Effects of Ultrasound-Assisted Vacuum Impregnation Antifreeze Protein on the Water-Holding Capacity and Texture Properties of the Yesso Scallop Adductor Muscle during Freeze–Thaw Cycles
Abstract
:1. Introduction
2. Materials and Methods
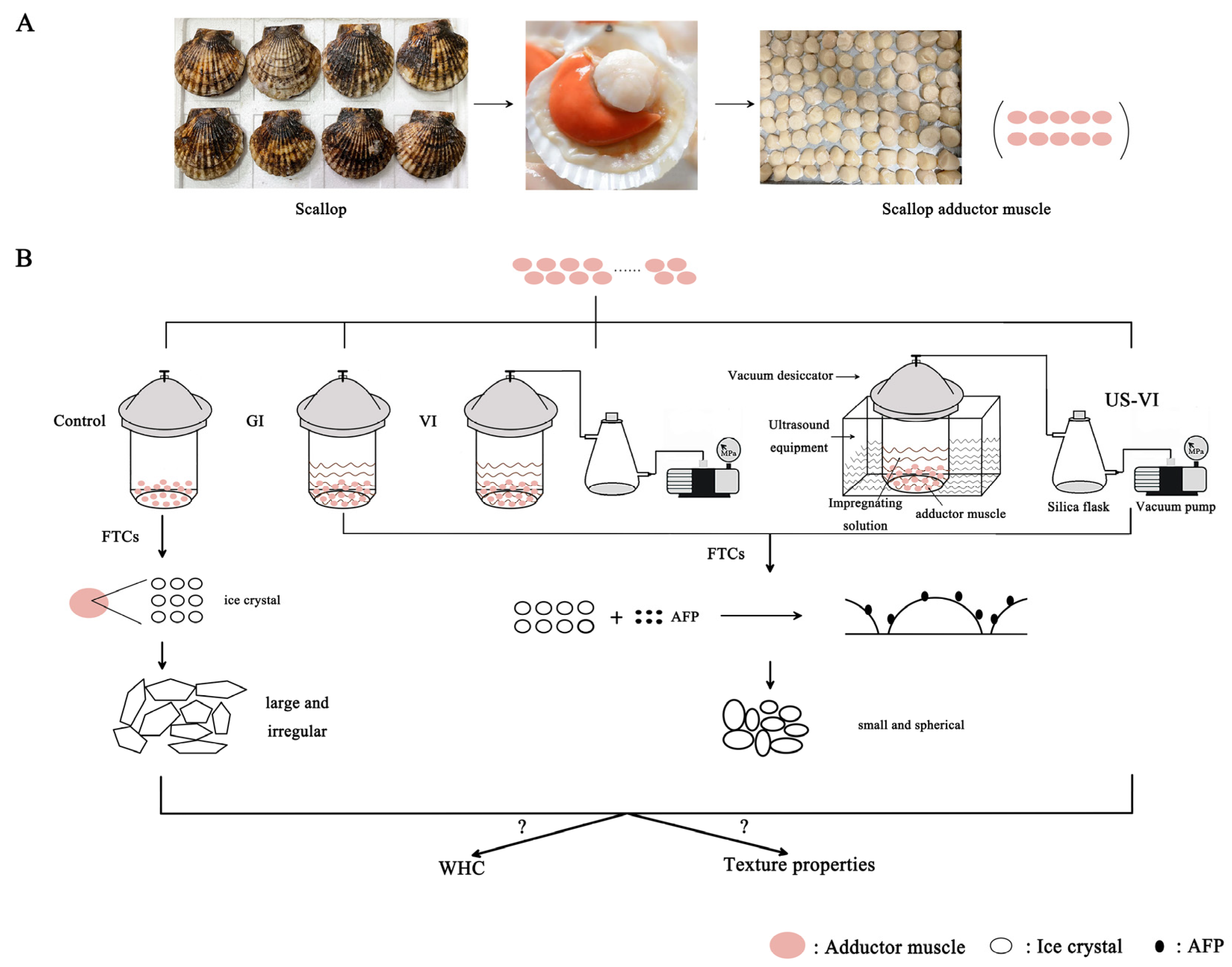
2.1. Sample Preparation
2.2. Methods
2.2.1. Yield and Moisture
2.2.2. Centrifugal Loss and Cooking Loss
2.2.3. Texture Profile Analysis (TPA) and Shear Force
2.2.4. Low-Field Nuclear Magnetic Resonance (LF-NMR) and Magnetic Resonance Image (MRI) Measurement
2.2.5. Observation and Determination of Ice Crystal Morphology
2.2.6. Scanning Electron Microscopy (SEM) Observation
2.2.7. Statistical Analysis
3. Results and Discussion
3.1. The Effects of AFP on the Water-Holding Capacity
3.1.1. Changes in WHC
3.1.2. Changes in Water States and Distribution
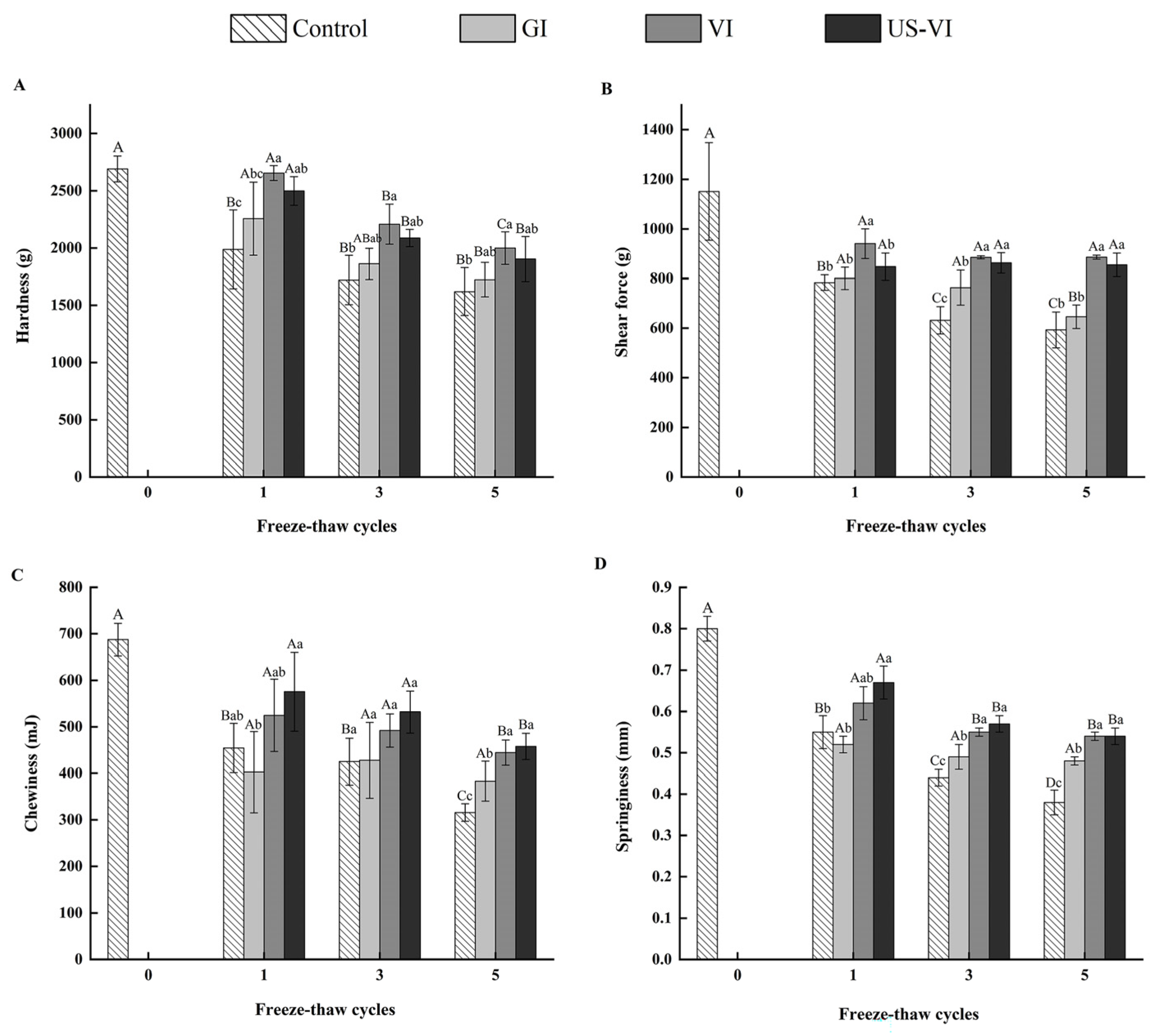
3.2. The Effects of AFP on the Texture Properties
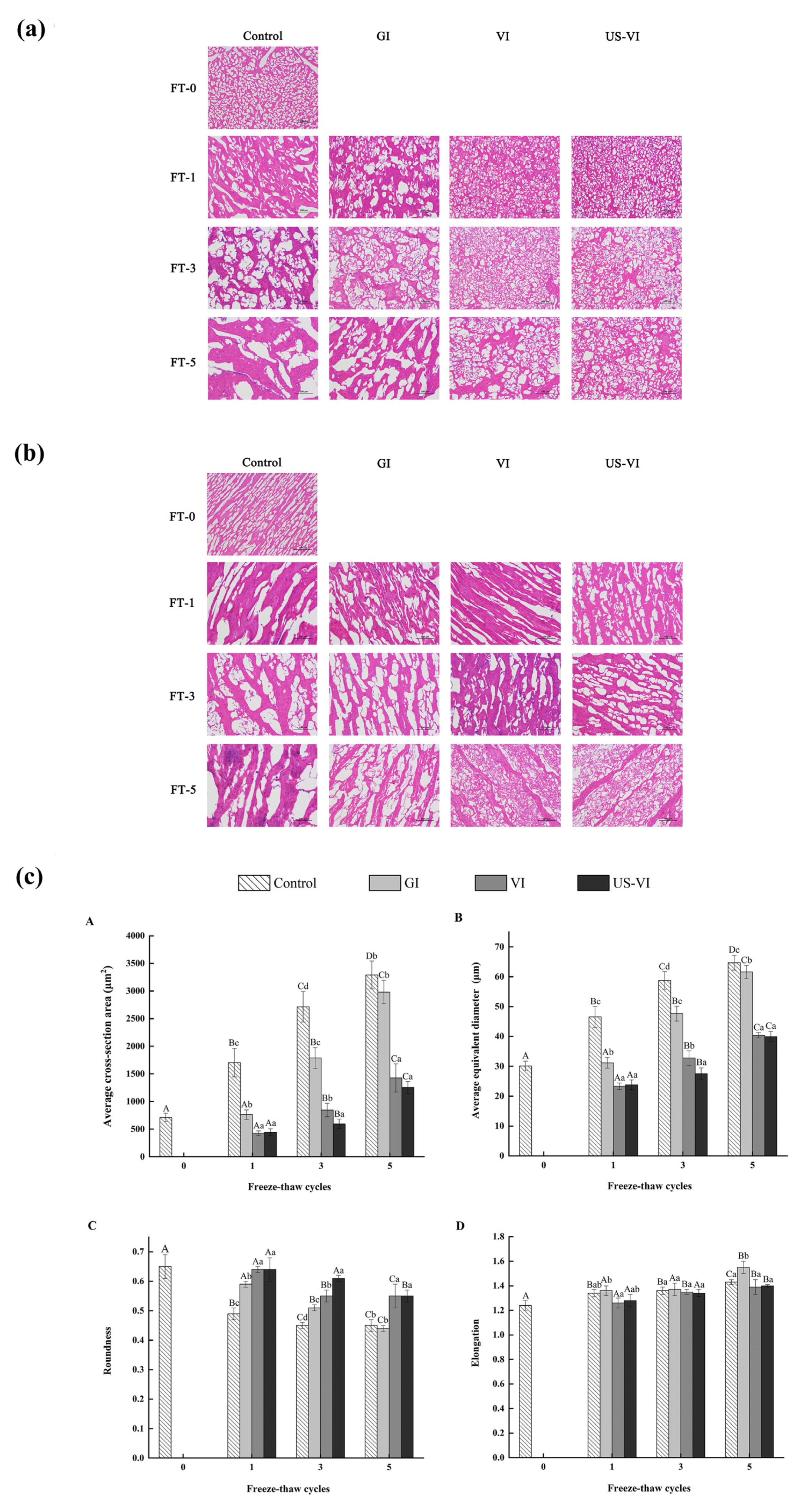
3.3. The Effects of AFP on the Microstructure
3.3.1. Changes in Ice Crystal Morphology
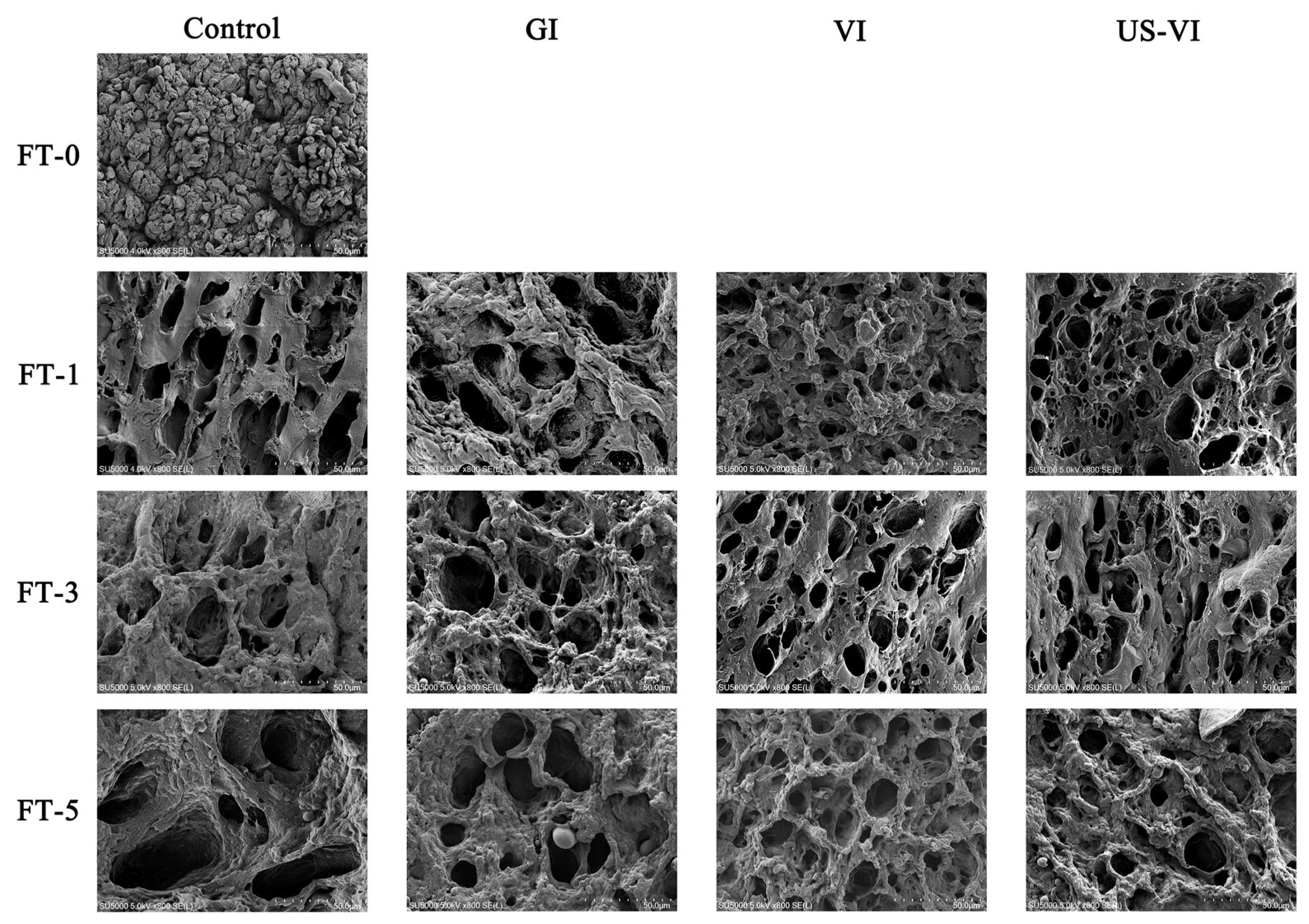
3.3.2. Changes in SEM Observation
3.4. The Partial Least Squares Regression (PLSR) Model
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mao, J.; Zhang, Q.; Yuan, C.; Zhang, W.; Hu, L.; Wang, X.; Liu, M.; Han, B.; Ding, J.; Chang, Y. Genome-wide identification, characterisation and expression analysis of the ALAS gene in the Yesso scallop (Patinopecten yessoensis) with different shell colours. Gene 2020, 757, 144925. [Google Scholar] [CrossRef] [PubMed]
- Hou, R.; Bao, Z.; Wang, S.; Su, H.; Li, Y.; Du, H.; Hu, J. Transcriptome sequencing and de novo analysis for yesso scallop (Patinopecten yessoensis) using 454 GS FLX. PLoS ONE 2011, 6, e21560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, X.; He, B.; Jiang, D.; Dong, X.; Qi, H. Postmortem biochemical and textural changes in the Patinopecten yessoensis adductor muscle (PYAM) during iced storage. Int. J. Food Prop. 2019, 22, 1024–1034. [Google Scholar] [CrossRef] [Green Version]
