Changes in Key Aroma Compounds and Esterase Activity of Monascus-Fermented Cheese across a 30-Day Ripening Period
Abstract
1. Introduction
2. Materials and Methods
2.1. Cheese Manufacture
2.2. Gas Chromatography–Olfactometry–Mass Spectrometry (GC-O-MS) Analysis
2.3. Esterase Activity
2.4. Sensory Evaluation
2.5. Statistical Analysis
3. Results and Discussion
3.1. Analysis of Volatile Aroma Compounds
3.1.1. Acids
3.1.2. Ketones
3.1.3. Esters
3.1.4. Alcohol and Aromatic Heterocyclic Compounds
3.2. The Analysis of Esterase Activity
3.3. The Analysis of Sensory Properties
3.4. Correlation Analysis between Volatile Aroma Compounds and Esterase Activity
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cheng, M.-J.; Wu, M.-D.; Chen, J.-J.; Chen, C.-Y.; Aung, T.; Wu, H.-C.; Lai, M.-H. Compounds from Monascus sanguineus. Chem. Nat. Compd. 2021, 57, 545–547. [Google Scholar] [CrossRef]
- Kim, D.; Ku, S. Beneficial Effects of Monascus sp. KCCM 10093 Pigments and Derivatives: A Mini Review. Molecules 2018, 23, 98. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Chen, F. Cocultivation Study of Monascus spp. and Aspergillus niger Inspired From Black-Skin-Red-Koji by a Double-Sided Petri Dish. Front. Microbiol. 2021, 12, 1076–1088. [Google Scholar] [CrossRef]
- Ren, X.; He, Z.; Lin, X.; Lin, X.; Liang, Z.; Liu, D.; Huang, Y.; Fang, Z. Screening and evaluation of Monascus purpureus FJMR24 for enhancing the raw material utilization rate in rice wine brewing. J. Sci. Food Agric. 2021, 101, 185–193. [Google Scholar] [CrossRef]
- Takuya, M.; Masaya, K.; Mayumi, S.; Masaya, Y.; Aiko, H.; Kana, I.; Shintaro, I.; Takumi, K.; Kenichi, S.; Akiko, H. Low dose red yeast rice with monacolin K lowers LDL cholesterol and blood pressure in Japanese with mild dyslipidemia: A multicenter, randomized trial. Asia Pac. J. Clin. Nutr. 2021, 30, 424–435. [Google Scholar] [CrossRef]
- Jia, S.C.; Zhang, J.; Su, Y.; Zhang, X.; Wang, M. Monascus vinegar-mediated alternation of gut microbiota and its correlation with lipid metabolism and inflammation in hyperlipidemic rats. J. Funct. Foods 2020, 74, 104152. [Google Scholar] [CrossRef]
