Effect of Peroxyl Radical-Induced Oxidation on Functional and Structural Characteristics of Walnut Protein Isolates Revealed by High-Resolution Mass Spectrometry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Walnut Protein Isolates (WPI) Preparation
2.3. Oxidation of WPI
2.4. Determination of Oxidation Degree of AAPH-Oxidized WPI (APPH-WPI)
2.4.1. Determination of Carbonyl Content
2.4.2. Determination of Sulfhydryl Group Content
2.4.3. Determination of Free Amino Groups Content
2.5. Determination of Structural Characteristics of APPH-WPI
2.5.1. Particle Size Distributions
2.5.2. Sodium Dodecyl Sulphate–Polyacrylamide Gel Electrophoresis (SDS-PAGE) Analysis
2.5.3. Intrinsic Fluorescence
2.5.4. Surface Hydrophobicity (H0)
2.5.5. Fourier Transformed Infrared Spectroscopy (FT-IR)
2.6. Determination of Physicochemical Properties of APPH-WPI
2.6.1. Solubility
2.6.2. Foaming ability (FA) and Foaming Stability (FS)
2.6.3. Water/Oil Holding Capacity (WHC/OHC)
2.7. Mass Spectrometry Analysis
2.8. Double Antibody Sandwich Enzyme-Linked Immunosorbent Assay (ELISA)
2.9. Statistical Analysis
3. Results and Discussion
3.1. Oxidative Indicators Analysis
3.1.1. Carbonyl Groups
3.1.2. Sulfhydryl Groups
3.1.3. Free Amino Groups
3.2. Structural Characteristics Analysis
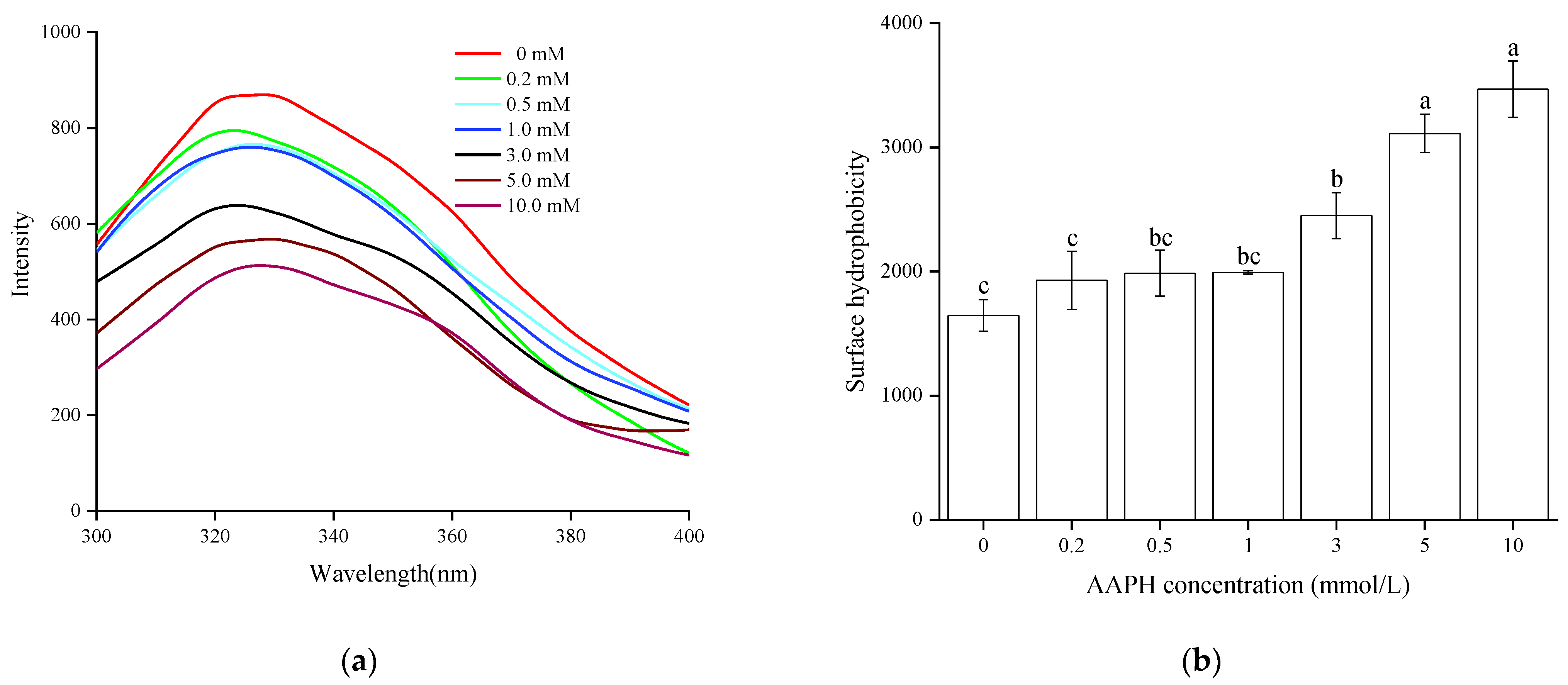
3.2.1. Endogenous Fluorescence
3.2.2. Surface Hydrophobicity
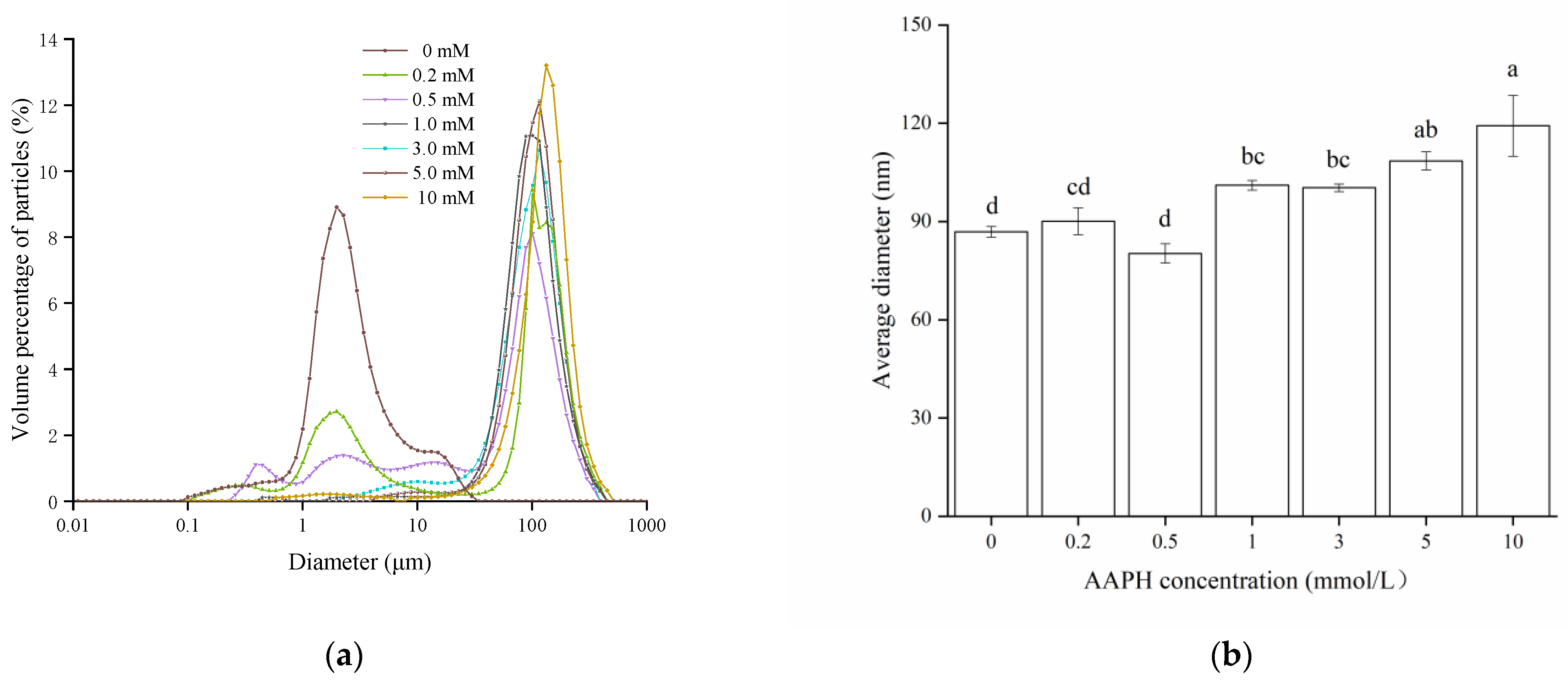
3.2.3. Particle Size Distributions
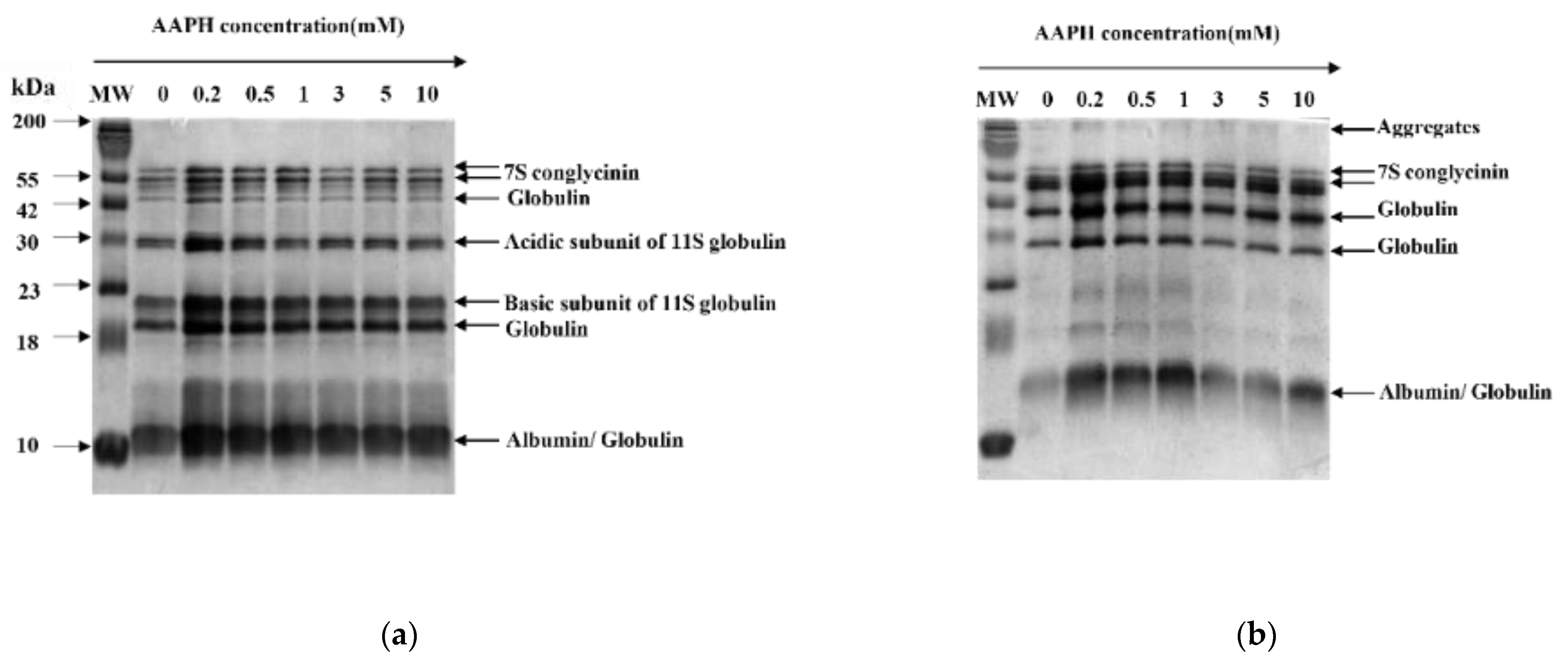
3.2.4. SDS-PAGE
3.2.5. Fourier Transform Infrared (FT-IR) Spectroscopy
3.3. Physicochemical Properties Analysis
3.3.1. Solubility
3.3.2. Foaming Ability (FA) and Foaming Stability (FS)
3.3.3. Water Holding Capacity (WHC) and Oil Holding Capacity (OHC)
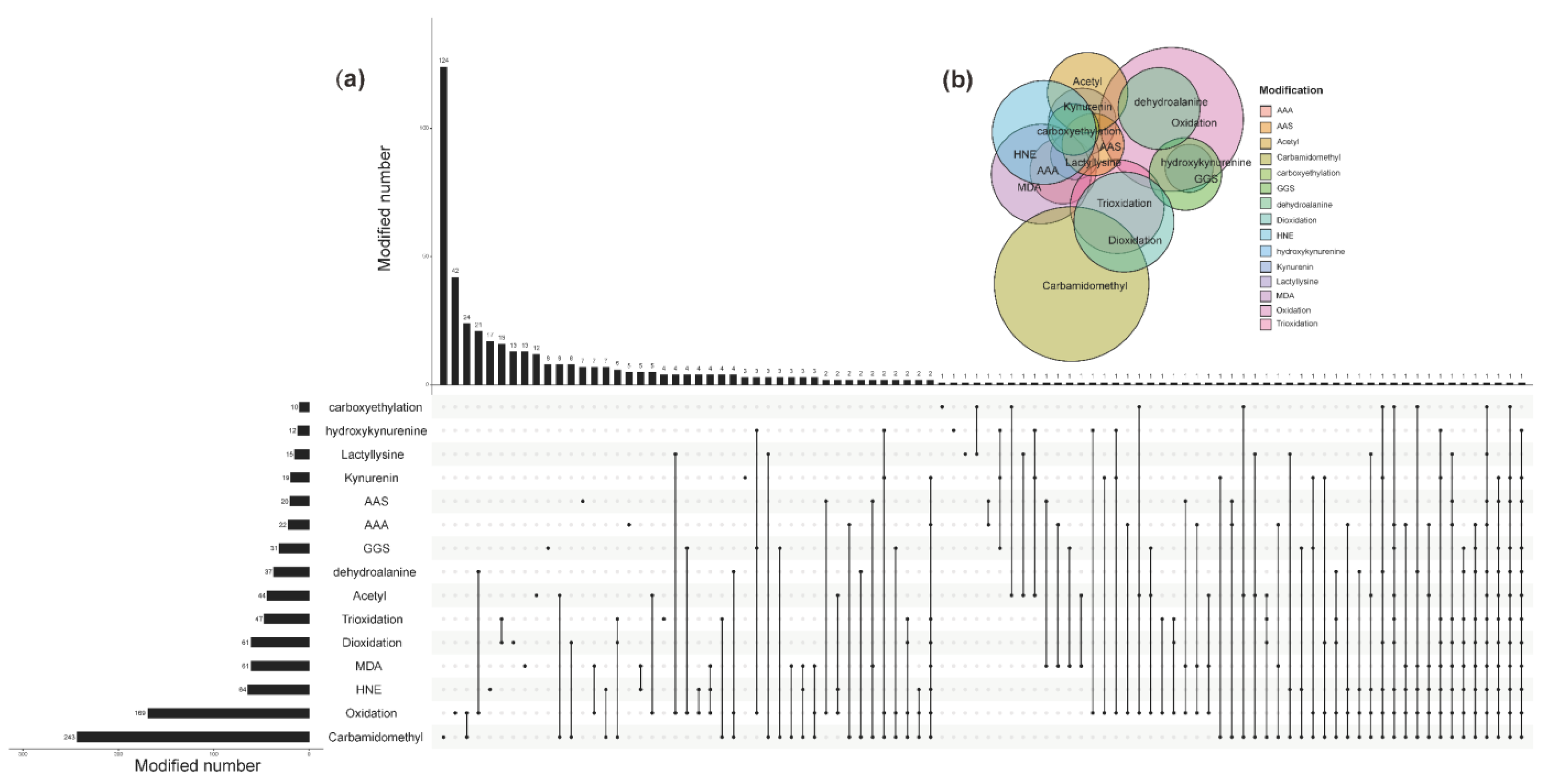
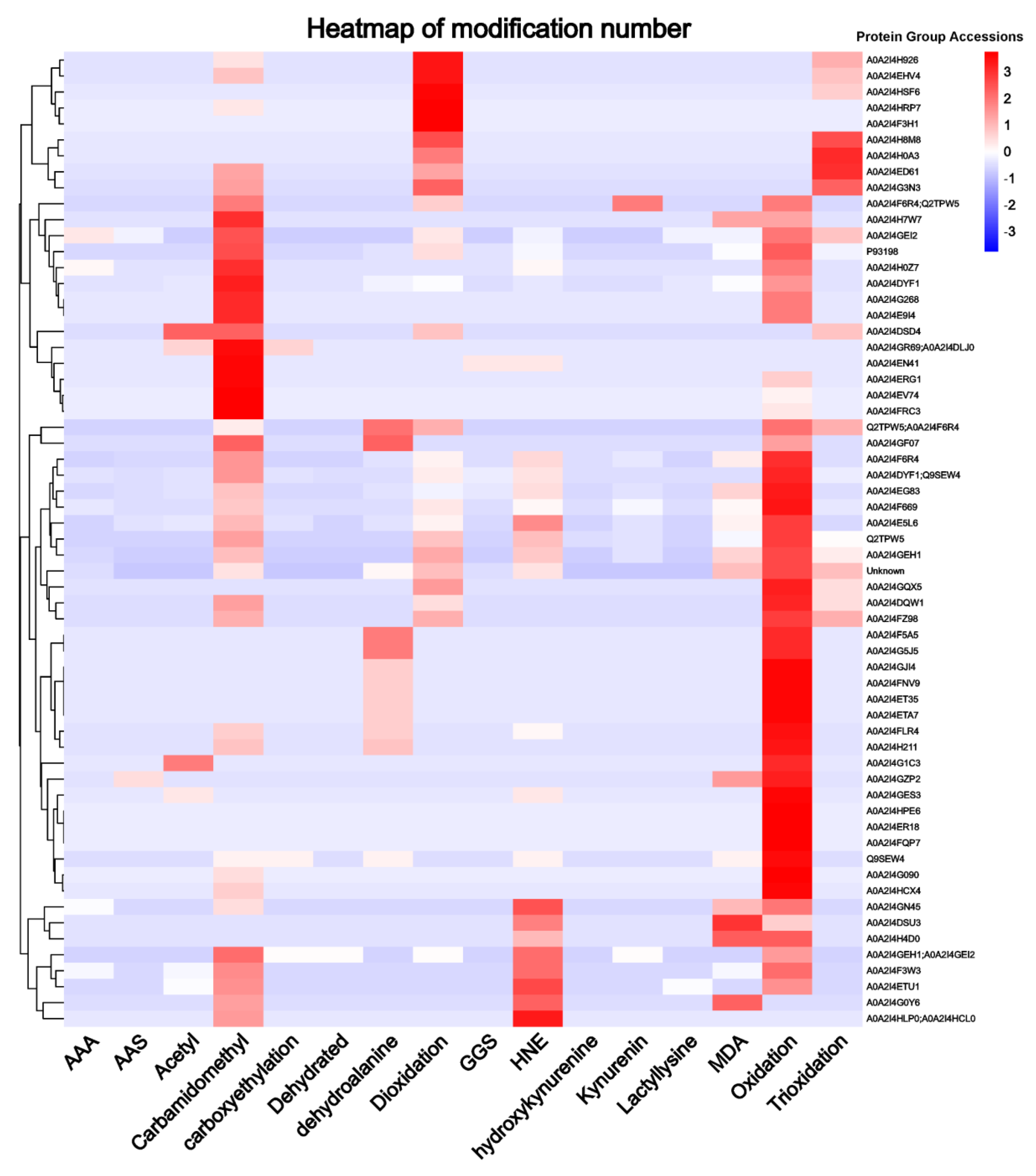
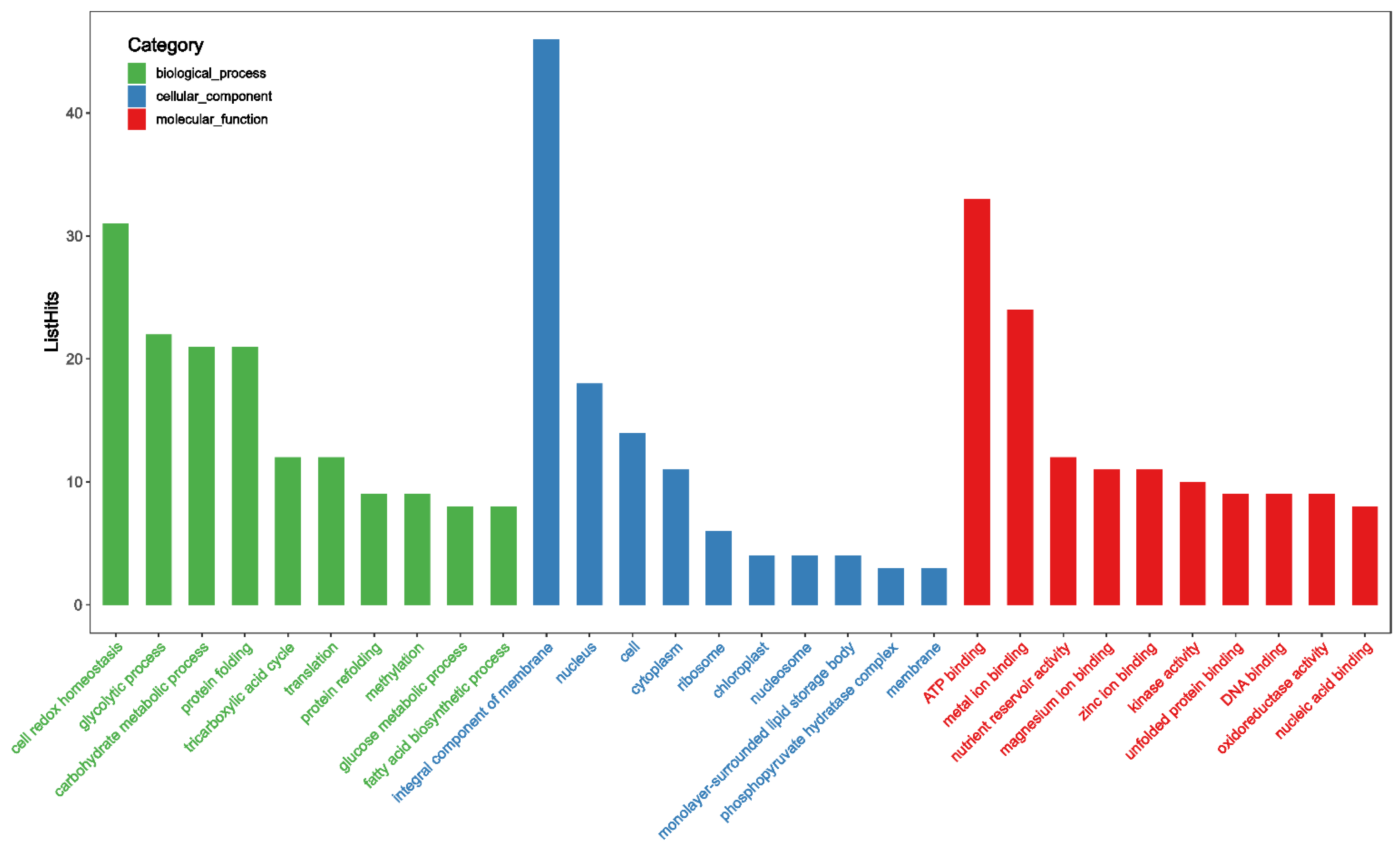
3.4. Proteomic Analysis of Oxidative Modified WPI
3.5. Antigenicity of Jug r 1
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mao, X.; Hua, Y. Composition, structure and functional properties of protein concentrates and isolates produced from walnut (Juglans regia L.). Int. J. Mol. Sci. 2012, 13, 1561–1581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, X.Z.; Zhang, L.N.; Lu, X.; Zhang, C.M.; Hua, Y.F.; Chen, Y.M. Effect of high-speed shearing treatment on dehulled walnut proteins. LWT Food Sci. Technol. 2019, 116, 108500. [Google Scholar] [CrossRef]
