Recombinant Tropomyosin from the Pacific Oyster (Crassostrea gigas) for Better Diagnosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Sample Preparation and Protein Extraction
2.3. Quantification of Total Protein
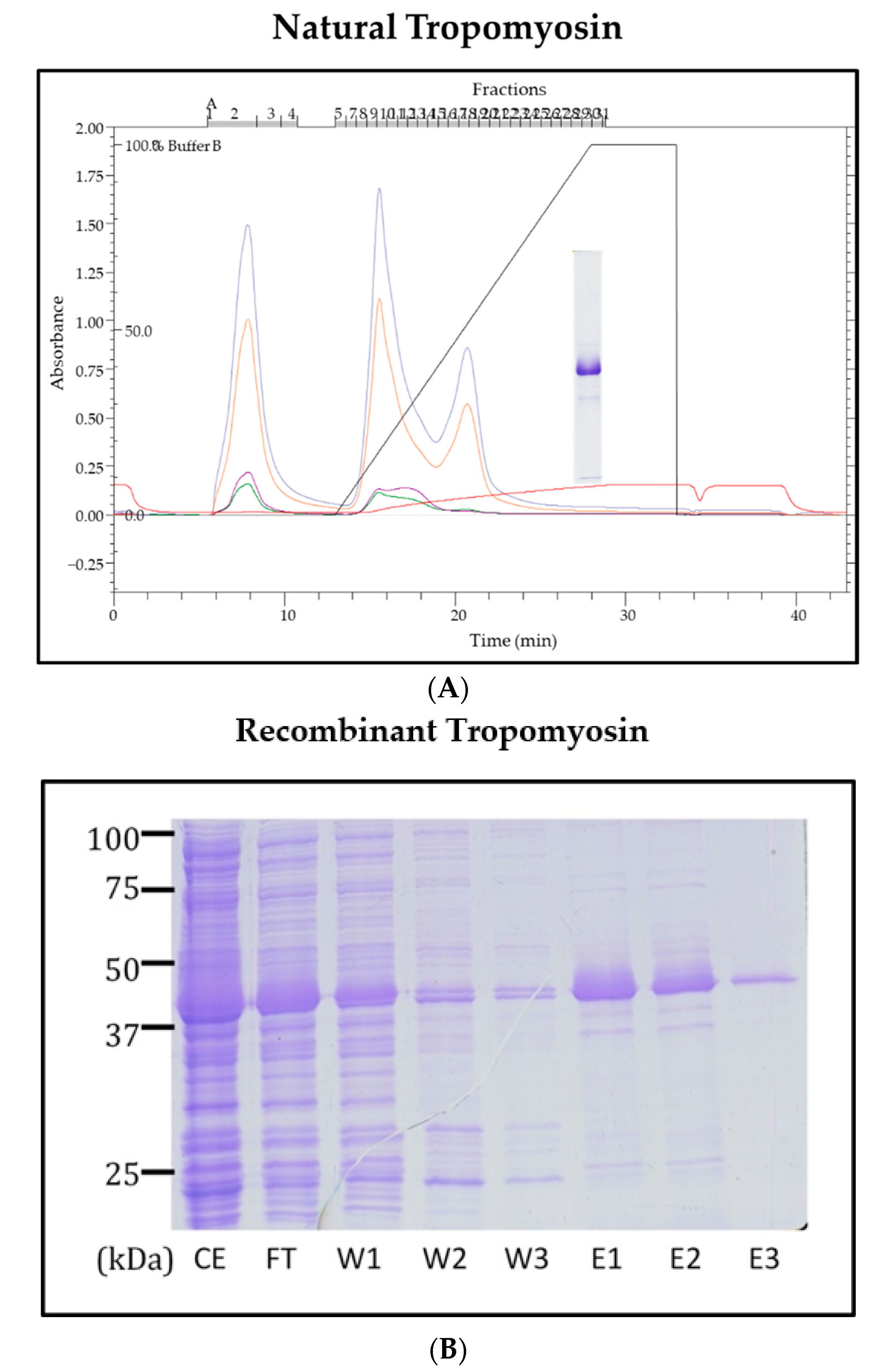
2.4. Purification of Natural TM from the Pacific Oyster
2.5. Cloning, Sequencing and Expression of Recombinant Oyster TM
2.6. SDS-PAGE and Immunoblotting Using Patient IgE-Antibody
2.7. Amino Acid Sequencing Using Mass Spectrometry
2.8. Analysis of Secondary Protein Structure Using CD Spectrometry
2.9. Evaluation of IgE-Reactivity Using ELISA
3. Results
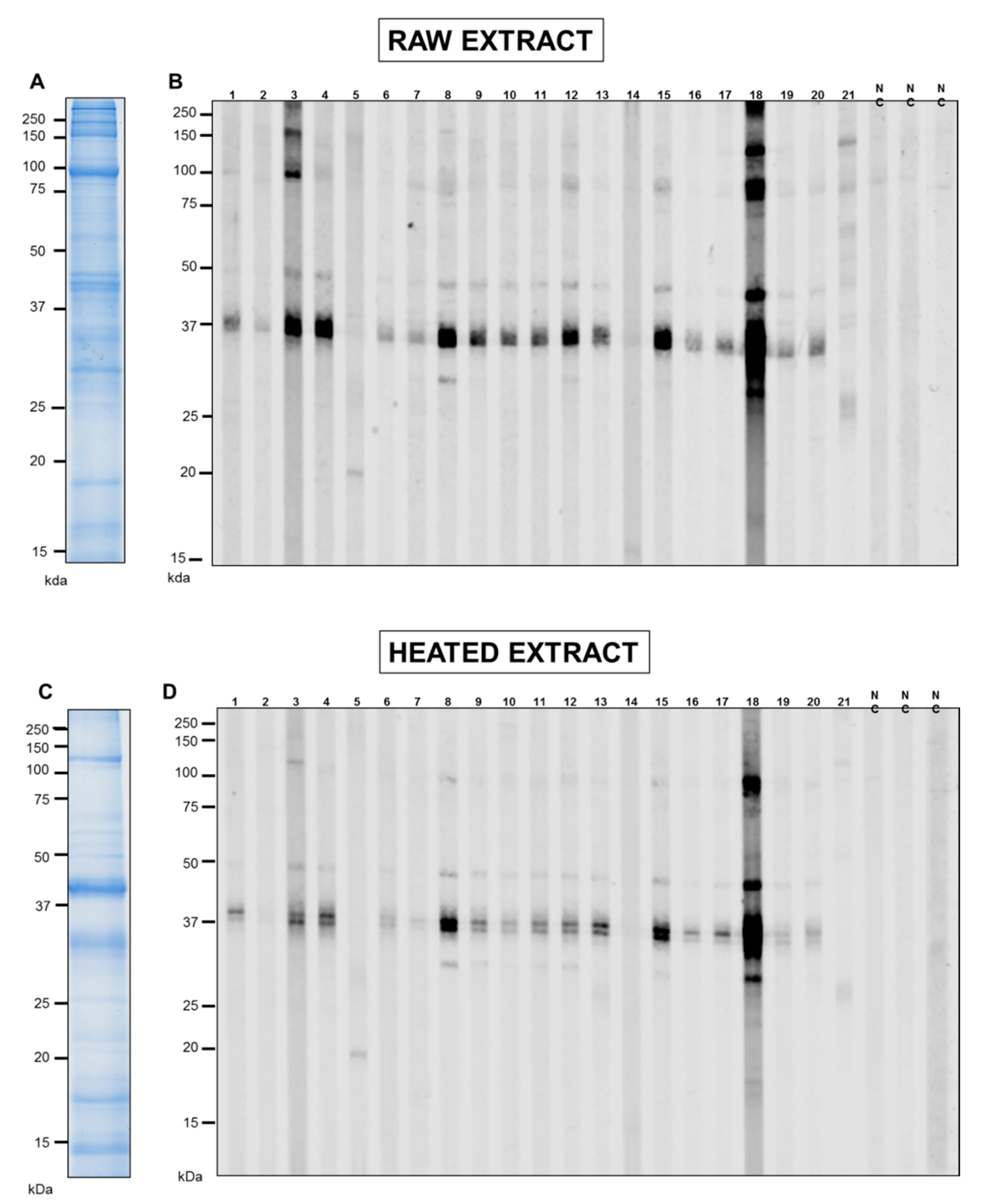
3.1. SDS-PAGE and IgE-Antibody Reactivity of Pacific Oyster Extracts
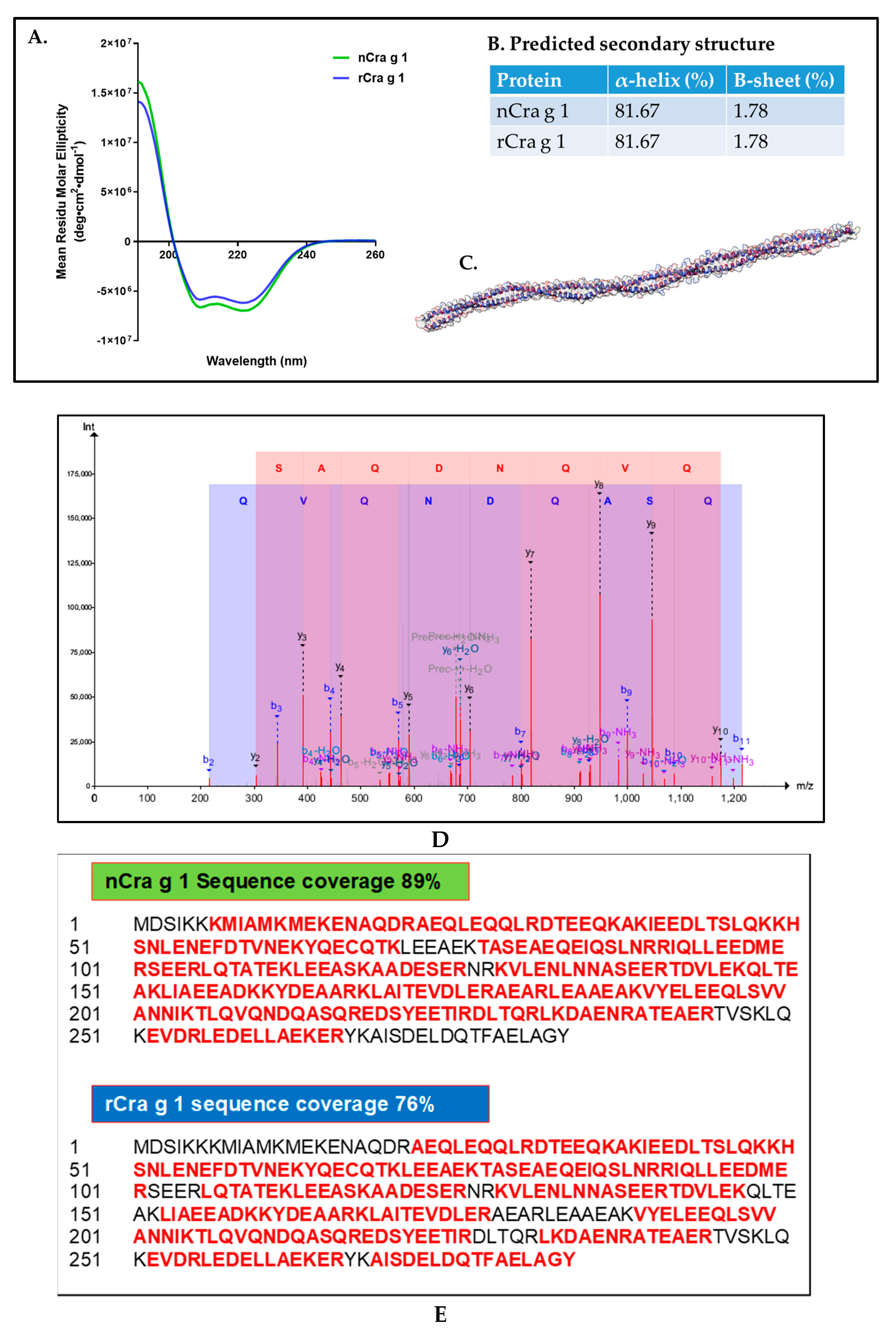
3.2. Sequencing and Characterisation of Pacific Oyster TM
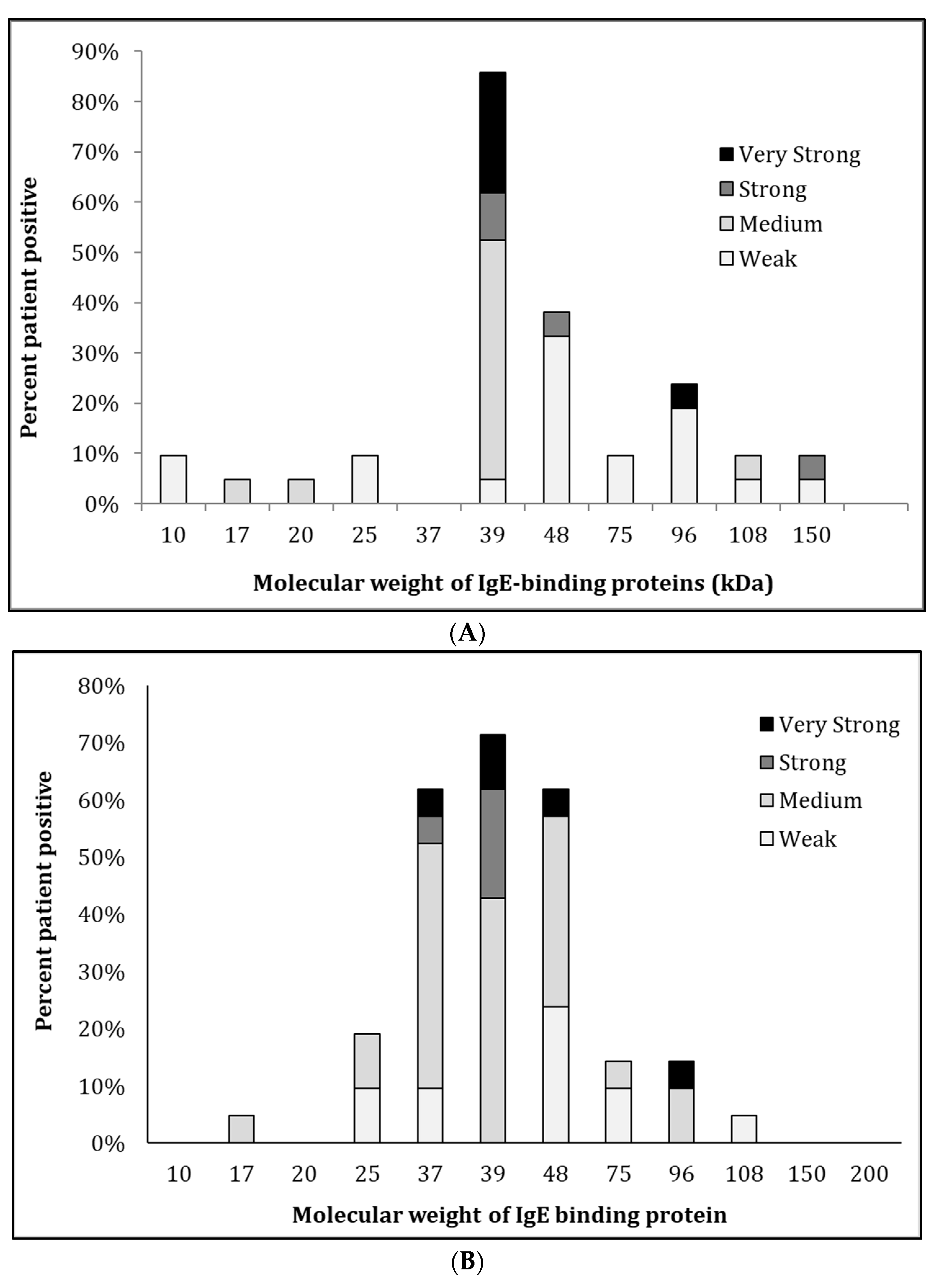
3.3. IgE-Recognition of Oyster-Sensitised Subjects to Natural and Recombinant TM
3.4. Immunological IgE-Reactivity Analysis Using ELISA
3.5. Tropomyosin Amino Acid Sequence Comparison and IgE-Binding Epitope Prediction
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guillen, J.; Natale, F.; Carvalho, N.; Casey, J.; Hofherr, J.; Druon, J.-N.; Fiore, G.; Gibin, M.; Zanzi, A.; Martinsohn, J.T. Global seafood consumption footprint. Ambio 2018, 48, 111–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, T.-C.; Tsai, T.-C.; Huang, C.-F.; Chang, F.-Y.; Lin, C.-C.; Chu, C.-H.; Lau, B.-H.; Wu, L.; Peng, H.-J.; Tang, R.-B. Prevalence of food allergy in Taiwan: A questionnaire-based survey. Intern. Med. J. 2012, 42, 1310–1315. [Google Scholar] [CrossRef] [PubMed]
