The Quantification of IgG Specific to α-Gal Could Be Used as a Risk Marker for Suffering Mammalian Meat Allergy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Ethical Approval
2.2. Determination of sIgE Antibodies against Different Allergens
2.3. Quantification of sIgE and sIgG Specific Antibodies against the α-Gal Epitope
2.4. Statistical Analysis
3. Results
3.1. Clinical and Demographic Data of Participants
3.1.1. AGS Patients
3.1.2. Risk-Population Group
3.1.3. Profiling of sIgE Antibodies in Risk- and Atopic Population Groups
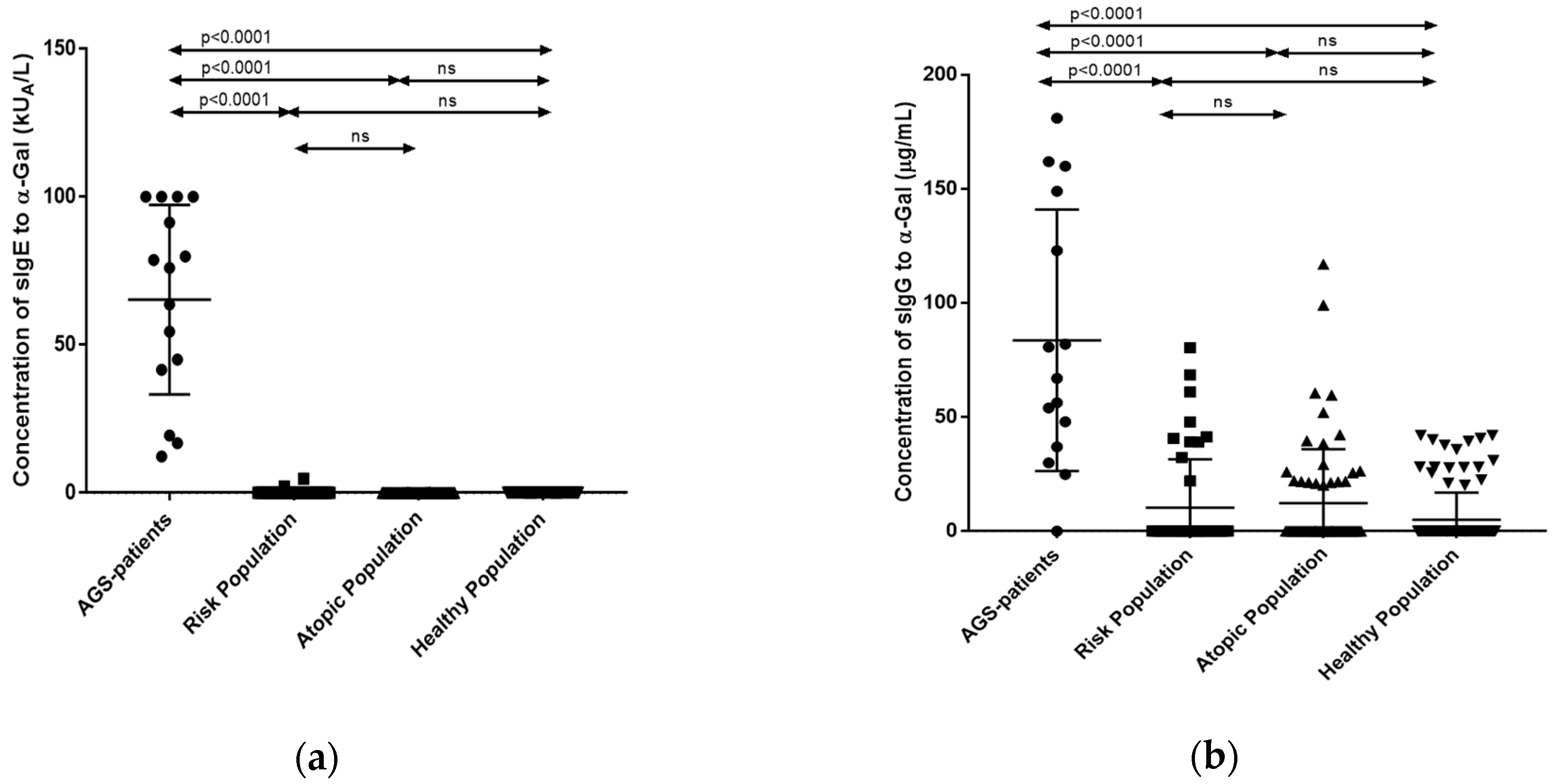
3.2. Quantification of sIgE and sIgG Antibodies against the α-Gal Epitope
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Commins, S.P. Diagnosis & management of alpha-gal syndrome: Lessons from 2500 patients. Expert Rev. Clin. Immunol. 2020, 16, 667–677. [Google Scholar] [CrossRef]
- Hilger, C.; Fischer, J.; Wolbing, F.; Biedermann, T. Role and mechanism of galactose-alpha-1,3-galactose in the elicitation of delayed anaphylactic reactions to red meat. Curr. Allergy Asthma Rep. 2019, 19, 3. [Google Scholar] [CrossRef] [Green Version]
- Commins, S.P.; James, H.R.; Stevens, W.; Pochan, S.L.; Land, M.H.; King, C.; Mozzicato, S.; Platts-Mills, T.A.E. Delayed clinical and ex vivo response to mammalian meat in patients with IgE to galactose-alpha-1,3- galactose. J. Allergy Clin. Immunol. 2014, 134, 108–115. [Google Scholar] [CrossRef] [Green Version]
- Fischer, J.; Hebsaker, J.; Caponetto, P.; Platts-Mills, T.A.E.; Biedermann, T. Galactose-alpha-1,3-galactose sensitization is a prerequisite for pork-kidney allergy and cofactor-related mammalian meat anaphylaxis. J. Allergy Clin. Immunol. 2014, 134, 755–759. [Google Scholar] [CrossRef] [PubMed]
- Platts-Mills, T.A.E.; Commins, S.P.; Biedermann, T.; van Hage, M.; Levin, M.; Beck, L.A.; Diuk-Wasser, M.; Jappe, U.; Apostolovic, D.; Minnicozzi, M.; et al. On the cause and consequences of IgE to galactose-α-1,3-galactose: A report from the national institute of allergy and infectious diseases workshop on understanding IgE-mediated mammalian meat allergy. J. Allergy Clin. Immunol. 2020, 145, 1061–1071. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.M.; Schuyler, A.J.; Workman, L.; Gupta, M.; James, H.R.; Posthumus, J.; McGowan, E.C.; Commins, S.P.; Platts-Mills, T.A.E. Investigation into the α-Gal syndrome: Characteristics of 261 children and adults reporting red meat allergy. J. Allergy Clin. Immunol. 2019, 7, 2348–2358. [Google Scholar] [CrossRef] [PubMed]
- Young, I.; Prematunge, C.; Pussegoda, K.; Corrin, T.; Waddell, L. Tick exposures and alpha-gal syndrome: A systematic review of the evidence. Ticks Tick-Borne Dis. 2021, 12, 101674. [Google Scholar] [CrossRef] [PubMed]