- Stanisic, J.; Ivkovic, T.; Romic, S.; Zec, M.; Culafic, T.; Stojiljkovic, M.; Koricanac, G. Beneficial effect of walnuts on vascular tone is associated with Akt signalling, voltage-dependent calcium channel LTCC and ATP-sensitive potassium channel Kv1.2. Int. J. Food Sci. Nutr. 2021, 72, 324–334. [Google Scholar] [CrossRef] [PubMed]
- Nuts & Dried Fruits Statistical Yearbook 2020/2021. Available online: https://www.nutfruit.org/files/tech/1625230833_INC_Stats_2021.pdf (accessed on 19 November 2021).
- Kong, X.; Zhang, L.; Song, W.; Zhang, C.; Hua, Y.; Chen, Y.; Li, X. Separation, identification and molecular binding mechanism of dipeptidyl peptidase IV inhibitory peptides derived from walnut (Juglans regia L.) protein. Food Chem. 2021, 347, 129062. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Fan, T.; Zhao, X.; Zhang, X.; Sun, Y.; Liu, H. Influence of pH and salt concentration on functional properties of walnut protein from different extraction methods. J. Food Sci. Tech. Mys. 2017, 54, 2833–2841. [Google Scholar] [CrossRef] [PubMed]
- Moghadam, M.; Salami, M.; Mohammadian, M.; Emam-Djomeh, Z.; Jahanbani, R.; Moosavi-Movahedi, A.A. Physicochemical and bio-functional properties of walnut proteins as affected by trypsin-mediated hydrolysis. Food Biosci. 2020, 36, 100611. [Google Scholar] [CrossRef]
- Sun, Q.; Ma, Z.F.; Zhang, H.X.; Ma, S.J.; Kong, L.M. Structural characteristics and functional properties of walnut glutelin as hydrolyzed: Effect of enzymatic modification. Int. J. Food Prop. 2019, 22, 265–279. [Google Scholar] [CrossRef] [Green Version]
- Qin, Z.; Guo, X.; Lin, Y.; Chen, J.; Liao, X.; Hu, X.; Wu, J. Effects of high hydrostatic pressure on physicochemical and functional properties of walnut (Juglans regia L.) protein isolate. J. Sci. Food Agric. 2013, 93, 1105–1111. [Google Scholar] [CrossRef]
- Hellwig, M. The chemistry of protein oxidation in food. Angew. Chem. Int. Ed. Engl. 2019, 58, 16742–16763. [Google Scholar] [CrossRef]