- Prescott, S.L.; Pawankar, R.; Allen, K.J.; Campbell, D.E.; Sinn, J.K.; Fiocchi, A.; Ebisawa, M.; Sampson, H.A.; Beyer, K.; Lee, B.-W. A global survey of changing patterns of food allergy burden in children. World Allergy Organ. J. 2013, 6, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le, T.T.K.; Nguyen, D.H.; Vu, A.T.L.; Ruethers, T.; Taki, A.C.; Lopata, A.L. A cross-sectional, population-based study on the prevalence of food allergies among children in two different socio-economic regions of Vietnam. Pediatr. Allergy Immunol. 2019, 30, 348–355. [Google Scholar] [CrossRef]
- Ruethers, T.; Taki, A.C.; Johnston, E.B.; Nugraha, R.; Le, T.T.K.; Kalic, T.; McLean, T.R.; Kamath, S.D.; Lopata, A.L. Seafood allergy: A comprehensive review of fish and shellfish allergens. Mol. Immunol. 2018, 100, 28–57. [Google Scholar] [CrossRef]
- Nugraha, R.; Kamath, S.D.; Johnston, E.; Zenger, K.R.; Rolland, J.M.; O’Hehir, R.E.; Lopata, A.L. Rapid and comprehensive discovery of unreported shellfish allergens using large-scale transcriptomic and proteomic resources. J. Allergy Clin. Immunol. 2018, 141, 1501–1504.e8. [Google Scholar] [CrossRef] [Green Version]
- Taylor, S.L. Molluscan shellfish allergy. Adv. Food Nutr. Res. 2008, 54, 139–177. [Google Scholar] [CrossRef]
