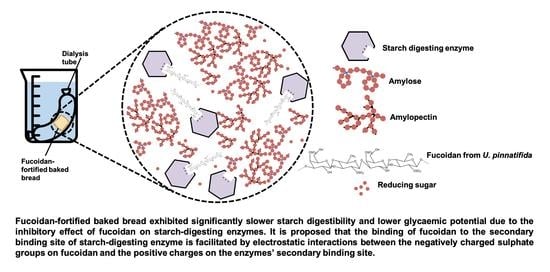
Fucoidan Regulates Starch Digestion: In Vitro and Mechanistic Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical and Samples
2.2. Preparation of Bread Samples
2.3. Quantification of Fucoidan
2.3.1. Fucoidan Extraction
2.3.2. Quantification of Fucoidan
2.4. In Vitro Digestibility Study
2.4.1. Oral Phase
2.4.2. Gastric Phase
2.4.3. Intestinal Phase
2.5. Reducing Sugar Release
2.6. Mathematical Modelling
2.7. Prediction of GI and GL (Glycemic Load)
2.7.1. Total Available Carbohydrate (TAC)
2.7.2. Estimation of GI and GL
2.8. Recovery of Fucoidan after Digestion
2.8.1. Sample Treatment
2.8.2. Fucoidan Extraction
2.8.3. Fucoidan Quantification
2.8.4. Determination of Potential Bioaccessibility and Bioavailability
2.9. Statistical Analysis
3. Results and Discussion
3.1. Recovery of Fucoidan after Baking
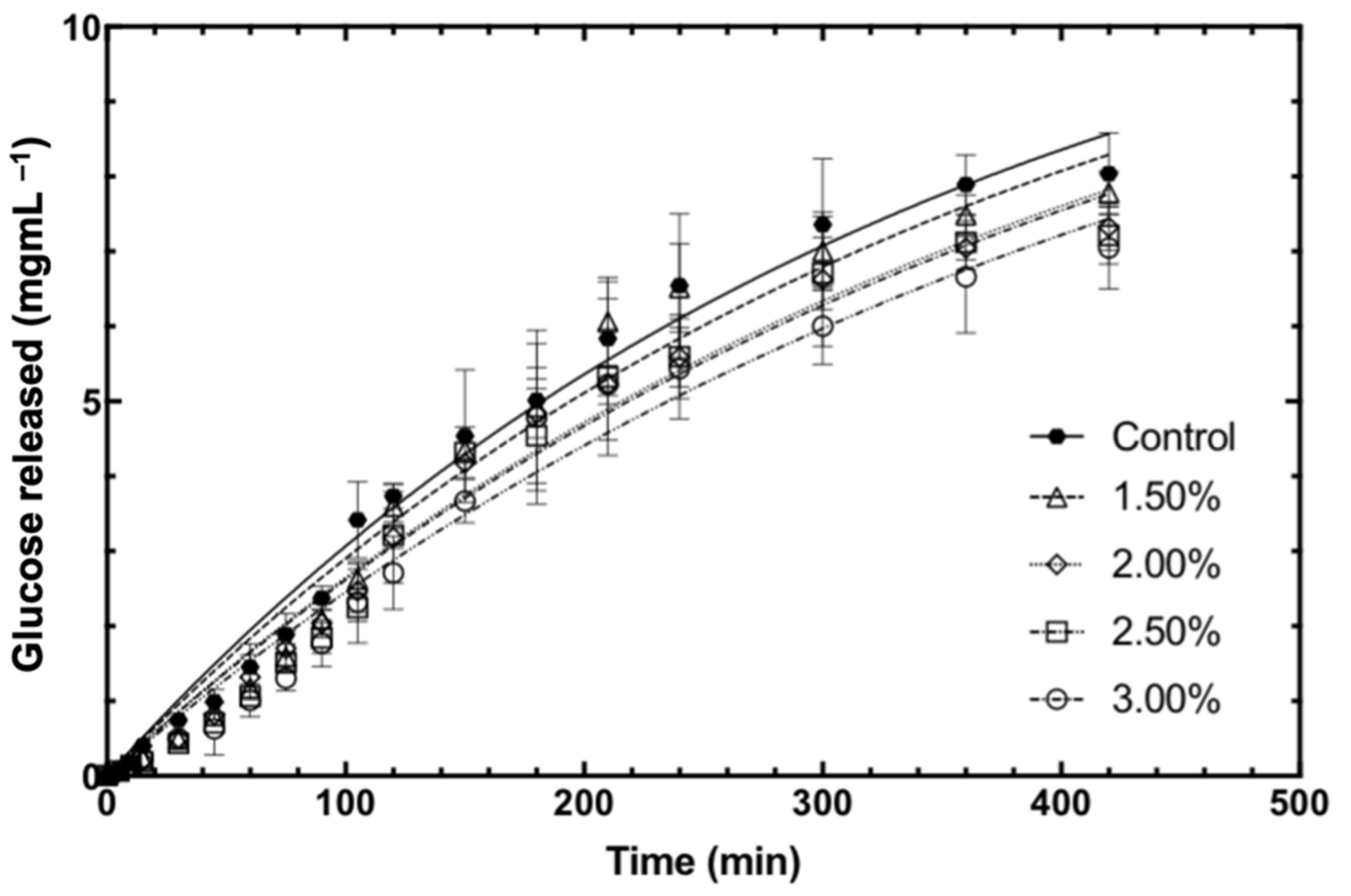
3.2. In Vitro Starch Digestibility of Bread
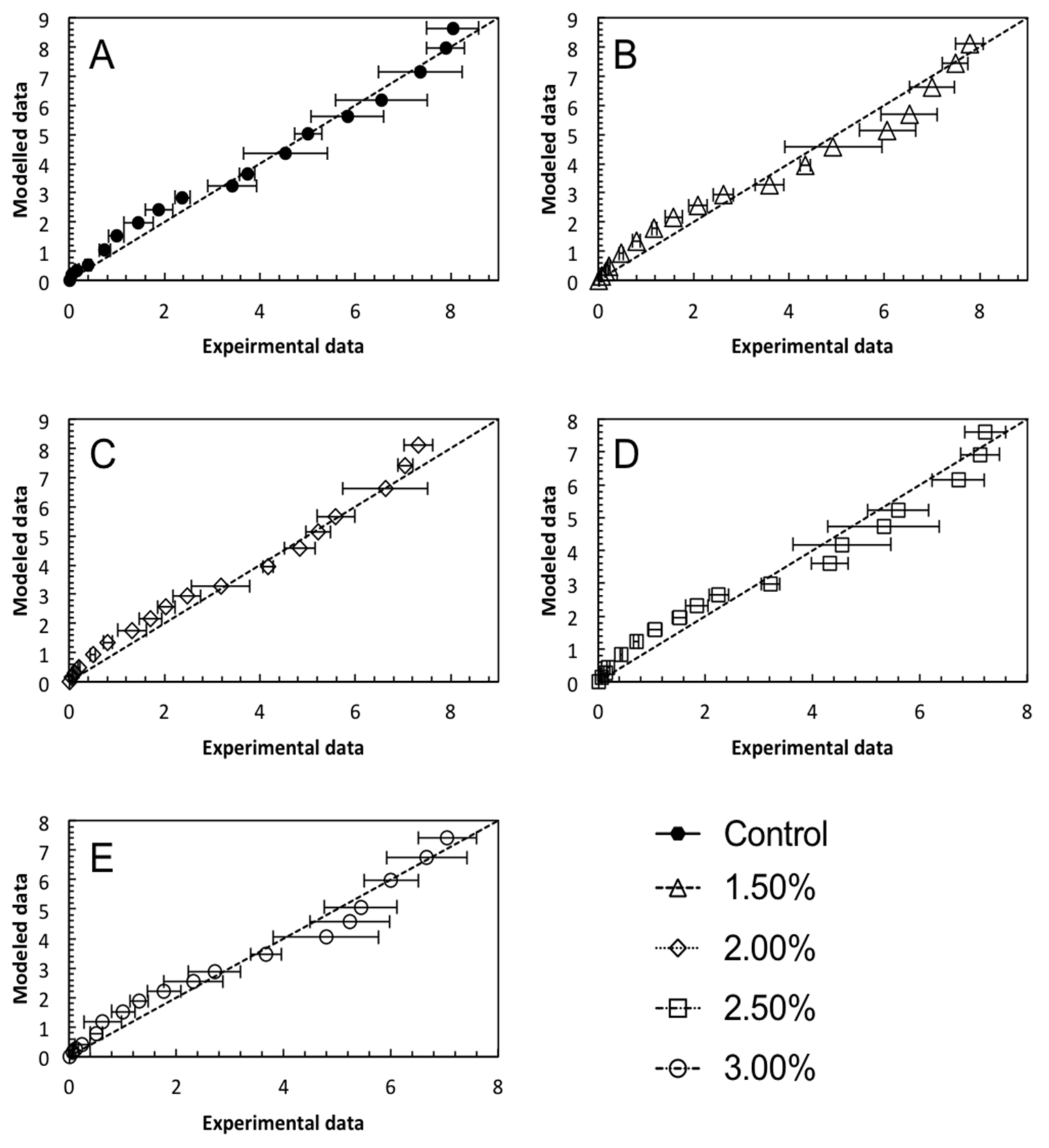
3.3. Mathematical Modelling
3.4. Prediction of GI and GL
3.5. Potential Bioaccessibility and Bioavailability of Fucoidan after Digestion
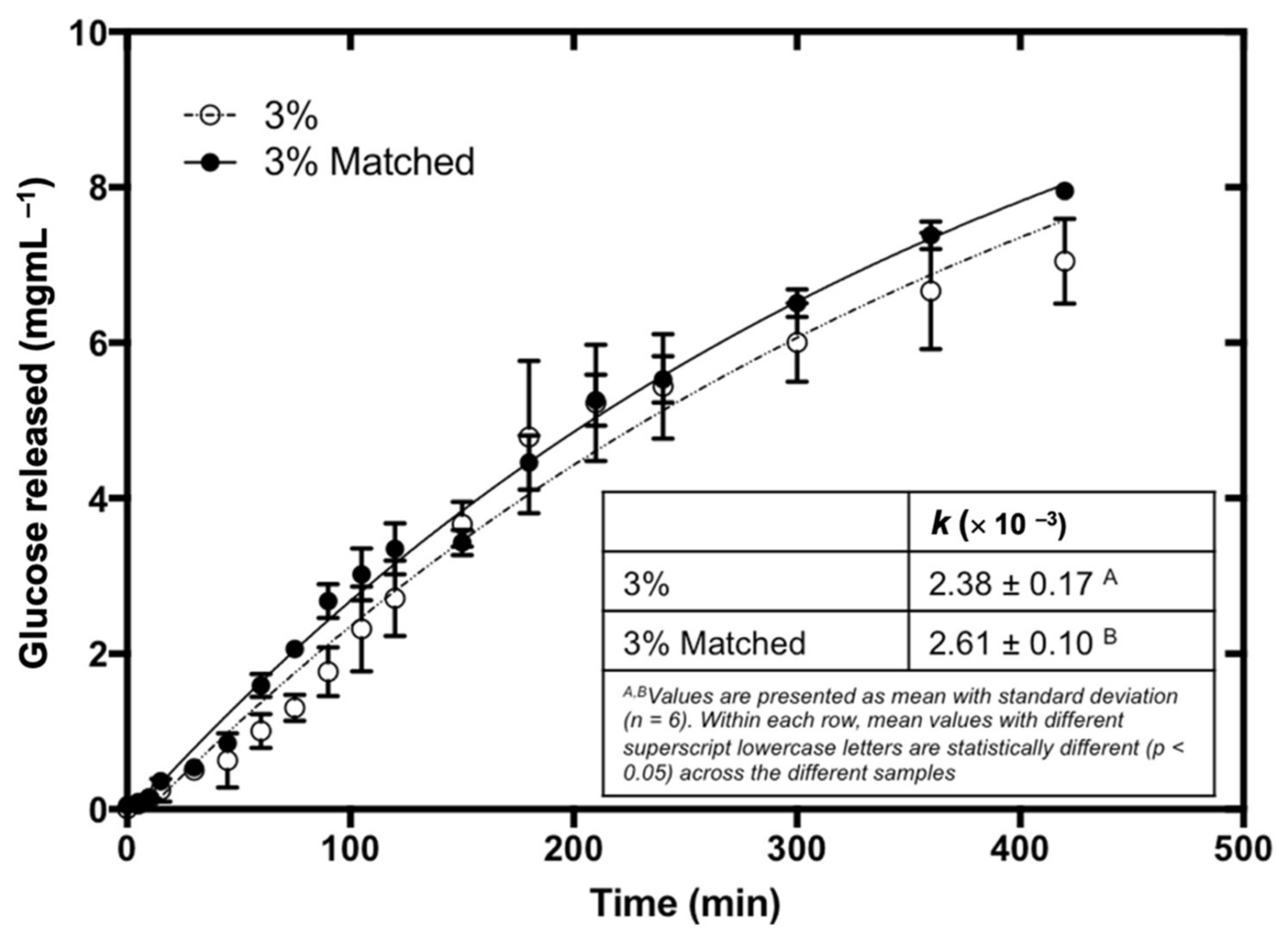
3.6. Effect of Bread Matrix on Fucoidan during Digestion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- ISO. Food Products-Determination of the Glycaemic Index (GI) and Recommendation for Food Classification; ISO: Geneva, Switzerland, 2010. [Google Scholar]
- Bhupathiraju, S.N.; Tobias, D.K.; Malik, V.S.; Pan, A.; Hruby, A.; Manson, J.E.; Willett, W.C.; Hu, F.B. Glycemic index, glycemic load, and risk of type 2 diabetes: Results from 3 large US cohorts and an updated meta-analysis. Am. J. Clin. Nutr. 2014, 100, 218–232. [Google Scholar] [CrossRef] [PubMed]
