Modified Atmosphere Packaging and 1-Methylcyclopropene Treatments Maintain the Fruit Quality of ‘Wonmi’ Persimmons during Export Simulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Simulation for Export
2.2. 1-MCP Treatments and Modified Atmosphere Packaging
2.3. Weight Loss Rate
2.4. Fruit Firmness
2.5. Soluble Solids Content (SSC)
2.6. Ethylene Production and the Respiration Rate
2.7. Color
2.8. RNA Isolation and Analysis of Gene Expression
2.9. Statistical Analysis
3. Results
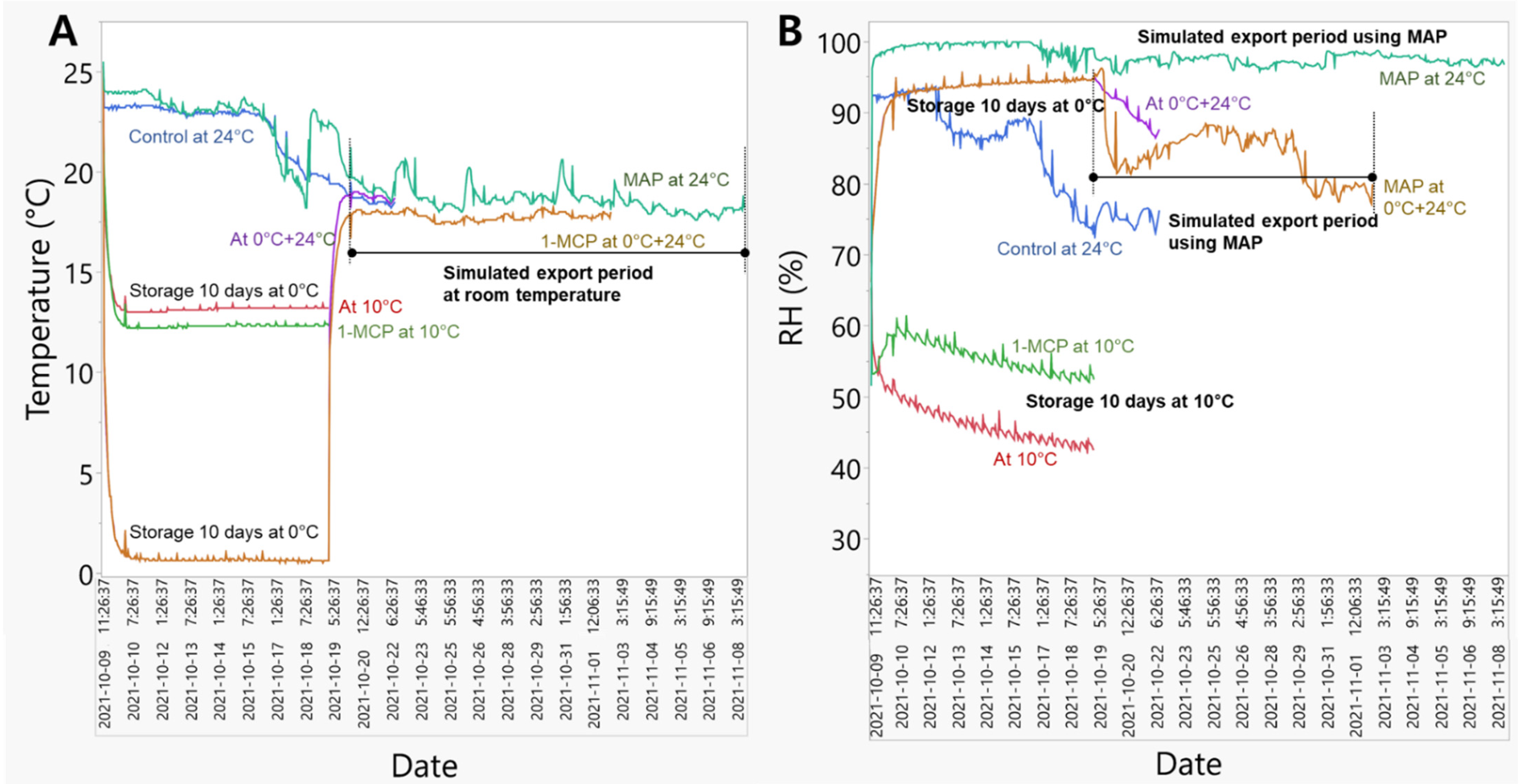
3.1. Change in Temperature and Relative Humidity during the Simulated Export Distribution Period
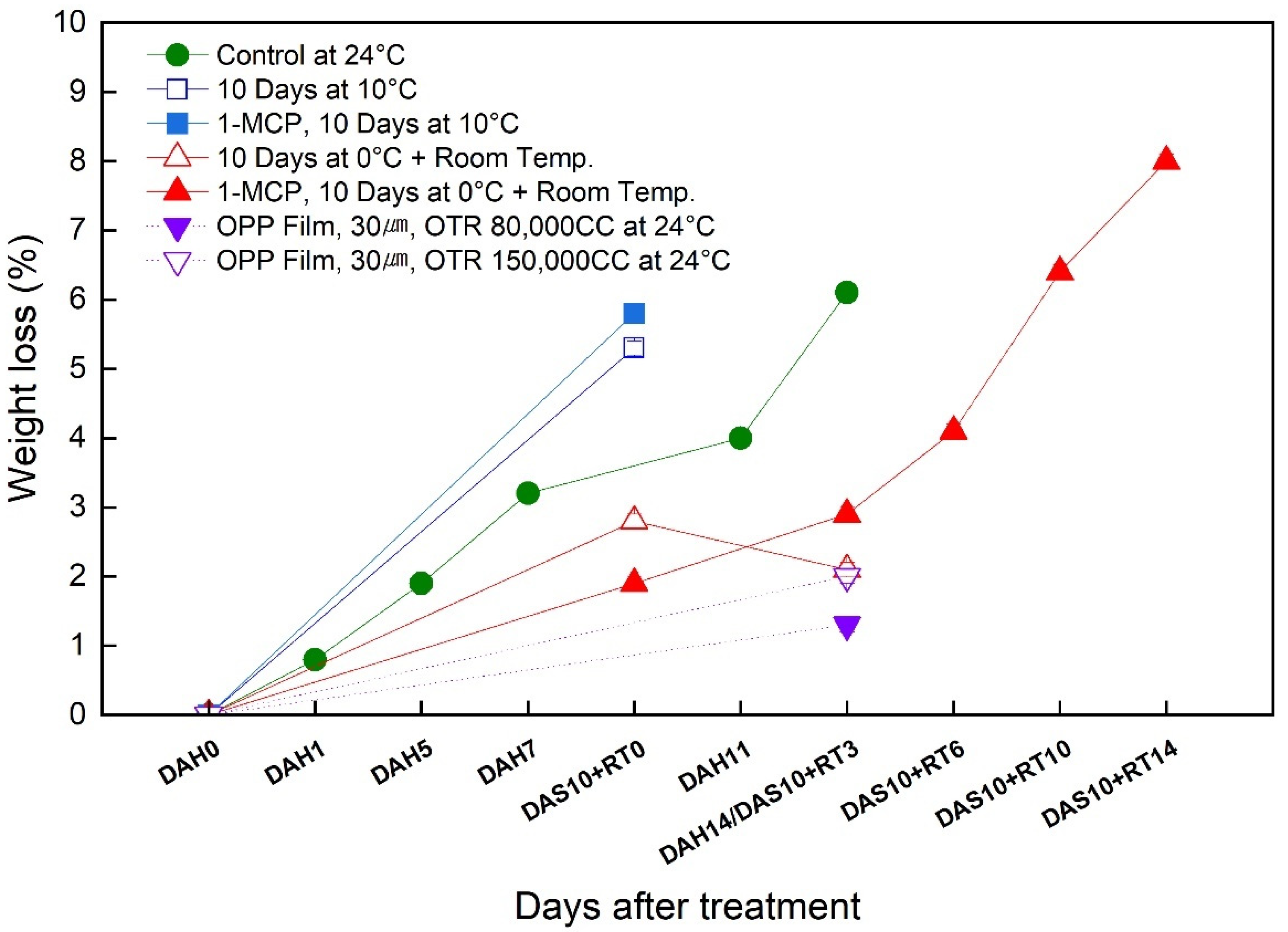
3.2. Change in the Weight Loss of the Sweet Persimmon ‘Wonmi’ during the Simulated Export Distribution Period
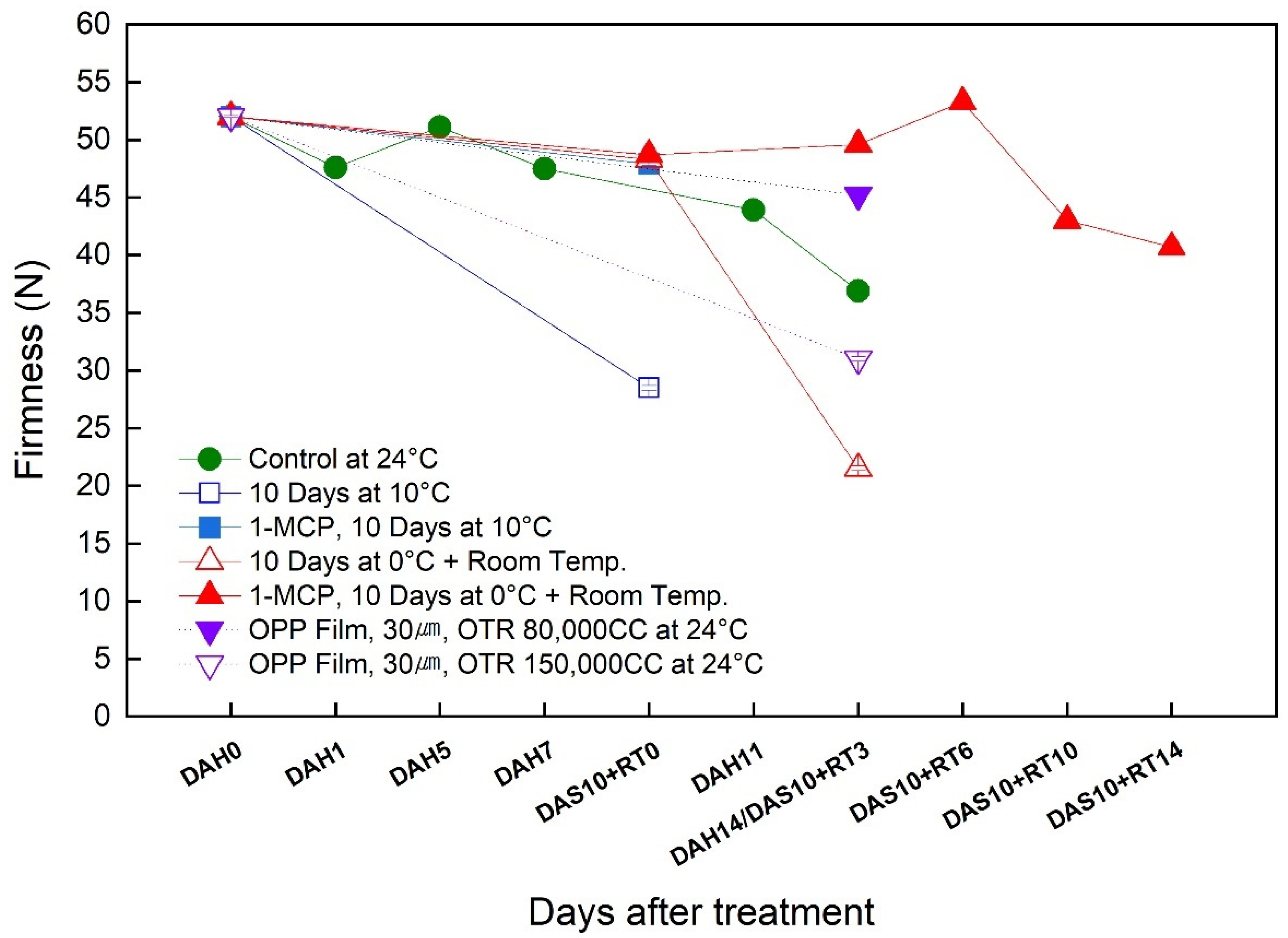