- Yu, H.; Liu, Z.; Hang, F.; Mo, B. Effect of Monascus sp. as an adjunct starter on physicochemical properties and proteolysis in semi-hard cheeses during ripening. Food Sci. Biotechnol. 2016, 25, 785–793. [Google Scholar] [CrossRef] [PubMed]
- Jiao, J.; Zheng, Z.; Liu, Z.; You, C. Study of the Compositional, Microbiological, Biochemical, and Volatile Profile of Red-Veined Cheese, an Internal Monascus-Ripened Variety. Front. Nutr. 2021, 8, 649611. [Google Scholar] [CrossRef]
- Huaning, S.; Liu, Z.; You, C. Influence of Monascus purpureus BD-M-4 on the physicochemical properties, proteolysis and volatile compounds of surface mould-ripened cheese. Food Sci. Biotechnol. 2019, 28, 129–138. [Google Scholar] [CrossRef]
- Xia, Y.; Yuan, R.; Weng, S.; Wang, G.; Ai, L. Proteolysis, lipolysis, texture and sensory properties of cheese ripened by Monascus fumeus. Food Res. Int. 2020, 137, 109657. [Google Scholar] [CrossRef]
- Batty, D.; Meunier-Goddik, L.; Waite-Cusic, J.G. Camembert-type cheese quality and safety implications in relation to the timing of high-pressure processing during aging. J. Dairy Sci. 2019, 102, 8721–8733. [Google Scholar] [CrossRef] [PubMed]
- Amiria, S.; Kohneshahrib, S.R.A.; Nabizadeh, F. The effect of unit operation and adjunct probiotic culture on physicochemical, biochemical, and textural properties of Dutch Edam cheese. LWT 2021, 155, 112859. [Google Scholar] [CrossRef]
- Jiang, C.; Liu, M.; Yan, X.; Bao, R.; Liu, A.; Wang, W.; Zhang, Z.; Liang, H.; Ji, C.; Zhang, S.; et al. Lipase Addition Promoted the Growth of Proteus and the Formation of Volatile Compounds in Suanzhayu, a Traditional Fermented Fish Product. Foods 2021, 10, 2529. [Google Scholar] [CrossRef] [PubMed]
- Ardö, Y. Enzymes in Cheese Ripening. In Agents of Change; Springer: Cham, Switzerland, 2021; pp. 363–395. [Google Scholar] [CrossRef]
- Karaca, O.B.; Güven, M. Effects of Proteolytic and Lipolytic Enzyme Supplementations on Lipolysis and Proteolysis Characteristics of White Cheeses. Foods 2018, 7, 125. [Google Scholar] [CrossRef]