- Nugraha, R.; Ruethers, T.; Johnston, E.B.; Rolland, J.M.; O’Hehir, R.E.; Kamath, S.D.; Lopata, A.L. Effects of Extraction Buffer on the Solubility and Immunoreactivity of the Pacific Oyster Allergens. Foods 2021, 10, 409. [Google Scholar] [CrossRef]
- Rolland, J.M.; Varese, N.P.; Abramovitch, J.B.; Anania, J.; Nugraha, R.; Kamath, S.; Hazard, A.; Lopata, A.L.; O’Hehir, R.E. Effect of Heat Processing on IgE Reactivity and Cross-Reactivity of Tropomyosin and Other Allergens of Asia-Pacific Mollusc Species: Identification of Novel Sydney Rock Oyster Tropomyosin Sac g 1. Mol. Nutr. Food Res. 2018, 62, e1800148. [Google Scholar] [CrossRef] [Green Version]
- Kamath, S.D.; Johnston, E.B.; Iyer, S.; Schaeffer, P.M.; Koplin, J.; Allen, K.; Lopata, A.L. IgE reactivity to shrimp allergens in infants and their cross-reactivity to house dust mite. Pediatr. Allergy Immunol. 2017, 28, 703–707. [Google Scholar] [CrossRef]
- Pascal, M.; Grishina, G.; Yang, A.C.; Sánchez-García, S.; Lin, J.; Towle, D.; Ibañez, M.D.; Sastre, J.; Sampson, H.A.; Ayuso, R. Molecular Diagnosis of Shrimp Allergy: Efficiency of Several Allergens to Predict Clinical Reactivity. J. Allergy Clin. Immunol. Pract. 2015, 3, 521–529.e10. [Google Scholar] [CrossRef] [PubMed]
- Gámez, C.; Sánchez-García, S.; Ibáñez, M.D.; López, R.; Aguado, E.; López, E.; Sastre, B.; Sastre, J.; del Pozo, V. Tropomyosin IgE-positive results are a good predictor of shrimp allergy. Allergy 2011, 66, 1375–1383. [Google Scholar] [CrossRef] [PubMed]
