1. Introduction
Game meat is a particularly appreciated product due to its high culinary [
1] and nutritional value (high protein and mineral content and low fat and cholesterol levels) [
2,
3,
4]. In developed countries, free game is perceived as an organic product [
5,
6] free of antibiotics and other pharmacological compounds [
7]. Sustainable hunting is seen as a way to maintain biodiversity and ecosystems [
8], being embraced even by the locavore movement [
9]. Rates of consumption are lower than those of domestic species, sometimes related to the difficulties in obtaining it [
10]. In Spain, meat from wild game species represents only 2% of the total meat intake [
11], and 59.6% of game meat is consumed by the hunters themselves or the hunters’ inner circle (relatives, friends, and neighbors) [
12].
Due to its origin and special processing circumstances, game meat faces its own challenges and may represent a higher sanitary risk in comparison with farm-origin meat. Wildlife does not have the sanitary status achieved by farm production; the animals are culled in the field [
13,
14] and, especially in domestic consumption, carcasses are often eviscerated and skinned under poor hygienic conditions [
14,
15,
16]. Some issues such as carcass contamination, promoted by bullet trajectory [
13,
17,
18], heavy metals from ammunition remains [
19,
20,
21,
22], or the time between death and skinning and dressing [
13,
23], are specific concerns in game meat use.
When animals are correctly shot and dressed, microbiological scores of fresh carcasses may be low, similar to those from domestic species slaughtered in controlled conditions [
6,
13,
17,
24,
25]. Notwithstanding this, levels of biotic contamination can be highly variable due to the shifting conditions in which wild game is killed and processed in the field and their carcasses transported, handled, chilled, and stored [
13,
14,
16,
17,
26]. External factors, such as warm/cold seasons, contribute to microbiological growth as well, being difficult to mitigate [
27].
Zoonotic viruses, bacteria, and parasites are commonly found in game carcasses. Health hazards will vary depending on the animal species, the degree of fecal contamination, the time elapsed from death to butchering, and the conditions of dressing and cooling [
28,
29,
30,
31,
32,
33,
34,