- Wang, J.; Yang, Z.J.; Xu, L.Y.; Wang, B.; Tan, L. Key aroma compounds identified in Cheddar cheese with different ripening times by aroma extract dilution analysis, odor activity value, aroma recombination, and omission. J. Dairy Sci. 2020, 104, 1576–1590. [Google Scholar] [CrossRef]
- Wang, J.; Yang, Z.J.; Wang, Y.D.; Cao, Y.P.; Liu, Y. The key aroma compounds and sensory characteristics of commercial Cheddar Cheeses. J. Dairy Sci. 2021, 104, 7555–7571. [Google Scholar] [CrossRef]
- Gemert, L.J.V. Compilations of Odour Threshold Values in Air, Water and Other Media; Oliemans Punter Partn. B.V.: Utrecht, The Netherlands, 2013. [Google Scholar]
- Grosch, W. Detection of potent odorants in foods by aroma extract dilution analysis. Trends Food Sci. Technol. 1993, 4, 68–73. [Google Scholar] [CrossRef]
- Trabue, S.L.; Anhalt, J.C.; Zahn, J.A. Bias of Tedlar Bags in the Measurement of Agricultural Odorants. J. Environ. Qual. 2006, 35, 1668–1677. [Google Scholar] [CrossRef]
- Mukdsi, M.C.A.; Medina, R.B.; Alvarez, M.D.F.; González, S.N. Ester synthesis by lactic acid bacteria isolated from goat’s and ewe’s milk and cheeses. Food Chem. 2009, 117, 241–247. [Google Scholar] [CrossRef]
- Majcher, U.; Klejborowska, G.; Kaik, M.; Maj, E.; Huczyński, A. Synthesis and Biological Evaluation of Novel Triple-Modified Colchicine Derivatives as Potent Tubulin-Targeting Anticancer Agents. Cells 2018, 7, 216. [Google Scholar] [CrossRef] [PubMed]
- De-La-Fuente-Blanco, A.; Ferreira, V. Gas Chromatography Olfactometry (GC-O) for the (Semi)Quantitative Screening of Wine Aroma. Foods 2020, 9, 1892. [Google Scholar] [CrossRef] [PubMed]
- Karagul-Yuceer, Y.; Isleten, M.; Uysal-Pala, C. Sensory characteristics of Ezine cheese. J. Sens. Stud. 2007, 22, 49–65. [Google Scholar] [CrossRef]
- Wyder, M.T.M.T.; Puhan, Z. Role of selected yeasts in cheese ripening: An evaluation in aseptic cheese curd slurries. Int. Dairy J. 1999, 9, 117–124. [Google Scholar] [CrossRef]
- Wang, B.; Wang, J.; Xu, L.Y.; Zhang, J.H.; Ai, N.S.; Cao, Y.P. Characterization of the key odorants in kurut with aroma recombination and omission studies. J. Dairy Sci. 2020, 103, 4164–4173. [Google Scholar] [CrossRef]