- Yang, F.; Jing, D.; Yu, D.; Xia, W.; Jiang, Q.; Xu, Y.; Yu, P. Differential roles of ice crystal, endogenous proteolytic activities and oxidation in softening of obscure pufferfish (Takifugu obscurus) fillets during frozen storage. Food Chem. 2019, 278, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Jing, D.; Yang, F.; Gao, P.; Jiang, Q.; Xu, Y.; Yu, P.; Xia, W. The factors influencing the flavor characteristics of frozen obscure pufferfish (Takifugu obscurus) during storage: Ice crystals, endogenous proteolysis and oxidation. Int. J. Refrig. 2021, 122, 147–155. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, L.; Li, Q.; Luo, Y. Comparison of gel properties and biochemical characteristics of myofibrillar protein from bighead carp (Aristichthys nobilis) affected by frozen storage and a hydroxyl radical-generation oxidizing system. Food Chem. 2017, 223, 96–103. [Google Scholar] [CrossRef]
- Jiang, Q.; Nakazawa, N.; Hu, Y.; Osako, K.; Okazaki, E. Changes in quality properties and tissue histology of lightly salted tuna meat subjected to multiple freeze-thaw cycles. Food Chem. 2019, 293, 178–186. [Google Scholar] [CrossRef]
- Nakazawa, N.; Okazaki, E. Recent research on factors influencing the quality of frozen seafood. Fish. Sci. 2020, 86, 231–244. [Google Scholar] [CrossRef] [Green Version]
- Sveinsdóttir, H.I.; Karlsdóttir, M.G.; Arason, S.; Stefánsson, G.; Sone, I.; Skåra, T.; Rustad, T.; Larsson, K.; Undeland, I.; Gudjónsdóttir, M. Effect of antioxidants on the sensory quality and physicochemical stability of Atlantic mackerel (Scomber scombrus) fillets during frozen storage. Food Chem. 2020, 321, 126744. [Google Scholar] [CrossRef]
