Plasma Metabolomic Profiling Reveals Preliminary Biomarkers of Pork Quality Based on pH Value
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animals and Handling
2.3. Sample Collection
2.4. Meat Quality Traits
2.5. Determination of Metabolite Concentration
2.6. Metabolite Extraction, Separation, and UHPLC-MS/MS Analysis
2.7. Data Processing and Metabolite Identification
2.8. Statistical Analysis
3. Results
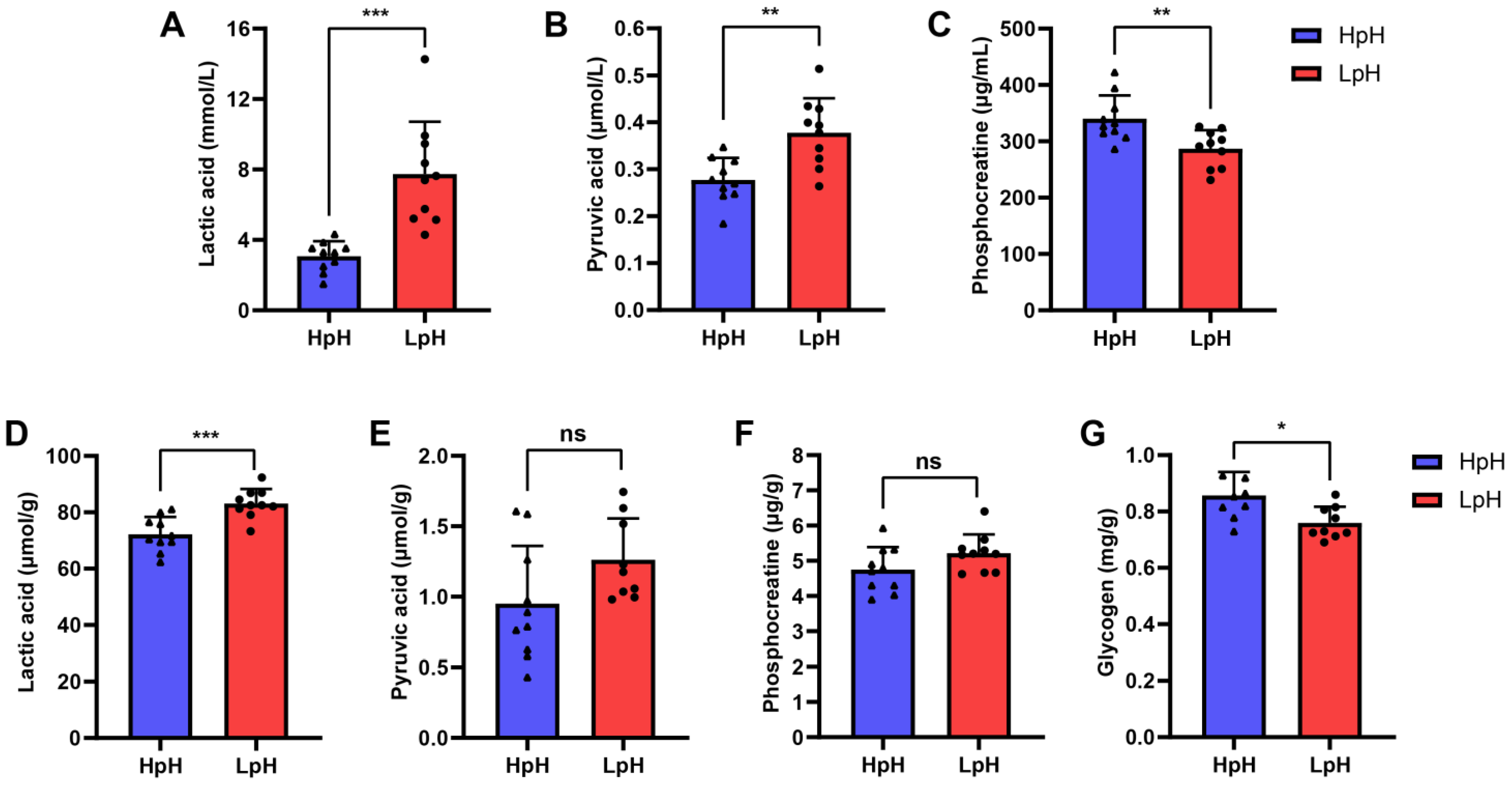
3.1. Correlation Analysis among Pork Meat Quality Traits
3.2. Comparison of Pork Meat with Different Quality
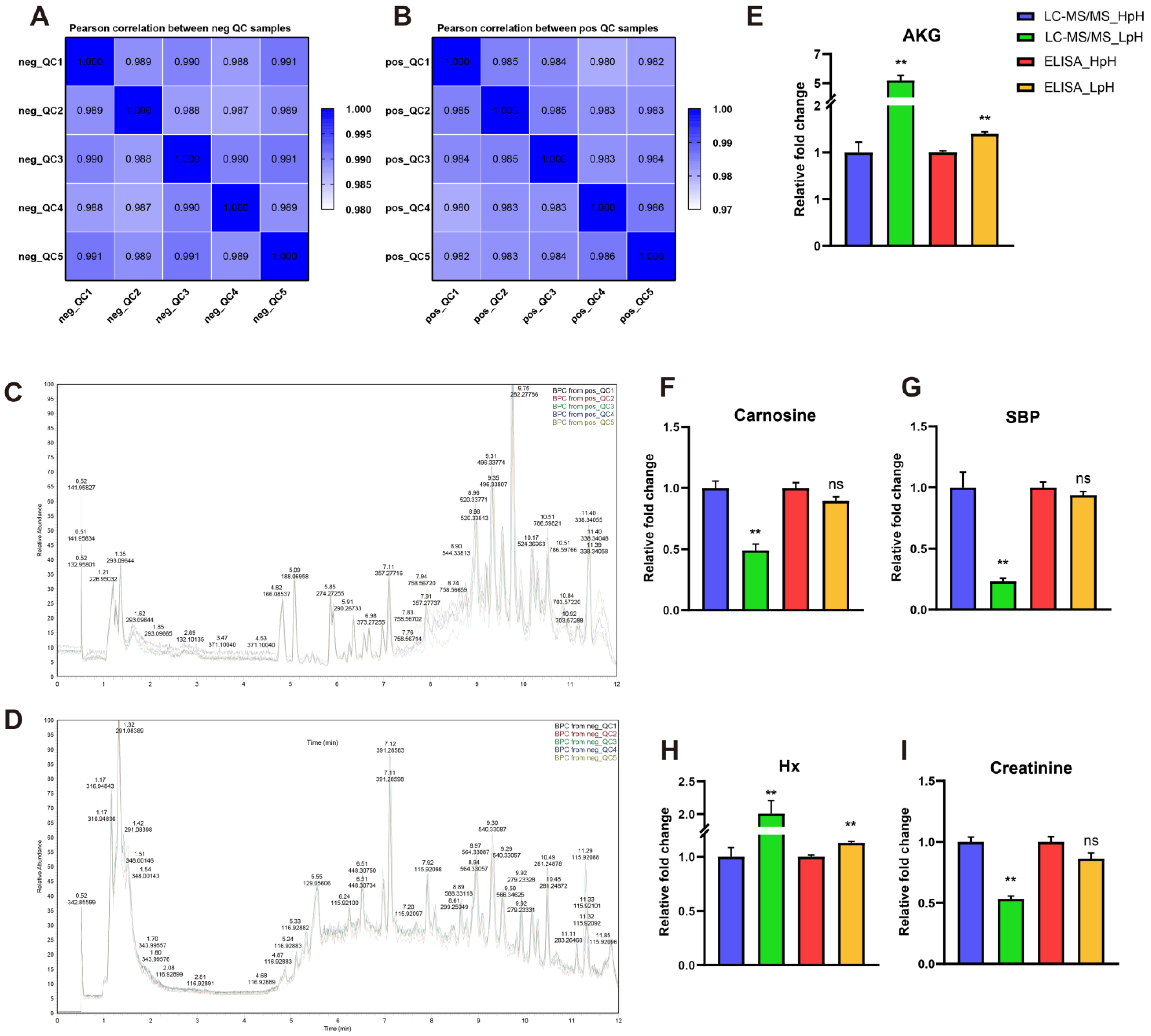
3.3. Relationship between Metabolite Profiles in Porcine Plasma and LTL Muscle
3.4. Quality Control of UHPLC-MS/MS Analysis
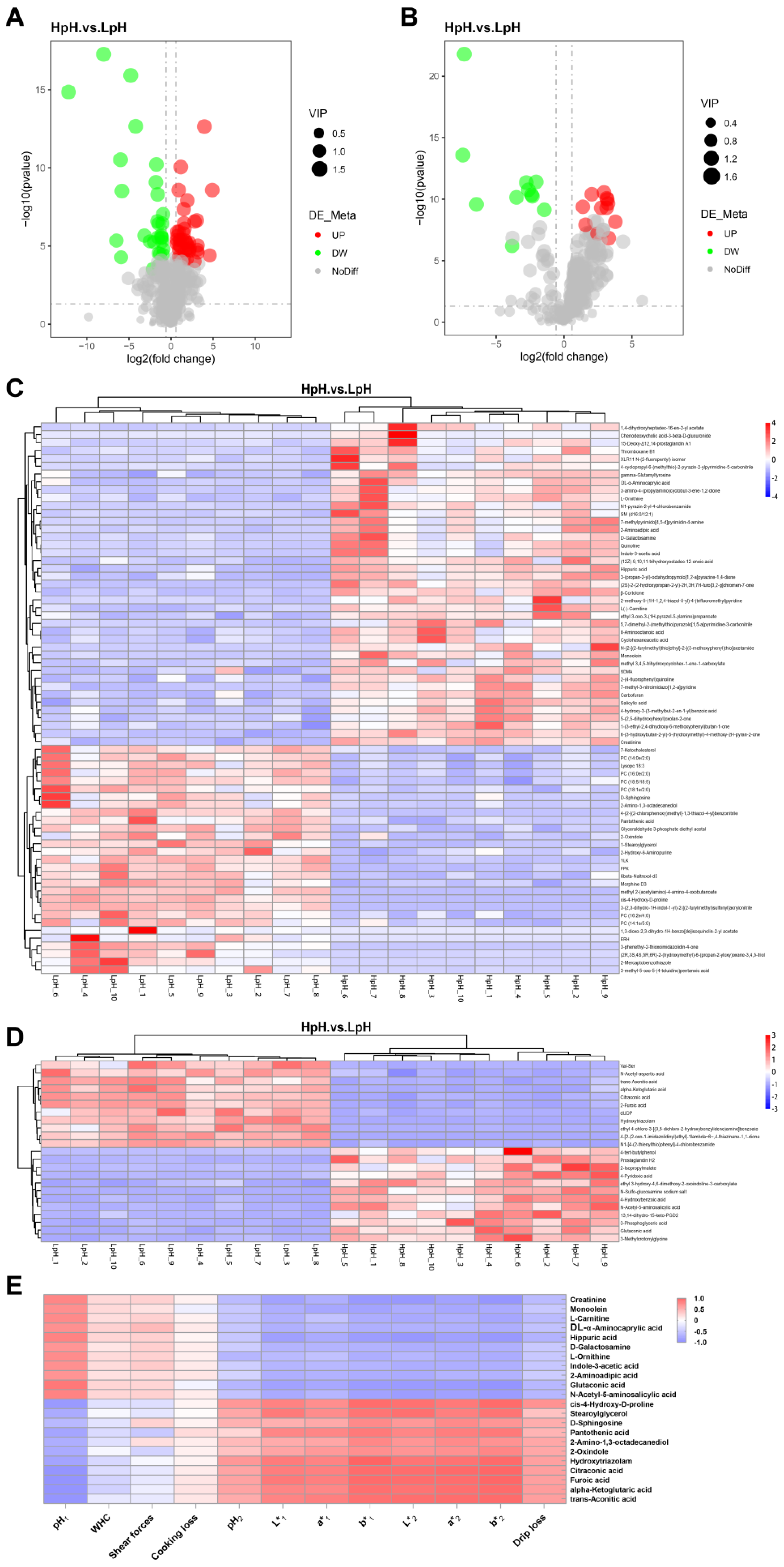
3.5. Multivariate Statistical Analysis
3.6. Screening and Identification of Differential Metabolites
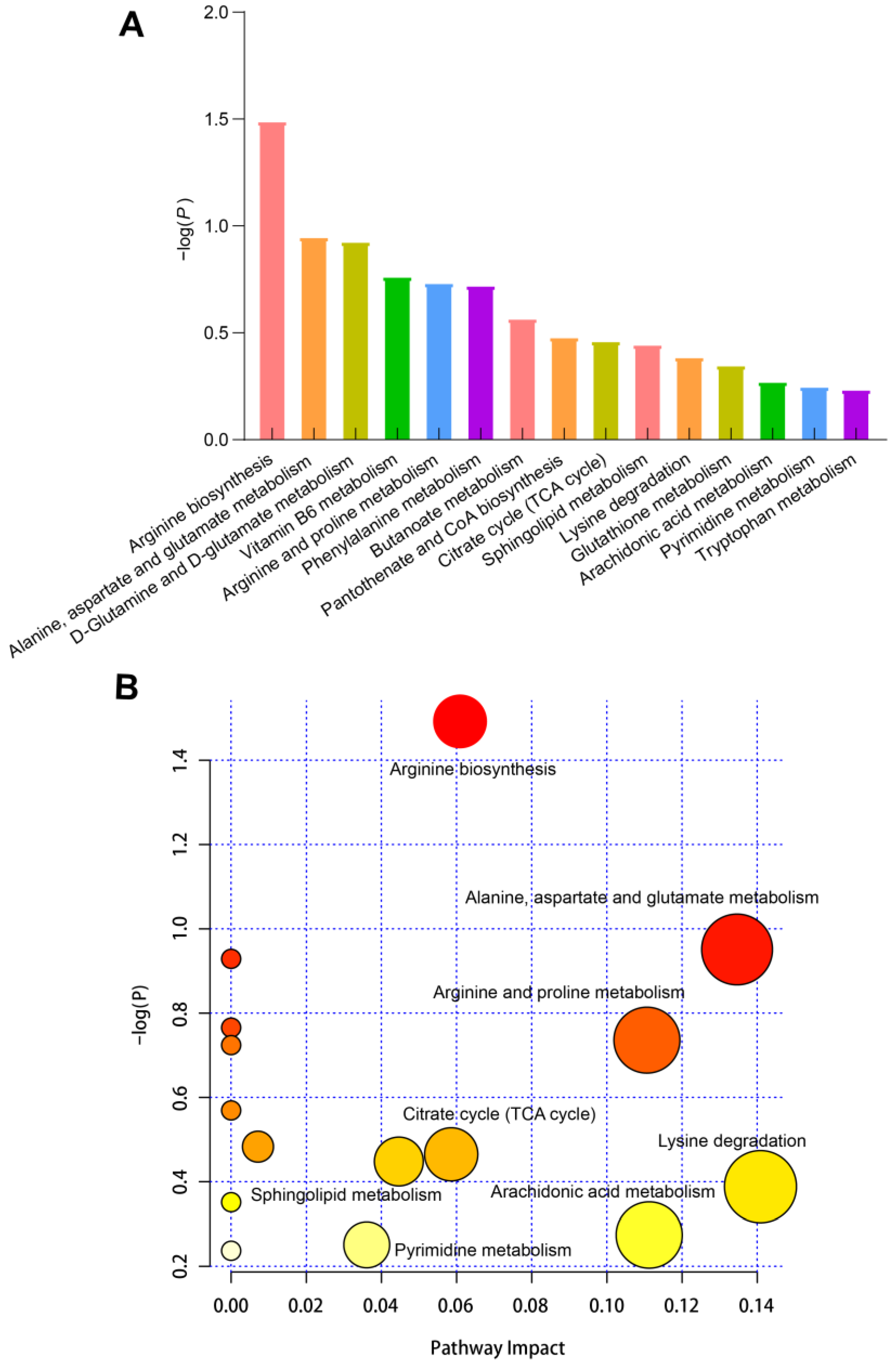
3.7. KEGG Enrichment Analysis of Differential Metabolites
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gan, M.; Shen, L.; Chen, L.; Jiang, D.; Jiang, Y.; Li, Q.; Chen, Y.; Ge, G.; Liu, Y.; Xu, X.; et al. Meat Quality, Amino Acid, and Fatty Acid Composition of Liangshan Pigs at Different Weights. Animals 2020, 10, 822. [Google Scholar] [CrossRef] [PubMed]
- Welzenbach, J.; Neuhoff, C.; Heidt, H.; Cinar, M.; Looft, C.; Schellander, K.; Tholen, E.; Große-Brinkhaus, C. Integrative Analysis of Metabolomic, Proteomic and Genomic Data to Reveal Functional Pathways and Candidate Genes for Drip Loss in Pigs. Int. J. Mol. Sci. 2016, 17, 1426. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, Y.; Ma, T.; Long, H.; Niu, L.; Zhang, X.; Lei, Y.; Wang, L.; Chen, Y.; Wang, Q.; et al. A Splicing Mutation in PHKG1 Decreased Its Expression in Skeletal Muscle and Caused PSE Meat in Duroc × Luchuan Crossbred Pigs. Anim. Genet. 2019, 50, 395–398. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, J.; Gong, H.; Cui, L.; Zhang, W.; Ma, J.; Chen, C.; Ai, H.; Xiao, S.; Huang, L.; et al. Genetic Correlation of Fatty Acid Composition with Growth, Carcass, Fat Deposition and Meat Quality Traits Based on GWAS Data in Six Pig Populations. Meat Sci. 2019, 150, 47–55. [Google Scholar] [CrossRef]
- Picard, B.; Lebret, B.; Cassar-Malek, I.; Liaubet, L.; Berri, C.; Le Bihan-Duval, E.; Hocquette, J.F.; Renand, G. Recent Advances in Omic Technologies for Meat Quality Management. Meat Sci. 2015, 109, 18–26. [Google Scholar] [CrossRef]
- Cui, L.; Lu, H.; Lee, Y.H. Challenges and Emergent Solutions for LC-MS/MS Based Untargeted Metabolomics in Diseases. Mass Spec. Rev. 2018, 37, 772–792. [Google Scholar] [CrossRef]
- Cevallos-Cevallos, J.M.; Reyes-De-Corcuera, J.I.; Etxeberria, E.; Danyluk, M.D.; Rodrick, G.E. Metabolomic Analysis in Food Science: A Review. Trends Food Sci. Technol. 2009, 20, 557–566. [Google Scholar] [CrossRef]
- Goldansaz, S.A.; Guo, A.C.; Sajed, T.; Steele, M.A.; Plastow, G.S.; Wishart, D.S. Livestock Metabolomics and the Livestock Metabolome: A Systematic Review. PLoS ONE 2017, 12, e0177675. [Google Scholar] [CrossRef]