- Galili, U.; Shohet, S.B.; Kobrin, E.; Stults, C.L.; Macher, B.A. Man, apes, and Old World monkeys differ from other mammals in the expression of alpha-galactosyl epitopes on nucleated cells. J. Biol. Chem. 1988, 263, 17755–17762. [Google Scholar] [CrossRef]
- Galili, U. Human Natural Antibodies to Mammalian Carbohydrate Antigens as Unsung Heroes Protecting against Past, Present, and Future Viral Infections. Antibodies 2020, 9, 25. [Google Scholar] [CrossRef]
- Apostolovic, D.; Mihailovic, J.; Commins, S.P.; Wijnveld, M.; Kazimirova, M.; Starkhammar, M.; Stockinger, H.; Platts-Mills, T.A.E.; Cirkovic Velickovic, T.; Hamsten, C.; et al. Allergenomics of the tick Ixodes ricinus reveals important α-Gal-carrying IgE-binding proteins in red meat allergy. Allergy 2020, 75, 217–220. [Google Scholar] [CrossRef]
- Araujo, R.N.; Franco, P.F.; Rodrigues, H.; Santos, L.C.B.; McKayç, C.S.; Sanhueza, C.A.; Nascimento Brito, C.R.; Araújo Azevedo, M.; Venuto, A.P.; Cowan, P.J.; et al. Amblyomma sculptum tick saliva: α-Gal identification, antibody response and possible association with red meat allergy in Brazil. Int. J. Parasitol. 2016, 46, 213–220. [Google Scholar] [CrossRef] [Green Version]
- Hamsten, C.; Starkhammar, M.; Tran, T.A.; Johansson, M.; Bengtsson, U.; Ahlén, G.; Sällberg, M.; Grönlund, H.; van Hage, M. Identification of galactose-a-1,3-galactose in the gastrointestinal tract of the tick Ixodes ricinus; Possible relationship with red meat allergy. Allergy Eur. J. Allergy Clin. Immunol. 2013, 68, 549–552. [Google Scholar] [CrossRef] [PubMed]
- Commins, S.P.; James, H.R.; Kelly, L.A.; Pochan, S.L.; Workman, L.J.; Perzanowski, M.S.; Kocan, K.M.; Fahy, J.V.; Nganga, L.W.; Ronmark, E.; et al. The relevance of tick bites to the production of IgE antibodies to the mammalian oligosaccharide galactose-α-1,3-galactose. J. Allergy Clin. Immunol. 2011, 127, 1286–1293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitchell, C.L.; Lin, F.C.; Vaughn, M.; Apperson, C.S.; Meshnick, S.R.; Commins, S.P. Association between lone star tick bites and increased alpha-gal sensitization: Evidence from a prospective cohort of outdoor workers. Parasites Vectors 2020, 13, 470. [Google Scholar] [CrossRef]
- Kwak, M.; Somerville, C.; van Nunen, S. A novel Australian tick Ixodes (Endopalpiger) australiensis inducing mammalian meat allergy after tick bite. Asia Pac. Allergy 2018, 8, 31. [Google Scholar] [CrossRef] [PubMed]