- Mcsweeney, P. Biochemistry of cheese ripening. Int. J. Dairy Technol. 2010, 57, 127–144. [Google Scholar] [CrossRef]
- Yxa, B.; Xw, A.; Xiao, L.A.; Xl, A.; Cz, A.; Wl, A.; Xs, A.; Ww, A.; Bsa, C. Discovery and development of a novel short-chain fatty acid ester synthetic biocatalyst under aqueous phase from Monascus purpureus isolated from Baijiu. Food Chem. 2020, 338, 128025. [Google Scholar] [CrossRef]
- Kranz, C.; Panitz, C.; Kunz, B. Biotransformation of free fatty acids in mixtures to methyl ketones by Monascus purpureus. Appl. Microbiol. Biotechnol. 1992, 36, 436–439. [Google Scholar] [CrossRef]
- Sophie Sablé, G.C. Current knowledge of soft cheeses flavor and related compounds. J. Agric. Food Chem. 1999, 47, 4825–4836. [Google Scholar] [CrossRef]
- Khattab, A.R.; Guirguis, H.A.; Tawfik, S.M.; Farag, M.A. Cheese ripening: A review on modern technologies towards flavor enhancement, process acceleration and improved quality assessment. Trends Food Sci. Technol. 2019, 88, 33–360. [Google Scholar] [CrossRef]
- Liu, S.Q.; Holland, R.; Crow, V.L. Esters and their biosynthesis in fermented dairy products: A review. Int. Dairy J. 2004, 14, 923–945. [Google Scholar] [CrossRef]
- Paiva, A.L.; Balcão, V.M.; Malcata, F.X. Kinetics and mechanisms of reactions catalyzed by immobilized lipases☆. Enzym. Microb. Technol. 2000, 27, 187–204. [Google Scholar] [CrossRef]
- Liu, H.; Sun, B. Effect of Fermentation Processing on the Flavor of Baijiu. J. Agric. Food Chem. 2018, 66, 5425–5432. [Google Scholar] [CrossRef]
- Xu, Y.; Zhao, J.; Liu, X.; Zhang, C.; Sun, B. Flavor mystery of Chinese traditional fermented baijiu: The great contribution of ester compounds. Food Chem. 2021, 369, 130920. [Google Scholar] [CrossRef] [PubMed]
- Hong, Q.; Liu, X.M.; Hang, F.; Zhao, J.X.; Zhang, H.; Chen, W. Screening of adjunct cultures and their application in ester formation in Camembert-type cheese. Food Microbiol. 2018, 70, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Chen, J.; Shi, X.; Wang, B.; Zheng, X. Characteristic physicochemical indexes and flavor compounds in Xinjiang Kazak cheese during ripening. Food Biosci. 2020, 35, 100586. [Google Scholar] [CrossRef]
