Food-Borne Nanocarriers for Calcium Delivery: A New Choice for Nutrient Supplements
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
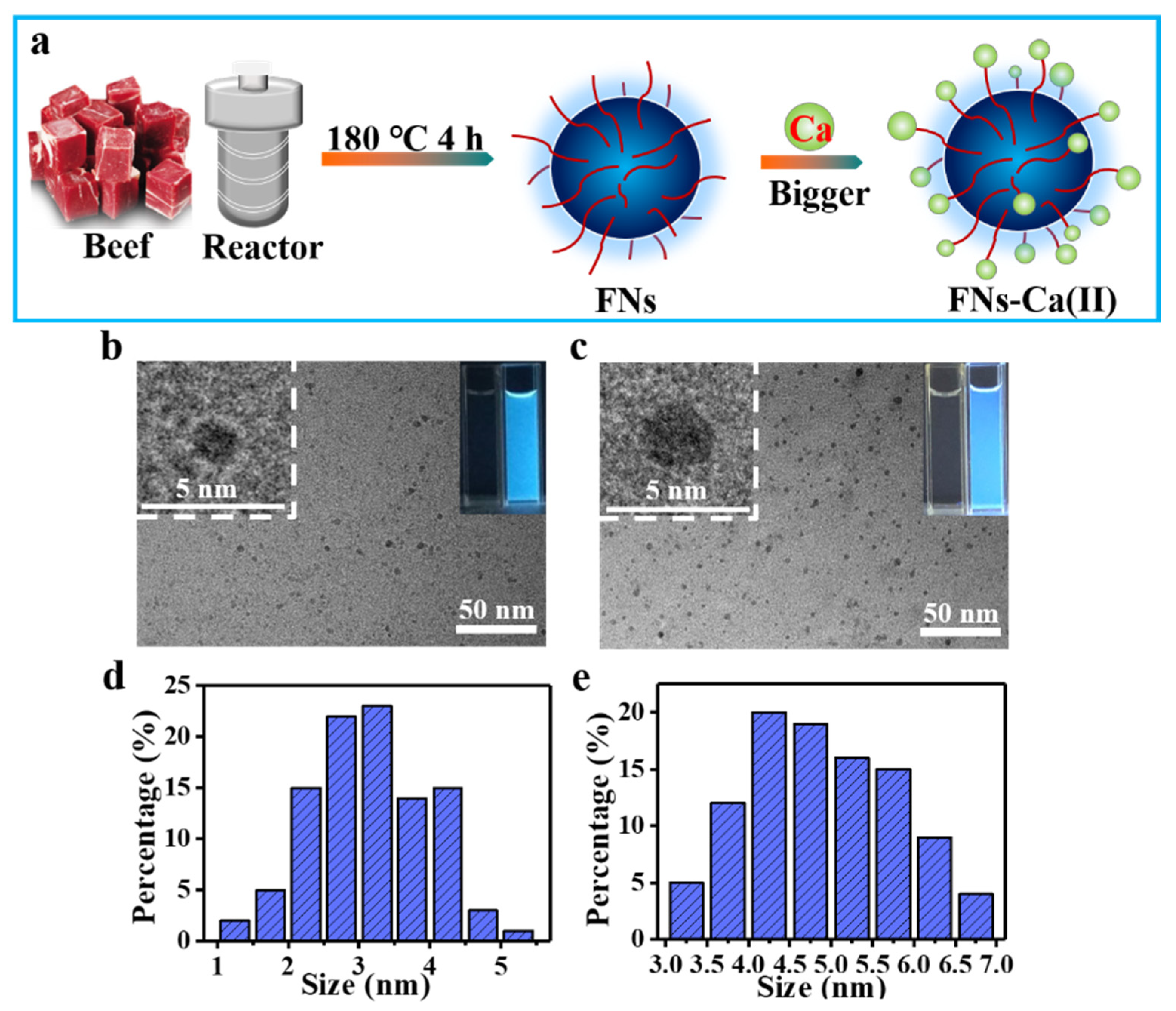
2.2. Synthesis and Purification of FNs
2.3. Preparation of the FNs-Ca(II) Complex
2.4. FN and FNs-Ca(II) Complex Analysis
2.5. Calcium-Binding Capacity Assay
2.6. Cytotoxicity and Biodistribution Analysis of FNs-Ca(II) in Caco-2 Cells
3. Results and Discussion
3.1. TEM Analysis of FNs-Ca(II)
3.2. Optical Property Analysis of FNs and FNs-Ca(II)
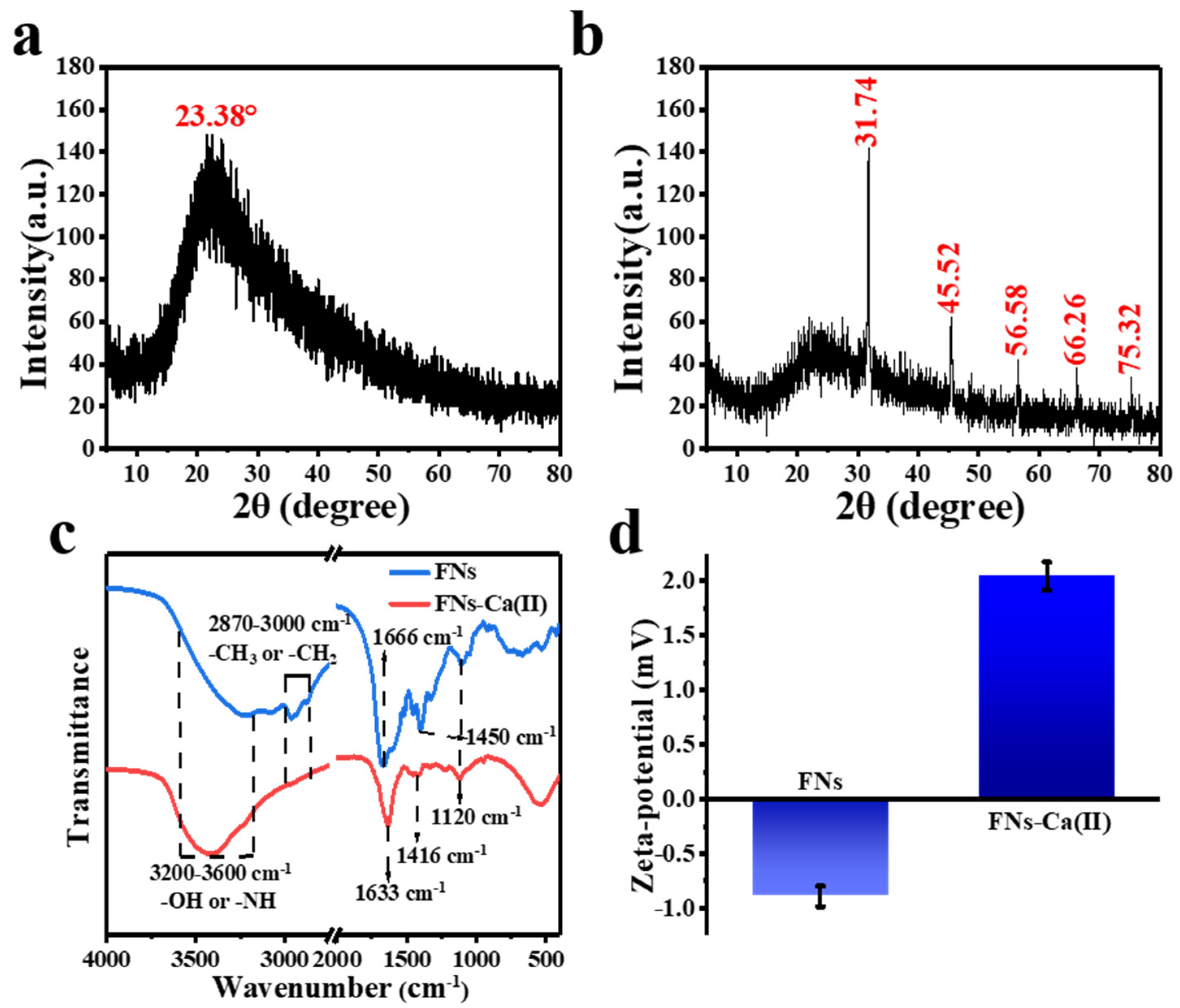
3.3. XRD and FT-IR Analysis
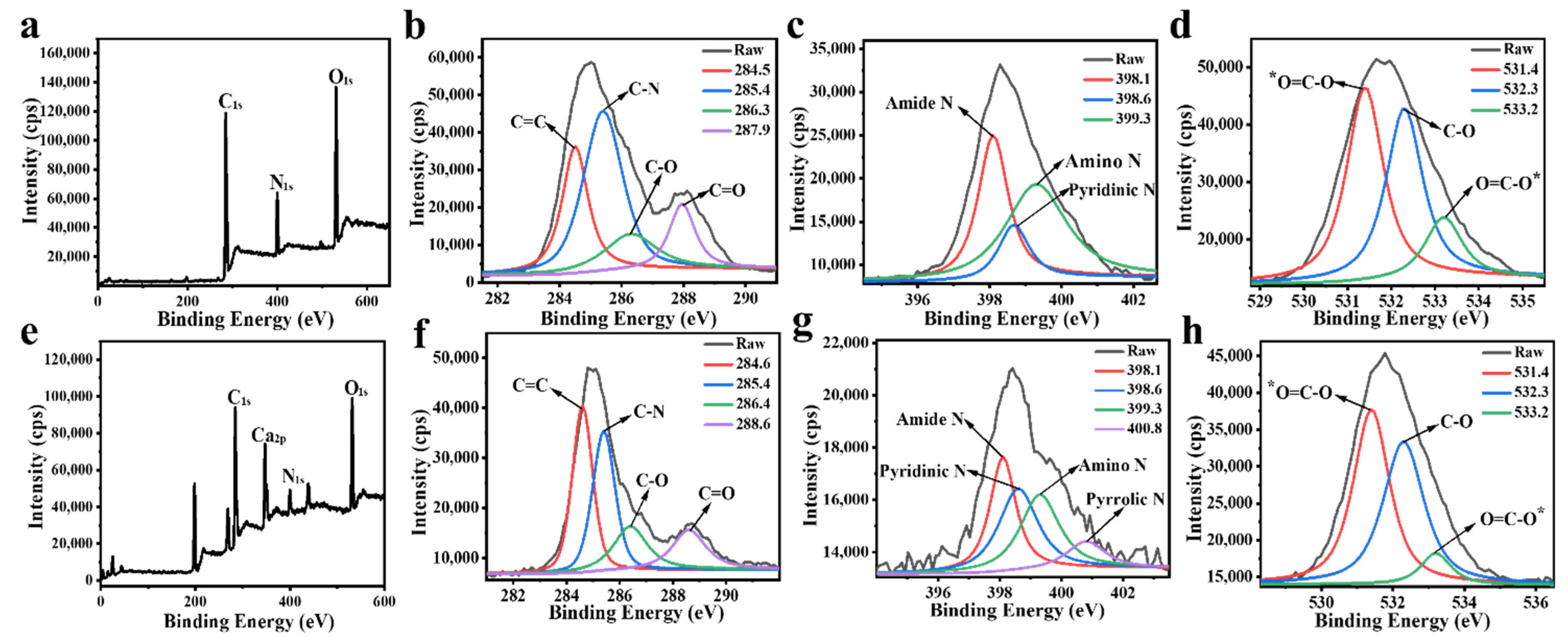
3.4. XPS Analysis of FNs and FNs-Ca(II)
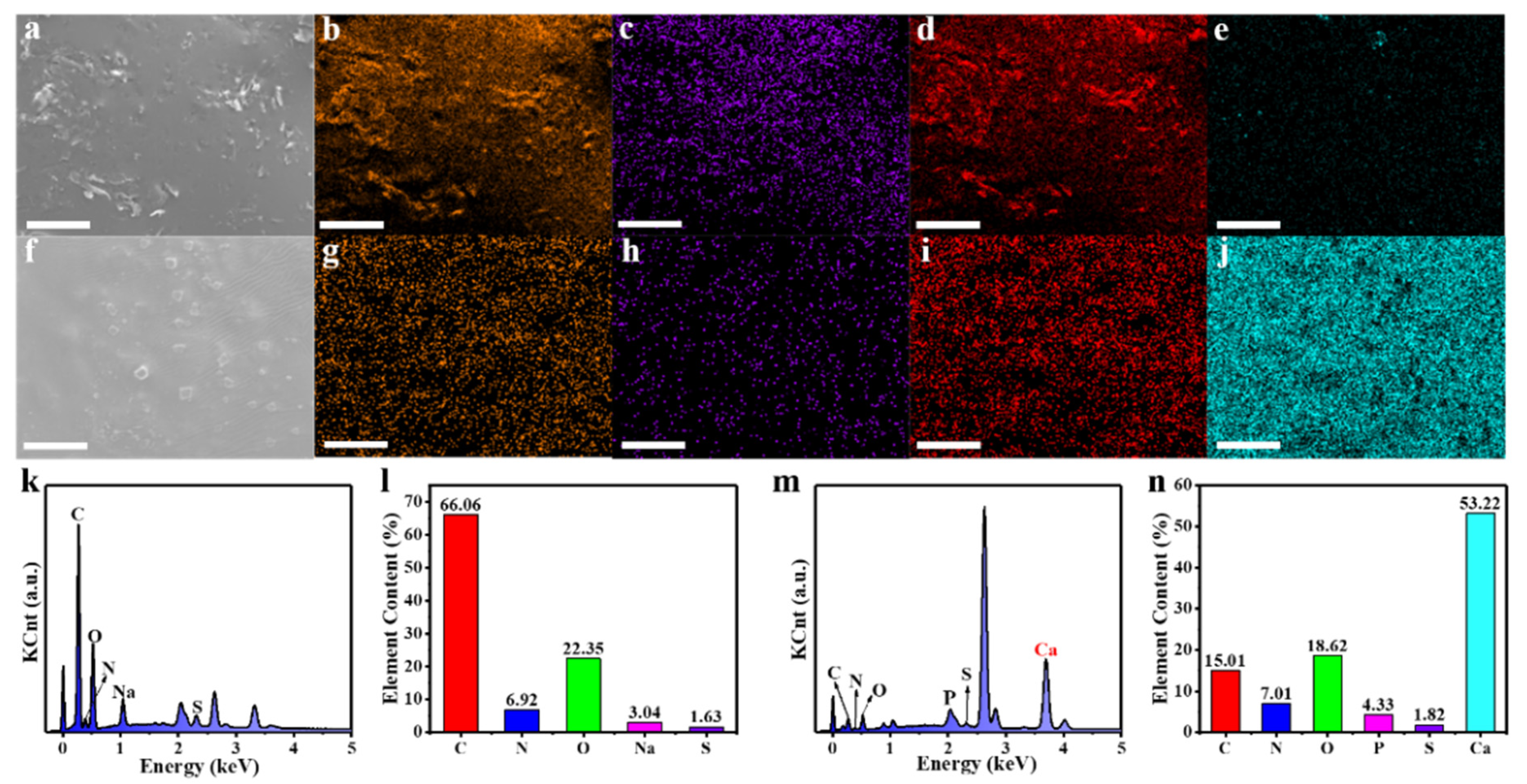
3.5. EDS Analysis of FNs and FNs-Ca(II)
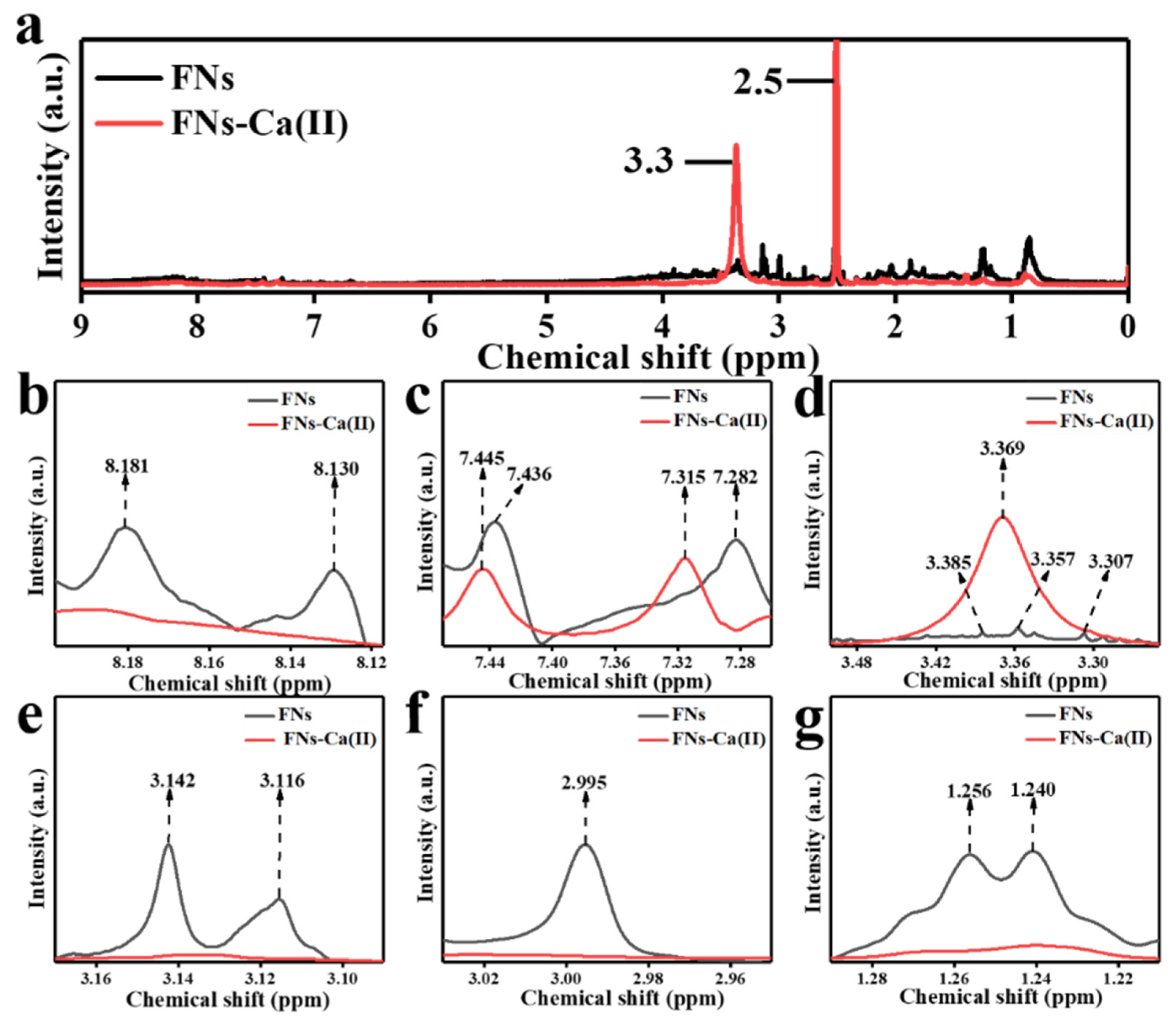
3.6. 1H NMR Analysis of FNs and FNs-Ca(II)
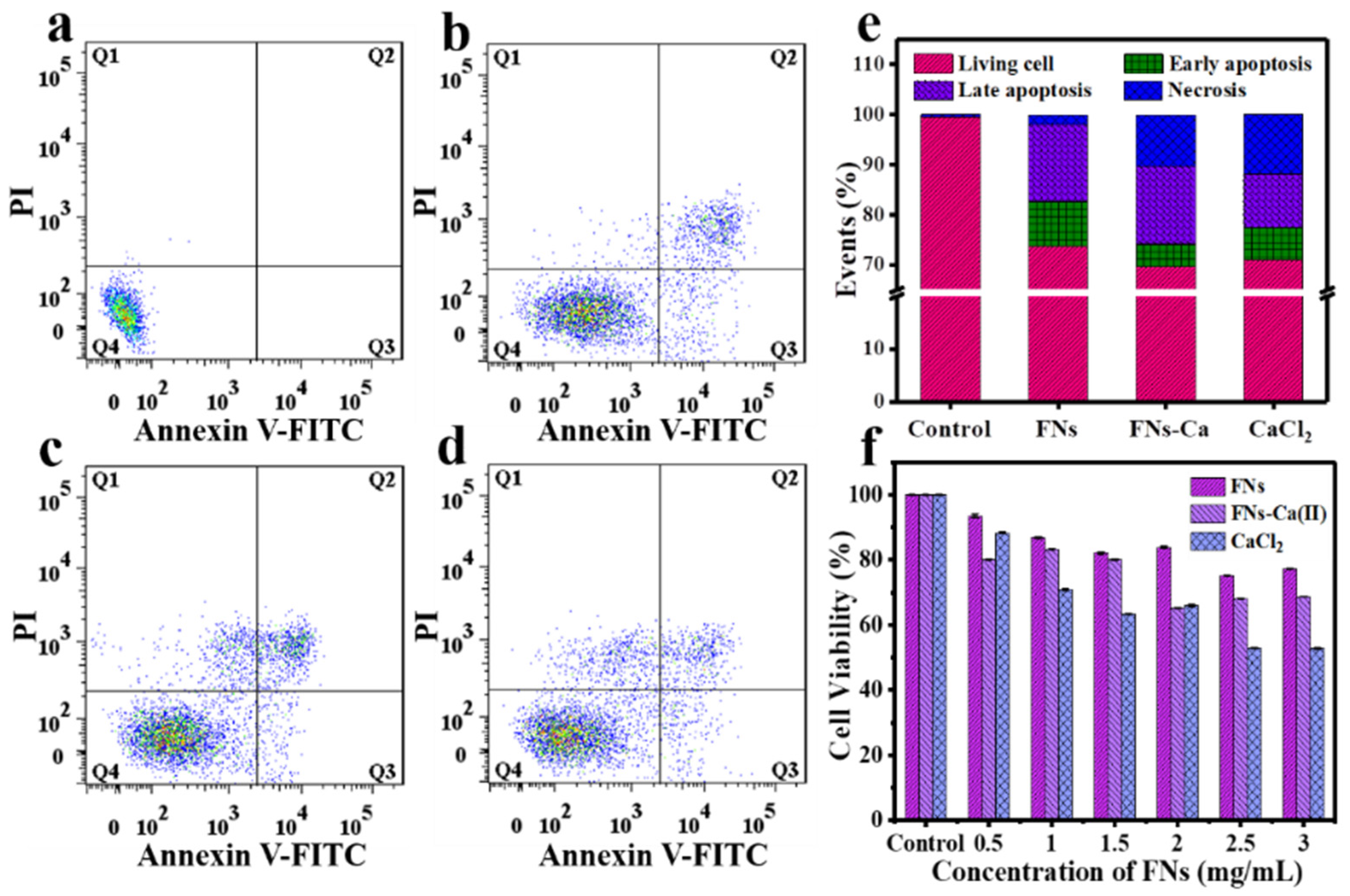
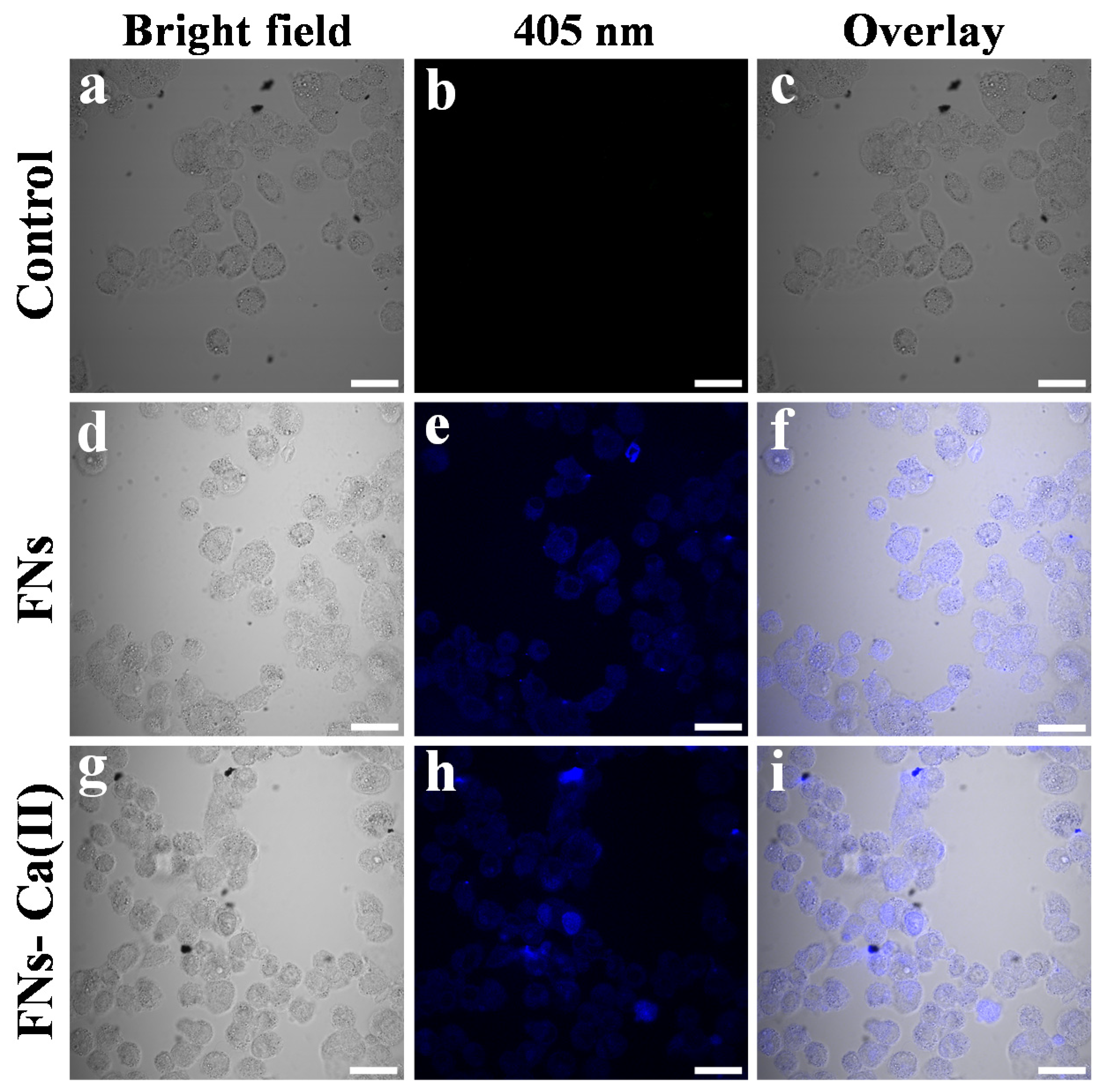
3.7. Cellular Bio-Distribution and Cytotoxicity of FNs and FNs-Ca(II)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mertz, W. The essential trace elements. Science 1981, 213, 1332–1338. [Google Scholar] [CrossRef]
- Xie, Y.; Yi, G.; Zhang, A. Effects of calcium in physiology and metabolism of fruit crops. J. Fruit Sci. 2003, 20, 369–373. [Google Scholar]
- Marie, P.J.; Pettifor, J.M.; Ross, F.P.; Glorieux, F.H. Histological osteomalacia due to dietary calcium deficiency in children. N. Engl. J. Med. 1982, 307, 584–588. [Google Scholar] [CrossRef] [PubMed]
