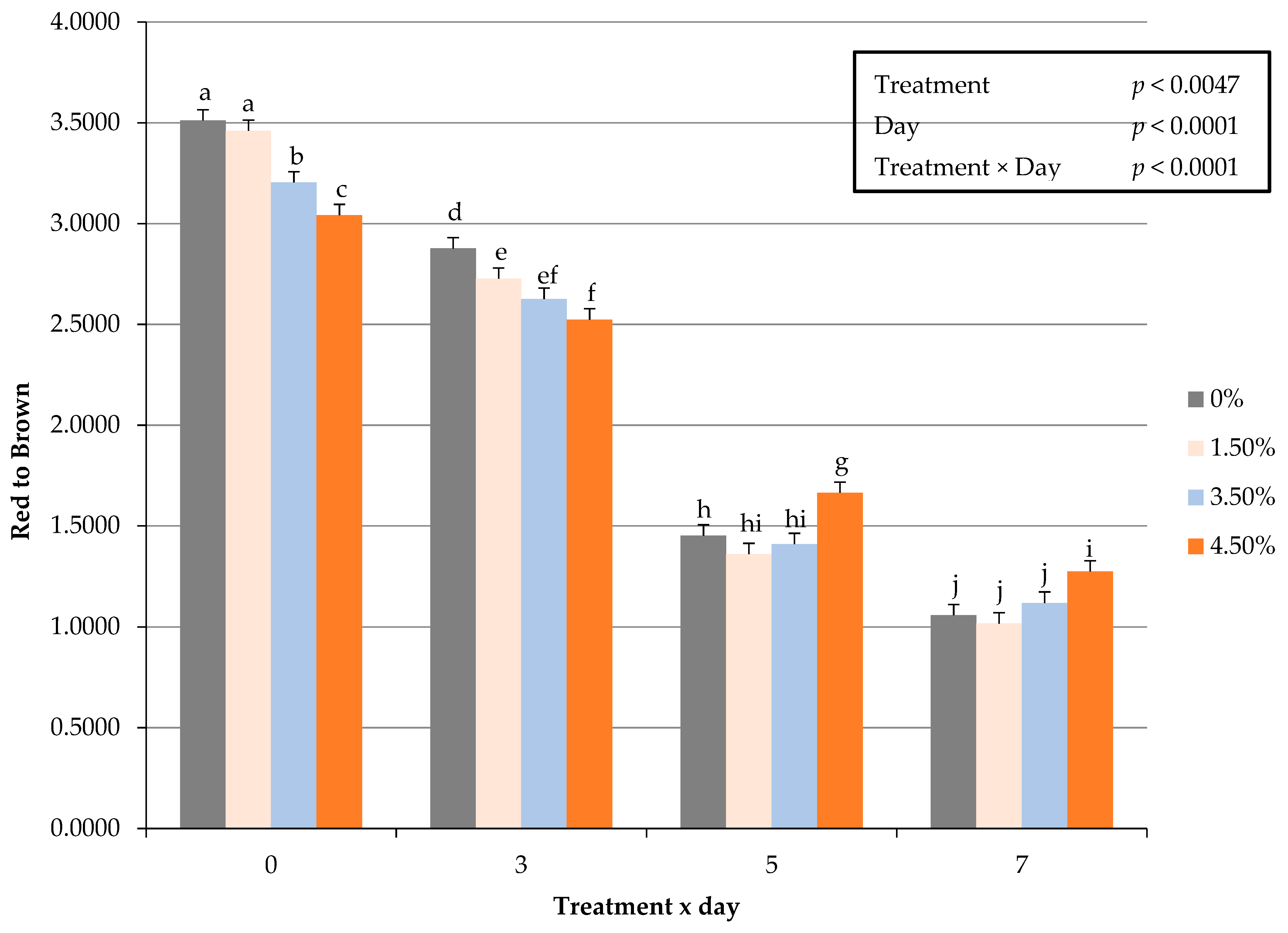
3.7. Instrumental Cooked Color
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barone, J.T. Drop in cattle expected to push beef prices up. Nation’s Restaur. News 2011, 44, 16. [Google Scholar]
- Jiménez-Colmenero, F.; Carballo, J.; Cofrades, S. Healthier meat and meat products: Their role as functional foods. Meat Sci. 2001, 59, 5–13. [Google Scholar] [CrossRef]
- Ishmael, W. Beef Industry Exhibits New Market Fundamentals. 2011. Available online: http://beefmagazine.com/business/wes-ishmael/0601-new-market-fundamentals (accessed on 10 March 2015).