35].
Opinion polls are a common tool to obtain information from stakeholders about many topics. Fish and wildlife management professionals use public opinion and attitude surveys to facilitate an understanding of their constituents [
36]. Those surveys have been used to consult attitudes towards hunting and fishing [
37,
38,
39], game meat consumption [
38,
40], or the opinion about governmental wildlife agencies [
41]. Public health authorities also use this methodology to obtain information about the food hygiene practices of their citizens [
42,
43].
We used opinion polls to assess the hygienic–sanitary aspects of game meat processing and self-consumption in the Valencian community, Spain, as a first step to obtain a human and environmental health risk assessment.
3. Results
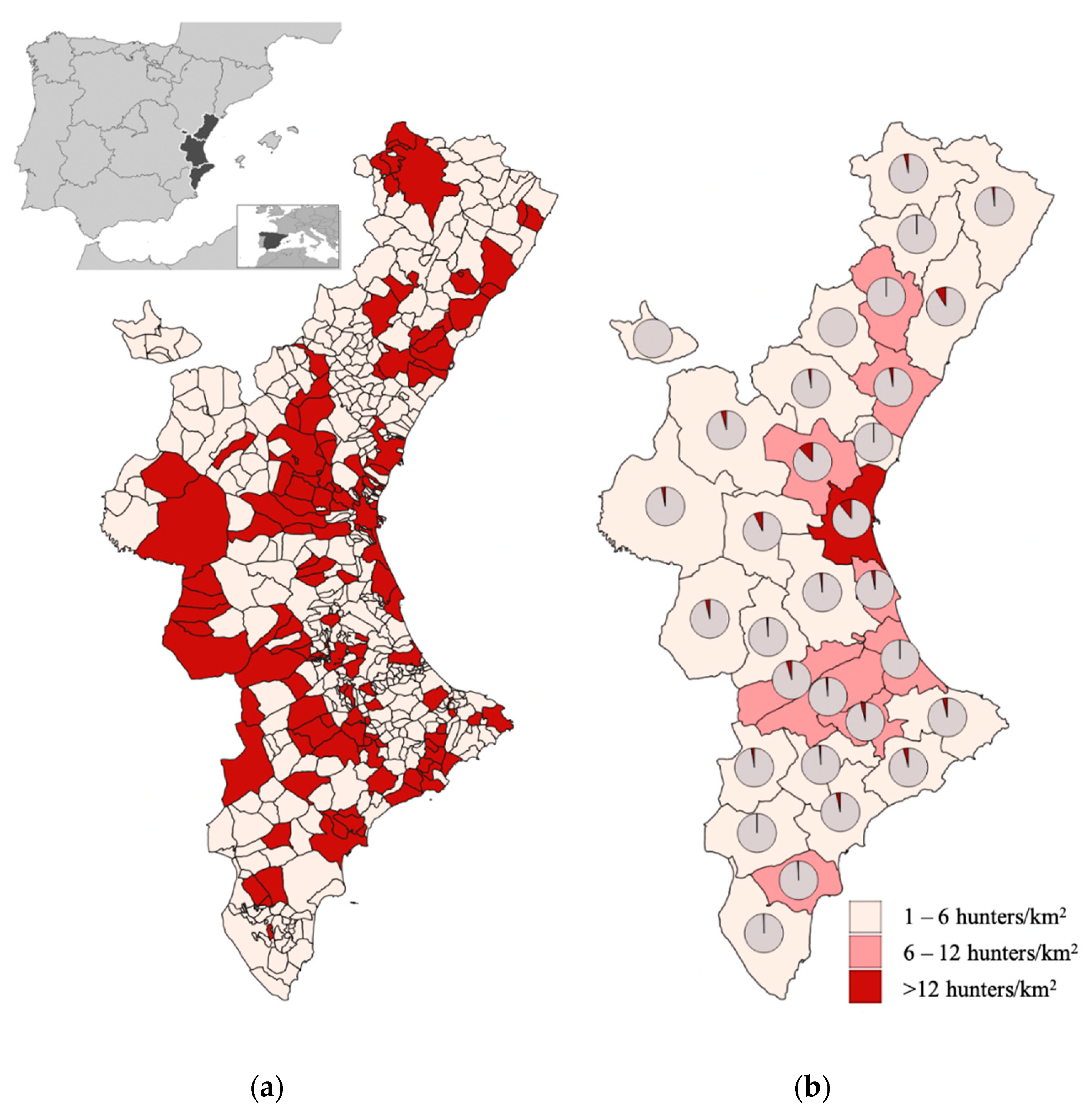
The survey period began in January 2018 and ended in February 2020. A total of 516 questionnaires were filled out, but 44 (8.52%) were rejected due to missing answers or because the respondents lived out of the surveyed area. The final amount of 472 valid interviews was considered enough for the targeted population. Questionnaires from 128 municipalities were held, representing 23.8% of the total number of municipalities into which the territory is divided (
Figure 1).
The maximum possible score, corresponding with optimal hygienic behavior, is 65 points. The average score achieved by the surveyed hunters was 29 points (highest 52, lowest 1) with a deviation of 8 points (median and interquartile range, because data distribution, studied through the Kolmogorov–Smirnov test, is not normal, p = 0.030). An overwhelming majority of participants in our survey were men (95.1%), but women achieved the best scores (p = 0.003). Among ages, 18.8% of the participants were between 18–30, 44.4% 31–50, 27.5% 51–65, and 9.3% >65 years old. Age-related differences in the hygiene score were marginally significant (p = 0.053). However, when comparing the results from >65 y.o. hunters with those from the rest of the interviews, the result was p = 0.007. Referring to educational background, 6.1% had no studies, 37.8% had primary education, 39.3% had secondary–professional education, and 16.6% had university degrees. The total score value increased significantly (p = 0.046) as the educational levels got higher.
