Oyster (Crassostrea gigas) Extract Attenuates Dextran Sulfate Sodium-Induced Acute Experimental Colitis by Improving Gut Microbiota and Short-Chain Fatty Acids Compositions in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animal Experimental Approval
2.3. Animal Experiment
2.4. Serum Biochemical Parameters
2.5. Histopathological Analysis
2.6. Fecal SCFA Compositions
2.7. 16S rRNA Amplicon Sequence and Bioinformatics
2.8. Statistical Analysis
3. Results
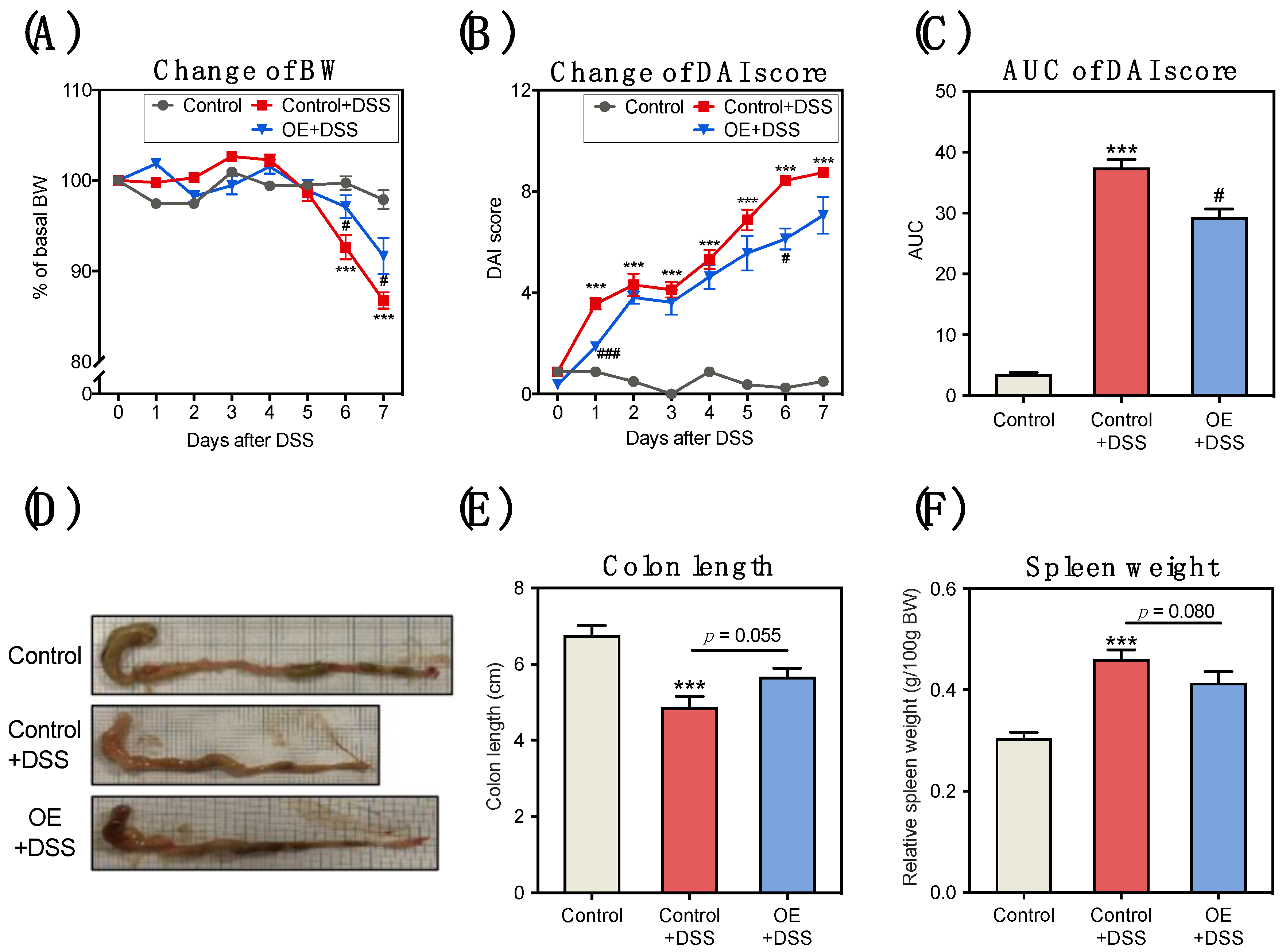
3.1. OE Intake Alleviates DSS-Induced Acute Experimental Colitis Symptoms
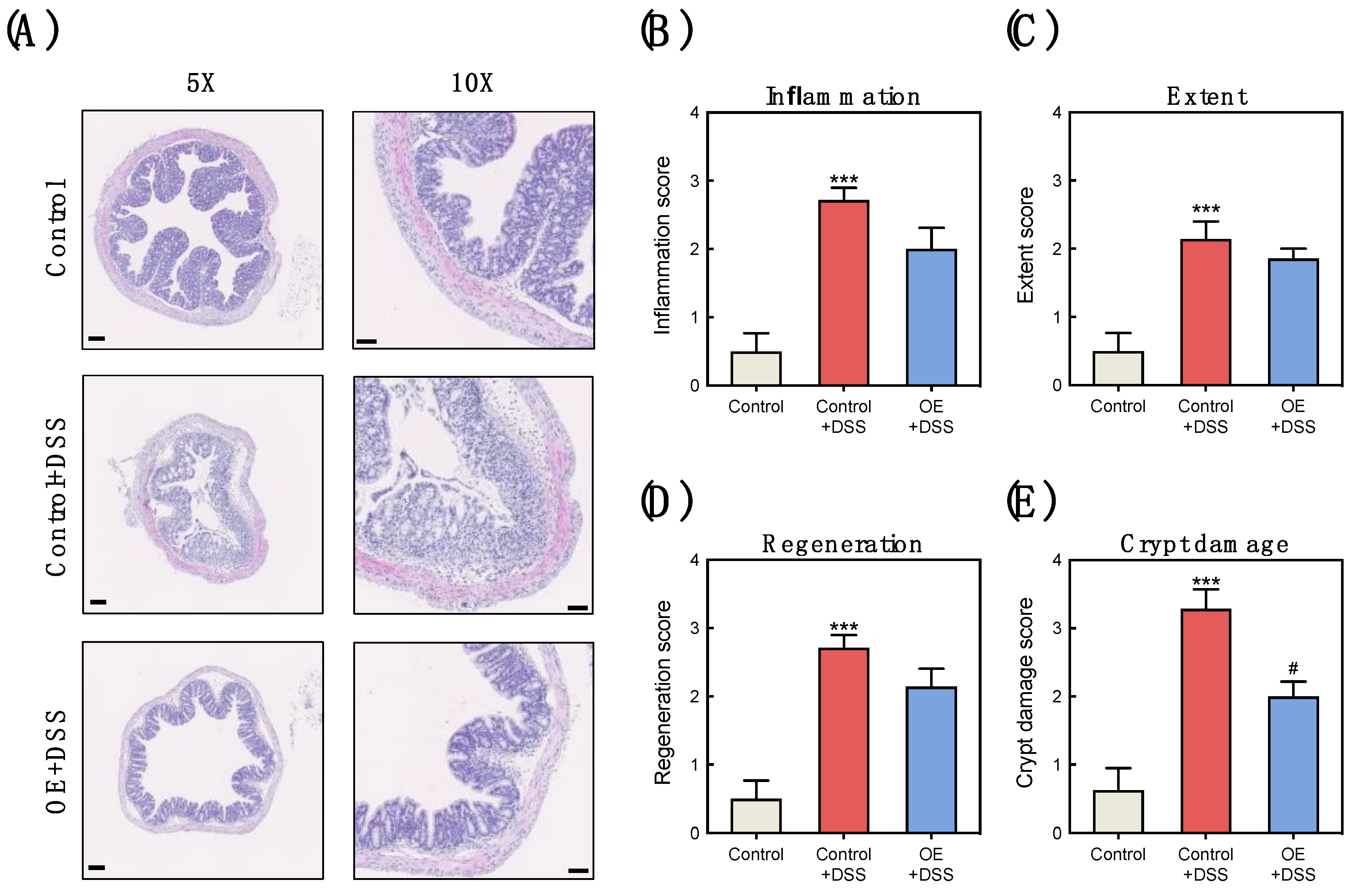
3.2. OE Intake Alleviates the Histological Damage in the Colon of DSS-Induced Acute Experimental Colitis Mice
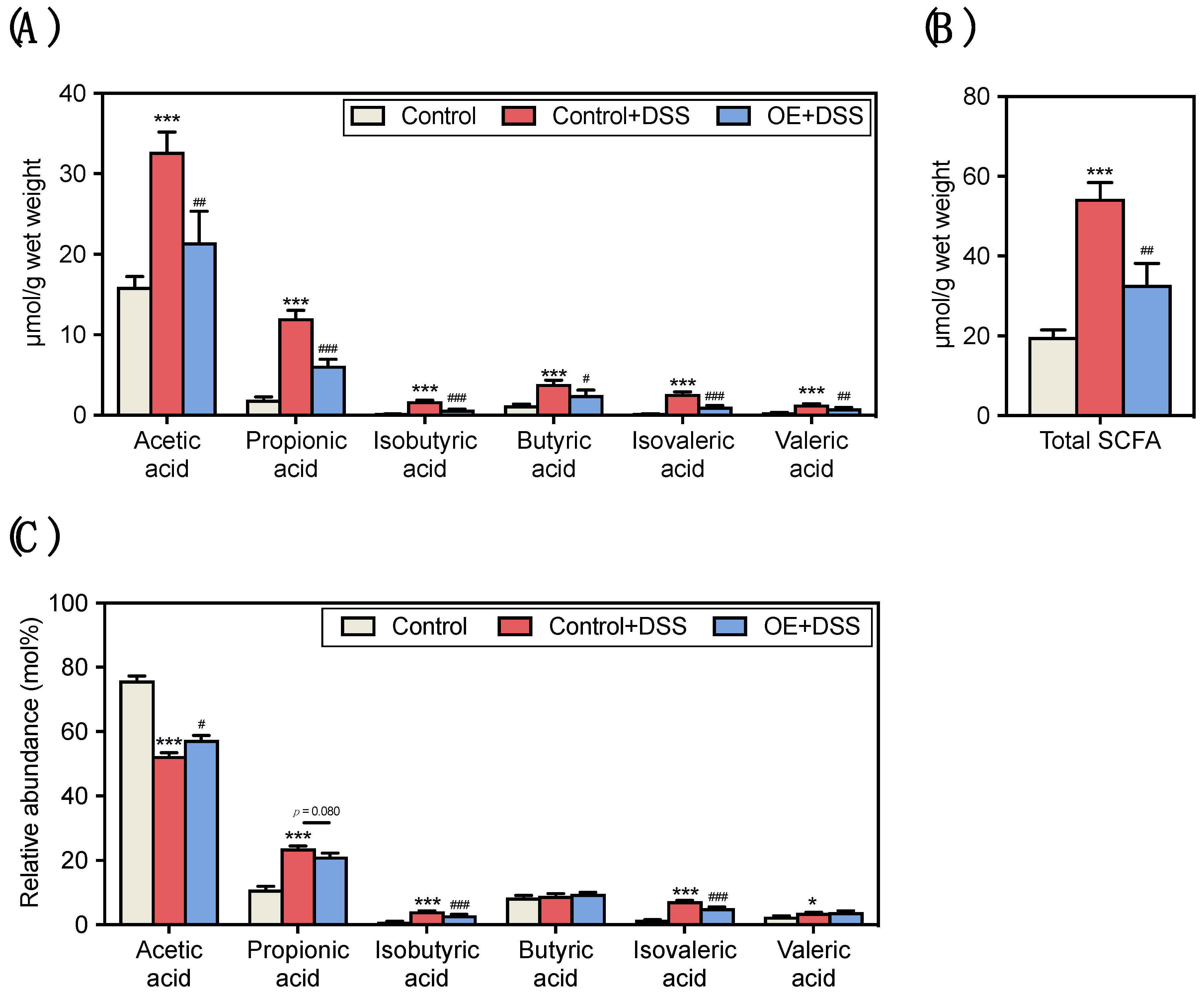
3.3. Effect of OE on SCFA Contents and Compositions in the Fecal of DSS-Induced Acute Experimental Colitis Mice
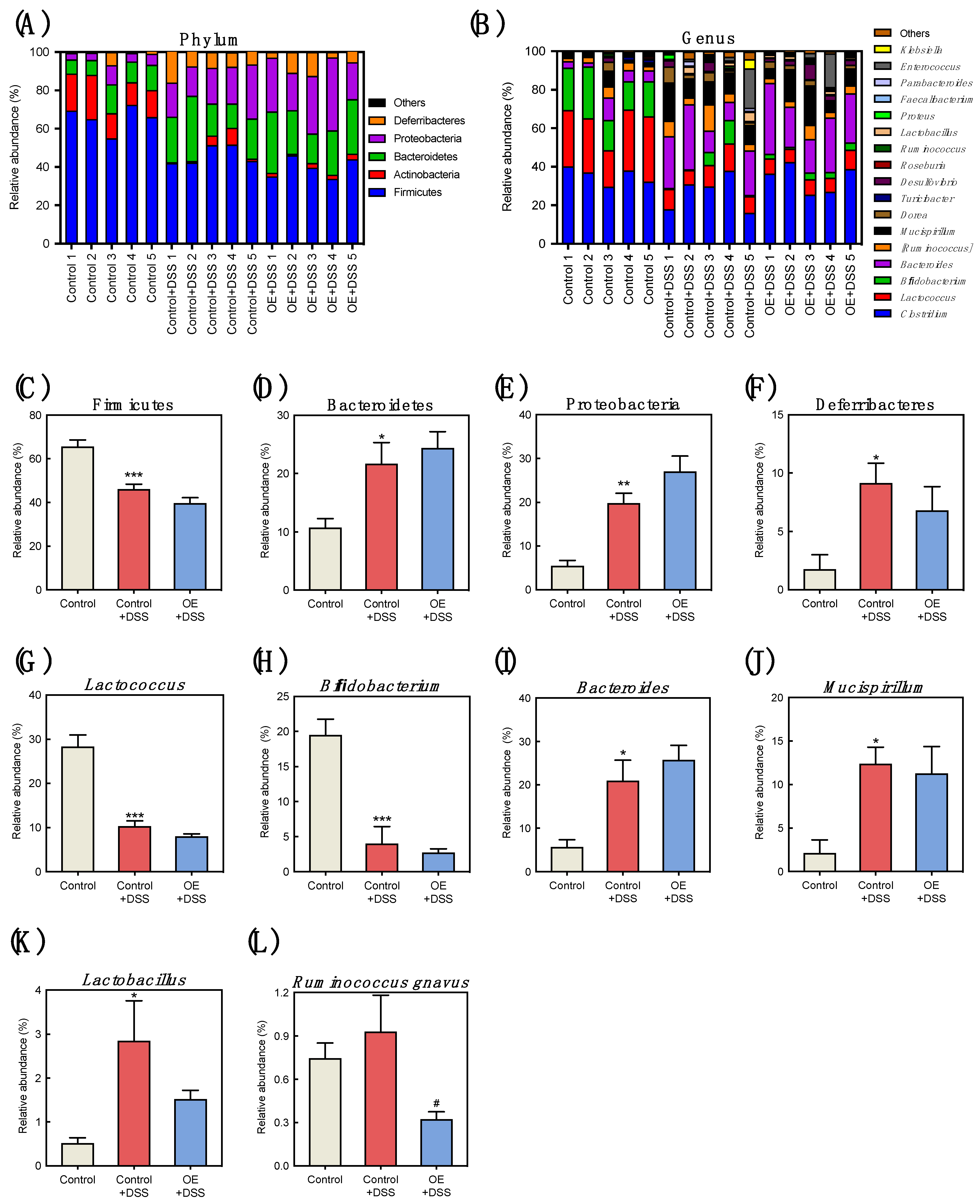
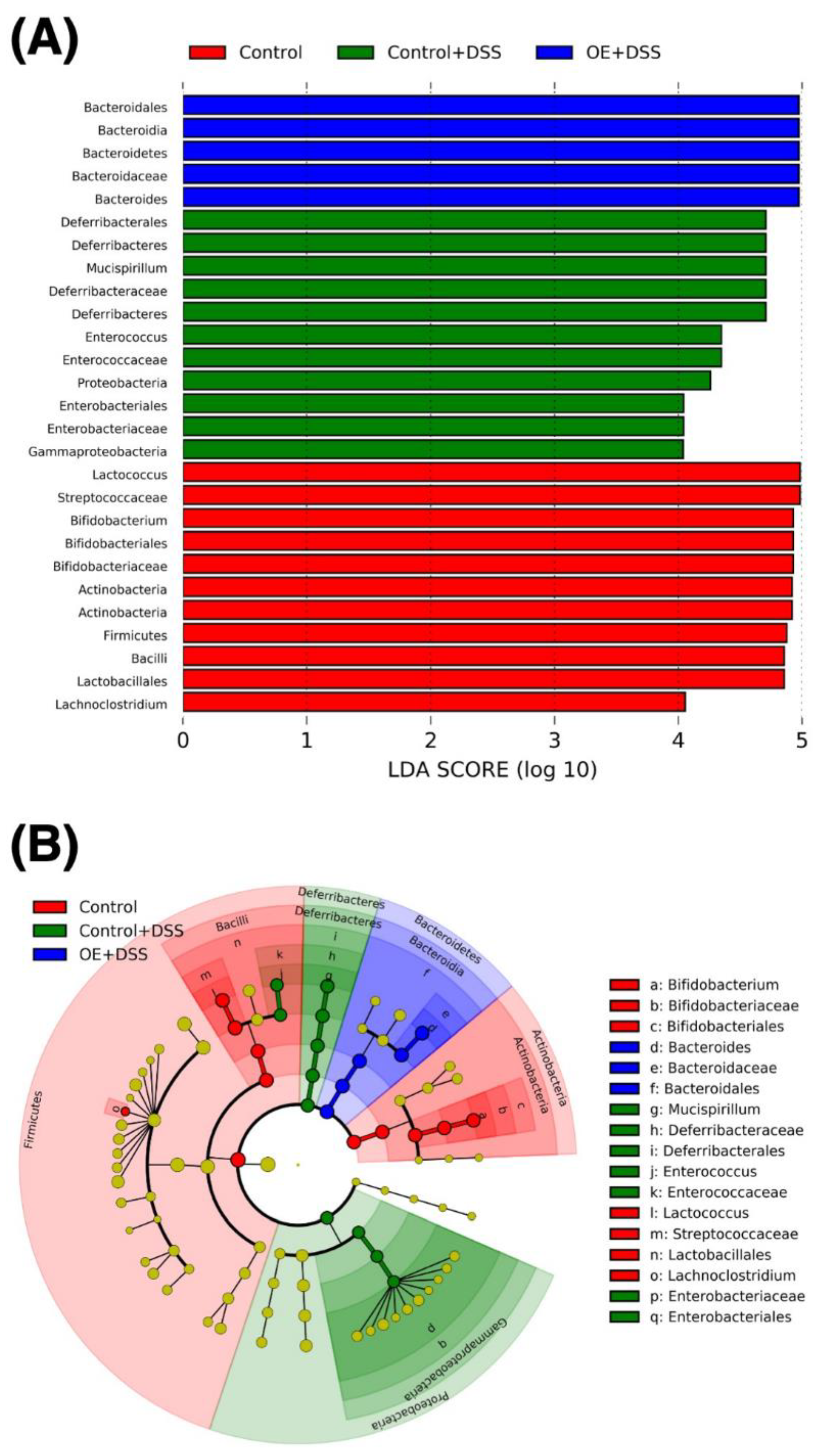
3.4. Effect of OE on Microbiota in the Feces of DSS-Induced Acute Experimental Colitis Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de Lange, K.M.; Barrett, J.C. Understanding inflammatory bowel disease via immunogenetics. J. Autoimmun. 2015, 64, 91–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dahlhamer, J.M.; Zammitti, E.P.; Ward, B.W.; Wheaton, A.G.; Croft, J.B. Prevalence of Inflammatory Bowel Disease Among Adults Aged ≥18 Years—United States, 2015. MMWR Morb. Mortal Wkly Rep. 2016, 65, 1166–1169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molodecky, N.A.; Soon, I.S.; Rabi, D.M.; Ghali, W.A.; Ferris, M.; Chernoff, G.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Barkema, H.W.; et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 2012, 142, 46–54.e42; quiz e30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, M.; He, J.; Shen, Y.; Zhang, C.; Wang, J.; Chen, Y. New Frontiers in Genetics, Gut Microbiota, and Immunity: A Rosetta Stone for the Pathogenesis of Inflammatory Bowel Disease. Biomed. Res. Int. 2017, 2017, 8201672. [Google Scholar] [CrossRef]
- Sandborn, W.J. Current directions in IBD therapy: What goals are feasible with biological modifiers? Gastroenterology 2008, 135, 1442–1447. [Google Scholar] [CrossRef]