3.3. Change in the Firmness during the Simulated Export Distribution Period
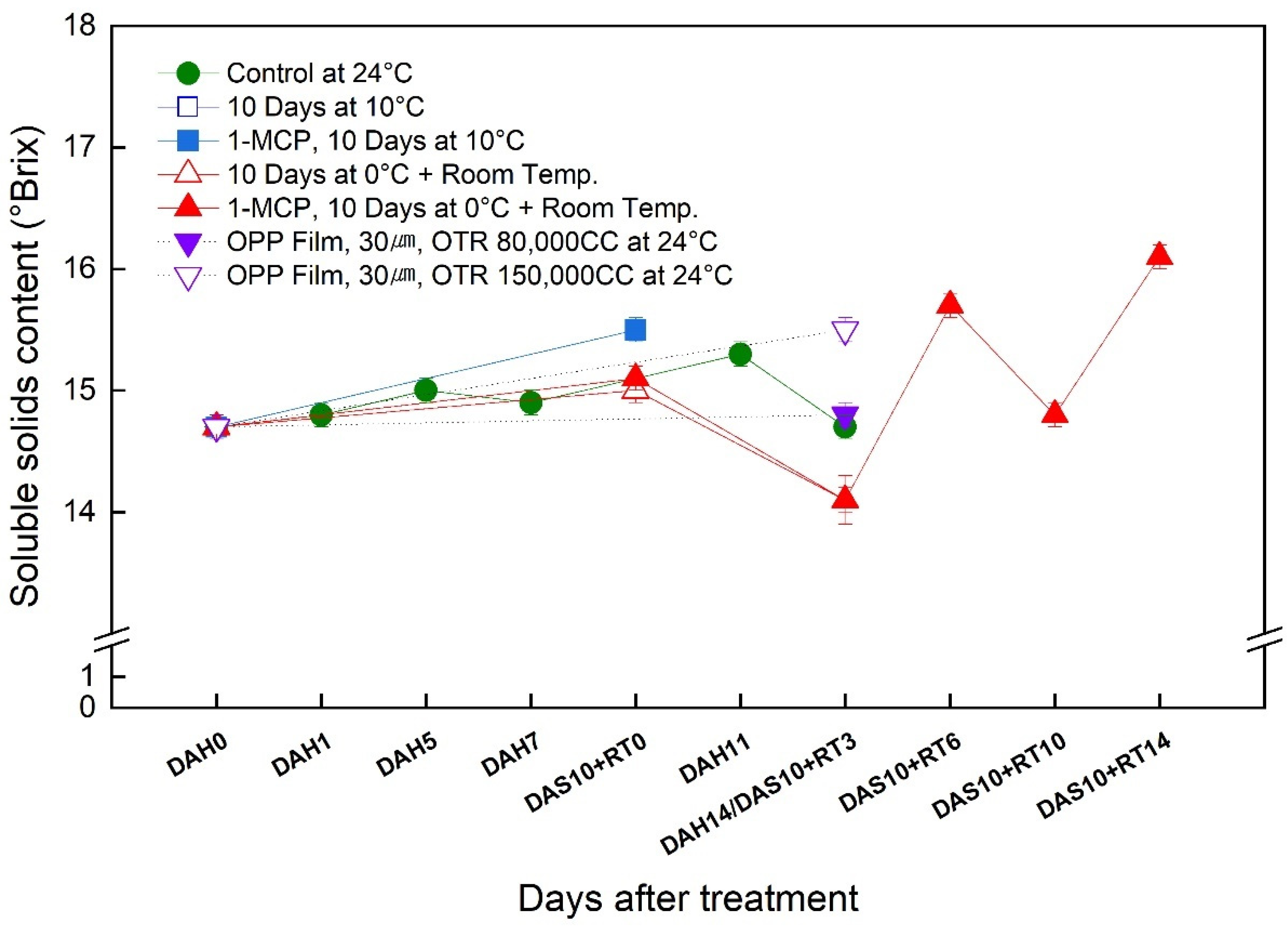
3.4. Change in the Soluble Solids Content (SSC) of the ‘Wonmi’ Persimmon during the Simulated Export Distribution Period
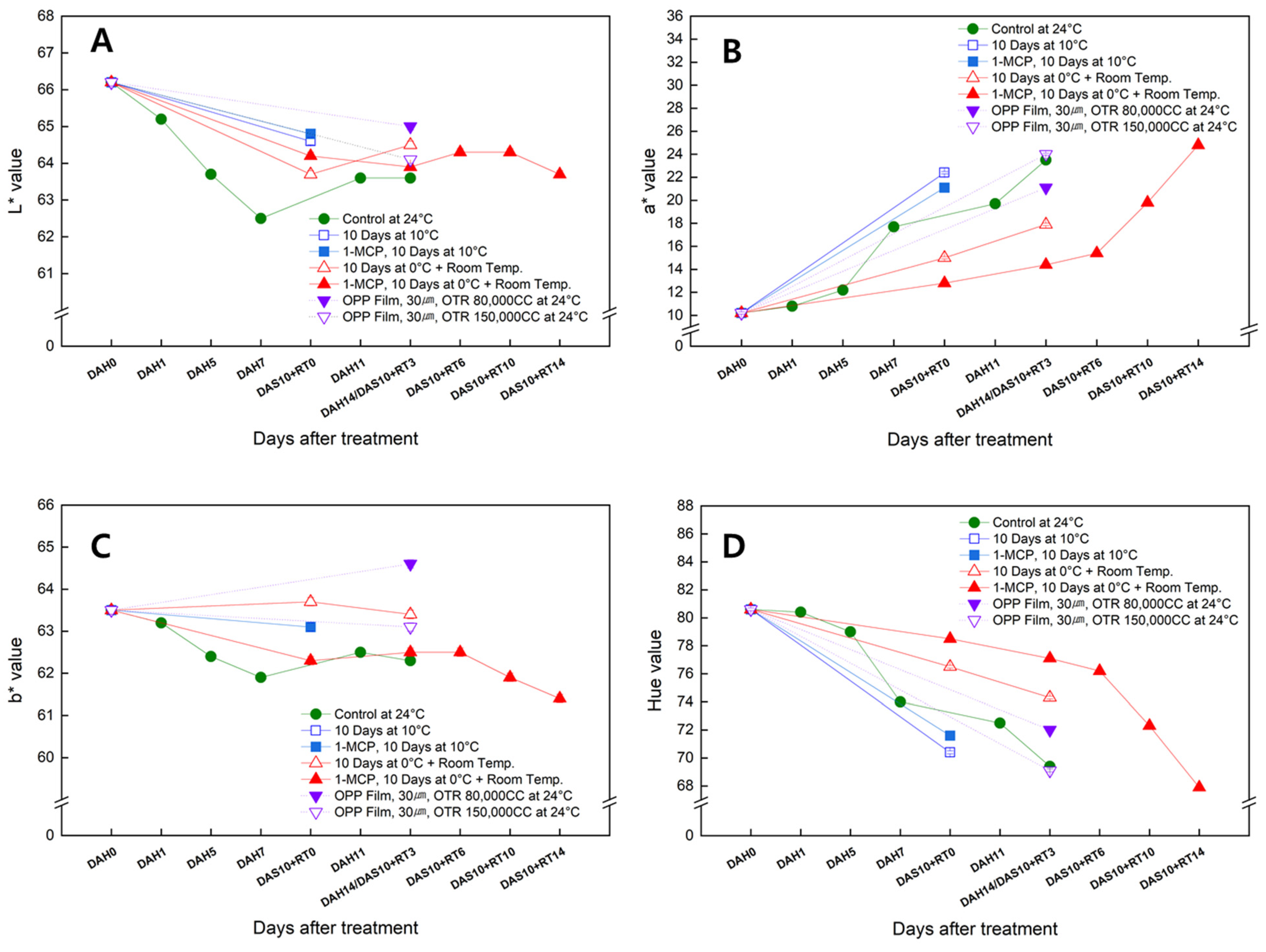
3.5. Changes in the Skin Color of the Sweet Persimmon ‘Wonmi’ during the Simulated Export Distribution Period
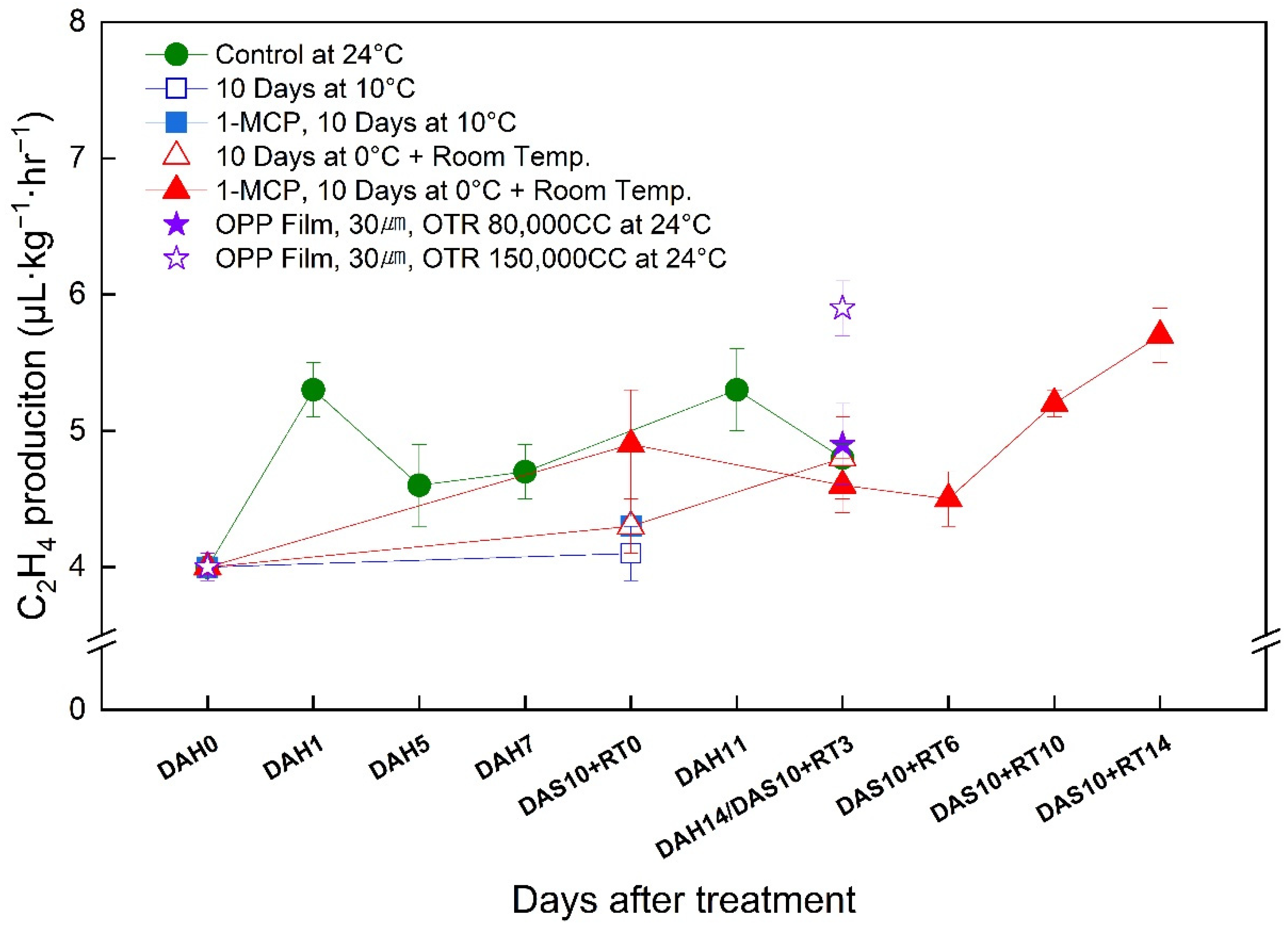
3.6. Changes in the Ethylene Production Rate of the ‘Wonmi’ Persimmon during the Simulated Export Distribution Period
3.7. Changes in the Respiration Rate of ‘Wonmi’ Persimmons during the Simulated Export Distribution Period
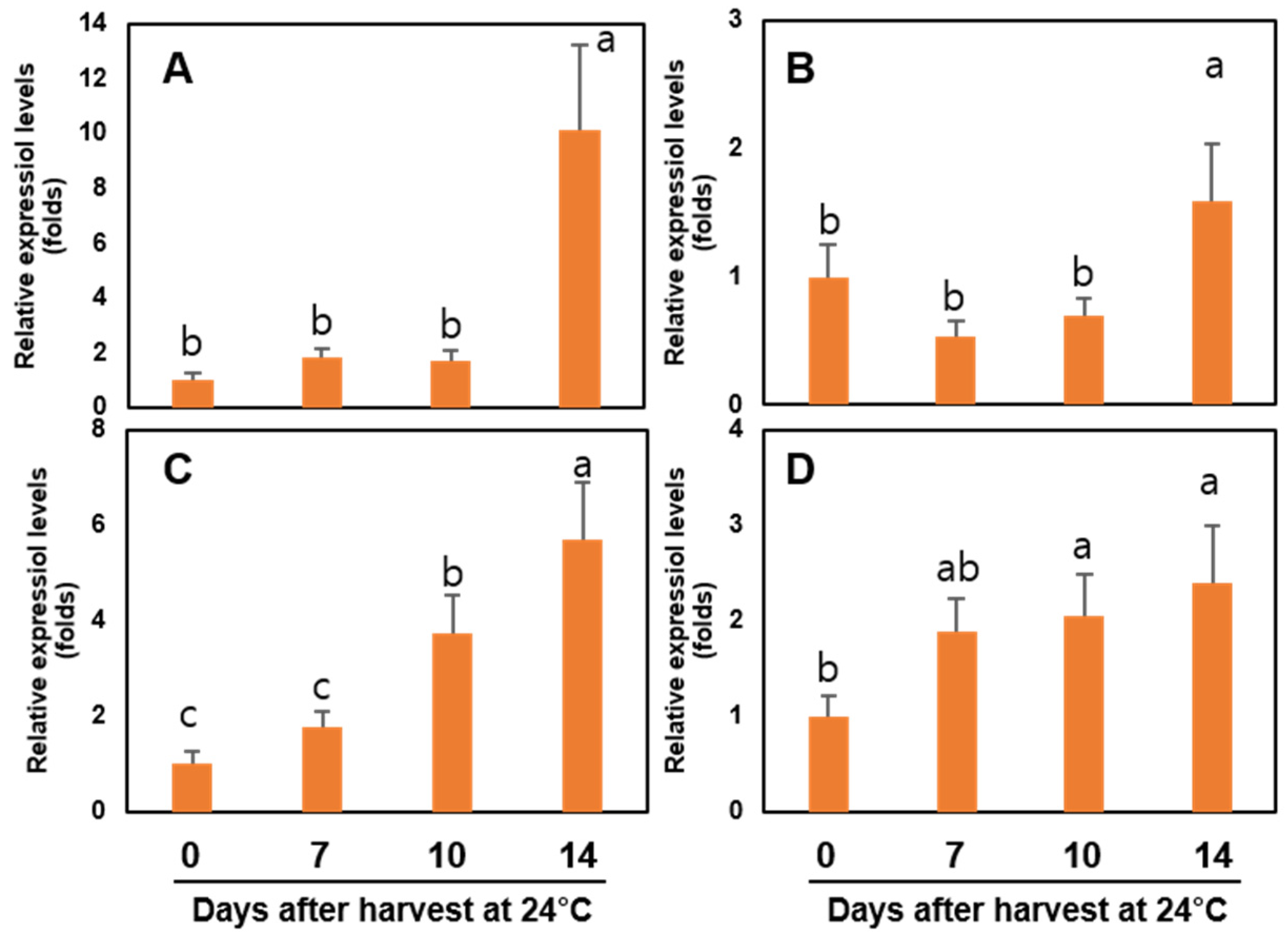
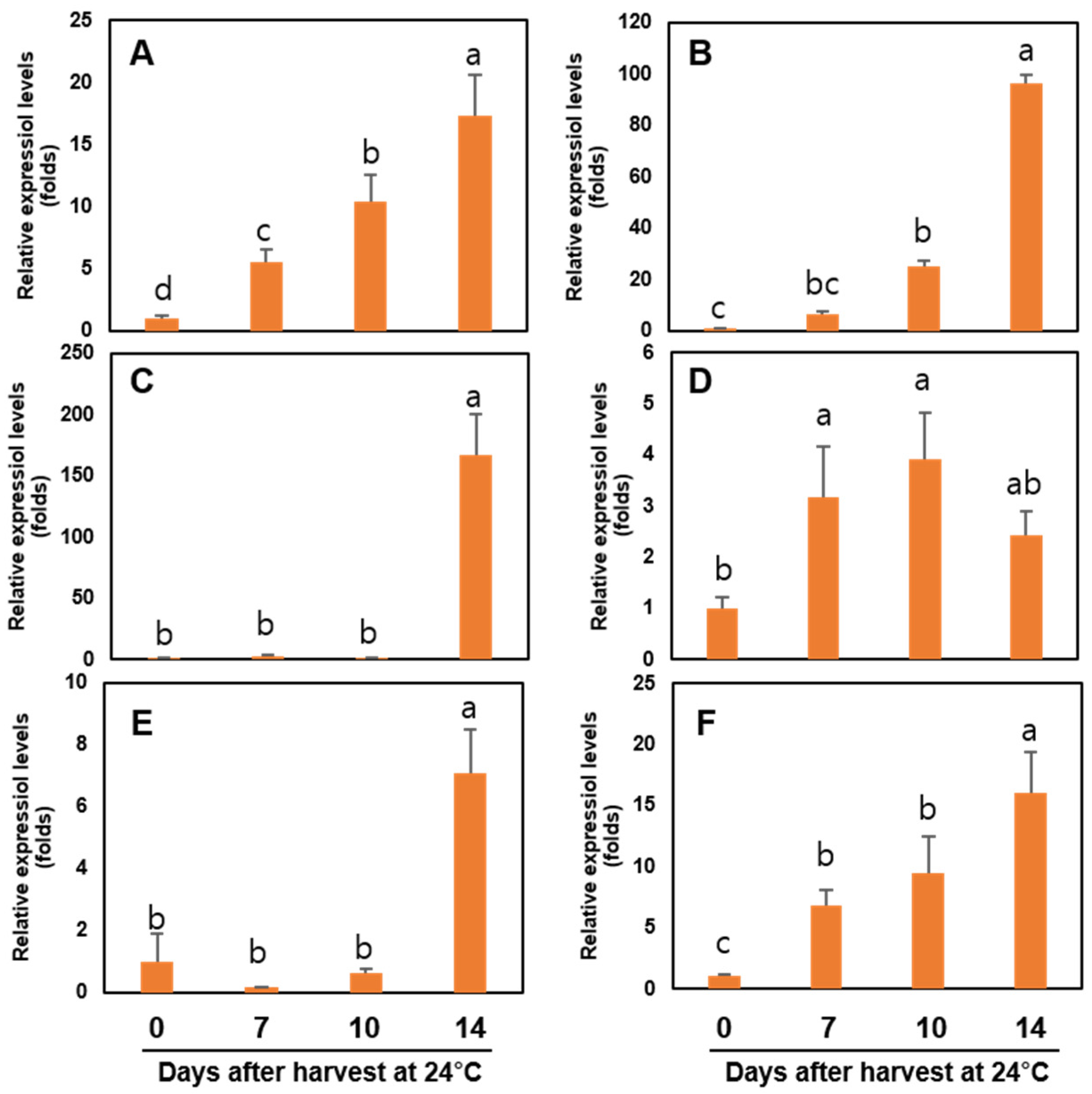
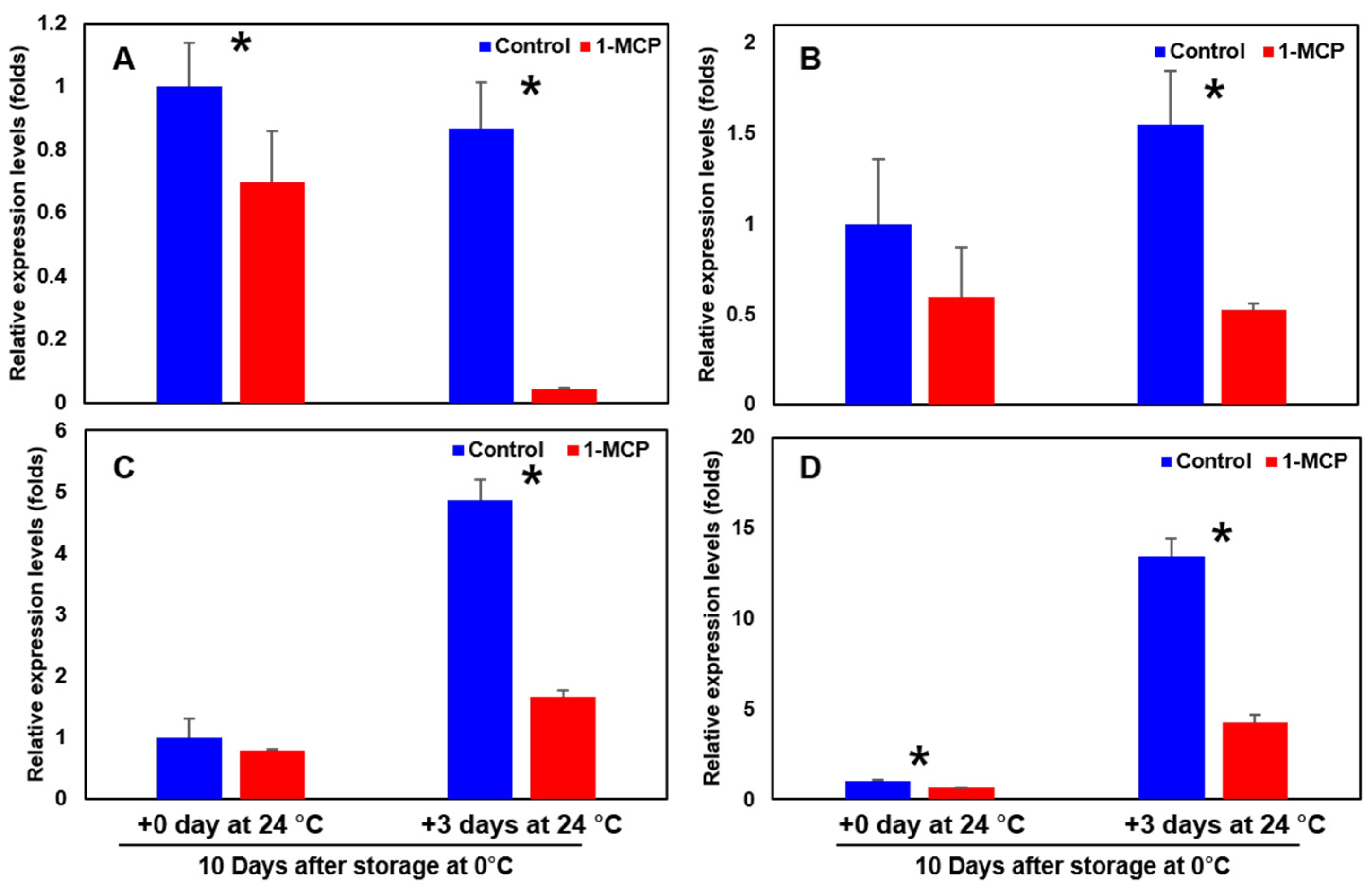