The largest consumed big game species in our survey was wild boar (Sus scrofa) (22.5%). Among small game, wild rabbit (Oryctolagus cuniculus) (24.4%) and red-legged partridge (Alectoris rufa) (20.7%) are the most popular ones. The preferred hunting modalities (90.6%) involve hunting dogs or other assistant animals (ferrets or raptors). In total, 92.1% of the collaborators consume the hunted prey; 40% of them allocate game meat to their own families, 9.4% give the meat to relatives, friends, or neighbors, and 48.4% to both previous assumptions. Only 2.1% of the surveyed hunters declared selling the meat. Most hunters (59.3%) cooked it for consumption. Preparations such as raw sausages or salt-cured meat are less common (16% and 10.1%, respectively). Pickled preparation (13.7%) is also common, especially among small game hunters, while other options are scarce (smoked (0.4%), pâté (0.1%), or raw (0.3%)). Among the 7.9% who do not consume their game meat, the main situation was the lack of time for meat dressing (69.5%), rejection (19.5%), lack of culinary interest (8.7%), or health problems (2.17% with uric acid rising).
Sanitary behaviors such as veterinary inspection or freezing were irregular among the participants. Only 22.4% always resort to a veterinarian, while 11.9% do it “usually” and 13.4% “not normally”. Most results (34%) are for those who approach a veterinarian only for Trichinella diagnosis after hunting wild boar, while 18.2% (essentially small game hunters) admitted to never consulting a veterinarian. The need to freeze game meat before consumption is felt as mandatory for some hunters; 40.6% declared freezing it “always” while 48.2% do it “usually”. A lower 7.6% admitted putting the meat in the freezer “occasionally” and only 3.5% “never” do it. Those hunters who declared freezing game meat were additionally asked about the period they let the meat freeze. Of these, 17.3% freeze the meat for less than 1 month, 39.1% between 1 to 2 months, 34.5% between 2 to 6 months, and 9.1% more than 6 months.
Focusing on cross-contaminations, several questions were asked about the possible weaknesses in meat processing, personal hygiene, cleaning protocols, or suitability of dressing facilities. About hunted-animal transport, most respondents (74.4%) declared carrying the animals in their personal vehicles; 13.1% prefer to move them in the back of an open pickup or open trailer; 8.5% transported the carcasses in the back of a pickup with a canopy or an enclosed trailer. The best option from a food safety point of view, an exclusive vehicle or trailer with a refrigeration system, was available for only 3.6% of the surveyed hunters. Only 3.2% of the interviewed hunters had authorization to transport animal by-products (ABP) not intended for human consumption. In fact, except for those involved in farming, the meat industry, or animal health, this terminology was unknown. About evisceration, 19.7% of the interviewed hunters declared dressing the prey immediately in the field. For 28.7%, the time elapsed was less than 2 h; 2 to 4 h for 27.7%, 4 to 6 h in 13.5%, 6 to 8 h in 4.1%, and 5.1% exceed over 8 h; 81.8% of respondents owned their own dressing facilities, 7% performed the process in another person’s place, 7.8% in a collective property (hunting clubs), 1.5% were done in public places (commonly obsolete municipal slaughterhouses granted by local authorities), and 1.9% of the answers indicated dressing the animals directly at the killing site. Of the dressing sites, 60.8% were outdoors (killing site, yards, or patios), while 39.3% were indoor installations. Only 32.3% of the facilities were specifically dedicated for game dressing, while the rest had shared uses with other activities (yard 19.3%, storage 15.7%, garage 15.5%, workshop 1.7%) or even with activities, allowing the contamination of food and meals or human contagion (pantry 5.7%, kitchen 5.3%, meeting place 3.2%). Of the hunters, 91.6% had access to safe water in their dressing facilities, but only 54.8% (mainly those who dress the animals in their own homes) had hot water. Of the facilities, 5.9% had water not coming from supply systems but from irrigation and rainwater tanks. In 2.5% of the situations, the facilities had no access to water at all. Regarding carcass position when dressing and cutting tool utilization, 53.8% of hunters declared suspending the animals with a hook or a similar device, 36.4% eviscerated the animals on a table or bench, 4.9% displayed the bodies on the ground over plastic sheeting, 3.4% on a pavement floor, and 1.5% directly on a dirt floor; 68.1% of the participants declared using a specific set of knives for dressing, while the remaining 31.9% admitted using the same knives for other purposes. Some questions were made regarding the light sources in the dressing facilities. Of the responders, 22.4% depended completely on sunlight, while the rest had access to artificial sources. Incandescent bulbs were the most common devices (34.9%), followed by LED lights (14.8%) and halogen tubes (14.6%), while 9.5% used a combination of natural and artificial sources and a marginal 3.8% used portable manual devices (such as torches and headlamps). Given the potential for insects and rodents to contaminate food [