- UN. Transfoming Our World: The 2030 Agenda for Sustainable Development; General Assembly of the United Nations: New York, NY, USA, 2015. [Google Scholar]
- Pintado, T.; Delgado-Pando, G. Towards More Sustainable Meat Products: Extenders as a Way of Reducing Meat Content. Foods 2020, 9, 1044. [Google Scholar] [CrossRef] [PubMed]
- Domingo, J.L.; Nadal, M. Carcinogenicity of consumption of red meat and processed meat: A review of scientific news since the IARC decision. Food Chem. Toxicol. 2017, 105, 256–261. [Google Scholar] [CrossRef]
- Micha, R.; Michas, G.; Mozaffarian, D. Unprocessed Red and Processed Meats and Risk of Coronary Artery Disease and Type 2 Diabetes—An Updated Review of the Evidence. Curr. Atheroscler. Rep. 2012, 14, 515–524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morach, B.; Witte, B.; Walker, D.; Von Koeller, E.; Grosse-Holz, F.; Rogg, J.; Brigl, M.; Dehnert, N.; Obkoj, P.; Koktenturk, S.; et al. Food for Thought: The Protein Transformation. Available online: https://www.bcg.com/en-gb/publications/2021/the-benefits-of-plant-based-meats/ (accessed on 16 November 2021).
- Ball, J.J.; Wyatt, R.P.; Lambert, B.D.; Smith, H.R.; Reyes, T.M.; Sawyer, J.T. Influence of Plant-Based Proteins on the Fresh and Cooked Characteristics of Ground Beef Patties. Foods 2021, 10, 1971. [Google Scholar] [CrossRef]
- Research and Markets 2020. Processed Poultry & Meat Market Analysis 2020–2025—Global Market Forecast to Grow at CAGR of 7.35% during 2020 and 2025. Available online: https://www.globenewswire.com/news-release/2020/06/08/2044720/0/en/Processed-Poultry-Meat-Market-Analysis-2020-2025-Global-Market-Forecast-to-Grow-at-a-CAGR-of-7-35-During-2020-and-2025.html (accessed on 26 October 2021).
- Mintel 2020 GNPD—Plant Proteins in Meat Substitues. Available online: https://www.gnpd.com/sinatra/analysis/chart_results/search/FlMf6yv1YN/?analysis_id=cc8808dc-ef15-4dc6-b442-bb162fc327f5t_tab=cc8808dc-ef15-4dc6-b442-bb162fc327f5 (accessed on 26 October 2021).