- Radulian, G.; Rusu, E.; Dragomir, A.; Posea, M. Metabolic effects of low glycaemic index diets. Nutr. J. 2009, 8, 5. [Google Scholar] [CrossRef]
- Goh, R.; Gao, J.; Ananingsih, V.K.; Ranawana, V.; Henry, C.J.; Zhou, W. Green tea catechins reduced the glycaemic potential of bread: An in vitro digestibility study. Food Chem. 2015, 180, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Sui, X.; Zhang, Y.; Zhou, W. Bread fortified with anthocyanin-rich extract from black rice as nutraceutical sources: Its quality attributes and in vitro digestibility. Food Chem. 2016, 196, 910–916. [Google Scholar] [CrossRef]
- Kim, K.-T.; Rioux, L.-E.; Turgeon, S.L. Alpha-amylase and alpha-glucosidase inhibition is differentially modulated by fucoidan obtained from Fucus vesiculosus and Ascophyllum nodosum. Phytochemistry 2014, 98, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Lu, F.; Wei, X.; Zhao, R. Fucoidan: Structure and bioactivity. Molecules 2008, 13, 1671–1695. [Google Scholar] [CrossRef] [PubMed]
- Koh, H.S.A.; Lu, J.; Zhou, W. Structure characterization and antioxidant activity of fucoidan isolated from Undaria pinnatifida grown in New Zealand. Carbohyr. Polym. 2019, 212, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Koh, H.S.A.; Lu, J.; Zhou, W. Structural Dependence of Sulfated Polysaccharide for Diabetes Management: Fucoidan from Undaria pinnatifida Inhibiting α-Glucosidase More Strongly Than α-Amylase and Amyloglucosidase. Front. Pharmacol. 2020, 11, 831. [Google Scholar] [CrossRef]
- Koh, H.S.A.; Lim, S.E.V.; Lu, J.; Zhou, W. Bioactivity enhancement of fucoidan through complexing with bread matrix and baking. LWT 2020, 130, 109646. [Google Scholar] [CrossRef]