50], the interviewed hunters were asked about the protective measures available in their dressing facilities. Regarding insects, the most common devices were insect gauzes in windows (34.5%), adhesive traps (8.7%), light traps (8.5%), and insecticide sprays (4.4%). Even so, 43.8% of the responders did not use any device to control insects. Concerning rodents, spring traps were the preferred devices (14.1%), followed by poison pellets (12.7%), glue traps (5.6%), cage traps (5%), and, finally, cats (0.8%). Most of the hunters referred to not using pest control strategies (61.8%). About cleaning protocols, 35.3% of users informed hosing down their facilities after dressing, while 24.5% prefer to mop the floor. The combination of both (hosing down and mopping) was also popular (14.9%). The use of scouring pads (9.3%) was referred when cleaning sinks, tables, and other surfaces. Dry brushing was the option for only 1.5% of hunters. Combinations of several of the previous procedures were reported in 10.5% of the answers, and 3.9% did not clean the dressing site in any way. About disinfectant use, the most common ones were bleach (27.4%), soap (8.4%), and floor cleaners (4.8%). Other options were marginal (ammonia 0.2%, caustic soda 0.4%). Almost half of the interviewed subjects (47.9%) do not use any kind of substance but water. Regarding the clothes they used during the dressing. 63.7% use the same clothes as in the field and 15.5% use covering clothes (such as a lab coat or a coverall) over the usual wear; 16.4% changed all their clothes to use specific clothing and only 4.4% use specific footwear (rubber boots). To evaluate the risk of suffering cuts, stabs, spills, or inhalations, a question was made about the use of personal protective equipment (PPE). The most common items were disposable gloves (42.1%), while other equipment was used by less than 5% of participants (leather gloves 4.6%, steel mesh gloves 4%, facial mask 2%, face shield 1.8%). Some hunters (5.5%) used more than one item simultaneously, disposable gloves and steel mesh gloves being the most common combination (1.7%). Most answers (44.9%) confessed to not using protective equipment at all. About personal hygiene, they were asked about the possibility of washing their own hands during and after meat processing in the slaughter facility; 45.4% declared having access to running water, 8.9% had access to water, but from an uncertain origin (e.g., rainwater, water well), and 2.3% had no option to wash their hands. In addition, 36.9% used soap and 6.4% hand sanitizers or disinfectants.
Regarding waste management, the most usual way to dispose of the remains was municipal garbage containers (41.3%). In 33.2% of cases, the answers showed the abandonment of offal and other remains directly in the field (11.1% were buried, 11.5% were left in an open area, and 10.6% were hidden in a pit cave or gully). Of the interviewed hunters, 12.8% declared to use offal to feed domestic animals (dogs 12.1%, cats 0.34%, pigs 0.34%). In 7.9% of the answers, the organic remains were disposed of in carcass containers (associated with the farming industry); 4.4% of users declared leaving the offal in vulture feeding stations, and a marginal 0.34% use incineration to dispose of the remains. Wastewater was eliminated through collective water networks by most hunters (53.5%); 30.2% released the contaminated water into rivers, ravines, or crop fields, 7.8% did not know the destination, 7.6% had access to a specific organic residue water treatment plant, and 0.8% stored the wastewater in septic tanks.
4. Discussion
This survey offers a wide spectrum view towards private game meat self-consumption, a scarcely studied topic concerning both wildlife management and public health perspectives. Although some key aspects cannot be evaluated by using opinion polls, such as the efficiency of dressing, hygiene and cleaning protocols, the time span needed to reach refrigeration/freezing temperatures, or the microbiological status, it still constitutes a useful tool for studying the social aspects implied, and it allows us to detect deep-seated procedural errors, training needs, and deficiencies in the facilities.