- Schoos, A.M.M.; Chawes, B.L.K.; Følsgaard, N.V.; Samandari, N.; Bønnelykke, K.; Bisgaard, H. Disagreement between skin prick test and specific IgE in young children. Allergy 2015, 70, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Köhler, J.; Blank, S.; Müller, S.; Bantleon, F.; Frick, M.; Huss-Marp, J.; Lidholm, J.; Spillner, E.; Jakob, T. Component resolution reveals additional major allergens in patients with honeybee venom allergy. J. Allergy Clin. Immunol. 2014, 133, 1383–1389.e6. [Google Scholar] [CrossRef] [Green Version]
- Liang, J.; Tan, C.C.; Taylor, S.L.; Baumert, J.L.; Lopata, A.L.; Lee, N.A. Quantitative analysis of species specificity of two anti-parvalbumin antibodies for detecting southern hemisphere fish species demonstrating strong phylogenetic association. Food Chem. 2017, 237, 588–596. [Google Scholar] [CrossRef]
- Ruethers, T.; Raith, M.; Sharp, M.F.; Koeberl, M.; Stephen, J.N.; Nugraha, R.; Le, T.T.K.; Quirce, S.; Nguyen, H.X.M.; Kamath, S.D.; et al. Characterization of Ras k 1 a novel major allergen in Indian mackerel and identification of parvalbumin as the major fish allergen in 33 Asia-Pacific fish species. Clin. Exp. Allergy 2018, 48, 452–463. [Google Scholar] [CrossRef]
- Ishikawa, M.; Shimakura, K.; Nagashima, Y.; Shiomi, K. Isolation and Properties of Allergenic Proteins in the Oyster Crassostrea gigas. Fish. Sci. 1997, 63, 610–614. [Google Scholar] [CrossRef] [Green Version]
- Ishikawa, M.; Ishida, M.; Shimakura, K.; Nagashima, Y.; Shiomi, K. Tropomyosin, the Major Oyster Crassostrea gigas Allergen and its IgE-binding Epitopes. J. Food Sci. 1998, 63, 44–47. [Google Scholar] [CrossRef]