- Harris, K.G.; Chang, E.B. The intestinal microbiota in the pathogenesis of inflammatory bowel diseases: New insights into complex disease. Clin. Sci. 2018, 132, 2013–2028. [Google Scholar] [CrossRef]
- Triantafyllidi, A.; Xanthos, T.; Papalois, A.; Triantafillidis, J.K. Herbal and plant therapy in patients with inflammatory bowel disease. Ann. Gastroenterol. 2015, 28, 210–220. [Google Scholar]
- Xu, Z.; Tang, H.; Huang, F.; Qiao, Z.; Wang, X.; Yang, C.; Deng, Q. Algal Oil Rich in n-3 PUFA Alleviates DSS-Induced Colitis via Regulation of Gut Microbiota and Restoration of Intestinal Barrier. Front. Microbiol. 2020, 11, 615404. [Google Scholar] [CrossRef]
- Hwang, S.; Hwang, S.; Jo, M.; Lee, C.G.; Rhee, K.J. Oral administration of Korean propolis extract ameliorates DSS-induced colitis in BALB/c mice. Int. J. Med. Sci. 2020, 17, 1984–1991. [Google Scholar] [CrossRef]
- Xiang, X.W.; Zhou, X.L.; Wang, R.; Shu, C.H.; Zhou, Y.F.; Ying, X.G.; Zheng, B. Protective Effect of Tuna Bioactive Peptide on Dextran Sulfate Sodium-Induced Colitis in Mice. Mar. Drugs 2021, 19, 127. [Google Scholar] [CrossRef]
- Wen, C.; Guo, Q.; Wang, W.; Duan, Y.; Zhang, L.; Li, J.; He, S.; Chen, W.; Li, F. Taurine Alleviates Intestinal Injury by Mediating Tight Junction Barriers in Diquat-Challenged Piglet Models. Front. Physiol. 2020, 11, 449. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Gao, X.; Nie, L.; Xie, J.; Dai, T.; Shi, C.; Tao, L.; Wang, Y.; Tian, Y.; Sheng, J. Astragalin Attenuates Dextran Sulfate Sodium (DSS)-Induced Acute Experimental Colitis by Alleviating Gut Microbiota Dysbiosis and Inhibiting NF-κB Activation in Mice. Front. Immunol. 2020, 11, 2058. [Google Scholar] [CrossRef] [PubMed]
- Tapiero, H.; Tew, K.D. Increased glutathione expression in cells induced by Crassostera gigas extract (JCOE). Biomed. Pharm. 1996, 50, 149–153. [Google Scholar] [CrossRef]
- Yoshikawa, T.; Naito, Y.; Masui, Y.; Fujii, T.; Boku, Y.; Yoshida, N.; Kondo, M. 30—Antioxidant Properties of Crassostera gigas Oyster Extract. In Antioxidant Food Supplements in Human Health; Packer, L., Hiramatsu, M., Yoshikawa, T., Eds.; Academic Press: San Diego, CA, USA, 1999; pp. 461–469. [Google Scholar] [CrossRef]