- Chen, X.; Wu, J.; Cai, X.; Wang, S. Production, structure-function relationships, mechanisms, and applications of antifreeze peptides. Compr. Rev. Food Sci. Food Saf. 2020, 20, 542–562. [Google Scholar] [CrossRef]
- Zhang, B.; Cao, H.-J.; Wei, W.-Y.; Ying, X.-G. Influence of temperature fluctuations on growth and recrystallization of ice crystals in frozen peeled shrimp (Litopenaeus vannamei) pre-soaked with carrageenan oligosaccharide and xylooligosaccharide. Food Chem. 2020, 306, 125641. [Google Scholar] [CrossRef] [PubMed]
- Nian, L.; Cao, A.; Cai, L. Investigation of the antifreeze mechanism and effect on quality characteristics of largemouth bass (Micropterus salmoides) during F-T cycles by hAFP. Food Chem. 2020, 325, 126918. [Google Scholar] [CrossRef] [PubMed]
- Xiang, H.; Yang, X.; Ke, L.; Hu, Y. The properties, biotechnologies, and applications of antifreeze proteins. Int. J. Biol. Macromol. 2020, 153, 661–675. [Google Scholar] [CrossRef] [PubMed]
- Fahy, G.M.; Wowk, B. 010 the control of ice nucleation and growth in tissues and organs. Cryobiology 2013, 67, 401. [Google Scholar] [CrossRef]
- Li, F.; Du, X.; Ren, Y.; Kong, B.; Wang, B.; Xia, X.; Bao, Y. Impact of ice structuring protein on myofibrillar protein aggregation behaviour and structural property of quick-frozen patty during frozen storage. Int. J. Biol. Macromol. 2021, 178, 136–142. [Google Scholar] [CrossRef]
- Yılmaz, F.M.; Bilek, S.E. Ultrasound-assisted vacuum impregnation on the fortification of fresh-cut apple with calcium and black carrot phenolics. Ultrason. Sonochem. 2018, 48, 509–516. [Google Scholar] [CrossRef]
- Aykın-Dinçer, E. Application of ultrasound-assisted vacuum impregnation for improving the diffusion of salt in beef cubes. Meat Sci. 2021, 176, 108469. [Google Scholar] [CrossRef]