- Leung, P.S.C.; Chu, K.H. cDNA cloning and molecular identification of the major oyster allergen from the Pacific oyster Crassostrea gigas. Clin. Exp. Allergy 2001, 31, 1287–1294. [Google Scholar] [CrossRef]
- Koeberl, M.; Kamath, S.D.; Saptarshi, S.R.; Smout, M.J.; Rolland, J.M.; O’Hehir, R.E.; Lopata, A.L. Auto-induction for high yield expression of recombinant novel isoallergen tropomyosin from King prawn (Melicertus latisulcatus) for improved diagnostics and immunotherapeutics. J. Immunol. Methods 2014, 415, 6–16. [Google Scholar] [CrossRef]
- Kamath, S.D.; Thomassen, M.R.; Saptarshi, S.R.; Nguyen, H.M.; Aasmoe, L.; Bang, B.E.; Lopata, A.L. Molecular and immunological approaches in quantifying the air-borne food allergen tropomyosin in crab processing facilities. Int. J. Hyg. Environ. Health 2014, 217, 740–750. [Google Scholar] [CrossRef] [PubMed]
- Louis-Jeune, C.; Andrade-Navarro, M.A.; Perez-Iratxeta, C. Prediction of protein secondary structure from circular dichroism using theoretically derived spectra. Proteins Struct. Funct. Bioinform. 2012, 80, 374–381. [Google Scholar] [CrossRef] [PubMed]
- James, J.K.; Pike, D.H.; Khan, I.J.; Nanda, V. Structural and Dynamic Properties of Allergen and Non-Allergen Forms of Tropomyosin. Structure 2018, 26, 997–1006.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matricardi, P.M.; Kleine-Tebbe, J.; Hoffmann, H.J.; Valenta, R.; Hilger, C.; Hofmaier, S.; Aalberse, R.C.; Agache, I.; Asero, R.; Ballmer-Weber, B.; et al. EAACI Molecular Allergology User’s Guide. Pediatr. Allergy Immunol. 2016, 27 (Suppl. 23), 1–250. [Google Scholar] [CrossRef]