- Yoshikawa, T.; Naito, Y.; Masui, K.; Fujii, T.; Boku, Y.; Nakagawa, S.; Yoshida, N.; Kondo, M. Free radical-scavenging activity of Crassostera gigas extract (JCOE). Biomed. Pharm. 1997, 51, 328–332. [Google Scholar] [CrossRef]
- Matsuda, Y.; Yoshida, M. Examination of the Anti-tumor Action of Oyster Extract for P388 Leukemia. J. Jpn. Health Med. Assoc. 2004, 13, 3–10. [Google Scholar] [CrossRef]
- Matsuda, Y.; Izuta, S.; Fujita, T.; Murata, M.; Doi, E.; Ishizu, H.; Ohta, T.; Nakatsuka, M.; Tsubouti, R.; Shibata, Y. Influence of oyster extract on the platelet aggregation, the nucleotides and minerals of blood in diabetes mellitus patients. Trace Nutr. Res. 1992, 9, 67–73. [Google Scholar]
- Hosomi, R.; Matsuda, Y.; Ishimaru, A.; Takemura, S.; Fukunaga, K.; Yoshida, M. Effect of Dietary Oyster Extract on the Colonic Aberrant Crypt Foci in Mice. Trace Nutr. Res. 2007, 24, 71–75. [Google Scholar]
- Hosomi, R.; Matsuda, Y.; Watanabe, Y.; Fukunaga, K.; Yoshida, M. Effect of Dietary Oyster Extract on the p-aminophenol-induced Nephrotoxicity in Rats. Trace Nutr. Res. 2008, 25, 91–95. [Google Scholar]
- Ikeda, Y.; Hosomi, R.; Matsuda, Y.; Arai, H.; Shimono, T.; Kanda, S.; Nishiyama, T.; Yoshida, M.; Fukunaga, K. Effects of Dietary Oyster Extract on the Gut Microbiome in Rats. Trace Nutr. Res. 2018, 35, 41–46. [Google Scholar]
- Nakamura, J.; Ikeda, Y.; Hosomi, R.; Ishida, T.; Matsui, H.; Matsuda, Y.; Shimono, T.; Kanda, S.; Nishiyama, T.; Yoshida, M.; et al. Effects of Dietary Oyster-Extract on the Composition of the Microbiota and Short-Chain Fatty Acids in the Feces of Mice. Trace Nutr. Res. 2019, 36, 21–28. [Google Scholar]
- Kau, A.L.; Ahern, P.P.; Griffin, N.W.; Goodman, A.L.; Gordon, J.I. Human nutrition, the gut microbiome and the immune system. Nature 2011, 474, 327–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waldschmitt, N.; Metwaly, A.; Fischer, S.; Haller, D. Microbial Signatures as a Predictive Tool in IBD-Pearls and Pitfalls. Inflamm. Bowel. Dis. 2018, 24, 1123–1132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khor, B.; Gardet, A.; Xavier, R.J. Genetics and pathogenesis of inflammatory bowel disease. Nature 2011, 474, 307–317. [Google Scholar] [CrossRef] [Green Version]
- O’Hara, A.M.; Shanahan, F. The gut flora as a forgotten organ. EMBO Rep. 2006, 7, 688–693. [Google Scholar] [CrossRef] [Green Version]