- Du, Z.; Bing, H.; Ma, X.; Cheng, Y.; Ruan, R. Catalytic pyrolysis of microalgae and their three major components: Carbohydrates, proteins, and lipids. Bioresour. Technol. 2012, 130C, 777–782. [Google Scholar] [CrossRef]
- Taboada, N.; Nieuwenhove, C.V.; Alzogaray, S.L.; Medina, R. Influence of autochthonous cultures on fatty acid composition, esterase activity and sensory profile of Argentinean goat cheeses. J. Food Compos. Anal. 2015, 40, 86–94. [Google Scholar] [CrossRef]
- Holland, R.; Liu, S.Q.; Crow, V.L.; Delabre, M.L.; Lubbers, M.; Bennett, M.; Norris, G. Esterases of lactic acid bacteria and cheese flavour: Milk fat hydrolysis, alcoholysis and esterification. Int. Dairy J. 2005, 15, 711–718. [Google Scholar] [CrossRef]
- Cain, W.S. Odor intensity: Mixtures and masking. Chem. Senses 1975, 1, 339–352. [Google Scholar] [CrossRef]
- Cheng, H. Volatile flavor compounds in yogurt: A review. Food Sci. Nutr. 2010, 50, 938–950. [Google Scholar] [CrossRef] [PubMed]
- Soda, M.E.; Law, J.; Tsakalidou, E.; Kalantzopoulos, G. Lipolytic activity of cheese related microorganisms and its impact on cheese flavour. Dev. Food Sci. 1995, 37, 1823–1847. [Google Scholar] [CrossRef]
- Muller, J.; Maceachran, D.; Burd, H.; Sathitsuksanoh, N.; Bi, C.; Yeh, Y.C.; Lee, T.S.; Hillson, N.J.; Chhabra, S.R.; Singer, S.W. Engineering of Ralstonia eutropha H16 for autotrophic and heterotrophic production of methyl ketones. Appl Environ. Microbiol 2013, 79, 4433–4439. [Google Scholar] [CrossRef] [PubMed]
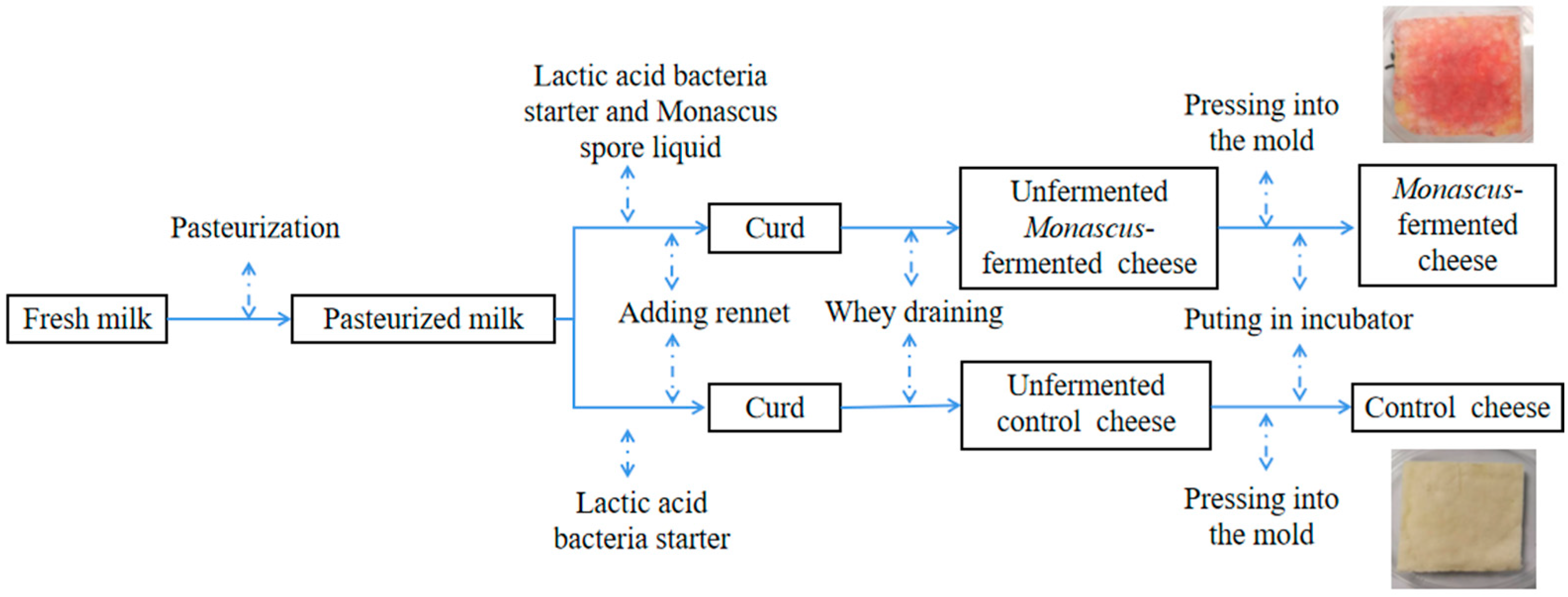
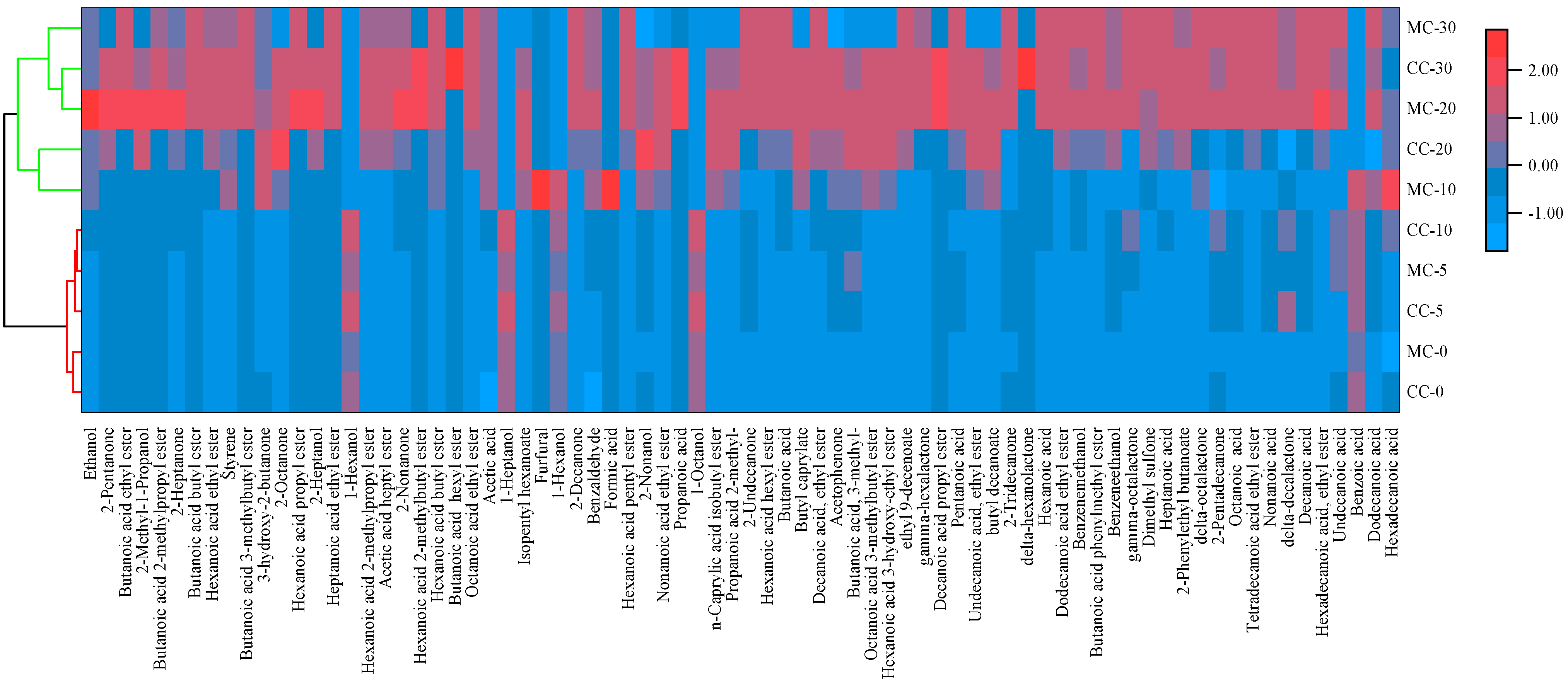

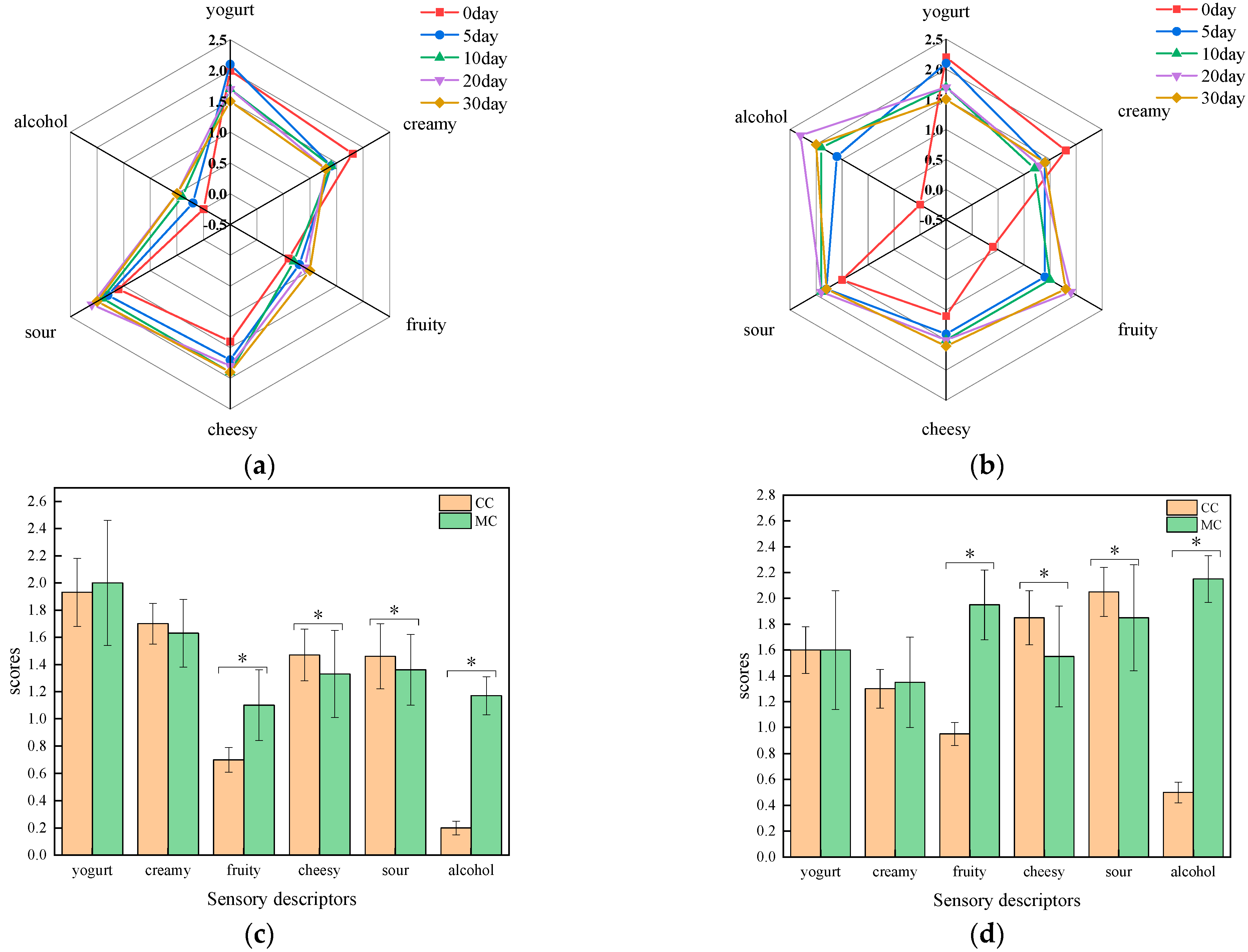
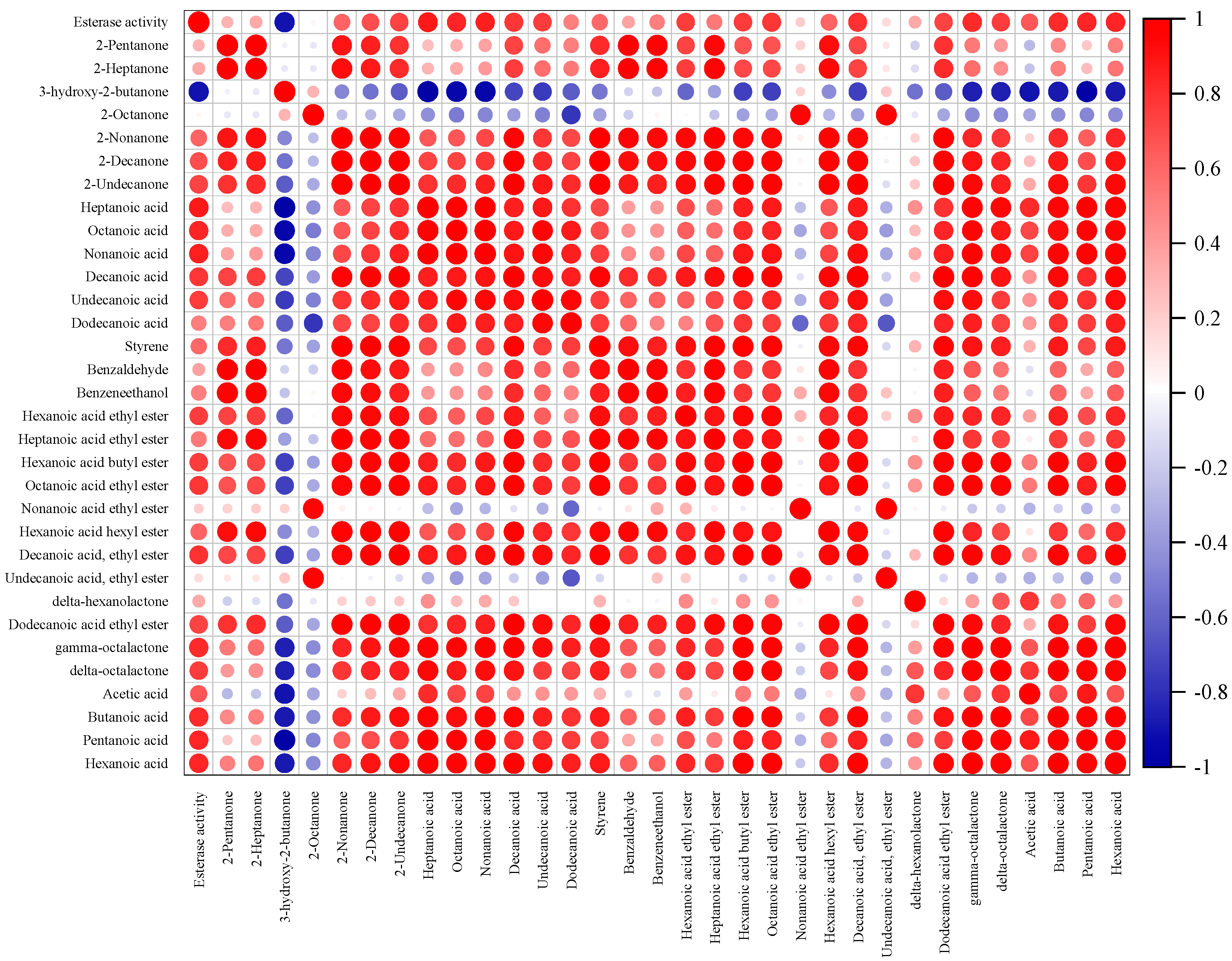
| No. | Compound | RI | Threshold [18] (μg/kg) | ROAV | Flavor Description | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Calc 1 | Ref 2 | ID 3 | ||||||||||||||
| Acids | CC-0 | CC-5 | CC-10 | CC-20 | CC-30 | MC-0 | MC-5 | MC-10 | MC-20 | MC-30 | ||||||
| 1 | Acetic acid | 1450 | 1449 | MS,RI,O,S | 700 | 1.20 b | 1.21 b | 1.23 b | 1.26 b | 1.59 a | 1.22 b | 1.23 b | 1.28 b | 1.76 a | 1.60 a | Pungent, sour, vinegar. |