- Clemmons, B.A.; Powers, J.B.; Campagna, S.R.; Seay, T.B.; Embree, M.M.; Myer, P.R. Rumen Fluid Metabolomics of Beef Steers Differing in Feed Efficiency. Metabolomics 2020, 16, 23. [Google Scholar] [CrossRef]
- Tan, C.; Selamat, J.; Jambari, N.N.; Sukor, R.; Murugesu, S.; Khatib, A. Muscle and Serum Metabolomics for Different Chicken Breeds under Commercial Conditions by GC–MS. Foods 2021, 10, 2174. [Google Scholar] [CrossRef]
- Xiao, R.-J.; Xu, Z.-R.; Chen, H.-L. Effects of Ractopamine at Different Dietary Protein Levels on Growth Performance and Carcass Characteristics in Finishing Pigs. Anim. Feed. Sci. Technol. 1999, 79, 119–127. [Google Scholar] [CrossRef]
- Gan, M.; Shen, L.; Fan, Y.; Guo, Z.; Liu, B.; Chen, L.; Tang, G.; Jiang, Y.; Li, X.; Zhang, S.; et al. High Altitude Adaptability and Meat Quality in Tibetan Pigs: A Reference for Local Pork Processing and Genetic Improvement. Animals 2019, 9, 1080. [Google Scholar] [CrossRef] [PubMed]
- Honikel, K.O. Reference Methods for the Assessment of Physical Characteristics of Meat. Meat Sci. 1998, 49, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Food Science and Technology. Muscle as Food; Bechtel, P.J., Ed.; Academic Press: Orlando, FL, USA, 1986; ISBN 978-0-12-084190-5. [Google Scholar]
- Cao, Z.; Wang, T.; Xia, W.; Zhu, B.; Tian, M.; Zhao, R.; Guan, D. A Pilot Metabolomic Study on Myocardial Injury Caused by Chronic Alcohol Consumption-Alcoholic Cardiomyopathy. Molecules 2021, 26, 2177. [Google Scholar] [CrossRef] [PubMed]
- Wen, B.; Mei, Z.; Zeng, C.; Liu, S. MetaX: A Flexible and Comprehensive Software for Processing Metabolomics Data. BMC Bioinform. 2017, 18, 183. [Google Scholar] [CrossRef]