- Zhao, X.; Wu, J.; Chen, L.; Yang, H. Effect of vacuum impregnated fish gelatin and grape seed extract on metabolite profiles of tilapia (Oreochromis niloticus) fillets during storage. Food Chem. 2019, 293, 418–428. [Google Scholar] [CrossRef]
- Awad, T.S.; Moharram, H.A.; Shaltout, O.E.; Asker, D.; Youssef, M.M. Applications of ultrasound in analysis, processing and quality control of food: A review. Food Res. Int. 2012, 48, 410–427. [Google Scholar] [CrossRef]
- Alarcon-Rojo, A.D.; Janacua, H.; Rodriguez, J.C.; Paniwnyk, L.; Mason, T.J. Power ultrasound in meat processing. Meat Sci. 2015, 107, 86–93. [Google Scholar] [CrossRef] [Green Version]
- McClements, D.J. Advances in the application of ultrasound in food analysis and processing. Trends Food Sci. Technol. 1995, 6, 293–299. [Google Scholar] [CrossRef]
- Zhang, C.; Li, X.-A.; Wang, H.; Xia, X.; Kong, B. Ultrasound-assisted immersion freezing reduces the structure and gel property deterioration of myofibrillar protein from chicken breast. Ultrason. Sonochem. 2020, 67, 105137. [Google Scholar] [CrossRef] [PubMed]
- Vaskoska, R.; Ha, M.; Ong, L.; Chen, G.; White, J.; Gras, S.; Warner, R. Myosin sensitivity to thermal denaturation explains differences in water loss and shrinkage during cooking in muscles of distinct fibre types. Meat Sci. 2021, 179, 108521. [Google Scholar] [CrossRef]
- Li, P.; Sun, L.; Wang, J.; Wang, Y.; Zou, Y.; Yan, Z.; Zhang, M.; Wang, D.; Xu, W. Effects of combined ultrasound and low-temperature short-time heating pretreatment on proteases inactivation and textural quality of meat of yellow-feathered chickens. Food Chem. 2021, 355, 129645. [Google Scholar] [CrossRef]