- Chinuki, Y.; Ishiwata, K.; Yamaji, K.; Takahashi, H.; Morita, E. Haemaphysalis longicornis tick bites are a possible cause of red meat allergy in Japan. Allergy 2016, 71, 421–425. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Quintela, A.; Dam Laursen, A.S.; Vidal, C.; Skaaby, T.; Gude, F.; Linneberg, A. IgE antibodies to alpha-gal in the general adult population: Relationship with tick bites, atopy, and cat ownership. Clin. Exp. Allergy 2014, 44, 1061–1068. [Google Scholar] [CrossRef]
- Mateo-Borrega, M.B.; Garcia, B.; Larramendi, C.H.; Azofra, J.; González-Mancebo, E.; Alvarado, M.I.; Alonso-Díaz-de-Durana, M.D.; Núñez-Orjales, R.; Diéguez, M.C.; Guilarte, M.; et al. IgE-Mediated Sensitization to Galactose-α-1,3- Galactose (α-Gal) in Urticaria and Anaphylaxis in Spain: Geographical Variations and Risk Factors. J. Investig. Allergol. Clin. Immunol. 2019, 29, 436–443. [Google Scholar] [CrossRef] [Green Version]
- Villalta, D.; Cecchi, L.; Farsi, A.; Chiarini, F.; Minale, P.; Voltolini, S.; Scala, E.; Quercia, O.; Muratore, L.; Pravettoni, V.; et al. Galactose-α-1,3-galactose syndrome: An Italian survey. Eur. Ann. Allergy Clin. Immunol. 2017, 49, 263–269. [Google Scholar] [CrossRef] [Green Version]
- Cabezas-Cruz, A.; Hodžić, A.; Román-Carrasco, P.; Mateos-Hernández, L.; Duscher, G.G.; Sinha, D.K.; Hemmer, W.; Swoboda, I.; Estrada-Peña, A.; de la Fuente, J. Environmental and Molecular Drivers of the α-Gal Syndrome. Front. Immunol. 2019, 31, 1210. [Google Scholar] [CrossRef] [Green Version]
- Commins, S.P. Invited Commentary: Alpha-Gal Allergy: Tip of the Iceberg to a Pivotal Immune Response. Curr. Allergy Asthma Rep. 2016, 16, 61. [Google Scholar] [CrossRef]
- Cunningham, D.; Humblet, Y.; Siena, S.; Khayat, D.; Bleiberg, H.; Santoro, A.; Bets, D.; Mueser, M.; Harstrick, A.; Verslype, C.; et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N. Engl. J. Med. 2004, 351, 337–345. [Google Scholar] [CrossRef] [Green Version]
- Chung, C.H.; Mirakhur, B.; Chan, E.; Le, Q.T.; Berlin, J.; Morse, M.; Murphy, B.A.; Satinover, S.M.; Hosen, J.; Mauro, D.; et al. Cetuximab-induced anaphylaxis and IgE specific for galactose-alpha-1,3-galactose. N. Engl. J. Med. 2008, 358, 1109–1117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Commins, S.P.; Satinover, S.M.; Hosen, J.; Mozena, J.; Borish, L.; Lewis, B.D.; Woodfolk, J.A.; Platts-Mills, T.A. Delayed anaphylaxis, angioedema, or urticaria after consumption of red meat in patients with IgE antibodies specific for galactose-alpha-1,3-galactose. J. Allergy Clin. Immunol. 2009, 123, 426–433. [Google Scholar] [CrossRef] [Green Version]
- Commins, S.P.; Jerath, M.R.; Cox, K.; Erickson, L.D.; Platts-Mills, T. Delayed anaphylaxis to alpha-gal, an oligosaccharide in mammalian meat. Allergol. Int. 2016, 65, 16–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steinke, J.W.; Platts-Mills, T.A.; Commins, S.P. The alpha-gal story: Lessons learned from connecting the dots. J. Allergy Clin. Immunol. 2015, 135, 589–596. [Google Scholar] [CrossRef] [Green Version]