- Hamoen, J.R.; Vollebregt, H.M.; van der Sman, R.G.M. Prediction of the Time Evolution of PH in Meat. Food Chem. 2013, 141, 2363–2372. [Google Scholar] [CrossRef]
- Ramanathan, R.; Mancini, R.A.; Dady, G.A. Effects of Pyruvate, Succinate, and Lactate Enhancement on Beef Longissimus Raw Color. Meat Sci. 2011, 88, 424–428. [Google Scholar] [CrossRef]
- Wang, C.; Matarneh, S.K.; Gerrard, D.; Tan, J. Contributions of Energy Pathways to ATP Production and PH Variations in Postmortem Muscles. Meat Sci. 2022, 189, 108828. [Google Scholar] [CrossRef]
- Hayat, M.N.; Kaka, U.; Sazili, A.Q. Assessment of Physicochemical Characteristics and Microbiological Quality in Broiler Chicken Breast Muscle (Pectoralis Major) Subjected to Different Temperatures and Lengths of Cold Transportation. Foods 2021, 10, 874. [Google Scholar] [CrossRef]
- Fang, L.H.; Jin, Y.H.; Do, S.H.; Hong, J.S.; Kim, B.O.; Han, T.H.; Kim, Y.Y. Effects of Dietary Energy and Crude Protein Levels on Growth Performance, Blood Profiles, and Carcass Traits in Growing-Finishing Pigs. J. Anim. Sci. Technol. 2019, 61, 204–215. [Google Scholar] [CrossRef]
- Thema, K.; Mlambo, V.; Snyman, N.; Mnisi, C.M. Evaluating Alternatives to Zinc-Bacitracin Antibiotic Growth Promoter in Broilers: Physiological and Meat Quality Responses. Animals 2019, 9, 1160. [Google Scholar] [CrossRef] [PubMed]
- Saeed, O.A.; Sazili, A.Q.; Akit, H.; Ebrahimi, M.; Alimon, A.R.; Samsudin, A.A. Effects of Corn Supplementation on Meat Quality and Fatty Acid Composition of Dorper Lambs Fed PKC-Urea Treated Rice Straw. BMC Vet. Res. 2019, 15, 233. [Google Scholar] [CrossRef] [PubMed]