- Bi, D.; Yu, B.; Han, Q.; Lu, J.; White, W.L.; Lai, Q.; Cai, N.; Luo, W.; Gu, L.; Li, S. Immune activation of RAW264. 7 macrophages by low molecular weight fucoidan extracted from New Zealand Undaria pinnatifida. J. Agric. Food Chem. 2018, 66, 10721–10728. [Google Scholar] [CrossRef]
- Ananingsih, V.K.; Gao, J.; Zhou, W. Impact of green tea extract and fungal alpha-amylase on dough proofing and steaming. Food Bioproc. Tech. 2013, 6, 3400–3411. [Google Scholar] [CrossRef][Green Version]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carriere, F.; Boutrou, R.; Corredig, M.; Dupont, D. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]
- Goñi, I.; Garcia-Alonso, A.; Saura-Calixto, F. A starch hydrolysis procedure to estimate glycemic index. Nutr. Res. 1997, 17, 427–437. [Google Scholar] [CrossRef]
- Lin, J.; Teo, L.M.; Leong, L.P.; Zhou, W. In vitro bioaccessibility and bioavailability of quercetin from the quercetin-fortified bread products with reduced glycemic potential. Food Chem. 2019, 286, 629–635. [Google Scholar] [CrossRef]
- Wolter, A.; Hager, A.-S.; Zannini, E.; Arendt, E.K. Influence of sourdough on in vitro starch digestibility and predicted glycemic indices of gluten-free breads. Food Funct. 2014, 5, 564–572. [Google Scholar] [CrossRef]
- Wolever, T.M.; Brand-Miller, J.C.; Abernethy, J.; Astrup, A.; Atkinson, F.; Axelsen, M.; Björck, I.; Brighenti, F.; Brown, R.; Brynes, A. Measuring the glycemic index of foods: Interlaboratory study. Am. J. Clin. Nutr. 2008, 87, 247S–257S. [Google Scholar] [CrossRef]
- Yoon, S.-H.; Mukerjea, R.; Robyt, J.F. Specificity of yeast (Saccharomyces cerevisiae) in removing carbohydrates by fermentation. Carbohydr. Res. 2003, 338, 1127–1132. [Google Scholar] [CrossRef]
- Wilkinson, J. The pathway of the adaptive fermentation of galactose by yeast. Biochem. J. 1949, 44, 460–467. [Google Scholar] [CrossRef]