- Li, X.; Liu, C.; Wang, J.; Zhou, K.; Yi, S.; Zhu, W.; Xu, Y.; Lin, H.; Li, J. Effect of hydroxyl radicals on biochemical and functional characteristics of myofibrillar protein from large yellow croaker (Pseudosciaena crocea). J. Food Biochem. 2020, 44, e13084. [Google Scholar] [CrossRef]
- Nyaisaba, B.M.; Liu, X.X.; Zhu, S.C.; Fan, X.J.; Sun, L.L.; Hatab, S.; Miao, W.H.; Chen, M.L.; Deng, S.G. Effect of hydroxyl-radical on the biochemical properties and structure of myofibrillar protein from Alaska pollock (Theragra chalcogramma). LWT Food Sci. Technol. 2019, 106, 15–21. [Google Scholar] [CrossRef]
- Chen, N.; Zhao, M.; Sun, W. Effect of protein oxidation on the in vitro digestibility of soy protein isolate. Food Chem. 2013, 141, 3224–3229. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.; Lin, H.; Li, Z.; Nayak, B.; Ahmed, I.; Tian, S.; Chen, G.; Lin, H.; Zhao, J. Structural changes of 2,2′-azobis(2-amidinopropane) dihydrochloride (AAPH) treated shrimp tropomyosin decrease allergenicity. Food Chem. 2019, 274, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Li, M.; Shao, J.; Chen, H.; Xu, X.; Jin, Z.; Liu, X. Effect of oxidative modification on structural and foaming properties of egg white protein. Food Hydrocoll. 2017, 75, 223–228. [Google Scholar] [CrossRef]
- Fu, Q.; Liu, R.; Wang, H.; Hua, C.; Song, S.; Zhou, G.; Zhang, W. Effects of Oxidation in Vitro on Structures and Functions of Myofibrillar Protein from Beef Muscles. J. Agric. Food Chem. 2019, 67, 5866–5873. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.J.; Tu, Z.C.; Wang, H.; Hu, Y.M.; Du, P.C.; Yang, Y.P. Mechanism of the effect of 2, 2′-azobis (2-amidinopropane) dihydrochloride simulated lipid oxidation on the IgG/IgE binding ability of ovalbumin. Food Chem. 2020, 327, 127037. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Xiong, Z.; Lu, S.; Walayat, N.; Hu, C.; Xiong, H. Effects of oxidative modification on the functional, conformational and gelling properties of myofibrillar proteins from Culter alburnus. Int. J. Biol. Macromol. 2020, 162, 1442–1452. [Google Scholar] [CrossRef]
- Guo, X.; Qiu, H.; Deng, X.; Mao, X.; Guo, X.; Xu, C.; Zhang, J. Effect of Chlorogenic Acid on the Physicochemical and Functional Properties of Coregonus Peled Myofibrillar Protein through Hydroxyl Radical Oxidation. Molecules 2019, 24, 3205. [Google Scholar] [CrossRef] [Green Version]
