Cyclodextrin Inclusion Complexes and Their Application in Food Safety Analysis: Recent Developments and Future Prospects
Abstract
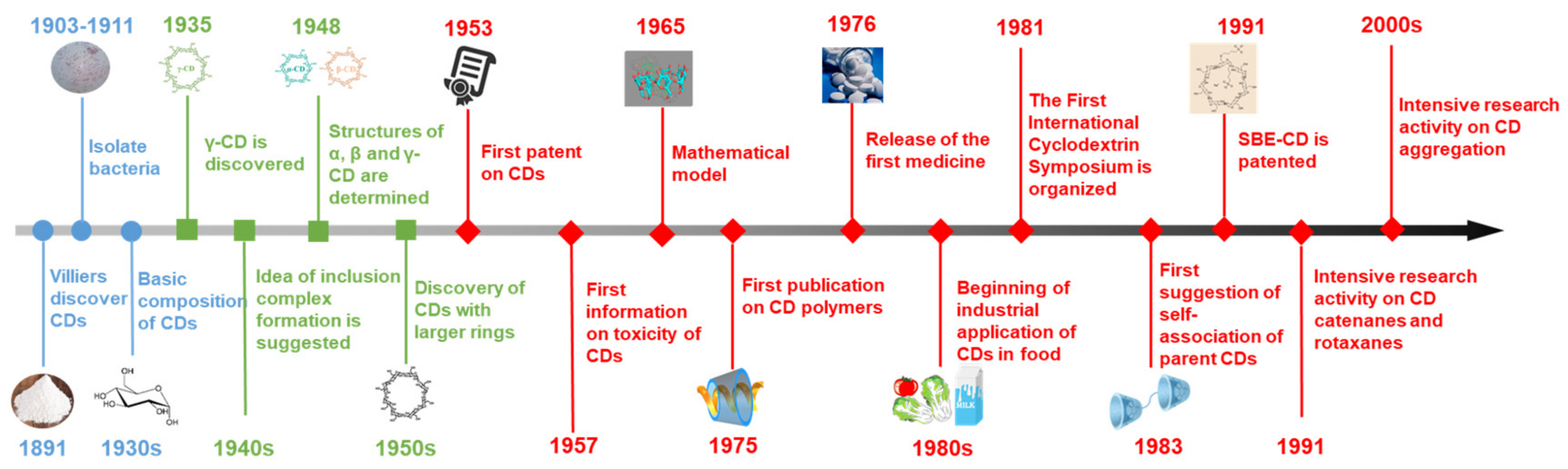
1. Introduction
2. Structure and Physicochemical Properties of Cyclodextrins
2.1. α-Cyclodextrin
2.2. β-Cyclodextrin
2.3. γ-Cyclodextrin
| Physicochemical Properties | α-CD | β-CD | γ-CD | References |
|---|---|---|---|---|
| Glucose unit | 6 | 7 | 8 | [1] |
| Chemical formula | C36H60O30 | C42H70O35 | C48H80O40 | [9] |
| Molecular weight (Da) | 972 | 1135 | 1297 | [18] |
| Diameter of central cavity (nm) | 0.57 | 0.78 | 0.95 | [10] |
| Outer diameter (nm) | 1.4–1.5 | 1.5–1.6 | 1.7–1.8 | [9] |
| Melting point (°C) | 275 | 280 | 275 | [9] |
| pKa at 25 °C | 12.3 | 12.2 | 12.1 | [9] |
| Internal water molecules | 6–8 | 11–12 | 13–17 | [9] |
| Solubility in water at 25 °C (mg/mL) | 145 | 18.5 | 232 | [18] |
2.4. Other Structures
3. Inclusion Complex Formation in CDs
4. Preparation Method of Cyclodextrins Inclusion Complex
4.1. Saturated Aqueous Solution Method
4.2. Kneading Method
4.3. Spray Drying Method
4.4. Freeze–Drying Method
4.5. Colloid Grinding Method
4.6. Supercritical Fluid Method
5. Application of CDs and Their Derivatives in the Food Safety Analysis
5.1. Additives
5.2. Foodborne Pathogens
5.3. Pesticides
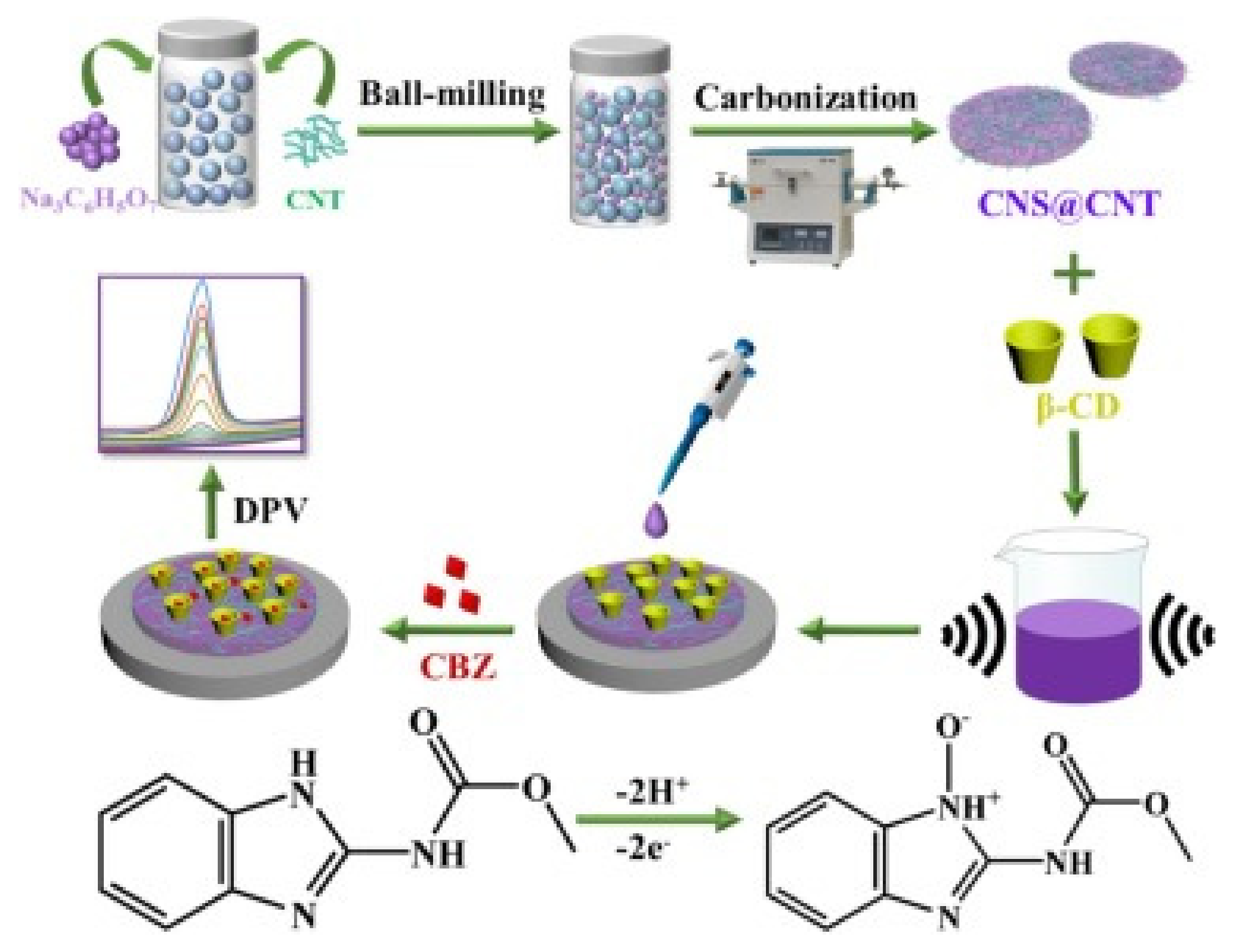
5.4. Antibiotics
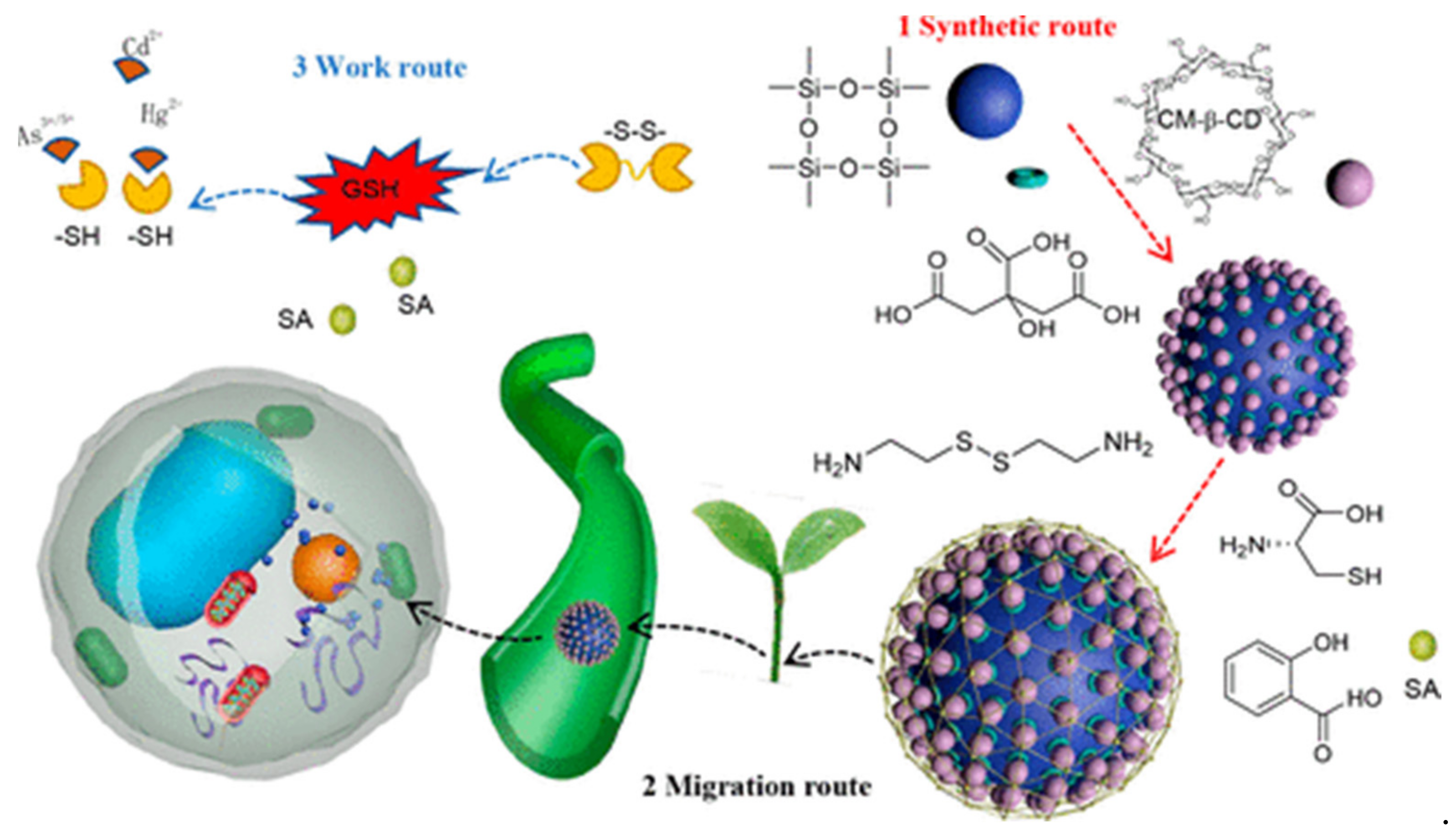
5.5. Heavy Metals
5.6. Others
6. Concluding Remarks and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jansook, P.; Ogawa, N.; Loftsson, T. Cyclodextrins: Structure, physicochemical properties and pharmaceutical applications. Int. J. Pharm. 2018, 535, 272–284. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Shuguli, C.; Vidal, C.P.; Cantero-Lopez, P.; Lopez-Polo, J. Encapsulation of plant extract compounds using cyclodextrin inclusion complexes, liposomes, electrospinning and their combinations for food purposes. Trends Food Sci. Technol. 2021, 108, 177–186. [Google Scholar] [CrossRef]