- Tan, M.; Lin, Z.; Zu, Y.; Zhu, B.; Cheng, S. Effect of multiple freeze-thaw cycles on the quality of instant sea cucumber: Emphatically on water status of by LF-NMR and MRI. Food Res. Int. 2018, 109, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Luan, L.; Wang, L.; Wu, T.; Chen, S.; Ding, T.; Hu, Y. A study of ice crystal development in hairtail samples during different freezing processes by cryosectioning versus cryosubstitution method. Int. J. Refrig. 2018, 87, 39–46. [Google Scholar] [CrossRef]
- Pearce, K.L.; Rosenvold, K.; Andersen, H.J.; Hopkins, D.L. Water distribution and mobility in meat during the conversion of muscle to meat and ageing and the impacts on fresh meat quality attributes—A review. Meat Sci. 2011, 89, 111–124. [Google Scholar] [CrossRef]
- Szmańko, T.; Lesiów, T.; Górecka, J. The water-holding capacity of meat: A reference analytical method. Food Chem. 2021, 357, 129727. [Google Scholar] [CrossRef]
- Puolanne, E. Chapter 8—Developments in our understanding of water-holding capacity in meat. In New Aspects of Meat Quality; Purslow, P.P., Ed.; Woodhead Publishing: Sawston, UK, 2017; pp. 167–190. [Google Scholar]
- Goula, A.M.; Thymiatis, K.; Kaderides, K. Valorization of grape pomace: Drying behavior and ultrasound extraction of phenolics. Food Bioprod. Process. 2016, 100, 132–144. [Google Scholar] [CrossRef]
- Gudjónsdóttir, M.; Lauzon, H.L.; Magnússon, H.; Sveinsdóttir, K.; Arason, S.; Martinsdóttir, E.; Rustad, T. Low field nuclear magnetic resonance on the effect of salt and modified atmosphere packaging on cod (Gadus morhua) during superchilled storage. Food Res. Int. 2011, 44, 241–249. [Google Scholar] [CrossRef]
- Cheng, J.-H.; Sun, D.-W.; Han, Z.; Zeng, X.-A. Texture and structure measurements and analyses for evaluation of fish and fillet freshness quality: A review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.; Lv, H.; Tang, W.; Zhang, X.; Sun, H. Effect of chitosan coating combined with pomegranate peel extract on the quality of Pacific white shrimp during iced storage. Food Control 2016, 59, 818–823. [Google Scholar] [CrossRef]