- Shen, H.; Stephen Elmore, J.; Zhao, M.; Sun, W. Effect of oxidation on the gel properties of porcine myofibrillar proteins and their binding abilities with selected flavour compounds. Food Chem. 2020, 329, 127032. [Google Scholar] [CrossRef]
- Trigui, I.; Zarai, Z.; Chevance, S.; Cheikh-Rouhou, S.; Attia, H.; Ayadi, M.A. Physicochemical properties, antioxidant activity and in vitro gastrointestinal digestion of purified proteins from black cumin seeds. Int. J. Biol. Macromol. 2019, 126, 454–465. [Google Scholar] [CrossRef]
- Labuckas, D.; Maestri, D.; Lamarque, A. Effect of different oil extraction methods on proximate composition and protein characteristics of walnut (Juglans regia L.) flour. LWT Food Sci. Technol. 2014, 59, 794–799. [Google Scholar] [CrossRef]
- Zhu, Z.; Mao, X.; Wu, Q.; Zhang, J.; Deng, X. Effects of oxidative modification of peroxyl radicals on the structure and foamability of chickpea protein isolates. J. Food Sci. 2021, 86, 824–833. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Li, F.; Wu, X. Effects of rice bran rancidity on oxidation, structural characteristics and interfacial properties of rice bran globulin. Food Hydrocoll. 2020, 110, 106123. [Google Scholar] [CrossRef]
- Abbou, A.; Kadri, N.; Dahmoune, F.; Chergui, A.; Remini, H.; Berkani, F.; Adel, K.; Boukhalfa, F.; Madani, K. Optimising functional properties and chemical composition of Pinus halepensis Mill. Seeds protein concentrates. Food Hydrocoll. 2020, 100, 105416. [Google Scholar] [CrossRef]
- Zhou, L.Y.; Zhang, Y.; Zhao, C.B.; Lin, H.J.; Wang, Z.J.; Wu, F. Structural and functional properties of rice bran protein oxidized by peroxyl radicals. Int. J. Food Prop. 2017, 20, 1456–1467. [Google Scholar] [CrossRef]
- Wang, J.; Tan, Y.; Xu, H.; Niu, S.; Yu, J. Effect of 2,2-azobis (2-amidinopropane) dihydrochloride oxidized casein on the microstructure and microrheology properties of emulsions. Food Sci. Biotechnol. 2016, 25, 1283–1290. [Google Scholar] [CrossRef]
- Li, F.; Wu, X.; Wu, W. Effects of oxidative modification by malondialdehyde on the in vitro digestion properties of rice bran protein. J. Cereal Sci. 2020, 97, 103158. [Google Scholar] [CrossRef]