- van der Veen, B.A.; Uitdehaag, J.C.M.; Penninga, D.; van Alebeek, G.; Smith, L.M.; Dijkstra, B.W.; Dijkhuizen, L. Rational design of cyclodextrin glycosyltransferase from Bacillus circulans strain 251 to increase alpha-cyclodextrin production. J. Mol. Biol. 2000, 296, 1027–1038. [Google Scholar] [CrossRef] [PubMed]
- Messner, M.; Kurkov, S.V.; Flavia-Piera, R.; Brewster, M.E.; Loftsson, T. Self-assembly of cyclodextrins: The effect of the guest molecule. Int. J. Pharm. 2011, 408, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Ermolinsky, B.; Peredelchuk, M.; Provenzano, D. alpha-Cyclodextrin decreases cholera toxin binding to GM(1)-gangliosides. J. Med. Microbiol. 2013, 62, 1011–1014. [Google Scholar]
- Liu, Y.; Sameen, D.E.; Ahmed, S.; Wang, Y.; Lu, R.; Dai, J.; Li, S.; Qin, W. Recent advances in cyclodextrin-based films for food packaging. Food Chem. 2022, 370, 131026. [Google Scholar] [CrossRef]
- Hu, Y.; Qiu, C.; Qin, Y.; Xu, X.M.; Fan, L.P.; Wang, J.P.; Jin, Z.Y. Cyclodextrin-phytochemical inclusion complexes: Promising food materials with targeted nutrition and functionality. Trends Food Sci. Technol. 2021, 109, 398–412. [Google Scholar] [CrossRef]
- Kolaric, L.; Simko, P. Application of beta-cyclodextrin in the production of low-cholesterol milk and dairy products. Trends Food Sci. Technol. 2022, 119, 13–22. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Y.N.; Gao, X.L.; Fu, J.J.; Hu, L.D. Application of cyclodextrin in food industry. Crit. Rev. Food Sci. Nutr. 2022, 62, 2627–2640. [Google Scholar] [CrossRef]