- Ni, J.; Wu, G.D.; Albenberg, L.; Tomov, V.T. Gut microbiota and IBD: Causation or correlation? Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 573–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C., Jr. AIN-93 purified diets for laboratory rodents: Final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [CrossRef] [PubMed]
- Ito, R.; Shin-Ya, M.; Kishida, T.; Urano, A.; Takada, R.; Sakagami, J.; Imanishi, J.; Kita, M.; Ueda, Y.; Iwakura, Y.; et al. Interferon-gamma is causatively involved in experimental inflammatory bowel disease in mice. Clin. Exp. Immunol. 2006, 146, 330–338. [Google Scholar] [CrossRef]
- Dieleman, L.A.; Palmen, M.J.; Akol, H.; Bloemena, E.; Peña, A.S.; Meuwissen, S.G.; Van Rees, E.P. Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin. Exp. Immunol. 1998, 114, 385–391. [Google Scholar] [CrossRef]
- Hosomi, R.; Matsudo, A.; Sugimoto, K.; Shimono, T.; Kanda, S.; Nishiyama, T.; Yoshida, M.; Fukunaga, K. Dietary Fat Influences the Expression of Genes Related to Sterol Metabolism and the Composition of Cecal Microbiota and Its Metabolites in Rats. J. Oleo. Sci. 2019, 68, 1133–1147. [Google Scholar] [CrossRef] [Green Version]
- Maeda, H.; Hosomi, R.; Yokoyama, T.; Ikeda, Y.; Nishimoto, A.; Tanaka, G.; Shimono, T.; Kanda, S.; Nishiyama, T.; Yoshida, M.; et al. Dietary Alaska pollock protein attenuates liver steatosis and alters gut microbiota in leptin-deficient ob/ob mice. J. Funct. Foods 2020, 75, 104266. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Axelsson, L.G.; Landström, E.; Bylund-Fellenius, A.C. Experimental colitis induced by dextran sulphate sodium in mice: Beneficial effects of sulphasalazine and olsalazine. Aliment. Pharmacol. Ther. 1998, 12, 925–934. [Google Scholar] [CrossRef] [PubMed]
- Lavelle, A.; Sokol, H. Gut microbiota-derived metabolites as key actors in inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 223–237. [Google Scholar] [CrossRef]
- Kim, B.R.; Shin, J.; Guevarra, R.; Lee, J.H.; Kim, D.W.; Seol, K.H.; Lee, J.H.; Kim, H.B.; Isaacson, R. Deciphering Diversity Indices for a Better Understanding of Microbial Communities. J. Microbiol. Biotechnol. 2017, 27, 2089–2093. [Google Scholar] [CrossRef] [Green Version]