| 2 | Butanoic acid | 1621 | 1637 | MS,RI,O,S | 410 | 5.52 e | 6.58 d,e | 10.49 d | 9.43 d | 15.84 c | 4.26 e | 9.37 d | 76.95 a | 82.32 a | 62.60 b | Sharp, rancid, butter. |
| 3 | Pentanoic acid | 1738 | 1762 | MS,RI,O,S | 1700 | 0 c | 0 c | 0 c | 0.01 c | 1.02 c | 0.01 c | 0.02 c | 1.43 b | 2.16 a | 1.85 a | Rancid |
| 4 | Hexanoic acid | 1852 | 1861 | MS,RI,O,S | 890 | 0.92 d | 1.00 d | 1.12 d | 1.12 d | 1.4 c | 1.28 cd | 2.46 b | 6.74 a | 6.27 a | 5.73 a | sour, fatty. |
| 5 | Heptanoic acid | 1954 | 1950 | MS,RI,O,S | 100 | 0.16 c | 0.11 c | 0.22 c | 0.22 c | 0.38 c | 0.14 c | 3.03 b | 22.18 a | 27.34 a | 28.24 a | Rancid, sour. |
| 6 | Nonanoic acid | 2065 | 2050 | MS,RI,O,S | 4600 | 0 c | 0 c | 0.12 c | 1.01 b | 0.09 c | 0 c | 0.01 c | 1.25 a | 1.18 b | 0.21 c | Waxy, fatty. |
| 7 | Octanoic acid | 2277 | 2279 | MS,RI,O,S | 500 | 1.26 b | 0.78 c | 1.76 c | 2.14 b | 3.04 b | 1.15 c | 3.44 b | 52.74 a | 51.18 a | 69.41 a | Rancid, soapy. |
| 8 | Decanoic acid | 2338 | 2356 | MS,RI,O,S | 130 | 3.25 c | 1.79 d | 4.51 c | 5.61 c | 7.69 c | 2.17 d | 8.72 c | 225.37 a | 143.67 b | 136.20 b | Rancid, sour, fatty. |
| 9 | Undecanoic acid | 2906 | 2910 | MS,RI,O,S | 1000 | 0 c | 0 c | 0 c | 0 c | 0.58 b | 1.01 a | 0 c | 0 c | 0.82 a | 1.08 a | Waxy, creamy. |
| 10 | Dodecanoic acid | 1450 | 1449 | MS,RI,O,S | 10,000 | 0.01 c | 0.1 c | 0.2 c | 1.02 b | 2.12 b | 2.04 b | 2.5 b | 2.01 b | 5.80 a | 3.60 b | Mild, fatty, coconut. |
| Ketones | ||||||||||||||||
| 11 | 2-Pentanone | 976 | 974 | MS,RI,O,S | 1380 | 0 c | 0 c | 0 c | 0 c | 0 c | 0 c | 0.08 b,c | 5.73 a | 0.4 b | 0.15 b | Wine, banana, woody. |
| 12 | 2-Heptanone | 1181 | 1184 | MS,RI,O,S | 140 | 0.71 e | 0.62 e | 0.76 e | 0.64 e | 1.27 d | 2.37 d | 5.74 c | 115.46 a | 17.26 b | 4.37 c | Fruity, coconut. |
| 13 | 3-Hydroxy-2-butanone | 1278 | 1281 | MS,RI,O,S | 80 | 2.85 c | 2.78 c | 2.72 c | 2.66 c | 3.94 c | 14.76 a | 10.58 ab | 6.76 b | 1.33 d | 1.13 d | Buttery, creamy, milky. |
| 14 | 2-Octanone | 1280 | 1283 | MS,RI,O,S | 50 | 0 d | 0 d | 0 d | 0 d | 0 d | 0.22 c | 8.56 a | 1.94 b | 2.01 b | 1.84 b | Woody, mushroom. |