- Van Nunen, S.A.; O’Connor, K.S.; Clarke, L.R.; Boyle, R.X.; Fernando, S.L. An association between ticks bite reactions and red meat allergy in humans. Med. J. Aust. 2009, 190, 510–514. [Google Scholar] [CrossRef]
- Van Nunen, S.A.; Fernando, S.L.; Clarke, L.R.; Boyle, R.X. The association between Ixodes holocyclus tick bite reactions and red meat allergy (abstract). Intern. Med. J. 2007, 37 (Suppl. 5), A132. [Google Scholar]
- Binder, A.M.; Commins, S.P.; Altrich, M.L.; Wachs, T.; Biggerstaff, B.J.; Beard, C.B.; Petersen, L.R.; Kersh, G.J.; Armstrong, P.A. Diagnostic testing for galactose-alpha-1,3-galactose. Ann. Allergy Asthma Immunol. 2021, 126, 411–416. [Google Scholar] [CrossRef]
- Hamsten, C.; Tran, T.A.T.; Starkhammar, M.; Brauner, A.; Commins, S.P.; Platts-Mills, T.A.E.; van Hage, M. Red meat allergy in Sweden: Association with tick sensitization and B-negative blood groups. J. Allergy Clin. Immunol. 2013, 132, 1431–1434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morisset, M.; Richard, C.; Astier, C.; Jacquenet, S.; Croizier, A.; Beaudouin, E.; Cordebar, V.; Morel-Codreanu, F.; Petit, N.; Moneret-Vautrin, D.A.; et al. Anaphylaxis to pork kidney is related to IgE antibodies specific for galactose-alpha-1,3-galactose. Allergy 2012, 67, 699–704. [Google Scholar] [CrossRef]
- Fernández-Soto, P.; Dávila, I.; Laffond, E.; Lorente, F.; Encinas-Grandes, A.; Pérz-Sánchez, R. Tick bite-induced anaphylaxis in Spain. Ann. Trop. Med. Parasitol. 2001, 95, 97–103. [Google Scholar] [CrossRef] [Green Version]
- Macher, B.A.; Galili, U. The Galα1,3Galβ1,4GlcNAc-R (α-Gal) epitope: A carbohydrate of unique evolution and clinical relevance. Biochim. Biophys. Acta 2008, 1780, 75–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minanov, O.P.; Itescu, S.; Neethling, F.A.; Morgenthau, A.S.; Kwiatkowski, P.; Cooper, D.K.; Michler, R.E. Anti-gal IgG antibodies in sera of newborn humans and baboons and its significance in pig xenotransplantation. Transplantation 1997, 63, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Montassier, E.; Al-Ghalith, G.A.; Mathe, C.; Le Bastard, Q.; Douillard, V.; Garnier, A.; Guimon, R.; Raimondeau, B.; Touchefeu, Y.; Duchalais, E.; et al. Distribution of bacterial alpha1,3-galactosyltransferase genes in the human gut microbiome. Front. Immunol. 2019, 10, 3000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galili, U.; Anaraki, F.; Thall, A.; Hill-Black, C.; Radic, M. One percent of human circulating B lymphocytes are capable of producing the natural anti-Gal antibody. Blood 1993, 82, 2485–2493. [Google Scholar] [CrossRef] [Green Version]