- Deng, X.; Lei, Y.; Liu, J.; Zhang, J.; Qin, J. Biochemical changes of Coregonus peled myofibrillar proteins isolates as affected by HRGS oxidation system. J. Food Biochem. 2019, 43, e12710. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Y.; Chen, L.; Xu, X.; Zhou, G.; Li, Z.; Feng, X. Dose-dependent effects of rosmarinic acid on formation of oxidatively stressed myofibrillar protein emulsion gel at different NaCl concentrations. Food Chem. 2018, 243, 50–57. [Google Scholar] [CrossRef]
- Li, X.P.; Liu, C.K.; Wang, J.X.; Li, W.X.; Lin, B.Y.; Zhu, W.H.; Xu, Y.X.; Yi, S.M.; Mi, H.B.; Li, J.R. Tea Polyphenols Affect Oxidative Modification and Solution Stability of Myofibrillar Protein from Grass Carp (Ctenopharyngodon idellus). Food Biophys. 2020, 15, 397–408. [Google Scholar] [CrossRef]
- Pan, J.F.; Lian, H.L.; Jia, H.; Hao, R.Y.; Wang, Y.J.; Ju, H.P.; Li, S.J.; Dong, X.P. Dose affected the role of gallic acid on mediating gelling properties of oxidatively stressed Japanese seerfish myofibrillar protein. LWT Food Sci. Technol. 2020, 118, 108849. [Google Scholar] [CrossRef]
- Zhu, Y.P.; Wang, Y.; Li, J.L.; Li, F.; Teng, C.; Li, X.T. Effects of Water-Extractable Arabinoxylan on the Physicochemical Properties and Structure of Wheat Gluten by Thermal Treatment. J. Agric. Food Chem. 2017, 65, 4728–4735. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Li, H.; Wang, Z.; Emara, A.M.; Hu, Y.; He, Z. Effects of in vitro oxidation on myofibrillar protein charge, aggregation, and structural characteristics. Food Chem. 2020, 332, 127396. [Google Scholar] [CrossRef] [PubMed]
- Heinonen, M.; Gürbüz, G.; Ertbjerg, P. Oxidation of proteins. In Chemical Changes during Processing and Storage of Foods; Rodriguez-Amaya, D.B., Amaya-Farfan, J., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 85–123. [Google Scholar]
- Akabas, M.H. Cysteine modification: Probing channel structure, function and conformational change. In Novel Chemical Tools to Study Ion Channel Biology; Ahern, C., Pless, S., Eds.; Springer New York: New York, NY, USA, 2015; pp. 25–54. [Google Scholar]