- Ally, M.; Tang, J.Y.; Joselyn, L.; Maria, A.R.; Melika, R.; Chanana, A.M.; Epstein, E.H. Calcium channel blockade and vismodegib-induced muscle cramps. Jama Dermatol. 2015, 151, 1132–1134. [Google Scholar] [CrossRef] [PubMed]
- Fievet, P.; Fournier, A.; De, B.A.; El, E.N.; Gregoire, I.; Westeel, P.F.; Renaud, H.; Makdassi, R. Atrial natriuretic factor in pregnancy-induced hypertension and preeclampsia: Increased plasma concentrations possibly explaining these hypovolemic states with paradoxical hyporeninism. Am. J. Hypertens. 1988, 1, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Lipschitz, A.D. Nutrition and health in the elderly. Curr. Opin. Gastroen. 1991, 7, 277–283. [Google Scholar] [CrossRef]
- Namkung, M.H.; Appleyard, R.; Jansen, J.; Lin, J.H.; Diamond, T. Osteoporosis influences the early period of fracture healing in a rat osteoporotic model. Bone 2001, 28, 80–86. [Google Scholar] [CrossRef]
- Mishra, B.; Sharma, G.; Shukla, D. Investigation of organoleptic characteristics in the development of soft chews of calcium carbonate as mineral supplement. J. Pharm. Soc. Jap. 2009, 129, 1537–1544. [Google Scholar] [CrossRef][Green Version]
- Behar, J.; Hitchings, M.; Smyth, R.D. Calcium stimulation of gastrin and gastric acid secretion: Effect of small doses of calcium carbonate. Gut 1977, 18, 442–448. [Google Scholar] [CrossRef]
- Heller, J.H. The role of calcium in the prevention of kidney stones. J. Am. Coll. Nutr. 1999, 18, 373S–378S. [Google Scholar] [CrossRef]