- Du, X.; Chang, P.; Tian, J.; Kong, B.; Sun, F.; Xia, X. Effect of ice structuring protein on the quality, thermal stability and oxidation of mirror carp (Cyprinus carpio L.) induced by freeze-thaw cycles. LWT 2020, 124, 109140. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhou, Q.; Sun, D.-W. Measuring and controlling ice crystallization in frozen foods: A review of recent developments. Trends Food Sci. Technol. 2019, 90, 13–25. [Google Scholar] [CrossRef]
- Du, X.; Li, H.; Dong, C.; Ren, Y.; Xia, X. Effect of ice structuring protein on the microstructure and myofibrillar protein structure of mirror carp (Cyprinus carpio L.) induced by freeze-thaw processes. LWT 2020, 139, 110570. [Google Scholar] [CrossRef]
- Van Westen, T.; Groot, R.D. Effect of temperature cycling on ostwald ripening. Cryst. Growth Des. 2018, 18, 4952–4962. [Google Scholar] [CrossRef] [Green Version]
- Hassas-Roudsari, M.; Goff, H.D. Ice structuring proteins from plants: Mechanism of action and food application. Food Res. Int. 2012, 46, 425–436. [Google Scholar] [CrossRef]
- Yeh, Y.; Feeney, R.E. Antifreeze proteins: Structures and mechanisms of function. Chem. Rev. 1996, 96, 601–618. [Google Scholar] [CrossRef]
- Chantarasataporn, P.; Yoksan, R.; Visessanguan, W.; Chirachanchai, S. Water-based nano-sized chitin and chitosan as seafood additive through a case study of Pacific white shrimp (Litopenaeus vannamei). Food Hydrocoll. 2013, 32, 341–348. [Google Scholar] [CrossRef]
- Huff-Lonergan, E.; Lonergan, S.M. Mechanisms of water-holding capacity of meat: The role of postmortem biochemical and structural changes. Meat Sci. 2005, 71, 194–204. [Google Scholar] [CrossRef]