- Ayuso, R.; Reese, G.; Leong-Kee, S.; Plante, M.; Lehrer, S.B. Molecular Basis of Arthropod Cross-Reactivity: IgE-Binding Cross-Reactive Epitopes of Shrimp, House Dust Mite and Cockroach Tropomyosins. Int. Arch. Allergy Immunol. 2002, 129, 38–48. [Google Scholar] [CrossRef]
- Lopata, A.L.; Kleine-Tebbe, J.; Kamath, S.D. Allergens and molecular diagnostics of shellfish allergy: Part 22 of the Series Molecular Allergology. Allergo J. Int. 2016, 25, 210–218. [Google Scholar] [CrossRef] [Green Version]
- FAO. The State of World Fisheries and Aquaculture, 2020; FAO: Rome, Italy, 2020. [Google Scholar]
- Ruethers, T.; Taki, A.C.; Nugraha, R.; Cao, T.T.; Koeberl, M.; Kamath, S.D.; Williamson, N.A.; O’Callaghan, S.; Nie, S.; Mehr, S.S.; et al. Variability of allergens in commercial fish extracts for skin prick testing. Allergy 2019, 74, 1352–1363. [Google Scholar] [CrossRef] [PubMed]
- Prester, L. Seafood Allergy, Toxicity, and Intolerance: A Review. J. Am. Coll. Nutr. 2016, 35, 271–283. [Google Scholar] [CrossRef]
- Vidal, C.; Bartolomé, B.; Rodríguez, V.; Armén, M.; Linneberg, A.; González-Quintela, A. Sensitization pattern of crustacean-allergic individuals can indicate allergy to molluscs. Allergy 2015, 70, 1493–1496. [Google Scholar] [CrossRef]