- Atsutane, O.; Masako, O.; Seigo, B.; Takashi, A.; Takashi, S.; El, S. Calcium and magnesium absorption from the colon and rectum are increased in rats fed fructooligosaccharides. J. Nutr. 1995, 125, 2417–2424. [Google Scholar]
- Mellander, O.; Isaksson, B. The physiological importance of the casein phosphopeptide calcium salts. I. intravenous and peroral calcium cosage in animal experiments. Acta. Soc. Bot. Pol. 1950, 55, 239–246. [Google Scholar]
- Wu, W.; Li, B.; Hou, H.; Zhang, H.; Zhao, X. Isolation and identification of calcium-chelating peptides from pacific cod skin gelatin and their binding properties with calcium. Food Funct. 2017, 8, 4441–4448. [Google Scholar] [CrossRef] [PubMed]
- Cui, P.; Songyi, L.; Ziqi, J.; Zhu, B.W.; Liang, S.; Na, S. In vitro digestion profile and calcium absorption studies of sea cucumber ovum derived heptapeptide-calcium complex. Food Funct. 2018, 9, 4582–4592. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.Y.; Wu, Y.Y.; Zhao, X.; Lai, B.; Sun, N.; Tan, M.Q. Food-borne nanocarriers from roast beef patties for iron delivery. Food Funct. 2019, 10, 6711–6719. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Bi, J.; Liu, S.; Wang, H.; Tan, M. Presence and formation of fluorescence carbon dots in grilled hamburger. Food Funct. 2017, 8, 2558–2565. [Google Scholar] [CrossRef]
- Bi, J.; Li, Y.; Wang, H.; Song, Y.; Cong, S.; Yu, C.; Zhu, B.W.; Tan, M. Presence and formation mechanism of foodborne carbonaceous nanostructures from roasted pike eel (Muraenesox cinereus). J. Agric. Food Chem. 2018, 66, 2862–2869. [Google Scholar] [CrossRef]