- Yu, P.B.; Holzknecht, Z.E.; Bruno, D.; Parker, W.; Platt, J.L. Modulation of natural IgM binding and complement activation by natural IgG antibodies: A role for IgG anti-Gal alpha1-3Gal antibodies. J. Immunol. 1996, 157, 5163–5168. [Google Scholar] [PubMed]
- Platts-Mills, T.A.E.; Li, R.C.; Keshavarz, B.; Smith, A.R.; Wilson, J.M. Diagnosis and Management of Patients with the α-Gal Syndrome. J. Allergy Clin. Immunol. Pract. 2020, 8, 15–23. [Google Scholar] [CrossRef]
- Fischer, J.; Yazdi, A.S.; Biedermann, T. Clinical spectrum of a-Gal syndrome: From immediate-type to delayed immediate-type reactions to mammalian innards and meat. Allergo J. Int. 2016, 25, 55–62. [Google Scholar] [CrossRef] [Green Version]
- Platts-Mills, T.A.E.; Schuyler, A.J.; Tripathi, A.; Commins, S.P. Anaphylaxis to the carbohy-drate side chain alpha-gal. Immunol. Allergy Clin. N. Am. 2015, 35, 247–260. [Google Scholar] [CrossRef] [Green Version]
- Kollmann, D.; Nagl, B.; Ebner, C.; Emminger, W.; Wöhrl, S.; Kitzmüller, C.; Vrtala, S.; Mangold, A.; Ankersmit, H.J.; Bohle, B. The quantity and quality of α-gal-specific antibodies differ in individuals with and without delayed red meat allergy. Allergy 2017, 72, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Kim, D.; Boorgula, G.D.; De Schutter, K.; Smagghe, G.; Šimo, L.; Archer-Hartmann, S.A.; Azadi, P. Alpha-Gal and Cross-Reactive Carbohydrate Determinants in the N-Glycans of Salivary Glands in the Lone Star Tick, Amblyomma americanum. Vaccines 2020, 8, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villar, M.; Pacheco, I.; Merino, O.; Contreras, M.; Mateos-Hernández, L.; Prado, E.; Barros-Picanço, D.K.; Lima-Barbero, J.F.; Artigas-Jerónimo, S.; Alberdi, P.; et al. Tick and Host Derived Compounds Detected in the Cement Complex Substance. Biomolecules 2020, 10, 555. [Google Scholar] [CrossRef] [Green Version]
- Chandrasekhar, J.L.; Cox, K.M.; Erickson, L.D. B Cell Responses in the Development of Mammalian Meat Allergy. Front. Immunol. 2020, 11, 1532. [Google Scholar] [CrossRef]
- Carvalho-Costa, T.M.; Mendes, M.T.; da Silva, M.V.; da Costa, T.A.; Tiburcio, M.G.; Anhê, A.C.; Rodrigues, V., Jr.; Oliveira, C.J.F. Immunosuppressive effects of Amblyomma cajennense tick saliva on murine bone marrow-derived dendritic cells. Parasites Vectors 2015, 8, 22. [Google Scholar] [CrossRef] [Green Version]
- Rispens, T.; Derksen, N.I.; Commins, S.P.; Platts-Mills, T.A.; Aalberse, R.C. IgE production to α-gal is accompanied by elevated levels of specific IgG1 antibodies and low amounts of IgE to blood group B. PLoS ONE 2013, 8, e55566. [Google Scholar] [CrossRef]