- Matsudo, A.; Nishimura, N.; Hosomi, R.; Harumatsu, S.; Fukuda, S.; Matsuda, Y.; Yoshida, M.; Fukunaga, K. Dietary Oyster-Extract Decreases Cholesterol Content by Suppressing hepatic HMG-CoA Reductase mRNA Expression Level and Enhancing Fecal Steroids Excretions in Rats Fed High-Fat Diet. Trace Nutr. Res. 2016, 33, 43–48. [Google Scholar]
- Jarnum, S.; Jensen, K.B. Fecal radioiodide excretion following intravenous injection of 131-I-albumin and 125-I-immunoglobulin G in chronic inflammatory bowel disease. An aid to topographic diagnosis. Gastroenterology 1975, 68, 1433–1444. [Google Scholar] [CrossRef]
- Saul, D.; Kosinsky, R.L. Dextran Sodium Sulfate-induced Colitis as a Model for Sarcopenia in Mice. Inflamm. Bowel. Dis. 2020, 26, 56–65. [Google Scholar] [CrossRef]
- Okayasu, I.; Hatakeyama, S.; Yamada, M.; Ohkusa, T.; Inagaki, Y.; Nakaya, R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology 1990, 98, 694–702. [Google Scholar] [CrossRef]
- Randhawa, P.K.; Singh, K.; Singh, N.; Jaggi, A.S. A review on chemical-induced inflammatory bowel disease models in rodents. Korean J. Physiol. Pharmacol. 2014, 18, 279–288. [Google Scholar] [CrossRef] [Green Version]
- Arpaia, N.; Campbell, C.; Fan, X.; Dikiy, S.; van der Veeken, J.; deRoos, P.; Liu, H.; Cross, J.R.; Pfeffer, K.; Coffer, P.J.; et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 2013, 504, 451–455. [Google Scholar] [CrossRef]
- Vital, M.; Karch, A.; Pieper, D.H. Colonic Butyrate-Producing Communities in Humans: An Overview Using Omics Data. mSystems 2017, 2, e00130-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelly, C.J.; Zheng, L.; Campbell, E.L.; Saeedi, B.; Scholz, C.C.; Bayless, A.J.; Wilson, K.E.; Glover, L.E.; Kominsky, D.J.; Magnuson, A.; et al. Crosstalk between Microbiota-Derived Short-Chain Fatty Acids and Intestinal Epithelial HIF Augments Tissue Barrier Function. Cell Host Microbe. 2015, 17, 662–671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huda-Faujan, N.; Abdulamir, A.S.; Fatimah, A.B.; Anas, O.M.; Shuhaimi, M.; Yazid, A.M.; Loong, Y.Y. The impact of the level of the intestinal short chain Fatty acids in inflammatory bowel disease patients versus healthy subjects. Open Biochem. J. 2010, 4, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Machiels, K.; Joossens, M.; Sabino, J.; De Preter, V.; Arijs, I.; Eeckhaut, V.; Ballet, V.; Claes, K.; Van Immerseel, F.; Verbeke, K.; et al. A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut 2014, 63, 1275–1283. [Google Scholar] [CrossRef]