- Jallow, D.B.; Chou Hsia, L. Effect of Sodium Bicarbonate Supplementation on Carcass Characteristics of Lambs Fed Concentrate Diets at Different Ambient Temperature Levels. Asian Australas. J. Anim. Sci. 2014, 27, 1098–1103. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Welzenbach, J.; Neuhoff, C.; Looft, C.; Schellander, K.; Tholen, E.; Große-Brinkhaus, C. Different Statistical Approaches to Investigate Porcine Muscle Metabolome Profiles to Highlight New Biomarkers for Pork Quality Assessment. PLoS ONE 2016, 11, e0149758. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, J.; Ma, J.; Yu, Q.; Han, L. ITRAQ-Mediated Analysis of the Relationship between Proteomic Changes and Yak Longissimus Lumborum Tenderness over the Course of Postmortem Storage. Sci. Rep. 2021, 11, 10450. [Google Scholar] [CrossRef]
- Muchenje, V.; Dzama, K.; Chimonyo, M.; Strydom, P.E.; Raats, J.G. Relationship between Pre-Slaughter Stress Responsiveness and Beef Quality in Three Cattle Breeds. Meat Sci. 2009, 81, 653–657. [Google Scholar] [CrossRef]
- Scheffler, T.L.; Gerrard, D.E. Mechanisms Controlling Pork Quality Development: The Biochemistry Controlling Postmortem Energy Metabolism. Meat Sci. 2007, 77, 7–16. [Google Scholar] [CrossRef]
- Otto, G.; Roehe, R.; Looft, H.; Thoelking, L.; Kalm, E. Comparison of Different Methods for Determination of Drip Loss and Their Relationships to Meat Quality and Carcass Characteristics in Pigs. Meat Sci. 2004, 68, 401–409. [Google Scholar] [CrossRef]