- Lopata, A.L.; O’Hehir, R.E.; Lehrer, S.B. Shellfish allergy. Clin. Exp. Allergy 2010, 40, 850–858. [Google Scholar] [CrossRef]
- Nugraha, R.; Kamath, S.D.; Johnston, E.; Karnaneedi, S.; Ruethers, T.; Lopata, A.L. Conservation Analysis of B-Cell Allergen Epitopes to Predict Clinical Cross-Reactivity Between Shellfish and Inhalant Invertebrate Allergens. Front. Immunol. 2019, 10, 2676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
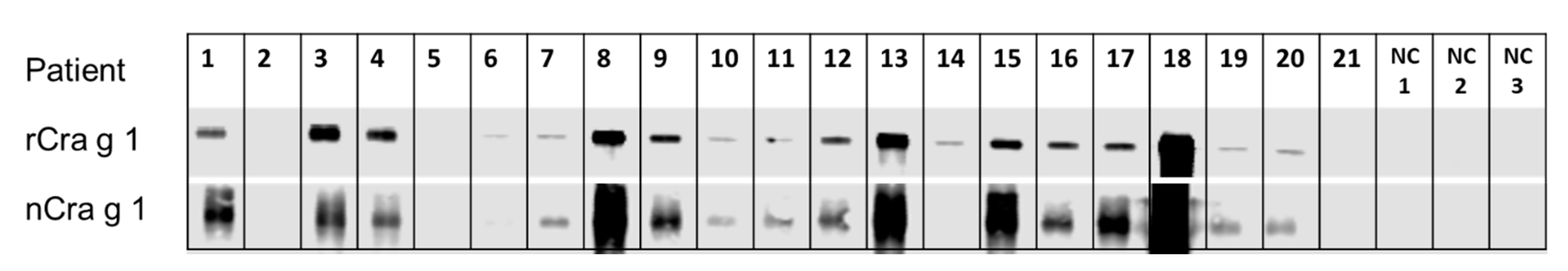
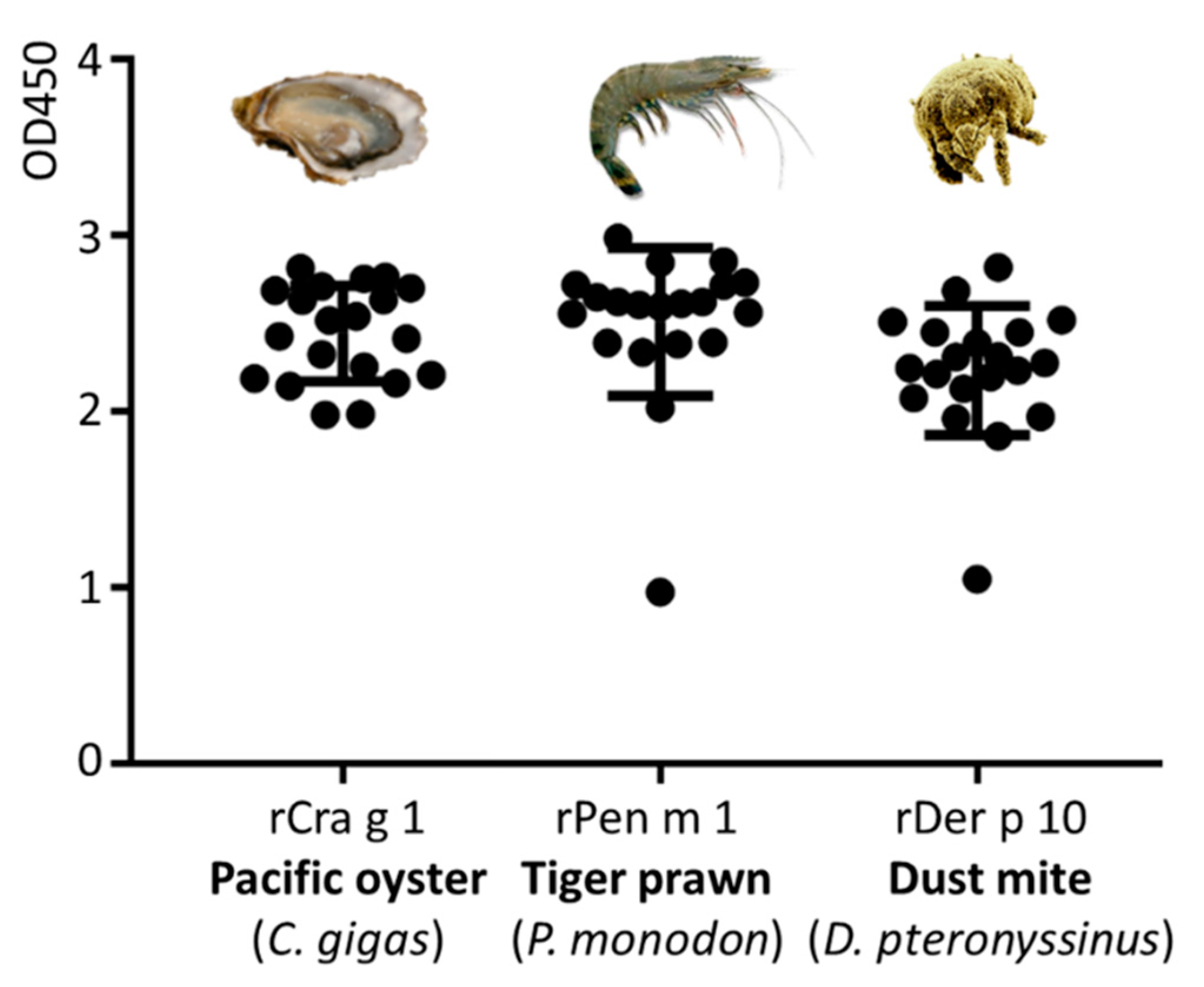
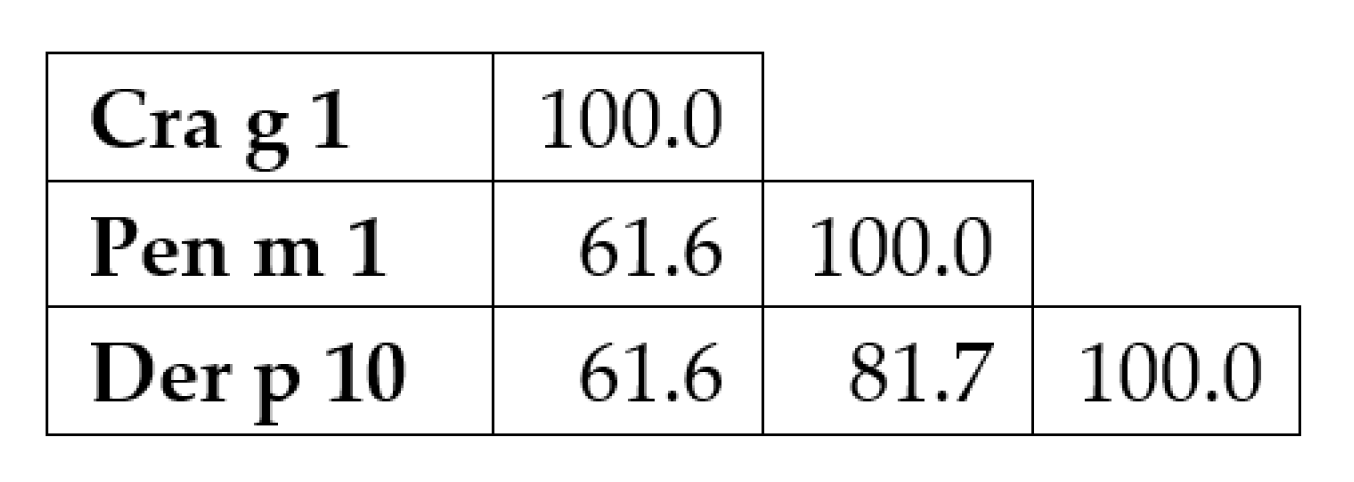
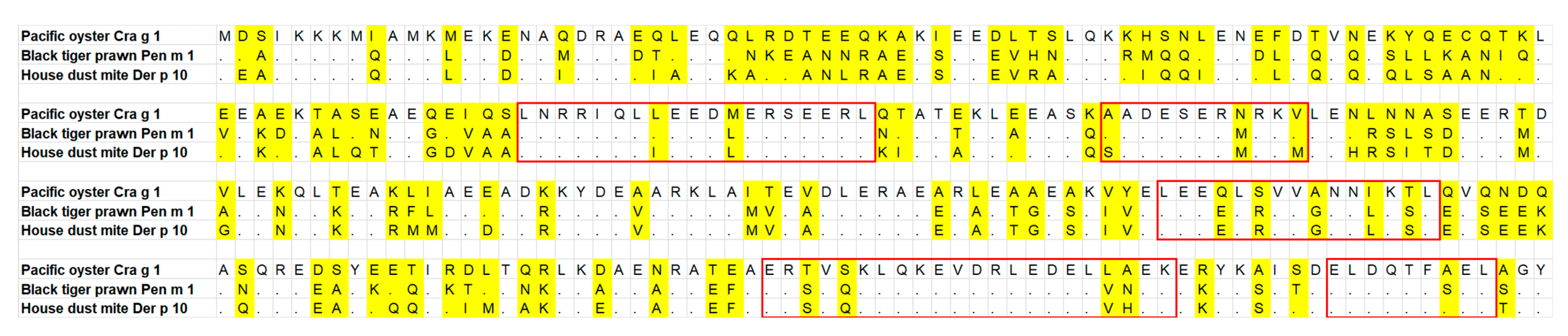
| Patient ID | Specific IgE (kUA L–1) | Implicated Species | Symptoms | ||
|---|---|---|---|---|---|
| Oyster | Prawn | HDM | |||
| 1 | 0.93 | 10.10 | 8.73 | Prawn | As, R, U |
| 2 | 0.92 | 9.50 | 14.10 | Prawn, crab, flounder | A, An, R |
| 3 | 2.04 | 9.03 | 13.60 | Calamari, snapper, tuna | R, An |
| 4 | 3.75 | 9.82 | 2.66 | Scallops, oyster, shellfish | An, U, pO |
| 5 | 4.29 | 0.20 | 9.49 | Sea perch, flake, rockling | An, pO |
| 6 | 1.04 | 6.84 | 31.70 | Mussel, scallops | An |
| 7 | 0.49 | 2.57 | 1.99 | Calamari, octopus | An |
| 8 | 5.99 | 32.40 | 6.47 | Shellfish | As, R, U, An |
| 9 | 2.41 | 8.98 | 2.36 | Shellfish | As, R, U, An |
| 10 | 1.11 | 3.63 | 5.03 | Crustaceans/molluscs | R, H, A |
| 11 | 1.19 | 4.30 | 57.5 | Prawn, crab meat and marinara mix | As |
| 12 | 6.68 | 17.2 | 13.50 | Salmon, crab, lobster, prawn | An |
| 13 | 2.59 | 9.81 | 16.80 | Prawns, calamari, fish | An |