- Taylor, E.V.; Holt, K.G.; Mahon, B.E.; Ayers, T.; Norton, D.; Gould, L.H. Ground Beef Consumption Patterns in the United States, FoodNet, 2006 through 2007. J. Food Prot. 2012, 75, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Impey, L. Health benefits raise oat demand. Farmers Weekly 2009, 150, 54–59. [Google Scholar]
- Mulrony, B.R.; Chaddad, F.R. Strategic Alliances in the U.S. beef supply chain. J. Food Distrib. Res. 2005, 36, 18–32. [Google Scholar] [CrossRef]
- Peel, D. Beef supply chains and the impact of the COVID-19 pandemic in the United States. Anim. Front. 2021, 11, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.; Gregory, P.J. Climate change and sustainable food production. Proc. Nutr. Soc. 2013, 72, 21–28. [Google Scholar] [CrossRef] [Green Version]
- Tiwari, U.; Cummins, E.; Brunton, N.; O’Donnell, C.; Gallagher, E. A comparison of oat flour and oat bran-based bread formulations. Br. Food J. 2013, 115, 300–313. [Google Scholar] [CrossRef]
- Gray, J. Dietary Fiber: Definition, Analysis, Physiology and Health; International Life Science Institute (ILSI): Washington, DC, USA, 2006. [Google Scholar]
- Rodríguez, R.; Jiménez, A.; Fernandez-Bolaños, J.; Guillén, R.; Heredia, A. Dietary fibre from vegetable products as source of functional ingredients. Trends Food Sci. Technol. 2006, 17, 3–15. [Google Scholar] [CrossRef]
- Weiss, J.; Gibis, M.; Schuh, V.; Salminen, H. Advances in ingredient and processing systems for meat and meat products. Meat Sci. 2010, 86, 196–213. [Google Scholar] [CrossRef]
- Rosell, C.M.; Santos, E.; Collar, C. Physico-chemical properties of commerical fibres from different sources: A compara-tive approach. Food Res. Int. 2009, 42, 176–184. [Google Scholar] [CrossRef] [Green Version]
- García, M.L.; Calvo, M.M.; Selgas, M.D. Beef hamburgers enriched in lycopene using dry tomato peel as an ingredient. Meat Sci. 2009, 83, 45–49. [Google Scholar] [CrossRef]
- Commision Internationale de l’ Eclairage. Recommendations on Uniform Color Spaces—Color Difference Equations, Psychometric Color Terms; C.I.E. Publication No. 15, Suppl. 2(E-1.3.1), 1971/(TC-1-3); Bureau Central de la CIE: Paris, France, 1978. [Google Scholar]
- American Meat Science Association (AMSA). Guidelines for meat color evaluation. In Proceedings of the 44th Annual Reciprocal Meat Conference, Champagne, IL, USA, 9–12 June 1991. [Google Scholar]
- Tapp, W.N.; Yancey, J.W.S.; Apple, J.K. How is the instrumental color of meat measured? Meat Sci. 2011, 89, 1–5. [Google Scholar] [CrossRef]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. Methods Enzymol. 1978, 52, 302–310. [Google Scholar] [CrossRef]
- Aleson-Carbonell, L.; Fernandez-Lopez, J.; Pérez-Alvarez, J.A.; Kuri, V. Characteristics of beef burger as influenced by various types of lemon albedo. Innov. Food Sci. Emerg. Technol. 2005, 6, 247–255. [Google Scholar] [CrossRef]
- Savell, J.W.; Cross, H.R.; Francis, J.J.; Wise, J.W.; Hale, D.S.; Wilkes, D.L.; Smith, G.C. National consumer retail beef study: Interaction of trim level, price and grade on consumer acceptance of beef steaks and roasts. J. Food Qual. 1989, 12, 251–274. [Google Scholar] [CrossRef]