- Cong, S.; Wang, N.Y.; Wang, K.; Wu, Y.; Li, D.; Song, Y.; Prakash, S.; Tan, M.Q. Fluorescent nanoparticles in the popular pizza: Properties, biodistribution and cytotoxicity. Food Funct. 2019, 10, 2408–2416. [Google Scholar] [CrossRef]
- Alam, A.; Park, B.; Ghouri, Z.K.; Kim, H.Y. Synthesis of carbon quantum dots from cabbage with down- and up-conversion photoluminescence properties: Excellent imaging agent for biomedical applications. Green Chem. 2015, 17, 3791–3797. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, H.S. Green synthesis of luminescent nitrogen-doped carbon dots from milk and its imaging application. Anal. Chem. 2014, 86, 8902–8905. [Google Scholar] [CrossRef]
- Song, Y.K.; Liu, K.J.; Su, W.T.; Hou, S.; Che, T.T.; Tan, M.Q. Construction and evaluation of an iron delivery system by ultra-small nanoparticles from roast sturgeon (Acipenser schrenckiid). Food Funct. 2021, 12, 1147–1155. [Google Scholar] [CrossRef] [PubMed]
- Geng, J.X.; Song, X.Y.; Zhang, X.D.; Tie, S.S.; Cao, L.; Tan, M.Q. Hydrophilic food-borne nanoparticles from beef broth as novel nanocarriers for zinc. J. Agric. Food Chem. 2019, 67, 6995–7004. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.T.; Xie, Y.S.; Na, X.K.; Bi, J.R.; Liu, S. Fluorescent carbon dots in baked lamb: Formation, cytotoxicity and scavenging capability to free radicals. Food Chem. 2019, 286, 405–412. [Google Scholar] [CrossRef]