- Ale, M.T.; Meyer, A.S. Fucoidans from brown seaweeds: An update on structures, extraction techniques and use of enzymes as tools for structural elucidation. Rsc. Adv. 2013, 3, 8131–8141. [Google Scholar] [CrossRef]
- Kim, D.-Y.; Shin, W.-S. Characterisation of bovine serum albumin–fucoidan conjugates prepared via the Maillard reaction. Food Chem. 2015, 173, 1–6. [Google Scholar] [CrossRef]
- Oudjeriouat, N.; Moreau, Y.; Santimone, M.; Svensson, B.; Marchis-Mouren, G.; Desseaux, V. On the mechanism of α-amylase: Acarbose and cyclodextrin inhibition of barley amylase isozymes. Eur. J. Biochem. 2003, 270, 3871–3879. [Google Scholar] [CrossRef]
- Cho, M.; Han, J.H.; You, S. Inhibitory effects of fucan sulfates on enzymatic hydrolysis of starch. LWT 2011, 44, 1164–1171. [Google Scholar] [CrossRef]
- De Melo, E.B.; da Silveira Gomes, A.; Carvalho, I. α-and β-Glucosidase inhibitors: Chemical structure and biological activity. Tetrahedron 2006, 62, 10277–10302. [Google Scholar] [CrossRef]
- Mohan, S.; Pinto, B.M. Zwitterionic glycosidase inhibitors: Salacinol and related analogues. Carbohydr. Res. 2007, 342, 1551–1580. [Google Scholar] [CrossRef] [PubMed]
- Rabasa-Lhoret, R.; Chiasson, J.L. α-Glucosidase Inhibitors. In International Textbook of Diabetes Mellitus; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2003. [Google Scholar]
- Heightman, T.D.; Vasella, A.T. Recent insights into inhibition, structure, and mechanism of configuration-retaining glycosidases. Angew. Chem. Int. Ed. 1999, 38, 750–770. [Google Scholar] [CrossRef]
- Krasikov, V.; Karelov, D.; Firsov, L. α-Glucosidases. Biochemistry 2001, 66, 267–281. [Google Scholar] [PubMed]