- Lao, Y.W.; Gungormusler-Yilmaz, M.; Shuvo, S.; Verbeke, T.; Spicer, V.; Krokhin, O.V. Chromatographic behavior of peptides containing oxidized methionine residues in proteomic LC-MS experiments: Complex tale of a simple modification. J. Proteom. 2015, 125, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Uchida, K.; Stadtman, E.R. Modification of histidine residues in proteins by reaction with 4-hydroxynonenal. Proc. Natl. Acad. Sci. USA 1992, 89, 4544–4548. [Google Scholar] [CrossRef] [Green Version]
- Estevez, M.; Ollilainen, V.; Heinonen, M. Analysis of protein oxidation markers alpha-aminoadipic and gamma-glutamic semialdehydes in food proteins using liquid chromatography (LC)-electrospray ionization (ESI)-multistage tandem mass spectrometry (MS). J. Agric. Food Chem. 2009, 57, 3901–3910. [Google Scholar] [CrossRef] [PubMed]
- Teuber, S.S.; Dandekar, A.M.; Peterson, W.R.; Sellers, C.L. Cloning and sequencing of a gene encoding a 2S albumin seed storage protein precursor from English walnut (Juglans regia), a major food allergen. J. Allergy Clin. Immunol. 1998, 101, 807–814. [Google Scholar] [CrossRef]
- Youle, R.J.; Huang, A.H. Albumin storage protein and allergens in cottonseeds. J. Agric. Food Chem. 1979, 27, 500–503. [Google Scholar] [CrossRef]
- Lourenço dos Santos, S.; Petropoulos, I.; Friguet, B. The Oxidized Protein Repair Enzymes Methionine Sulfoxide Reductases and Their Roles in Protecting against Oxidative Stress, in Ageing and in Regulating Protein Function. Antioxidants 2018, 7, 191. [Google Scholar] [CrossRef]
- Song, Y.; Li, Z.; Lin, H.; Du, S.; Hao, Z.; Lin, H.; Zhu, Z. Effect of malondialdehyde treatment on the IgE binding capacity and conformational structure of shrimp tropomyosin. Food Chem. 2015, 175, 374–380. [Google Scholar] [CrossRef]
- Lv, L.; Lin, H.; Li, Z.; Wang, J.; Ahmed, I.; Chen, H. Changes of structure and IgE binding capacity of shrimp (Metapenaeus ensis) tropomyosin followed by acrolein treatment. Food Funct. 2017, 8, 1028–1036. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Chen, Z.J.; Han, D.; Li, Y.Y.; Sun, X.T.; Wang, Z.J.; Jin, H. Structural and Functional Properties Changes of β-Conglycinin Exposed to Hydroxyl Radical-Generating Systems. Molecules 2017, 22, 1893. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| AAPH (mmol/L) | Carbonyl (nmol/mg) | Free Sulfhydryl (nmol/mg) | Total Sulfhydryl (nmol/mg) | Free Amino (nmol/mg) |
|---|---|---|---|---|
| 0.0 | 0.61 ± 0.08 c | 6.66 ± 0.10 a | 172.43 ± 16.17 ab | 0.67 ± 0.07 a |