- Cid-Samamed, A.; Rakmai, J.; Mejuto, J.C.; Simal-Gandara, J.; Astray, G. Cyclodextrins inclusion complex: Preparation methods, analytical techniques and food industry applications. Food Chem. 2022, 384, 132467. [Google Scholar] [CrossRef]
- Sabadini, E.; Cosgrove, T.; Egidio, F.C. Solubility of cyclomaltooligosaccharides (cyclodextrins) in H2O and D2O: A comparative study. Carbohydr. Res. 2006, 341, 270–274. [Google Scholar] [CrossRef]
- Li, Z.F.; Wang, M.; Wang, F.; Gu, Z.B.; Du, G.C.; Wu, J.; Chen, J. gamma-cyclodextrin: A review on enzymatic production and applications. Appl. Microbiol. Biotechnol. 2007, 77, 245–255. [Google Scholar] [CrossRef]
- Li, Z.; Chen, S.; Gu, Z.; Chen, J.; Wu, J. Alpha-cyclodextrin: Enzymatic production and food applications. Trends Food Sci. Technol. 2014, 35, 151–160. [Google Scholar] [CrossRef]
- Zhu, W.; Zhang, K.; Chen, Y.M.; Xi, F. Simple, Clean Preparation Method for Cross-Linked alpha-Cyclodextrin Nanoparticles via Inclusion Complexation. Langmuir 2013, 29, 5939–5943. [Google Scholar] [CrossRef]
- Kulkarni, A.; DeFrees, K.; Schuldt, R.A.; Hyun, S.H.; Wright, K.J.; Yerneni, C.K.; VerHeul, R.; Thompson, D.H. Cationic alpha-Cyclodextrin: Poly(ethylene glycol) Polyrotaxanes for siRNA Delivery. Mol. Pharm. 2013, 10, 1299–1305. [Google Scholar] [CrossRef][Green Version]
- Kurkov, S.V.; Loftsson, T. Cyclodextrins. Int. J. Pharm. 2013, 453, 167–180. [Google Scholar] [CrossRef]
- Freudenberg, K.; Jacobi, R. Über schardingers dextrine aus Stärke. Justus Liebigs Ann. Chem. 1935, 518, 102–108. [Google Scholar] [CrossRef]
- Loftsson, T.; Duchene, D. Cyclodextrins and their pharmaceutical applications. Int. J. Pharm. 2007, 329, 1–11. [Google Scholar] [CrossRef]
- Saokham, P.; Loftsson, T. gamma-Cyclodextrin. Int. J. Pharm. 2017, 516, 278–292. [Google Scholar] [CrossRef]