- Leygonie, C.; Britz, T.J.; Hoffman, L.C. Impact of freezing and thawing on the quality of meat: Review. Meat Sci. 2012, 91, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Haji-Akbari, A. Rating antifreeze proteins: Not a breeze. Proc. Natl. Acad. Sci. USA 2016, 113, 201602196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
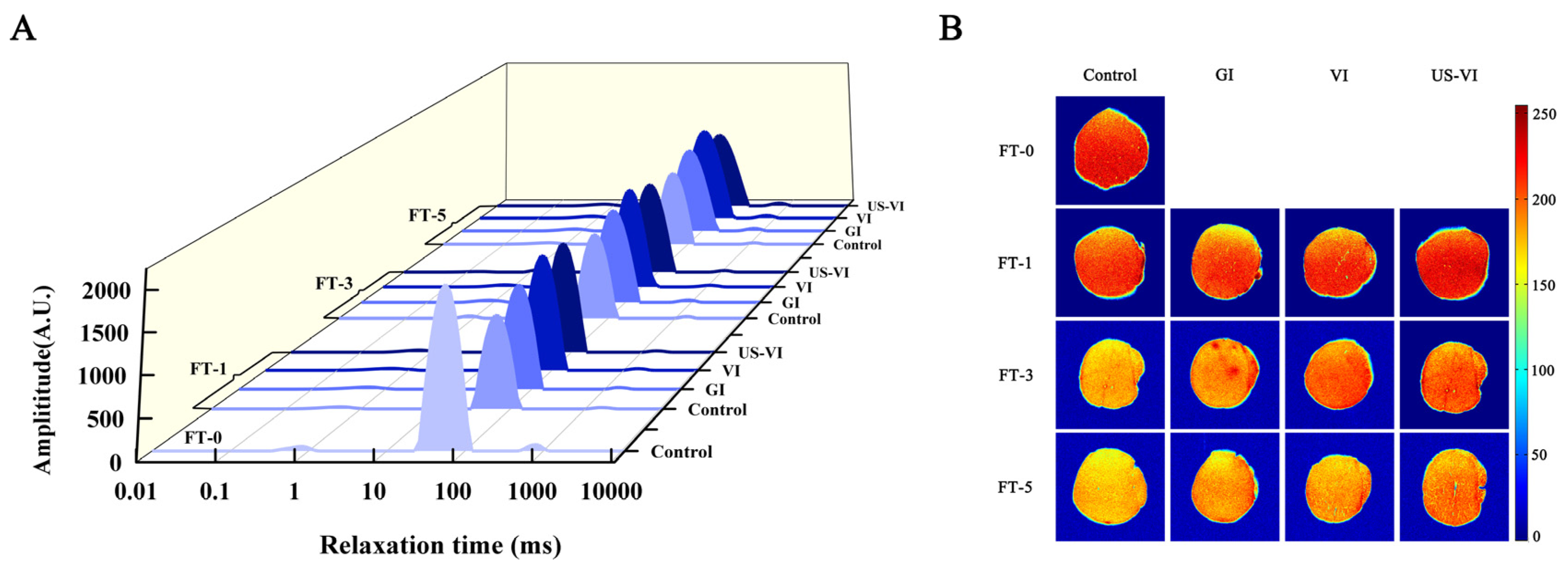

| FTCs | Impregnation Method | Yield (%) | Moisture (%) | Centrifugal Loss (%) | Cooking Loss (%) | T2 Relaxation Times (ms) | ||
|---|---|---|---|---|---|---|---|---|
| T2b | T21 | T22 | ||||||
| 0 | Fresh | - | 80.02 ± 0.28 A | 6.92 ± 0.51 A | 3.99 ± 0.94 A | 0.73 ± 0.07 A | 52.51 ± 0.54 A | 759.36 ± 4.51 A |
| 1 | Control | 98.81 ± 0.16 Ad | 78.52 ± 0.12 Bb | 27.98 ± 1.99 Bc | 16.41 ± 1.20 Bb | 0.71 ± 0.11 Aa | 69.05 ± 6.16 Ba | 1387.10 ± 100.83 Bc |
| GI | 101.80 ± 0.74 Ac | 80.40 ± 0.15 Aa | 24.80 ± 2.48 Abc | 17.79 ± 2.57 Ab | 0.71 ± 0.06 Aa | 75.18 ± 7.46 Aa | 813.71± 96.27 Ab | |
| VI | 106.76± 0.58 Ab | 80.52 ± 0.38 Aa | 20.92 ± 0.69 Aab | 9.89 ± 2.90 Aa | 0.67 ± 0.09 Aa | 66.85 ± 5.29 Aa | 771.46 ± 141.98 Aab | |
| US-VI | 109.71± 0.58 Aa | 80.81 ± 0.16 Aa | 18.32 ± 0.96 Aa | 8.90 ± 1.16 Aa | 0.65 ± 0.04 Aa | 63.03 ± 4.58 Aa | 644.72 ± 38.81 Aa | |
| 3 | Control | 94.06 ± 0.49 Bd | 78.74 ± 0.21 Bc | 35.27 ± 2.50 Cb | 34.41 ± 2.55 Cb | 0.84 ± 0.08 Ab | 73.42 ± 6.09 Bb | 1538.35 ± 0.00 Cc |
| GI | 95.17 ± 0.91 Bc | 79.63 ± 0.58 Bb | 33.07 ± 2.80 Bb | 31.66 ± 1.42 Bab | 0.90 ± 0.00 Bb | 68.65 ± 2.52 Aab | 1019.21 ± 56.57 Bb | |
| VI | 98.10 ± 0.70 Bb | 80.52 ± 0.10 Aa | 33.73 ± 2.05 Bb | 28.14 ± 2.66 Ba | 0.73 ± 0.07 Aa | 65.33 ± 6.48 Aab | 838.60 ± 66.37 Ba | |
| US-VI | 101.01 ± 0.58 Ba | 80.18 ± 0.27 Aab | 29.04 ± 0.58 Ca | 25.68 ± 1.80 Ba | 0.70 ± 0.04 Aa | 65.19 ± 4.59 Aa | 803.18 ± 42.68 Ba | |