| 0.2 | 0.85 ± 0.05 b | 6.03 ± 0.21 b | 173.09 ± 11.10 a | 0.65 ± 0.05 ab |
| 0.5 | 0.90 ± 0.10 b | 5.11 ± 0.04 c | 165.66 ± 4.59 ab | 0.60 ± 0.03 b |
| 1.0 | 0.93 ± 0.05 b | 4.18 ± 0.13 d | 155.70 ± 4.76 bc | 0.54 ± 0.04 c |
| 3.0 | 1.30 ± 0.15 a | 3.16 ± 0.04 e | 148.00 ± 3.69 cd | 0.48 ± 0.03 d |
| 5.0 | 1.42 ± 0.01 a | 2.34 ± 0.04 f | 135.64 ± 2.17 d | 0.43 ± 0.02 e |
| 10.0 | 1.48 ± 0.12 a | 1.25 ± 0.09 g | 114.26 ± 4.35 e | 0.31 ± 0.04 f |
| AAPH (mmol/L) | Solubility (%) | WHC (g/g) | OHC (g/g) | FA (%) | FS (%) |
|---|---|---|---|---|---|
| 0.0 | 80.64 ± 3.41 a | 3.34 ± 0.05 b | 4.55 ± 0.07 d | 22.66 ± 3.65 e | 72.22 ± 6.80 c |
| 0.2 | 69.25 ± 3.72 b | 2.61 ± 0.03 d | 4.63 ± 0.08 d | 27.78 ± 2.72 cde | 74.58 ± 8.72 cb |
| 0.5 | 59.65 ± 2.96 c | 2.98 ± 0.15 c | 4.87 ± 0.07 bc | 28.89 ± 3.44 bcd | 78.50 ± 5.48 abc |
| 1.0 | 46.87 ± 2.41 d | 3.31 ± 0.12 bc | 5.00 ± 0.04 b | 36.67 ± 3.65 a | 83.33 ± 3.65 a |
| 3.0 | 39.71 ± 3.07 e | 4.05 ± 0.17 a | 5.84 ± 0.19 a | 33.33 ± 4.22 ab | 81.39 ± 5.00 ab |
| 5.0 | 31.48 ± 3.79 g | 3.86 ± 0.15 a | 4.74 ± 0.03 cd | 31.11 ± 3.44 bc | 78.33 ± 2.58 abc |
| 10.0 | 20.44 ± 2.49 h | 3.35 ± 0.29 b | 4.64 ± 0.07 d | 25.33 ± 2.98 de | 72.36 ± 6.46 c |
| Amino Acid | Peptide Sequence | Modification Types | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CAM | OX | DI | DHA | TRI | HNE | KYN | 3-HK | GGS | CAE | ACE | AAS | AAA | ||
| Cysteine | CFDGSLFEYCAK | √ | ||||||||||||
| Cysteine | GKLNFGRALECFFLSSCSSPCFCMNSMESQDEFETR | √ | ||||||||||||
| Cysteine | CFDGSLFEYCAK | √ | ||||||||||||
| Cysteine | GLHGAAIPGCPETFQSESSSQFR | √ | ||||||||||||
| Cysteine | MCCWNAPPCGFFYLNVDGAIFFYIHKAGVGAVVR | √ | ||||||||||||
| Methionine | CQDEMR | √ | ||||||||||||
| Methionine | QCCQQLSQMDEQCQCEGLR | √ | ||||||||||||
| Proline | CTNNAEKIPPGTVR | √ | ||||||||||||
| Proline | CGDQVGCPCIGFDGLDVASECGPSCECGLECGNRSTQR | √ | ||||||||||||
| Phenylalanine | FRPGTVALR | √ | ||||||||||||
| Phenylalanine | NFYLAGNPDDEFRPQGQQEYEQHRR | √ | ||||||||||||
| Phenylalanine | FMFWFACGVGGLEIICIFLVWCLLSR | √ | ||||||||||||
| Histidine | HNLDTQTESDVFSR | √ | ||||||||||||
| Histidine | ILRPVSVPGHFEAFHGSGGEDPESFYR | √ | ||||||||||||
| Histidine | ILRPVSVPGHFEAFHGSGGEDPESFYR | √ | ||||||||||||
| Histidine | QLHVILK | √ | ||||||||||||