- Wu, H.; Li, X.X.; Ji, H.Y.; Svensson, B.; Bai, Y.X. Improved production of gamma-cyclodextrin from high-concentrated starch using enzyme pretreatment under swelling condition. Carbohydr. Polym. 2022, 284, 119124. [Google Scholar] [CrossRef]
- Dexter French, A.O.P.; Effenberger, J.A.; Rougvie, M.A.; Abdullah, M. Studies on the Schardinger dextrins XII. The molecular size and structure of the δ-, ϵ-, ζ-, and η-dextrins. Arch. Biochem. Biophys. 1965, 111, 153–160. [Google Scholar]
- Pulley, A.O.; French, D. Studies on the Schardinger dextrins. XI. The isolation of new Schardinger dextrins. Biochem. Biophys. Res. Commun. 1961, 5, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Ikuta, D.; Hirata, Y.; Wakamori, S.; Shimada, H.; Tomabechi, Y.; Kawasaki, Y.; Ikeuchi, K.; Hagimori, T.; Matsumoto, S.; Yamada, H. Conformationally supple glucose monomers enable synthesis of the smallest cyclodextrins. Science 2019, 364, 674–677. [Google Scholar] [CrossRef] [PubMed]
- Astray, G.; Gonzalez-Barreiro, C.; Mejuto, J.C.; Rial-Otero, R.; Simal-Gandara, J. A review on the use of cyclodextrins in foods. Food Hydrocoll. 2009, 23, 1631–1640. [Google Scholar] [CrossRef]
- Yang, L.J.; Chang, Q.; Zhou, S.Y.; Yang, Y.H.; Xia, F.T.; Chen, W.; Li, M.Y.; Yang, X.D. Host-guest interaction between brazilin and hydroxypropyl-beta-cyclodextrin: Preparation, inclusion mode, molecular modelling and characterization. Dyes Pigm. 2018, 150, 193–201. [Google Scholar] [CrossRef]
- Liu, M.S.; Liao, R.Q.; Zhao, Y.L.; Yang, B. Host-Guest Inclusion System of Luteolin with Polyamine—Cyclodextrin: Preparation, Characterisation, Anti-oxidant and Anti-cancer Activity. Aust. J. Chem. 2016, 69, 174–182. [Google Scholar] [CrossRef]