| 15 | 2-Nonanone | 1385 | 1386 | MS,RI,O,S | 41 | 4.82 e | 3.94 e | 5.92 e | 6.07 e | 9.59 e | 8.73 e | 27.83 d | 610.2 a | 306.38 b | 176.91 c | Fruity, buttery. |
| 16 | 2-Decanone | 1489 | 1496 | MS,RI,O,S | 8.3 | 0 e | 0 e | 0 e | 0 e | 0 e | 0 e | 1.20 d | 19.09 s | 10.82 b,c | 7.16 c | Orange, floral, fatty. |
| 17 | 2-Undecanone | 1608 | 1615 | MS,RI,O,S | 5.5 | 18.75 d | 12.40 d | 25.91 d | 30.65 c | 38.72 c | 17.92 d | 54.15 c | 785.93 a | 472.95 b | 398.46 b | Fruity, creamy floral. |
| Alcohols | ||||||||||||||||
| 18 | 2-Nonanol | 1521 | 1528 | MS,RI,O,S | 58 | 1.65 b | 1.60 b | 1.46 b | 1.85 b | 1.68 b | 1.42 b | 1.53 b | 3.81 a | 1.77 b | 1.48 b | Creamy, citrus, orange. |
| 19 | Benzene ethanol | 1915 | 1922 | MS,RI,O,S | 560 | 0.02 d | 0.03 d | 0.06 d | 0.06 d | 0.12 d | 0.02 d | 0.85 c | 3.45 a | 1.14 b,c | 0.61 c | Floral, rose, bready. |
| Esters | ||||||||||||||||
| 20 | Hexanoic acid, ethyl ester | 1032 | 1041 | MS,RI,O,S | 50 | 0 d | 0 d | 0 d | 0 d | 0 d | 0.14 d | 200.71 c | 544.61 b | 645.39 a | 224.83 c | Pineapple, banana. |
| 21 | Heptanoic acid, ethyl ester | 1133 | 1131 | MS,RI,O,S | 300 | 0 d | 0 d | 0 d | 0 d | 0 d | 0 d | 0 d | 3.23 b | 8.11 a | 1.56 c | Fruity, pineapple. |
| 22 | Hexanoic acid, butyl ester | 1157 | 1161 | MS,RI,O,S | 1000 | 0 d | 0 d | 0 d | 0 d | 0 d | 0.06 c | 0.07 c | 4.41 ab | 5.17 a | 2.67 b | Winey, berry, soapy. |
| 23 | Octanoic acid, ethyl ester | 1216 | 1245 | MS,RI,O,S | 960 | 0 d | 0 d | 0 d | 0 d | 0 d | 0 d | 3.11 c | 36.05 ab | 43.72 a | 23.66 b | Wine, brandy, pear. |
| 24 | Nonanoic acid, ethyl ester | 1231 | 1246 | MS,RI,O,S | 390 | 0 c | 0 c | 0 c | 0 c | 0 c | 0.13 c | 4.41 a | 1.89 b | 2.31 b | 0 c | Fruity, rose, waxy. |
| 25 | Hexanoic acid hexyl ester | 1262 | 1256 | MS,RI,O,S | 0.5 | 0 d | 0 d | 0 d | 0 d | 0 d | 0 d | 30.44 c | 76.34 b | 221.40 a | 83.39 b | Herbal, vegetable. |
| 26 | Decanoic acid, ethyl ester | 1338 | 1341 | MS,RI,O,S | 530 | 0 d | 0.01 d | 0.01 d | 0.02 d | 0.04 d | 0 d | 3.87 c | 56.29 b | 81.22 a | 50.06 b | Oily, brandy, apple. |