Despite the openness of game activity towards women in recent decades, it continues to be a mainly masculine practice [
51,
52], and only 1.15% of hunters from our study area are women [
53]. This fact explains why only 4.9% of the surveyed people were huntresses. The marks achieved by women in our study were significantly higher than those of men. Several previous surveys have demonstrated a greater efficiency and dedication from women towards hygienic procedures [
43] and a bigger concern about potential food safety risks and safer practices than men [
42,
54,
55]. Our survey only detected marginally significant differences among age groups when compared altogether. However, in accordance with previous research [
56], which has found more food safety malpractices in older adults, we compared the score achieved by the >65 y.o. group with the rest of the participants, finding a significantly lower total score in the oldest group. Consistent with previous studies, the level of education is a relevant factor in the final achieved score. People with very little or no educational background have a low understanding of food safety issues [
57]. A higher level of formal education creates awareness in ensuring safer and more hygienic practices [
58,
59]. A higher awareness of potential risks is reached by people with university studies, with increased knowledge among those from health-related fields [
55,
60]. Experience can play an important role too, and it has been shown that training leads to better results in microbiologic contamination in game meat processing [
25].
In reference to hunted species, our results are consistent with the official game statistics, showing that the most consumed game is also the most abundant one. Wild rabbit is the most hunted and consumed small game species (430,931 individuals—24.4% answers), red-legged partridges for birds (89,049—20.7%), and wild boar for big game (26,832—22.5%) [
61]. In Spain, most authorized hunting modalities involve the utilization of assistant animals. Ferrets, raptors, and, especially, different dog breeds are used to help the hunters track, chase, and catch wild prey. Bite-borne microbiological contamination is a collateral effect of this utilization and a concern to food hygiene goals [
62]. Unlike other parts of the country, the Valencia region does not have a culinary tradition in preparing sausages or cured game meat. This cultural behavior works as a self-protective strategy against some pathogens (such as the hepatitis E virus, toxoplasmosis, or trichinellosis) that can be transmitted by raw and undercooked meat [
31,
63,
64,
65]. Specific concerns between wild boar meat consumption and
Trichinella spp. transmission were expressed by some hunters. Different strategies (or combinations of several procedures) are commonly carried out to reduce the risk of transmission, such as cooking the meat, freezing the carcasses for at least 2 months, and veterinary checking for
Trichinella larvae.
Geographic locations where the animals have been shot can be notably far from the dressing facilities. Thus, transport conditions can be challenging to assure meat safety. The safer option (i.e., an exclusive vehicle with refrigeration) was selected by only 3.6% of the surveyed hunters, thus evidencing the lack of proper transport systems, comprising a potential risk for meat contamination. Furthermore, the most popular option (i.e., personal vehicles) may represent a supplementary public health risk due to the difficulties of cleaning up fluid spills, the utilization of the vehicle for private purposes, and the contamination of personal items sharing the space. European regulations show some legal uncertainties regarding the scope of the rules on ABP from wild game. Regulation (EC) No 853/2004 admits that, if good hunting practices are observed, intestines and other body parts of healthy wild game hunted in their natural habitat may be disposed of on-site. To ensure the health status of the animal, a new figure of “trained hunter” was created, understood as a person with additional training, able to make an on-site evaluation of animal health before veterinary inspection [
66]. The Valencia Community Regional Order 3/2019 excluded this possibility, declaring graduates in veterinary medicine as the only professionals able to check the sanitary status of game animals [
67]. Regulation (EC) No 1069/2009 considers the wild animals when suspected of being infected with diseases transmissible to humans or animals as Category 1, while the carcasses and body parts of healthy game animals which are fit for human consumption but not intended for commercial sale are classified as Category 3 [
68]. All those regulations are focused on game meat commercialization, while self-consumption is not mentioned and could be considered a loophole and a threat to human health. The low rate (3.2%) of hunters with the authorization to transport ABP shows an extensive lack of knowledge about this regulation. The time elapsed between the death of the animal and the evisceration can be highly variable, depending on different factors such as the distance from the field to the dressing facilities or the hunting modality (it is longer in collective hunting, where participants are not allowed to leave their positions until the end of the hunt due to safety reasons [
69]); 22.7% of hunters declared exceeding the time span considered to be critical for limiting bacterial spread from unbroken guts [
70]. If fecal contamination is present because of abdominal shoots, the combination with high carcass temperatures for several hours presents a potential risk for human health. In fact, the contamination of game carcasses by feces and subsequent invasion by enteric pathogenic bacteria is rather common in game because, in general, animals are eviscerated and skinned under insufficient hygienic conditions [
15,
70,
71]. Butchering and dressing animals are potentially risky processes in which the handler comes into contact with organic material and body fluids, potentially carrying disease-producing microorganisms [