- Gong, L.; Li, T.T.; Chen, F.; Duan, X.W.; Yuan, Y.F.; Zhang, D.D.; Jiang, Y.M. An inclusion complex of eugenol into beta-cyclodextrin: Preparation, and physicochemical and antifungal characterization. Food Chem. 2016, 196, 324–330. [Google Scholar] [CrossRef]
- Dong, Q.L.; Wang, Y.P.; Wen, J.H.; Huang, M.; Yuan, E.D.; Zheng, J.X. Inclusion complex of neohesperidin dihydrochalcone and glucosyl-beta-cyclodextrin: Synthesis, characterization, and bitter masking properties in aqueous solutions. J. Mol. Liq. 2017, 241, 926–933. [Google Scholar] [CrossRef]
- Li, Y.; Li, F.; Cai, H.Y.; Chen, X.; Sun, W.; Shen, W.Y. Structural characterization of inclusion complex of arbutin and hydroxypropyl-beta-cyclodextrin. Trop. J. Pharm. Res. 2016, 15, 2227–2233. [Google Scholar] [CrossRef]
- Niu, H.; Chen, W.J.; Chen, W.X.; Yun, Y.H.; Zhong, Q.P.; Fu, X.; Chen, H.M.; Liu, G. Preparation and Characterization of a Modified-beta-Cyclodextrin/beta-Carotene Inclusion Complex and Its Application in Pickering Emulsions. J. Agric. Food. Chem. 2019, 67, 12875–12884. [Google Scholar] [CrossRef]
- Garrido, P.F.; Calvelo, M.; Garcia-Fandino, R.; Pineiro, A. Rings, Hexagons, Petals, and Dipolar Moment Sink-Sources: The Fanciful Behavior of Water around Cyclodextrin Complexes. Biomolecules 2020, 10, 431. [Google Scholar] [CrossRef]
- Matencio, A.; Bermejo-Gimeno, M.J.; Garcia-Carmona, F.; Lopez-Nicolas, J.M. Separating and Identifying the Four Stereoisomers of Methyl Jasmonate by RP-HPLC and using Cyclodextrins in a Novel Way. Phytochem. Anal. 2017, 28, 151–158. [Google Scholar] [CrossRef]
- Matencio, A.; Hernandez-Garcia, S.; Garcia-Carmona, F.; Lopez-Nicolas, J.M. A Way to Increase the Bioaccesibility and Photostability of Roflumilast, a COPD Treatment, by Cyclodextrin Monomers. Polymers 2019, 11, 801. [Google Scholar] [CrossRef]