- Apostolovic, D.; Rodrigues, R.; Thomas, P.; Starkhammar, M.; Hamsten, C.; van Hage, M. Immunoprofile of α-Gal- and B-antigen-specific responses differentiates red meat-allergic patients from healthy individuals. Allergy 2018, 73, 1525–1531. [Google Scholar] [CrossRef]
- Tomlinson, S.; Nussenzweig, V. Human alternative complement pathway-mediated lysis of rabbit erythrocytes is enhanced by natural anti-Galalpha1-3Gal antibodies. J. Immunol. 1997, 159, 5606–5609. [Google Scholar]
- Obukhova, P.; Rieben, R.; Bovin, N. Normal human serum contains high levels of anti-Gala1-4GlcNAc antibodies. Xenotransplantation 2007, 14, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Zappe, A.; Rosenlöcher, J.; Kohla, G.; Hinderlich, S.; Parr, M.K. Purification and Characterization of Antibodies Directed against the -Gal Epitope. BioChem 2021, 1, 81–97. [Google Scholar] [CrossRef]
- van Hage, M.; Schmid-Grendelmeier, P.; Skevaki, C.; Plebani, M.; Canonica, W.; Kleine-Tebbe, J.; Nystrand, M.; Jafari-Mamaghani, M.; Jakob, T. Performance evaluation of ImmunoCAP® ISAC 112: A multi-site study. Clin. Chem. Lab. Med. 2017, 55, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Sastre, J. Molecular diagnosis in allergy. Clin. Exp. Allergy 2010, 40, 1442–1460. [Google Scholar] [CrossRef] [PubMed]
- Viñas, M.; Postigo, I.; Suñen, E.; Martınez, J. Urticaria and silent parasitism by Ascaridoidea: Component-resolved diagnosis reinforces the significance of this association. PLoS Negl. Trop. Dis. 2020, 14, e0008177. [Google Scholar] [CrossRef] [Green Version]
- Cooper, D.K. Depletion of natural antibodies in non-human primates-a step towards successful discordant xenografting in humans. Clin. Transpl. 1992, 6, 178–183. [Google Scholar]
- Kobayashi, T.; Cooper, D.K. Anti-Gal, alpha-Gal epitopes, and xenotransplantation. Subcell. Biochem. 1999, 32, 229–257. [Google Scholar] [CrossRef]
- Commins, S.P.; Platts-Mills, T.A. Tick bites and read meat allergy. Curr. Opin. Allergy Clin. Immunol. 2013, 13, 354–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Nunen, S.A. Tick-induced allergies: Mammalian meat allergy, tick anaphylaxis and their significance. Asia Pac. Allergy 2015, 5, 3–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barral, M.; García-Pérez, A.L.; Juste, R.A.; de Luco, D.F.; Dehesa, V. Distribución y Actividad de los Ixódidos Presentes en la Vegetación de la Comunidad Autónoma Vasca; Informe Técnico N°54; Servicio Central de Publicaciones del Gobierno Vasco: Vitoria-Gasteiz, Spain, 1993. [Google Scholar]