- Lillelund, V.H.; Jensen, H.H.; Liang, X.; Bols, M. Recent developments of transition-state analogue glycosidase inhibitors of non-natural product origin. Chem. Rev. 2002, 102, 515–554. [Google Scholar] [CrossRef] [PubMed]
- Maki, K.; Galant, R.; Samuel, P.; Tesser, J.; Witchger, M.; Ribaya-Mercado, J.; Blumberg, J.; Geohas, J. Effects of consuming foods containing oat β-glucan on blood pressure, carbohydrate metabolism and biomarkers of oxidative stress in men and women with elevated blood pressure. Eur. J. Clin. Nutr. 2007, 61, 786–795. [Google Scholar] [CrossRef]
- Ou, S.; Kwok, K.C.; Li, Y.; Fu, L. In vitro study of possible role of dietary fiber in lowering postprandial serum glucose. J. Agric. Food Chem. 2001, 49, 1026–1029. [Google Scholar] [CrossRef]
- Rioux, L.E.; Turgeon, S.L.; Beaulieu, M. Rheological characterisation of polysaccharides extracted from brown seaweeds. J. Sci. Food Agric. 2007, 87, 1630–1638. [Google Scholar] [CrossRef]
- Sui, X.; Zhang, Y.; Zhou, W. In vitro and in silico studies of the inhibition activity of anthocyanins against porcine pancreatic α-amylase. J. Funct. Foods 2016, 21, 50–57. [Google Scholar] [CrossRef]
- Barclay, A.W.; Brand-Miller, J.C.; Wolever, T.M. Glycemic index, glycemic load, and glycemic response are not the same. Diabetes Care 2005, 28, 1839–1840. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, F.S.; Foster-Powell, K.; Brand-Miller, J.C. International tables of glycemic index and glycemic load values: 2008. Diabetes care 2008, 31, 2281–2283. [Google Scholar] [CrossRef]
- Coe, S.; Ryan, L. White bread enriched with polyphenol extracts shows no effect on glycemic response or satiety, yet may increase postprandial insulin economy in healthy participants. Nutr. Res. 2016, 36, 193–200. [Google Scholar] [CrossRef]