- Roediger, W.E. The colonic epithelium in ulcerative colitis: An energy-deficiency disease? Lancet 1980, 2, 712–715. [Google Scholar] [CrossRef]
- Vernia, P.; Caprilli, R.; Latella, G.; Barbetti, F.; Magliocca, F.M.; Cittadini, M. Fecal lactate and ulcerative colitis. Gastroenterology 1988, 95, 1564–1568. [Google Scholar] [CrossRef]
- Karakoyun, B.; Ertaş, B.; Yüksel, M.; Akakın, D.; Çevik, Ö.; Şener, G. Ameliorative effects of riboflavin on acetic acid-induced colonic injury in rats. Clin. Exp. Pharmacol. Physiol. 2018, 45, 563–572. [Google Scholar] [CrossRef]
- Verbeke, K.A.; Boobis, A.R.; Chiodini, A.; Edwards, C.A.; Franck, A.; Kleerebezem, M.; Nauta, A.; Raes, J.; van Tol, E.A.F.; Tuohy, K.M. Towards microbial fermentation metabolites as markers for health benefits of prebiotics. Nutr. Res. Rev. 2015, 28, 42–66. [Google Scholar] [CrossRef] [Green Version]
- Fan, S.; Zhang, Z.; Zhong, Y.; Li, C.; Huang, X.; Geng, F.; Nie, S. Microbiota-related effects of prebiotic fibres in lipopolysaccharide-induced endotoxemic mice: Short chain fatty acid production and gut commensal translocation. Food Funct. 2021, 12, 7343–7357. [Google Scholar] [CrossRef]
- Thomas, A.M.; Manghi, P.; Asnicar, F.; Pasolli, E.; Armanini, F.; Zolfo, M.; Beghini, F.; Manara, S.; Karcher, N.; Pozzi, C.; et al. Metagenomic analysis of colorectal cancer datasets identifies cross-cohort microbial diagnostic signatures and a link with choline degradation. Nat. Med. 2019, 25, 667–678. [Google Scholar] [CrossRef] [Green Version]
- Macfarlane, G.T.; Gibson, G.R.; Beatty, E.; Cummings, J.H. Estimation of short-chain fatty acid production from protein by human intestinal bacteria based on branched-chain fatty acid measurements. FEMS Microbiol. Lett. 1992, 101, 81–88. [Google Scholar] [CrossRef]