- Mabelane, T.; Basera, W.; Botha, M.; Thomas, H.F.; Ramjith, J.; Levin, M.E. Predictive values of alpha-gal IgE levels and alpha-gal IgE: Total IgE ratio and oral food challenge-proven meat allergy in a population with a high prevalence of reported red meat allergy. Pediatr. Allergy Immunol. 2018, 29, 841–849. [Google Scholar] [CrossRef]
- Fischer, J.; Huynh, H.N.; Hebsaker, J.; Forchhammer, S.; Yazdi, A.S. Prevalence and Impact of Type I Sensitization to Alpha-Gal in Patients Consulting an Allergy Unit. Int. Arch. Allergy Immunol. 2020, 18, 119–127. [Google Scholar] [CrossRef]
- Avila, J.L.; Rojas, M.; Galili, U. Immunogenic Gal alpha 1—3Gal carbohydrate epitopes are present on pathogenic American Trypanosoma and Leishmania. J. Immunol. 1989, 142, 2828–2834. [Google Scholar]
- Hamanova, M.; Chmelikova, M.; Nentwich, I.; Thon, V.; Lokaj, J. Anti-Gal IgM, IgA and IgG natural antibodies in childhood. Immunol. Lett. 2015, 164, 40–43. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhao, C.; Wang, W.; Jin, R.; Li, Q.; Ge, Q.; Guan, Y.; Zhang, Y. Prostaglandins E2 signal mediated by receptor subtype EP2 promotes IgE production in vivo and contributes to asthma development. Sci. Rep. 2016, 6, 20505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira, C.J.; Sá-Nunes, A.; Francischetti, I.M.; Carregaro, V.; Anatriello, E.; Silva, J.S.; Santos, I.K.; Ribeiro, J.M.; Ferreira, B.R. Deconstructing tick saliva: Non-protein molecules with potent immunomodulatory properties. J. Biol. Chem. 2011, 286, 10960–10969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, J.M.; Keshavarz, B.; James, H.R.; Retterer, M.K.C.; Schuyler, A.J.; Knoedler, A.; Workman, L.J.; Nganga, L.; Chico, M.E.; Rönmark, E.; et al. α-Gal specific-IgE prevalence and levels in Ecuador and Kenya: Relation to diet, parasites, and IgG4. J. Allergy Clin. Immunol. 2021, 147, 1393–1401.e7. [Google Scholar] [CrossRef]

| Patient | Gender | Total IgE (kUA/L) | IgE Specific to α-Gal [kUA/L] | Clinical Symptoms |
|---|---|---|---|---|
| 1 | m | 738 | >100 | Recurrent Urticaria |
| 2 | m | 569 | >100 | Anaphylaxis |
| 3 | m | 452 | >100 | Anaphylaxis |
| 4 | f | 325 | >100 | Recurrent Urticaria |
| 5 | m | 523 | 91.3 | Recurrent Urticaria |
| 6 | m | 238 | 79.9 | Anaphylaxis |
| 7 | m | 267 | 78.7 | Anaphylaxis |
| 8 | m | 461 | 76.0 | Anaphylaxis |
| 9 | m | 269 | 63.7 | Anaphylaxis |
| 10 | m | 230 | 54.4 | Anaphylaxis |
| 11 | m | 91.7 | 45.0 | Acute Urticaria |
| 12 | m | 671 | 41.6 | Recurrent Urticaria |
| 13 | m | 102 | 19.4 | Recurrent Urticaria |
| 14 | m | 139 | 16.8 | Acute Urticaria |
| 15 | m | 311 | 12.3 | Anaphylaxis |
| Percentage (%) | ||
|---|---|---|
| Male | 34 | |
| Female | 66 | |
| Demographic data | Age (20–60 yrs.) | 90 |
| Urban | 83 | |