72] that can be transferred to surfaces and tools while handling meat [
73]. There is an evident need for training in hunter communities about personal hygiene, protective measures, and the use of specific protective gear during game meat processing. The lack of use of these protective supplies has been demonstrated as a risk factor to people involved in dead animal handling [
74,
75,
76]. Due to their high exposure to wild animals, hunters and butchers of wild game (among other collectives) are commonly exposed to animal pathogens, being the origin of contagion to other people. Public health monitoring programs looking after wildlife diseases should focus on those groups [
77,
78]. Lighting is a relevant factor for proper animal dressing in order to detect alterations that lead to discarding affected areas and performing the operations in a safe way. Nearly a quarter of the surveyed users (normally associated with outdoor facilities) depended totally on sunlight, which should be a matter of concern. Additionally, there is room for improvement in those facilities using incandescent bulbs, which should be properly enclosed to prevent glass contamination in meat in case of breakage [
79]. Taking as a reference the European standard UNE 12464-1:2011 about the lighting of workplaces, the indoor dressing facilities must reach a 500 LUX light level [
80], an unknown factor for self-consumers. There is room for improvement in dressing facilities because most of them are located outdoors; the facilities are then exposed to contamination by insects and dust [
48] and are also used for other activities, in close contact with food and people. Insects and rodents are the origin or vehicle of microorganisms that are able to contaminate food and processing sites, being able to transmit
Salmonella spp. [
81,
82],
Campylobacter spp. [
83],
Yersinia spp. [
84], and
Cryptosporidium parvum [
85], among others. For this reason, it is considered essential to implement pest control protocols, not only by access prevention or direct persecution but also by ensuring that control methods (traps, poison, cats) do not constitute a significant health hazard in themselves. Under proper maintenance and control, some measures are considered desirable, such as insect gauzes, insect light traps, or rat poison, while others should be discarded due to their implicit risk of becoming a source of contamination. Most common insecticide sprays used in domestic dressing facilities are not focused on the food industry, so the chemicals could remain on surfaces where raw food materials are displayed because of the absence of further studies about suspension periods and a lack of proper training in the users. This situation constitutes a potential source of toxic exposure through food intake [
86]. Adhesive traps could be useful for early insect presence detection [
87] but should not be used as a device to control pest populations. On a general basis, all animal access to dressing facilities must be avoided, thus impeding the entry of domestic cats and disqualifying felines as valid rodent controllers [
88]. The quality of cleaning water access and lack of heating devices should be matters of concern. Surface and tool cleaning with hot water is a common method to reduce the pathogenic bacteria related to meat processing [
89,
90]. However, of the 91.5% of respondents who declared having water in their dressing facilities, only 54.8% had hot water and this does not imply reaching temperatures high enough to reduce bacterial presence in an effective way. The common practice of cleaning the carcass during dressing increases some bacterial counts [
25]. Future actions should be taken to train the target population, aiming at domestic infrastructure improvement. Inadequate decontamination procedures applied to potentially contaminated utensils may present further risks of microbial contamination in subsequent meal preparations, thereby reducing the shelf life and product safety [
56,
91,
92]. Almost one-third of the surveyed hunters declared using the dressing knives in other activities. The position of the carcasses when dressing can create additional contamination of the derived meat. Most surveyed hunters (90.2%) displayed behaviors considered appropriate, hanging up the carcasses (53.8%) [
48] or putting them on a specific table or bench (36.4%). Animal dressing on the floor (on a plastic sheeting (4.9%) or pavement surface (3.4%)) can be considered minor options, with the worst-case scenario (evisceration on a dirt floor) [
15] as anecdotical (1.5%). Cleaning protocols are key aspects of hygienic meat processing. It is essential to analyze the procedures to detect weaknesses that may signify a cross-contamination risk from inadequately sanitized facilities, health hazards due to bacterial proliferation, or the apparition of persistent bacteria [
93]. Floor cleaning through hosing down or mopping may be seen as correct practices, but this can generate droplets and therefore should be discouraged during and immediately prior to production periods [
94]. Using only water (the case of almost half of the interviewed users) would be useful only if high pressure is used. A proper combination of kinetic energy through the impact of water droplets, time, and temperature is enough to remove biofilms [
95]. However, if those conditions are not achieved, then protein and fatty organic residues will remain, allowing the persistence of microorganisms. Bleach and its derivates are some of the most effective home sanitizers available, and they are also used in the meat industry [
96,
97]. Thus, its popularity in being used alone or combined with other disinfectants is evaluated as desirable. As commented previously about insecticides, disinfectants used for cleaning surfaces or tools in contact with food should be authorized for the food industry to guarantee the safety of the derived products.