- Savell, J.W.; Harris, J.J.; Cross, H.R.; Hale, D.S.; Beasley, L.C. National beef market basket survey. J. Anim. Sci. 1991, 69, 2883–2893. [Google Scholar] [CrossRef]
- Anderson, E.T. Fat Retention Performance of Inner Pea Fiber and Its Potential Application in High and Low Fat Ground Beef. Master’s Thesis, University of Maryland, College Park, MD, USA, 1998. [Google Scholar]
- Sheridan, P.; Shilton, N. Analysis of yield while cooking beefburger patties using far infrared radiation. J. Food Eng. 2002, 51, 3–11. [Google Scholar] [CrossRef]
- Cross, H.R.; Berry, B.W.; Wells, L.H. Effects of fat level and source on the chemical, sensory and cooking properties of ground beef patties. J. Food Sci. 1980, 45, 791–794. [Google Scholar] [CrossRef]
- Berry, B.W.; Leddy, K.F. Effects of Fat Level and Cooking Method on Sensory and Textural Properties of Ground Beef Patties. J. Food Sci. 1984, 49, 870–875. [Google Scholar] [CrossRef]
- Kregel, K.K.; Prusa, K.J.; Hughes, K.V. Cholesterol Content and Sensory Analysis of Ground Beef as Influenced by Fat Level, Heating, and Storage. J. Food Sci. 1986, 51, 1162–1165. [Google Scholar] [CrossRef]
- Hoelscher, L.M.; Savell, J.; Smith, S.; Cross, H. Subcellular Distribution of Cholesterol within Muscle and Adipose Tissues of Beef Loin Steaks. J. Food Sci. 1988, 53, 718–722. [Google Scholar] [CrossRef]
- Troutt, E.; Hunt, M.; Johnson, D.; Claus, J.; Kastner, C.; Kropf, D. Characteristics of Low-fat Ground Beef Containing Texture-modifying Ingredients. J. Food Sci. 1992, 57, 19–24. [Google Scholar] [CrossRef]
- Keeton, J. Low-fat meat products—technological problems with processing. Meat Sci. 1994, 36, 261–276. [Google Scholar] [CrossRef]
- Piñero, M.; Parra, K.; Huerta-Leidenz, N.; de Moreno, L.A.; Ferrer, M.; Araujo, S.; Barboza, Y. Effect of oat’s soluble fibre (β-glucan) as a fat replacer on physical, chemical, microbiological and sensory properties of low-fat beef patties. Meat Sci. 2008, 80, 675–680. [Google Scholar] [CrossRef]
- Lynch, A.; Buckley, D.J.; Galvin, K.; Mullen, A.M.; Troy, D.J.; Kerry, J.P. Evaluation of rib steak colour from Friesian, Hereford and Charolais heifers pastured or overwintered prior to slaughter. Meat Sci. 2002, 61, 227–232. [Google Scholar] [CrossRef]
- Bruce, H.; Stark, J.; Beilken, S. The effects of finishing diet and postmortem ageing on the eating quality of the M. longissimus thoracis of electrically stimulated Brahman steer carcasses. Meat Sci. 2004, 67, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Hunt, M.C.; Mancini, R.A.; Hachmeister, K.A.; Kropf, D.H.; Merriman, M.; De Lduca, G.; Milliken, G. Carbon Monoxide in Modified Atmosphere Packaging Affects Color, Shelf Life, and Microorganisms of Beef Steaks and Ground Beef. J. Food Sci. 2004, 69, FCT45–FCT52. [Google Scholar] [CrossRef]
- Serdaroğlu, M.; Turp, G.Y.; Abrodímov, K. Quality of low-fat meatballs containing Legume flours as extenders. Meat Sci. 2005, 70, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Kanner, J. Oxidative processes in meat and meat products: Quality implications. Meat Sci. 1994, 36, 169–189. [Google Scholar] [CrossRef]