- Cardoso, C.; Afonso, C.; Lourenço, H.; Costa, S.; Nunes, M.L. Bioaccessibility assessment methodologies and their consequences for the risk–benefit evaluation of food. Trends. Food Sci. Technol. 2015, 41, 5–23. [Google Scholar] [CrossRef]
- Fernández-García, E.; Carvajal-Lérida, I.; Pérez-Gálvez, A. In vitro bioaccessibility assessment as a prediction tool of nutritional efficiency. Nutr. Res. 2009, 29, 751–760. [Google Scholar] [CrossRef]
- Etcheverry, P.; Grusak, M.A.; Fleige, L.E. Application of in vitro bioaccessibility and bioavailability methods for calcium, carotenoids, folate, iron, magnesium, polyphenols, zinc, and vitamins B6, B12, D, and E. Front. Physiol. 2012, 3, 317. [Google Scholar] [CrossRef]
- Zhao, X.; Guo, F.; Hu, J.; Zhang, L.; Xue, C.; Zhang, Z.; Li, B. Antithrombotic activity of oral administered low molecular weight fucoidan from Laminaria Japonica. Thromb. Res. 2016, 144, 46–52. [Google Scholar] [CrossRef]
- Nagamine, T.; Hayakawa, K.; Nakazato, K.; Iha, M. Determination of the active transport of fucoidan derived from Okinawa Mozuku across the human intestinal Caco-2 cells as assessed by size-exclusion chromatography. J. Chromatogr. B. Biomed. Appl. 2015, 997, 187–193. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Zaporozhets, T.; Besednova, N.; Kuznetsova, T.; Zvyagintseva, T.; Makarenkova, I.; Kryzhanovsky, S.; Melnikov, V. The prebiotic potential of polysaccharides and extracts of seaweeds. Russ. J. Mar. Biol. 2014, 40, 1–9. [Google Scholar] [CrossRef]


| Control | 1.50% | 2.00% | 2.50% | 3.00% | |
|---|---|---|---|---|---|
| k (×10−3) | 3.03 ± 0.23 a | 2.91 ± 0.18 a | 2.58 ± 0.23 b | 2.57 ± 0.12 b | 2.38 ± 0.17 b |
| C∞ (mg/mL) | 12.16 ± 0.44 | 12.16 ± 0.44 | 12.16 ± 0.44 | 12.16 ± 0.44 | 12.16 ± 0.44 |
| R2 | 0.963 | 0.961 | 0.971 | 0.955 | 0.945 |