Surfactants present in soap, detergents, and floor cleaners enable the removal of fatty organic remains and affect the cellular membranes of the microorganisms [
98]. The combined use with a sanitizer such as bleach is the best practice. Inappropriate practices, such as dry brushing, which often results in the generation of bioaerosols, transferring floor microorganisms to food preparation surfaces [
99,
100]; the total absence of cleaning interest is a minor point in this survey.
Waste management is one of the main problems detected in the survey. Trash containers were the most common way to dispose of solid residues derived from game dressing and butchering (41.3%), but those structures are accessible to synanthropic mammal species such as brown rats (
Rattus norvegicus) [
101] or red foxes (
Vulpes vulpes) [
102], who can feed on the remains, thus being a source of disease spreading. It is known that free-living cats (
Felis catus) tend to visit rubbish bins regularly [
103], closing the life cycle of toxoplasmosis. Environmental contamination with oocysts of
Toxoplasma gondii has been found surrounding rubbish deposits associated with feral cats [
104]. Toxoplasmosis infection rates in local wild boar (the most killed big game) is 14.1% [
105]; hence, organic remains are potentially risky; 33.2% of answers disclosed the abandonment of offal and other remains directly in the field. This method, being legal in some EU states (Regulation (EC) No 853/2004), is a source of contamination and a way to help parasites complete their life cycles [
15,
106,
107,
108]. Three species of
Trichinella have been found in Spain (
T. spiralis, T. britovi, and
T. pseudospiralis) [
109]. Under proper climatic conditions, infective
Trichinella larvae can remain viable in carrion for a long period [
110,
111], acting as a source of infection for mammal scavengers [
33] and synanthropic carnivores [
112]; 12.8% of surveyed hunters use the remains to feed domestic animals (essentially dogs but also cats and pigs). Wildlife is a reservoir of a plethora of pathogens that is able to affect domestic species, for example,
T. gondii [
105], Aujeszky’s virus [
113],
Alaria alata [
114,
115],
Echinococcus granulosus, transmitted from ruminants’ offal [
116], and
E. ortleppi from wild boar viscera, accessible to dogs (
Canis lupus familiaris) [
117]. Weaknesses in biosecurity protocols, particularly in small pig farms, can facilitate disease transmission from wild boar populations. African swine fever, because of its virulence and the current epidemiologic situation in continental Europe, is one of the most relevant concerns in this regard [
118]. Some waste management practices, such as offal incineration [
119], safe disposal in carcass containers [
116] (Jiménez et al., 2002), or avian scavengers [
120], are considered effective strategies to control disease dispersal. Only 7.9% had access to carcass containers (normally associated with farm activity). Although Spain hosts the most important vulture breeding populations of all European countries [
121], only 4.4% of collaborators use vulture feeding stations as places to leave the remains of game dressing. Vultures may contribute to the removal of ungulate infectious diseases due to their resistance to most pathogenic microorganisms [
120]. Although being very effective in reducing biological contamination, incineration (0.34%) can be considered anecdotal. About wastewater, only 7.6% of the hunters interviewed declared having access to a specific organic residue water treatment plant. Most of the cases (53.5%) used the collective water network, and 30.2% released the polluted water to bodies of surface water or farmlands. Water has been proved to be an effective vehicle for animal–human shared pathogens such as
Mycobacterium spp. [
122,
123,
124]
Toxoplasma gondii [
125], or hepatitis E virus [
126,
127]. Uncontrolled wastewater coming from slaughterhouses can pollute water bodies with a plethora of parasites [
128].