- Moon, C.T.; Yancey, J.W.S.; Apple, J.K.; Hollenbeck, J.J.; Johnson, T.M.; Winters, A.R. Incorporation of Lean Finely Tex-Tured Beef Improved Select Quality Characteristics of Ground Beef Patties; Arkansas Animal Science Department Report; University of Arkansas: Fayetteville, AR, USA, 2012; pp. 100–103. [Google Scholar]

| Treatment | ||||||
|---|---|---|---|---|---|---|
| 0% | 1.5% | 3.5% | 4.5% | SEM * | p-Value | |
| Allo-Kramer Shear Foce, N 1 | 394.19 b | 410.42 b | 474.54 a | 496.99 a | 8.854 | 0.0001 |
| Cook Yield, % 2 | 85.01 c | 88.87 b | 88.85 b | 90.47 a | 0.364 | 0.0001 |
| Cooking Time, s 3 | 585.20 c | 593.80 c | 702.55 a | 652.55 b | 12.951 | 0.0001 |
| Moisture Loss, % 4 | 0.5604 | 0.5905 | 0.5830 | 0.5598 | 0.167 | 0.7496 |
| Treatment | ||||||
|---|---|---|---|---|---|---|
| 0% | 1.5% | 3.5% | 4.5% | SEM * | p-Value | |
| Lightness (L*) 1 | 53.5732 c | 54.0109 b | 54.3325 b | 55.7153 a | 0.1382 | 0.0001 |
| Yellowness (b*) 2 | 21.2451 a | 20.7982 b | 19.9741 c | 20.0435 c | 0.1465 | 0.0001 |
| Day of Display | ||||||
|---|---|---|---|---|---|---|
| 0 | 3 | 5 | 7 | SEM * | p-Value | |
| Lightness (L*) 1 | 56.144 a | 54.367 b | 53.559 c | 53.559 c | 0.1382 | 0.0001 |
| Yellowness (b*) 2 | 25.246 a | 21.205 b | 17.804 c | 17.804 c | 0.1465 | 0.0001 |
| Treatment | ||||||
| 0% | 1.5% | 3.5% | 4.5% | SEM * | p-Value | |
| TBARS 1 | 1.6941 | 1.7293 | 1.8354 | 1.8832 | 0.0789 | 0.2995 |
| Day | ||||||
| 0 | 3 | 5 | 7 | SEM * | p-Value | |
| TBARS 1 | 2.421 a | 1.531 b | -- | 1.404 b | 0.0683 | 0.0001 |
| Treatment | ||||||
|---|---|---|---|---|---|---|
| 0% | 1.5% | 3.5% | 4.5% | SEM * | p-Value | |
| Lightness (L*) 1 | 55.3082 | 55.5011 | 55.4110 | 55.3773 | 0.1950 | 0.9165 |
| Redness (a*) 2 | 17.1937 a | 15.8252 b | 15.3455 b | 14.4602 c | 0.2436 | 0.0001 |
| Yellowness (b*) 3 | 16.3984 | 16.7297 | 16.5903 | 16.7103 | 0.1803 | 0.5507 |
| Hue Angle 4 | 1.5525 c | 1.5543 b | 1.5546 b | 1.5557 a | 0.0002 | 0.0001 |
| Chroma 5 | 23.7627 a | 23.0341 a,b | 22.6082 b,c | 22.1023 c | 0.2543 | 0.0004 |
| Red to Brown 6 | 1.9873 a | 1.8161 b | 1.8018 b | 1.6704 c | 0.0448 | 0.0002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ball, J.J.; Wyatt, R.P.; Coursen, M.M.; Lambert, B.D.; Sawyer, J.T. Meat Substitution with Oat Protein Can Improve Ground Beef Patty Characteristics. Foods 2021, 10, 3071. https://doi.org/10.3390/foods10123071
Ball JJ, Wyatt RP, Coursen MM, Lambert BD, Sawyer JT. Meat Substitution with Oat Protein Can Improve Ground Beef Patty Characteristics. Foods. 2021; 10(12):3071. https://doi.org/10.3390/foods10123071
Chicago/Turabian StyleBall, Jase J., Ross P. Wyatt, Madison M. Coursen, Barry D. Lambert, and Jason T. Sawyer. 2021. "Meat Substitution with Oat Protein Can Improve Ground Beef Patty Characteristics" Foods 10, no. 12: 3071. https://doi.org/10.3390/foods10123071
APA StyleBall, J. J., Wyatt, R. P., Coursen, M. M., Lambert, B. D., & Sawyer, J. T. (2021). Meat Substitution with Oat Protein Can Improve Ground Beef Patty Characteristics. Foods, 10(12), 3071. https://doi.org/10.3390/foods10123071