| 5 | Control | 87.44 ± 0.35 Cd | 78.38 ± 0.23 Bc | 32.05 ± 2.66 Cb | 47.44 ± 1.87 Da | 0.82 ± 0.07 Aab | 65.81 ± 4.71 Ba | 1623.72 ± 145.30 Ca |
| GI | 88.83 ± 0.55 Cc | 79.33 ± 0.13 Bb | 33.07 ± 0.88 Bb | 46.64 ± 2.75 Ca | 0.84 ± 0.06 Bb | 68.38 ± 3.74 Aa | 1615.71 ± 133.99 Ca | |
| VI | 92.77 ± 0.29 Cb | 79.22 ± 0.06 Bb | 32.35 ± 0.45 Bb | 42.06 ± 4.30 Ca | 0.71 ± 0.09 Aa | 62.01 ± 1.73 Aa | 1538.79 ± 73.90 Ca | |
| US-VI | 94.07 ± 0.07 Ca | 80.25 ± 0.23 Aa | 26.55 ± 0.72 Ba | 44.87 ± 3.25 Ca | 0.70 ± 0.05 Aa | 63.76 ± 2.67 Aa | 1437.52 ± 142.59 Ca | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Y.; Wang, H.; Zheng, Y.; Qiu, Z.; Wang, X. Effects of Ultrasound-Assisted Vacuum Impregnation Antifreeze Protein on the Water-Holding Capacity and Texture Properties of the Yesso Scallop Adductor Muscle during Freeze–Thaw Cycles. Foods 2022, 11, 320. https://doi.org/10.3390/foods11030320
Shi Y, Wang H, Zheng Y, Qiu Z, Wang X. Effects of Ultrasound-Assisted Vacuum Impregnation Antifreeze Protein on the Water-Holding Capacity and Texture Properties of the Yesso Scallop Adductor Muscle during Freeze–Thaw Cycles. Foods. 2022; 11(3):320. https://doi.org/10.3390/foods11030320
Chicago/Turabian StyleShi, Yuyao, Hongli Wang, Yao Zheng, Zehui Qiu, and Xichang Wang. 2022. "Effects of Ultrasound-Assisted Vacuum Impregnation Antifreeze Protein on the Water-Holding Capacity and Texture Properties of the Yesso Scallop Adductor Muscle during Freeze–Thaw Cycles" Foods 11, no. 3: 320. https://doi.org/10.3390/foods11030320
APA StyleShi, Y., Wang, H., Zheng, Y., Qiu, Z., & Wang, X. (2022). Effects of Ultrasound-Assisted Vacuum Impregnation Antifreeze Protein on the Water-Holding Capacity and Texture Properties of the Yesso Scallop Adductor Muscle during Freeze–Thaw Cycles. Foods, 11(3), 320. https://doi.org/10.3390/foods11030320