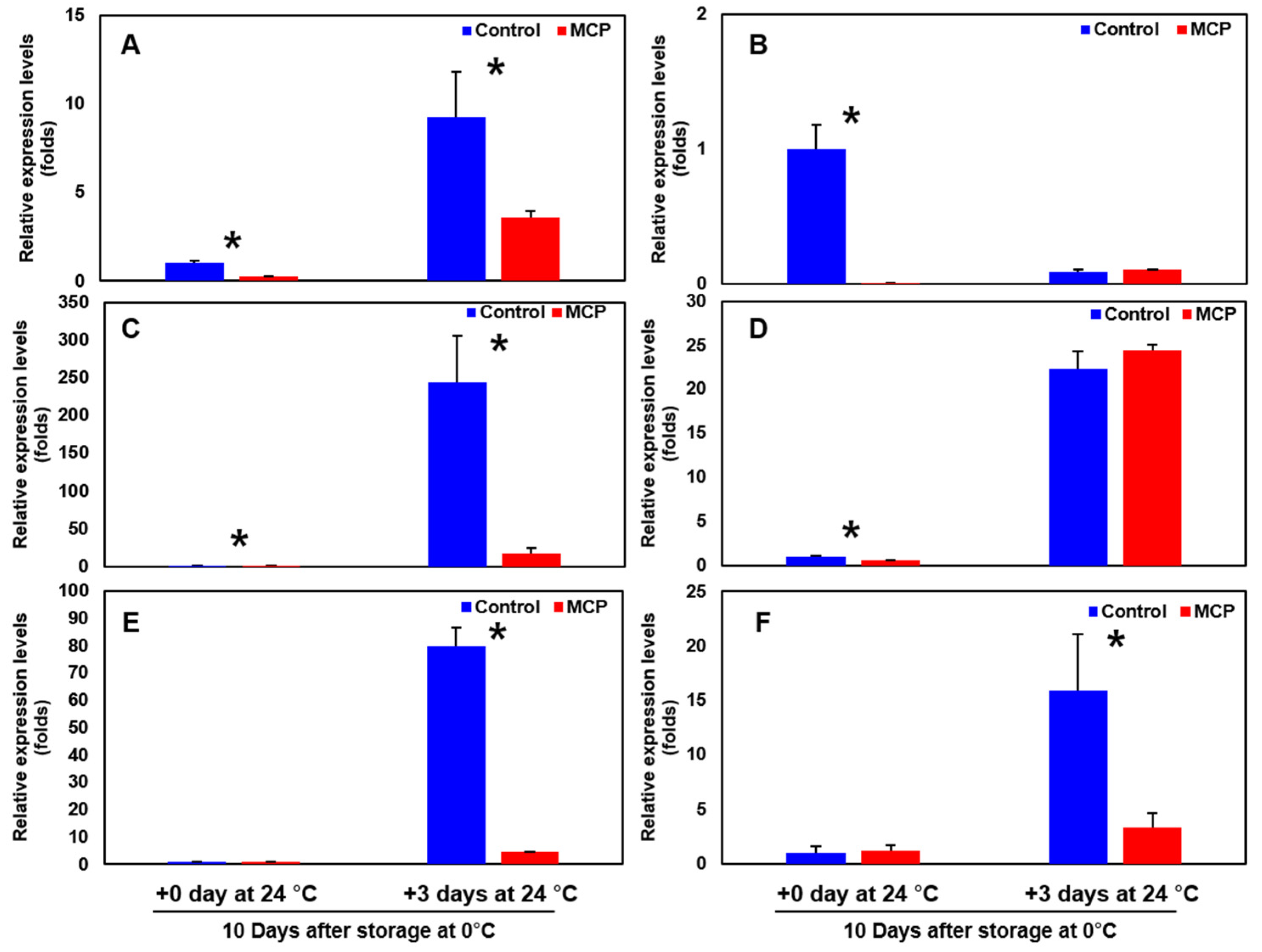
3.8. Expression of Ethylene Biosynthesis-, and Cell Wall Modification-Related Genes of the Sweet Persimmon ‘Wonmi’ during the Simulated Export Distribution Period
4. Discussion
4.1. Effects of Temperature and Relative Humidity on ‘Wonmi’ Persimmon Fruit Quality Attributes during the Simulated Export Distribution Period
4.2. Effects of 1-MCP Treatment on ‘Wonmi’ Persimmon Fruit Quality Attributes during the Simulated Export Distribution Period
4.3. Effects of MAP Treatment on ‘Wonmi’ Persimmon Fruit Quality Attributes during the Simulated Export Distribution Period
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kou, J.; Wei, C.; Zhao, Z.; Guan, J.; Wang, W. Effects of ethylene and 1-methylcyclopropene treatments on physiological changes and ripening-related gene expression of ‘Mopan’ persimmon fruit during storage. Postharvest Biol. Technol. 