| Rural | 17 | |
| Dog | 41 | |
| Animal contact | Cat | 24 |
| Others | 20 | |
| Daily meat consumption | 78 | |
| Meat-consumption related symptoms | None | 90 |
| Intolerance | 9 | |
| Lyme | 10 | |
| Chronic diseases | Asthma | 10 |
| Fibromyalgia | 4 | |
| Arthritis | 4 | |
| Others | 9 |
| Percentage (%) | |||
| Allergen | Main Source of Allergen | Risk Population (n = 46) | Atopic Population (n = 64) |
| Phl p 1 | Grass pollen | 15 | 53.1 |
| Ole e 1 | Olive tree pollen | 8.6 | 37 |
| Cup a 1 | Cypress tree pollen | 4.3 | 34 |
| Cry j 1 | Japanese cedar pollen | 2.1 | 26 |
| Bet v 2 | Birch pollen | 4.3 | 21.8 |
| Der 1 | Mites Group 1 allergens | 30 | 30 |
| Der 2 | Mites Group 2 allergens | 30 | 37.5 |
| Der p 10 | Mites Tropomyosin | 0 | 4.6 |
| Alt a 1 | Alternaria alternata | 0 | 12.5 |
| Asp f 1 | Aspergillus fumigatus | 0 | 7.8 |
| Pru p 3 | Apricot | 0 | 20 |
| Cor a 8 | Hazel | 0 | 7.8 |
| Act d 1 | Kiwifruit | 0 | 7.8 |
| Pen m 1 | Shrimp | 0 | 4.6 |
| Gad c 1 | Egg | 0 | 4.6 |
| Fel d 1 | Cat (uteroglobin) | 13 | 26 |
| Fel d 4 | Cat (lipocalin) | 6.5 | 6.25 |
| Can f 1 | Dog (lipocalin) | 4.3 | 4.6 |
| Mus m 1 | Mouse (lipocalin) | 2.1 | 6.2 |
| Equ c 1 | Horse (lipocalin) | 0 | 3.1 |
| Api m 1 | Bee venom (phospholipase A2) | 0 | 1.5 |
| Pol d 5 (array) | Wasp venom (Antigen 5) | 4.3 | 0 |
| Mux F3 (array) | Carbohydrates (CCDs) | 3.7 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joral, A.; Azketa, N.; Sanchez, P.; Vélez-del-Burgo, A.; Aranzabal-Soto, M.-A.; Lizarza, S.; Martínez, J.; Postigo, I. The Quantification of IgG Specific to α-Gal Could Be Used as a Risk Marker for Suffering Mammalian Meat Allergy. Foods 2022, 11, 466. https://doi.org/10.3390/foods11030466
Joral A, Azketa N, Sanchez P, Vélez-del-Burgo A, Aranzabal-Soto M-A, Lizarza S, Martínez J, Postigo I. The Quantification of IgG Specific to α-Gal Could Be Used as a Risk Marker for Suffering Mammalian Meat Allergy. Foods. 2022; 11(3):466. https://doi.org/10.3390/foods11030466
Chicago/Turabian StyleJoral, Alejandro, Nahikari Azketa, Patricia Sanchez, Ainara Vélez-del-Burgo, María-Ascensión Aranzabal-Soto, Susana Lizarza, Jorge Martínez, and Idoia Postigo. 2022. "The Quantification of IgG Specific to α-Gal Could Be Used as a Risk Marker for Suffering Mammalian Meat Allergy" Foods 11, no. 3: 466. https://doi.org/10.3390/foods11030466
APA StyleJoral, A., Azketa, N., Sanchez, P., Vélez-del-Burgo, A., Aranzabal-Soto, M.-A., Lizarza, S., Martínez, J., & Postigo, I. (2022). The Quantification of IgG Specific to α-Gal Could Be Used as a Risk Marker for Suffering Mammalian Meat Allergy. Foods, 11(3), 466. https://doi.org/10.3390/foods11030466