- Bararpour, N.; Gilardi, F.; Carmeli, C.; Sidibe, J.; Ivanisevic, J.; Caputo, T.; Augsburger, M.; Grabherr, S.; Desvergne, B.; Guex, N.; et al. DBnorm as an R Package for the Comparison and Selection of Appropriate Statistical Methods for Batch Effect Correction in Metabolomic Studies. Sci. Rep. 2021, 11, 5657. [Google Scholar] [CrossRef]
- Song, B.; Zheng, C.; Zheng, J.; Zhang, S.; Zhong, Y.; Guo, Q.; Li, F.; Long, C.; Xu, K.; Duan, Y.; et al. Comparisons of Carcass Traits, Meat Quality, and Serum Metabolome between Shaziling and Yorkshire Pigs. Anim. Nutr. 2022, 8, 125–134. [Google Scholar] [CrossRef]
- Koeth, R.A.; Wang, Z.; Levison, B.S.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L.; et al. Intestinal Microbiota Metabolism of L-Carnitine, a Nutrient in Red Meat, Promotes Atherosclerosis. Nat. Med. 2013, 19, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Sidwick, K.L.; Johnson, A.E.; Adam, C.D.; Pereira, L.; Thompson, D.F. Use of Liquid Chromatography Quadrupole Time-of-Flight Mass Spectrometry and Metabonomic Profiling To Differentiate between Normally Slaughtered and Dead on Arrival Poultry Meat. Anal. Chem. 2017, 89, 12131–12136. [Google Scholar] [CrossRef] [PubMed]


| Meat Quality Trait | High pH | Low pH | p Value |
|---|---|---|---|
| Body weight | 128.5 ± 14.74 | 138 ± 15.04 | ns |
| pH1 | 6.75 ± 0.08 | 6.16 ± 0.22 | ** |
| pH2 | 5.40 ± 0.05 | 5.51 ± 0.07 | * |
| L*1 | 39.07 ± 0.98 | 44.40 ± 2.10 | * |
| a*1 | 3.96 ± 0.90 | 6.89 ± 1.29 | ** |