- Van, D.B.; Nie, H.; Ju, B.; Marino, E.; Paulusse, J.M.J.; Schall, P.; Li, M.; Dohnalová, K. Excitation-dependent photoluminescence from single-carbon dots. Small 2017, 13, 1702098. [Google Scholar]
- Wu, H.; Yang, Z.; Chen, C.; Zhang, J.; Zhang, H.; Peng, H.; Wang, F. Synthesis, crystal structures, antioxidant activities, and DNA-binding studies of two silver (I) complexes with 1,3-bis(1-ethylbenzimidazol-2-yl)-2-thiapropane, and α,β-unsaturated carboxylates. J. Coord. Chem. 2016, 69, 1076–1087. [Google Scholar] [CrossRef]
- Scandola, F.; Indelli, M.T.; Chiorboli, C.; Bignozzi, C.A. Photoinduced electron transfer II. Cheminform 1990, 22, 73–149. [Google Scholar]
- Zhao, C.; Jiao, Y.; Hu, F.; Yang, Y. Green synthesis of carbon dots from pork and application as nanosensors for uric acid detection. Spectrochim. Acta. A Mol. Biomol. Spectrosc. 2018, 190, 360–367. [Google Scholar] [CrossRef]
- Wang, F.; Xu, Z.; Zhang, Y.; Li, J.; Nian, S.; Zhou, N. Green synthesis and bioactivity of vaterite-doped beta-dicalcium silicate bone cement. Ceram. Int. 2016, 42, 1856–1861. [Google Scholar] [CrossRef]
- Peng, Z.; Hou, H.; Zhang, K.; Li, B. Effect of calcium-binding peptide from pacific cod (Gadus macrocephalus) bone on calcium bioavailability in rats. Food Chem. 2017, 221, 373–378. [Google Scholar] [CrossRef]
- Zhao, X.; Shan, S.; Li, J.; Cao, L.; Tan, M. Assessment of potential toxicity of foodborne fluorescent nanoparticles from roasted pork. Nanotoxicology 2019, 13, 1310–1323. [Google Scholar] [CrossRef]