| RMSE | 0.322 | 0.457 | 0.363 | 0.413 | 0.383 |
| TAC (g/5 g bread) | 2.50 ± 0.36 a | 2.48 ± 0.31 a | 2.49 ± 0.36 a | 2.43 ± 0.34 a | 2.48 ± 0.38 a |
| pGIbread | 100 a | 89.2 ± 6.1 b | 87.3 ± 6.3 b | 86.7 ± 6.2 b | 82.3 ± 6.4 b |
| pGIglucose | 70 a | 62.5 ± 4.3 b | 61.1 ± 4.4 b | 60.7 ± 4.3 b | 57.6 ± 4.5 b |
| pGL | 17.5 ± 2.5 a | 15.4 ± 0.9 a,b | 15.1 ± 1.3 a,b | 14.6 ± 1.2 b | 14.1 ± 1.3 b |
| Dialysate (mg Fucoidan/5 g Bread) | Digesta (mg Fucoidan/5 g Bread) | Sediment (mg Fucoidan/5 g Bread) | Overall Recovery (%) | FAV (%) | FAC (%) | |
|---|---|---|---|---|---|---|
| Control | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| 1.50% | N.D. | 27.3 ± 1.4 a | 5.3 ± 0.6 a | 92.7 ± 2.4 a | N.D. | 77.6 ± 3.9 a |
| 2.00% | N.D. | 38.1 ± 1.3 b | 6.7 ± 1.4 b | 93.8 ± 3.5 a | N.D. | 79.7 ± 2.7 a |
| 2.50% | N.D. | 52.7 ± 4.4 c | 8.3 ± 1.5 c | 89.2 ± 5.4 a | N.D. | 77.1 ± 6.5 a |
| 3.00% | N.D. | 61.1 ± 3.1 d | 9.0 ± 0.7 c | 91.7 ± 3.8 a | N.D. | 79.8 ± 4.1 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koh, H.S.A.; Chong, J.E.L.; Lu, J.; Zhou, W. Fucoidan Regulates Starch Digestion: In Vitro and Mechanistic Study. Foods 2022, 11, 427. https://doi.org/10.3390/foods11030427
Koh HSA, Chong JEL, Lu J, Zhou W. Fucoidan Regulates Starch Digestion: In Vitro and Mechanistic Study. Foods. 2022; 11(3):427. https://doi.org/10.3390/foods11030427
Chicago/Turabian StyleKoh, Hui Si Audrey, Jin Er Leonard Chong, Jun Lu, and Weibiao Zhou. 2022. "Fucoidan Regulates Starch Digestion: In Vitro and Mechanistic Study" Foods 11, no. 3: 427. https://doi.org/10.3390/foods11030427
APA StyleKoh, H. S. A., Chong, J. E. L., Lu, J., & Zhou, W. (2022). Fucoidan Regulates Starch Digestion: In Vitro and Mechanistic Study. Foods, 11(3), 427. https://doi.org/10.3390/foods11030427