| 27 | Undecanoic acid, ethyl ester | 1349 | 1451 | MS,RI,O,S | 3 | 0 d | 0 d | 0 d | 0 d | 0 d | 5.76 c | 134.45 a | 52.00 b | 59.02 b | 0 d | Soapy, waxy, fatty. |
| 28 | δ-hexanolactone | 1419 | 1427 | MS,RI,O,S | 20 | 0 b | 0 b | 0 b | 0 b | 0 b | 0 b | 0 b | 4.12 a | 0 b | 0 b | Herbal, coconut. |
| 29 | Dodecanoic acid ethyl ester | 1421 | 1422 | MS,RI,O,S | 2 | 0 a | 0 a | 0 a | 0 a | 0 a | 0 a | 110.49 a | 119.95 a | 225.84 a | 123.15 a | Creamy, coconut. |
| 30 | γ-octalactone | 1433 | 1440 | MS,RI,O,S | 20 | 0 b | 0 b | 0 b | 0.24 b | 0.47 b | 0 b | 0 b | 10.94 a | 12.07 a | 10.01 a | Fatty, buttery, milky. |
| 31 | δ-octalactone | 1532 | 1526 | MS,RI,O,S | 0.42 a | 0 f | 0 f | 0 f | 0 f | 0 f | 23.14 d | 11.49 e | 142.11 b | 177.55 a | 113.55 c | Buttery, fruity. |
| Others | ||||||||||||||||
| 32 | Styrene | 1513 | 1528 | MS,RI,O,S | 65 a | 6.83 e | 7.16 e | 7.00 e | 7.31 e | 7.16 e | 10.66 d | 4.87 f | 53.63 a | 36.96 b | 21.94 c | Balsam, floral, plastic. |
| 33 | Benzaldehyde | 1247 | 1254 | MS,RI,O,S | 750 c | 0.43 c | 0.46 c | 0.47 c | 0.51 c | 0.53 c | 0.57 c | 0.44 c | 2.16 a | 0.89 bc | 0.59 c | Bitter, almond, cherry. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, H.; Wang, Y.; Han, H.; Cao, Y.; Wang, B. Changes in Key Aroma Compounds and Esterase Activity of Monascus-Fermented Cheese across a 30-Day Ripening Period. Foods 2022, 11, 4026. https://doi.org/10.3390/foods11244026
Zeng H, Wang Y, Han H, Cao Y, Wang B. Changes in Key Aroma Compounds and Esterase Activity of Monascus-Fermented Cheese across a 30-Day Ripening Period. Foods. 2022; 11(24):4026. https://doi.org/10.3390/foods11244026
Chicago/Turabian StyleZeng, Hong, Yadong Wang, Haoying Han, Yanping Cao, and Bei Wang. 2022. "Changes in Key Aroma Compounds and Esterase Activity of Monascus-Fermented Cheese across a 30-Day Ripening Period" Foods 11, no. 24: 4026. https://doi.org/10.3390/foods11244026
APA StyleZeng, H., Wang, Y., Han, H., Cao, Y., & Wang, B. (2022). Changes in Key Aroma Compounds and Esterase Activity of Monascus-Fermented Cheese across a 30-Day Ripening Period. Foods, 11(24), 4026. https://doi.org/10.3390/foods11244026