- Yang, Y.; Faheem, M.; Wang, L.; Meng, Q.; Sha, H.; Yang, N.; Yuan, Y.; Zhu, G. Surface pore engineering of covalent organic frameworks for ammonia capture through synergistic multivariate and open metal site approaches. ACS Cent. Sci. 2018, 4, 748–754. [Google Scholar] [CrossRef] [PubMed]
- Manuel, S.; Simone, B.; Harpreet, K.L.; Rita, B.; Elena, B. Energy dispersive X-ray (EDX) microanalysis: A powerful tool in biomedical research and diagnosis. Eur. J. Histochem. EJH 2018, 62, 89–99. [Google Scholar]
- Colboc, H.; Moguelet, P.; Bazin, D.; Bachmeyer, C.; Frochot, V.W. Physicochemical characterization of inorganic deposits associated with granulomas in cutaneous sarcoidosis. J. Eur. Acad. Dermatol. 2019, 33, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Hinton, J.F.; Amis, E.S. Nuclear magnetic resonance studies of ions in pure and mixed solvents. Chem. Rev. 1967, 67, 367–425. [Google Scholar] [CrossRef]
- Bergana, M.M.; Adams, K.M.; Harnly, J.; Moore, J.C.; Xie, Z. Non-targeted detection of milk powder adulteration by 1H NMR spectroscopy and conformity index analysis. J. Food Compos. Anal. 2019, 78, 49–58. [Google Scholar] [CrossRef]
- Corcilius, L.; Liu, D.Y.; Ochoa, J.L.; Linington, R.G.; Payne, R.J. Synthesis and evaluation of analogues of the glycinocin family of calcium-dependent antibiotics. Org. Biomol. Chem. 2018, 16, 5310–5320. [Google Scholar] [CrossRef]