| b*1 | 3.11 ± 0.62 | 9.25 ± 0.91 | ** |
| L*2 | 43.07 ± 2.54 | 56.57 ± 2.70 | ** |
| a*2 | 5.85 ± 1.00 | 10.27 ± 0.99 | ** |
| b*2 | 4.40 ± 1.08 | 11.95 ± 0.47 | ** |
| WHC (%) | 73.57 ± 1.99 | 71.64 ± 3.53 | ns |
| Drip loss (%) | 2.26 ± 0.60 | 4.03 ± 1.43 | * |
| Shear forces (N) | 7.16 ± 1.26 | 6.39 ± 2.09 | ns |
| Cooking loss (%) | 35.40 ± 3.90 | 36.04 ± 2.37 | ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, L.; Ma, J.; Zhou, H.; Chen, L.; Tang, J.; Zhang, K.; Zhao, Y.; Niu, L.; Zhang, S.; Jiang, A.; et al. Plasma Metabolomic Profiling Reveals Preliminary Biomarkers of Pork Quality Based on pH Value. Foods 2022, 11, 4005. https://doi.org/10.3390/foods11244005
Shen L, Ma J, Zhou H, Chen L, Tang J, Zhang K, Zhao Y, Niu L, Zhang S, Jiang A, et al. Plasma Metabolomic Profiling Reveals Preliminary Biomarkers of Pork Quality Based on pH Value. Foods. 2022; 11(24):4005. https://doi.org/10.3390/foods11244005
Chicago/Turabian StyleShen, Linyuan, Jianfeng Ma, Haodi Zhou, Lei Chen, Jie Tang, Kaige Zhang, Ye Zhao, Lili Niu, Shunhua Zhang, Anan Jiang, and et al. 2022. "Plasma Metabolomic Profiling Reveals Preliminary Biomarkers of Pork Quality Based on pH Value" Foods 11, no. 24: 4005. https://doi.org/10.3390/foods11244005
APA StyleShen, L., Ma, J., Zhou, H., Chen, L., Tang, J., Zhang, K., Zhao, Y., Niu, L., Zhang, S., Jiang, A., Wang, J., Guo, Z., Li, X., Chen, Y., Gan, M., & Zhu, L. (2022). Plasma Metabolomic Profiling Reveals Preliminary Biomarkers of Pork Quality Based on pH Value. Foods, 11(24), 4005. https://doi.org/10.3390/foods11244005