2020, 166, 111185. [Google Scholar] [CrossRef]
- Bibi, F.; Muhammad, A.; Khalid, M.U.; Shaukat, M.; Ahmad, U.; Batool, Z.; Khan, M.; Farooq, B.; Anjum, R.; Nishat, Z. Effect of hypobaric treatment and storage temperature on shelf life of persimmon fruit. Life Sci. J. 2021, 18, 55–60. [Google Scholar]
- Song, W.D.; Choo, Y.D.; Kim, T.C.; Kang, S.M. Science and industry of persimmon in the Republic of Korea. In III International Symposium on Persimmon, Jinju, Republic of Korea, 5 October 2004; Park, Y.M., Kang, S.M., Eds.; Acta Horticulturae; ISHS: Jinju, Republic of Korea, 2005; Volume 685, pp. 21–27. [Google Scholar]
- Ahn, G.H.; Choi, S.J.; Shin, G.H.; Kim, J.T. Quality assessment of ‘Fuyu’ persimmon treated by a combination of γ-irradiation and 1-methylcyclopropene. Korean J. Food Preserv. 2018, 25, 411–416. [Google Scholar] [CrossRef]
- Choi, S.T.; Kim, S.C.; Ahn, G.H.; Park, D.S.; Son, J.Y.; Kim, E.S. Introduction to sweet persimmon industry and advances in cultural practices in the Republic of Korea. In Proceedings of the VI International Symposium on Persimmon, Valencia, Spain, 16 October 2016; Badenes, M.L., Naval, M.M., Eds.; Acta Horticulturae. ISHS: Valencia, Spain, 2018; Volume 1195, pp. 17–22. [Google Scholar]
- Ma, K.B.; Yang, S.J.; Jo, Y.S.; Cho, K.S.; Kang, S.S. ‘Wonmi’, an early maturing, high-quality, sweet persimmon cultivar. Korean J. Breed. Sci. 2020, 52, 258–262. [Google Scholar] [CrossRef]