| 14 | 0.65 | 3.75 | 33.80 | Oyster | GI |
| 15 | 5.47 | 21.60 | 10.7 | Calamari | U, As |
| 16 | 1.35 | 5.42 | 40.20 | Mollusc | An, A, U |
| 17 | 1.08 | 6.73 | 6.90 | Shellfish | U, A |
| 18 | 35.8 | >100 | 22.00 | Shellfish | U, As |
| 19 | 0.45 | 9.74 | 1.29 | Shellfish | As |
| 20 | 1.08 | 5.05 | 1.97 | Pipis, squid | pO |
| 21 | 7.32 | 2.84 | 1.95 | Oyster | NI |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nugraha, R.; Ruethers, T.; Taki, A.C.; Johnston, E.B.; Karnaneedi, S.; Kamath, S.D.; Lopata, A.L. Recombinant Tropomyosin from the Pacific Oyster (Crassostrea gigas) for Better Diagnosis. Foods 2022, 11, 404. https://doi.org/10.3390/foods11030404
Nugraha R, Ruethers T, Taki AC, Johnston EB, Karnaneedi S, Kamath SD, Lopata AL. Recombinant Tropomyosin from the Pacific Oyster (Crassostrea gigas) for Better Diagnosis. Foods. 2022; 11(3):404. https://doi.org/10.3390/foods11030404
Chicago/Turabian StyleNugraha, Roni, Thimo Ruethers, Aya C. Taki, Elecia B. Johnston, Shaymaviswanathan Karnaneedi, Sandip D. Kamath, and Andreas L. Lopata. 2022. "Recombinant Tropomyosin from the Pacific Oyster (Crassostrea gigas) for Better Diagnosis" Foods 11, no. 3: 404. https://doi.org/10.3390/foods11030404
APA StyleNugraha, R., Ruethers, T., Taki, A. C., Johnston, E. B., Karnaneedi, S., Kamath, S. D., & Lopata, A. L. (2022). Recombinant Tropomyosin from the Pacific Oyster (Crassostrea gigas) for Better Diagnosis. Foods, 11(3), 404. https://doi.org/10.3390/foods11030404