- Shen, Z.-H.; Zhu, C.-X.; Quan, Y.-S.; Yang, Z.-Y.; Wu, S.; Luo, W.-W.; Tan, B.; Wang, X.-Y. Relationship between intestinal microbiota and ulcerative colitis: Mechanisms and clinical application of probiotics and fecal microbiota transplantation. World J. Gastroenterol. 2018, 24, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids from Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Margolles, A.; Sánchez, B. Selection of a Bifidobacterium animalis subsp. lactis strain with a decreased ability to produce acetic acid. Appl. Environ. Microbiol. 2012, 78, 3338–3342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Özcelik, S.; Kuley, E.; Özogul, F. Formation of lactic, acetic, succinic, propionic, formic and butyric acid by lactic acid bacteria. LWT 2016, 73, 536–542. [Google Scholar] [CrossRef]
- Crost, E.H.; Tailford, L.E.; Le Gall, G.; Fons, M.; Henrissat, B.; Juge, N. Utilisation of mucin glycans by the human gut symbiont Ruminococcus gnavus is strain-dependent. PLoS ONE 2013, 8, e76341. [Google Scholar] [CrossRef] [Green Version]
- Willing, B.P.; Dicksved, J.; Halfvarson, J.; Andersson, A.F.; Lucio, M.; Zheng, Z.; Järnerot, G.; Tysk, C.; Jansson, J.K.; Engstrand, L. A pyrosequencing study in twins shows that gastrointestinal microbial profiles vary with inflammatory bowel disease phenotypes. Gastroenterology 2010, 139, 1844–1854.e1. [Google Scholar] [CrossRef]
- Henke, M.T.; Kenny, D.J.; Cassilly, C.D.; Vlamakis, H.; Xavier, R.J.; Clardy, J. Ruminococcus gnavus, a member of the human gut microbiome associated with Crohn’s disease, produces an inflammatory polysaccharide. Proc. Natl. Acad. Sci. USA 2019, 116, 12672–12677. [Google Scholar] [CrossRef] [Green Version]
- Furuyashiki, T.; Takata, H.; Kojima, I.; Kuriki, T.; Fukuda, I.; Ashida, H. Metabolic fate of orally administered enzymatically synthesized glycogen in rats. Food Funct. 2011, 2, 183–189. [Google Scholar] [CrossRef]
- Shimizu, M.; Zhao, Z.; Ishimoto, Y.; Satsu, H. Dietary taurine attenuates dextran sulfate sodium (DSS)-induced experimental colitis in mice. Adv. Exp. Med. Biol. 2009, 643, 265–271. [Google Scholar] [CrossRef]

| Oyster Extract | |
|---|---|
| g/100 g | |
| Carbohydrate | 50.6 |
| Glycogen | 34.5 |
| Crude protein | 28.1 |
| Amino acid composition | |
| Alanine | 1.10 |
| Arginine | 0.52 |
| Aspartic acid 1 | 1.09 |
| Cystine | 0.14 |
| Glutamic acid 2 | 2.35 |
| Glycine | 1.24 |
| Histidine | 0.25 |
| Isoleucine | 0.25 |
| Leucine | 0.40 |
| Lysine | 0.51 |
| Methionine | 0.17 |
| Phenylalanine | 0.23 |
| Proline | 1.22 |