- Do, H.H.; Hauptmann, R.; Villinger, A.; Surkus, A.E.; Lochbrunner, S.; Ehlers, P.; Langer, P. Palladium-catalyzed synthesis and fluorescence study of 2,3-diaryl-5-ethynylbenzo [e] indoles. Tetrahedron 2017, 73, 3407–3414. [Google Scholar] [CrossRef]
- Gottlieb, H.E.; Kotlyar, V.; Nudelman, A. NMR chemical shifts of common laboratory solvents as trace impurities. J. Org. Chem. 1997, 62, 7512–7515. [Google Scholar] [CrossRef]
- Francisco, H.U.; Nuria, A.I.C.; Miguel, N.M.C.; Antonio, L.P.C.; René, F. Ni(II) and Cu(II) complexes with the dianionic N,N,O-tridentate schiff base 6-amino-5-formyl-1,3-dimethyluracil-benzoylhydrazone: Crystal structure of the monodimensionally hydrogen-bonded aqua-(6-amino-1,3-dimethyl-uracilato-benzoylhydrazone(2-)-N6,N51,O5. Polyhedron 2000, 19, 689–693. [Google Scholar]
- Trivedi, A.R.; Dodiya, D.K.; Ravat, N.R.; Shah, V.H. Synthesis and biological evaluation of some new pyrimidines via a novel chalcone series. Arkivoc 2008, 11, 131–141. [Google Scholar] [CrossRef]
- Steven, A.R.; Gordon, L. Downfield displacement of the NMR signal of water in deuterated dimethylsulfoxide by the addition of deuterated trifluoroacetic acid. Tetrahedron Lett. 2000, 41, 3225–3227. [Google Scholar]
- Grigoras, M.; Sava, M.; Colotin, G.; Simionescu, C.I. Synthesis and thermal behavior of some anthracene-based copolymers obtained by diels–alder cycloaddition reactions. J. Appl. Polym. Sci. 2008, 107, 846–853. [Google Scholar] [CrossRef]