- Ma, K.B.; Yang, S.J.; Jo, Y.S.; Kang, S.S. Current status of persimmon industry and prospect of the Korea-bred new persimmon varieties in Australia. J. Korean Soc. Int. Agric. 2020, 32, 377–380. [Google Scholar] [CrossRef]
- Besada, C.; Gil, R.; Fathi, A.; Navarro, P.; Salvador, A. Effect of transport temperature on persimmon fruit quality. In Proceedings of the VI International Conference Postharvest Unlimited, Madrid, Spain, 17 October 2017; Valero, D., Serrano, M., Gil, M., Eds.; Acta Horticulturae. ISHS: Madrid, Spain, 2019; Volume 1256, pp. 355–360. [Google Scholar]
- Iqbal, M.; Bibi, F.; Naeem, M.; Khan, M.A.; Khan, R. Effect of various temperatures on the postharvest quality and storage life of persimmon fruit. J. Postharvest Technol. 2019, 7, 9–24. [Google Scholar]
- Tu, K.; Nicolaıï, B.; Baerdemaeker, J.D. Effects of relative humidity on apple quality under simulated shelf temperature storage. Sci. Hortic. 2000, 85, 217–229. [Google Scholar] [CrossRef]
- Salvador, A.; Arnal, L.; Monterde, A.; Martínez-Jávega, J.M. Influence of ripening stage at harvest on chilling injury symptoms of persimmon cv. Rojo Brillante stored at different temperatures. Food Sci. Technol. Int. 2005, 11, 359–365. [Google Scholar] [CrossRef]
- Fahmy, K.; Nakano, K. Effective transport and storage condition for preserving the quality of ‘Jiro’ persimmon in export market. Agric. Agric. Sci. Procedia 2016, 9, 279–290. [Google Scholar] [CrossRef][Green Version]
- Crisosto, C.H. Persimmon Postharvest Quality Maintenance Guidelines. University of California. Davis, CA, 95616 USA. 1999. Available online: http://www.uckac.edu/postharv/PDF%20files/Guidelines/persimmon.pdf.