| Lysine | KEDIEMALTK | √ | ||||||||||||
| Lysine | RVNALENVVKPR | √ | ||||||||||||
| Lysine | KGSLGCIKYILK | √ | ||||||||||||
| Lysine | VGESPSPATASSKPEQAR | √ | ||||||||||||
| Lysine | EAAYNLHLIYK | √ | ||||||||||||
| Tyrosine | CDGCVRSIFPPFYTCAQCGFFLHKSCVELSR | √ | ||||||||||||
| Tyrosine | CLKLYCECFAAGIYCVGSCACETCFNKPEYEDLVLDTR | √ | ||||||||||||
| Tyrosine | ACGTCCARCDCVPPGTSGNYDACPCYANMTTHGGR | √ | ||||||||||||
| Tyrosine | VVQCTEGERSYHIFYQLCAGAPAALR | √ | ||||||||||||
| Tryptophan | FVSVCYHNYEHVYCFWHRGMICVCCHTWPEILYYK | √ | ||||||||||||
| Tryptophan | FLIGDDEHCWSENGVSNIEGGCYAK | √ | ||||||||||||
| Tryptophan | FMLWFACGVGGFEIICIFLVWCLLSR | √ | ||||||||||||
| Tryptophan | ANRMGWFQLQR | √ | ||||||||||||
| Tryptophan | GALYSDALYVPHWNLNAHSVVYALRGR | √ | ||||||||||||
| Arginine | RATGEGFEWVSFK | √ | ||||||||||||
| Arginine | RQEEAEWEEEEAR | √ | ||||||||||||
| Proline | ESFNLECGDVIRVPAGATVYVINQDSNERLEMVK | √ | ||||||||||||
| Proline | ESFNLECGDVIRVPAGATVYVINQDSNERLEMVK | □ | √ | □ | □ | □ | □ | □ | □ | □ | □ | □ | □ | □ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Yang, X.; Li, Y.; Wang, Z.; He, X.; Sun, J. Effect of Peroxyl Radical-Induced Oxidation on Functional and Structural Characteristics of Walnut Protein Isolates Revealed by High-Resolution Mass Spectrometry. Foods 2022, 11, 385. https://doi.org/10.3390/foods11030385
Zhang X, Yang X, Li Y, Wang Z, He X, Sun J. Effect of Peroxyl Radical-Induced Oxidation on Functional and Structural Characteristics of Walnut Protein Isolates Revealed by High-Resolution Mass Spectrometry. Foods. 2022; 11(3):385. https://doi.org/10.3390/foods11030385
Chicago/Turabian StyleZhang, Xuechun, Xi Yang, Yunqian Li, Zhenxing Wang, Xuemei He, and Jian Sun. 2022. "Effect of Peroxyl Radical-Induced Oxidation on Functional and Structural Characteristics of Walnut Protein Isolates Revealed by High-Resolution Mass Spectrometry" Foods 11, no. 3: 385. https://doi.org/10.3390/foods11030385
APA StyleZhang, X., Yang, X., Li, Y., Wang, Z., He, X., & Sun, J. (2022). Effect of Peroxyl Radical-Induced Oxidation on Functional and Structural Characteristics of Walnut Protein Isolates Revealed by High-Resolution Mass Spectrometry. Foods, 11(3), 385. https://doi.org/10.3390/foods11030385