| Serine | 0.41 |
| Threonine | 0.45 |
| Tryptophan | 0.06 |
| Tyrosine | 0.19 |
| Valine | 0.33 |
| Taurine | 5.5 |
| Crude fat | 2.0 |
| Ash | 15.4 |
| Sodium chloride | 8.2 |
| Moisture | 3.9 |
| Experimental Diets 1 | ||
|---|---|---|
| Control | OE | |
| g/100 g | ||
| Dextrinized corn starch | 132 | 132 |
| Corn starch | 397.486 | 366.486 |
| Sucrose | 95.9 | 100 |
| Cellulose | 50 | 50 |
| Casein | 200 | 182.3 |
| L-Cystine | 3 | 3 |
| Choline bitartrate | 2.5 | 2.5 |
| Sodium chloride-free AIN-93G mineral mixture | 35 | 35 |
| AIN-93 vitamin mixture | 10 | 10 |
| Soybean oil | 70 | 68.7 |
| Oyster extract powder | - | 50 |
| Sodium chloride | 4.1 | - |
| tert-Butylhydroquinone | 0.014 | 0.014 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishida, T.; Matsui, H.; Matsuda, Y.; Hosomi, R.; Shimono, T.; Kanda, S.; Nishiyama, T.; Fukunaga, K.; Yoshida, M. Oyster (Crassostrea gigas) Extract Attenuates Dextran Sulfate Sodium-Induced Acute Experimental Colitis by Improving Gut Microbiota and Short-Chain Fatty Acids Compositions in Mice. Foods 2022, 11, 373. https://doi.org/10.3390/foods11030373
Ishida T, Matsui H, Matsuda Y, Hosomi R, Shimono T, Kanda S, Nishiyama T, Fukunaga K, Yoshida M. Oyster (Crassostrea gigas) Extract Attenuates Dextran Sulfate Sodium-Induced Acute Experimental Colitis by Improving Gut Microbiota and Short-Chain Fatty Acids Compositions in Mice. Foods. 2022; 11(3):373. https://doi.org/10.3390/foods11030373
Chicago/Turabian StyleIshida, Tatsuya, Hiroyuki Matsui, Yoshikazu Matsuda, Ryota Hosomi, Takaki Shimono, Seiji Kanda, Toshimasa Nishiyama, Kenji Fukunaga, and Munehiro Yoshida. 2022. "Oyster (Crassostrea gigas) Extract Attenuates Dextran Sulfate Sodium-Induced Acute Experimental Colitis by Improving Gut Microbiota and Short-Chain Fatty Acids Compositions in Mice" Foods 11, no. 3: 373. https://doi.org/10.3390/foods11030373
APA StyleIshida, T., Matsui, H., Matsuda, Y., Hosomi, R., Shimono, T., Kanda, S., Nishiyama, T., Fukunaga, K., & Yoshida, M. (2022). Oyster (Crassostrea gigas) Extract Attenuates Dextran Sulfate Sodium-Induced Acute Experimental Colitis by Improving Gut Microbiota and Short-Chain Fatty Acids Compositions in Mice. Foods, 11(3), 373. https://doi.org/10.3390/foods11030373