- Khalturina, V.; Shklyaev, Y.V.; Aliev, Z.; Maslivets, A. Five-membered 2, 3-dioxoheterocycles: LXIV. reactions of 1-methyl-3, 4-dihydroisoquinolines with 5-arylfuran-2, 3-diones and (Z)-alkyl 4-aryl-2-hydroxy-4-oxobut-2-enoates. crystal and molecular structure of (2Z,5Z)-3-hydroxy-5-{8,8-dimethyl-2,3,8,9-tetrahydro [1,4] dioxino [2,3-g] isoquinolin-6 (7H)-ylidene}-1-phenylpent-2-ene-1, 4-dione. Russ. J. Org. Chem. 2009, 45, 728–734. [Google Scholar]
- Ferrari, M. Frontiers in cancer nanomedicine: Directing mass transport through biological barriers. Trends Biotechnol. 2010, 28, 181–188. [Google Scholar] [CrossRef]
- Verkhratsky, A.; Toescu, E. Endoplasmic reticulum Ca2+ homeostasis and neuronal death. J. Cell. Mol. Med. 2003, 7, 351–361. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, N.; Chen, Y.; Song, Y.; Yu, D.; Tan, M. Food-Borne Nanocarriers for Calcium Delivery: A New Choice for Nutrient Supplements. Foods 2022, 11, 308. https://doi.org/10.3390/foods11030308
Wang N, Chen Y, Song Y, Yu D, Tan M. Food-Borne Nanocarriers for Calcium Delivery: A New Choice for Nutrient Supplements. Foods. 2022; 11(3):308. https://doi.org/10.3390/foods11030308
Chicago/Turabian StyleWang, Nanying, Yannan Chen, Yukun Song, Deyang Yu, and Mingqian Tan. 2022. "Food-Borne Nanocarriers for Calcium Delivery: A New Choice for Nutrient Supplements" Foods 11, no. 3: 308. https://doi.org/10.3390/foods11030308
APA StyleWang, N., Chen, Y., Song, Y., Yu, D., & Tan, M. (2022). Food-Borne Nanocarriers for Calcium Delivery: A New Choice for Nutrient Supplements. Foods, 11(3), 308. https://doi.org/10.3390/foods11030308