- Vilhena, N.Q.; Gil, R.; Vendrell, M.; Salvador, A. Effect of preharvest 1-mcp treatment on the flesh firmness of ‘Rojo Brillante’ persimmon. Horticulturae 2022, 8, 350. [Google Scholar] [CrossRef]
- Ramin, A.A. Shelf-life extension of ripe non-astringent persimmon fruit using 1-MCP. Asian J. Plant Sci. 2008, 7, 218–222. [Google Scholar] [CrossRef]
- Karmoker, P.; Obatake, W.; Tanaka, F.; Tanaka, F. Efficacy of 1-MCP for modulating the ethylene sensitivity of Japanese persimmon in relation with internal structure. Environ. Control Biol. 2018, 56, 177–185. [Google Scholar] [CrossRef]
- Park, D.S.; Tilahun, S.; Heo, J.Y.; Park, K.C.; Jeong, C.S. Effect of 1-MCP on persimmon fruit quality and expression of ethylene response genes during ripening. J. Am. Pomol. Soc. 2017, 71, 103–111. [Google Scholar]
- He, Y.; Xue, J.; Li, H.; Han, S.; Jiao, J.; Rao, J. Ethylene response factors regulate ethylene biosynthesis and cell wall modification in persimmon (Diospyros kaki L.) fruit during ripening. Postharvest Biol. Technol. 2020, 168, 111255. [Google Scholar] [CrossRef]
- Jeong, M.; An, D.S.; Ahn, G.H.; Lee, D.S. Master packaging system for sweet persimmon applicable to produce supply chains. Postharvest Biol. Technol. 2013, 86, 141–146. [Google Scholar] [CrossRef]
- Ahmed, D.M.; Yousef, A.R.M.; Sarrwy, S.M.A. Modified atmosphere packaging for maintaining quality and shelf life extension of persimmon fruits. Asian J. Agric. Sci. 2011, 3, 308–316. [Google Scholar]

| Gene Name | Forward Primer | Reverse Primer |
| ACS | AGAATCCGGACGTTCGTGGATGA | AAGCATAGGGGAGTGAGGCGACAAC |
| ACO | TGGCAATGATGCTGTTATCTATC | CGAACTATTACAAATAACATGTGTC |
| ERS | GCGGATCTCATTGGACATAAGC | GAGCTTAAGCTTACAGGCACAC |
| ETR | AGATGCCTGCAGATTGGCATGA | GCAAGGCTGTGTTCACCATGGC |
| β-Gal | TACCAAACTTACCGGCTTGG | TTAGCCCCCTCTACCTTCGT |
| EGase | TCATGATATTGAACACCTCTGG | CAACACTTCAACAGCCCTACA |
| PE | ATTACATCGATTGTGGGTTTTG | TGCATGCATATTTACCATCAA |
| PG | GCCCCATTGTCCTTGAATCAGAGA | AAATGCACCACACTACGCTT |
| PL | CTTTGAATTTTCCCCGGCCC | TGCTATCTCGGGGCCAAAAG |
| XTH | ACGCCAAGTTCTGCGACAC | GGGTATCGCTTCCTGTCG |
| Actin | TTGGAGCAGAGAGATTCCGC | TTGCTCATCCGGTCAGCAAT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumarihami, H.M.P.C.; Shin, M.H.; Jang, K.E.; Kim, Y.-H.; Ma, K.B.; Kim, J.G. Modified Atmosphere Packaging and 1-Methylcyclopropene Treatments Maintain the Fruit Quality of ‘Wonmi’ Persimmons during Export Simulation. Foods 2022, 11, 4004. https://doi.org/10.3390/foods11244004
Kumarihami HMPC, Shin MH, Jang KE, Kim Y-H, Ma KB, Kim JG. Modified Atmosphere Packaging and 1-Methylcyclopropene Treatments Maintain the Fruit Quality of ‘Wonmi’ Persimmons during Export Simulation. Foods. 2022; 11(24):4004. https://doi.org/10.3390/foods11244004
Chicago/Turabian StyleKumarihami, H. M. Prathibhani C., Mi Hee Shin, Kyeong Eun Jang, Yun-Hee Kim, Kyeong Bok Ma, and Jin Gook Kim. 2022. "Modified Atmosphere Packaging and 1-Methylcyclopropene Treatments Maintain the Fruit Quality of ‘Wonmi’ Persimmons during Export Simulation" Foods 11, no. 24: 4004. https://doi.org/10.3390/foods11244004
APA StyleKumarihami, H. M. P. C., Shin, M. H., Jang, K. E., Kim, Y.-H., Ma, K. B., & Kim, J. G. (2022). Modified Atmosphere Packaging and 1-Methylcyclopropene Treatments Maintain the Fruit Quality of ‘Wonmi’ Persimmons during Export Simulation. Foods, 11(24), 4004. https://doi.org/10.3390/foods11244004