- Matencio, A.; Hernandez-Gil, C.J.G.; Garcia-Carmona, F.; Lopez-Nicolas, J.M. Physicochemical, thermal and computational study of the encapsulation of rumenic acid by natural and modified cyclodextrins. Food Chem. 2017, 216, 289–295. [Google Scholar] [CrossRef]
- Rabello, M.M.; Rolim, L.A.; Neto, P.J.R.; Hernandes, M.Z. CycloMolder software: Building theoretical cyclodextrin derivatives models and evaluating their host:guest interactions. J. Inclusion Phenom. Macrocycl. Chem. 2019, 93, 301–308. [Google Scholar] [CrossRef]
- Tao, X.; Huang, Y.K.; Wang, C.; Chen, F.; Yang, L.L.; Ling, L.; Che, Z.M.; Chen, X.G. Recent developments in molecular docking technology applied in food science: A review. Int. J. Food Sci. Technol. 2020, 55, 33–45. [Google Scholar] [CrossRef]
- Liu, X.W.; Shi, D.F.; Zhou, S.Y.; Liu, H.L.; Liu, H.X.; Yao, X.J. Molecular dynamics simulations and novel drug discovery. Expert Opin. Drug Discov. 2018, 13, 23–37. [Google Scholar] [CrossRef]
- Tian, B.R.; Xiao, D.; Hei, T.T.; Ping, R.; Hua, S.Y.; Liu, J.Y. The application and prospects of cyclodextrin inclusion complexes and polymers in the food industry: A review. Polym. Int. 2020, 69, 597–603. [Google Scholar] [CrossRef]
- Charumanee, S.; Okonogi, S.; Sirithunyalug, J.; Wolschann, P.; Viernstein, H. Effect of Cyclodextrin Types and Co-Solvent on Solubility of a Poorly Water Soluble Drug. Sci. Pharm. 2016, 84, 694–704. [Google Scholar] [CrossRef]
- Hernandez-Sanchez, P.; Lopez-Miranda, S.; Guardiola, L.; Serrano-Martinez, A.; Gabaldon, J.A.; Nunez-Delicado, E. Optimization of a method for preparing solid complexes of essential clove oil with beta-cyclodextrins. J. Sci. Food Agric. 2017, 97, 420–426. [Google Scholar] [CrossRef]
- Jiang, L.W.; Yang, J.D.; Wang, Q.; Ren, L.; Zhou, J. Physicochemical properties of catechin/beta-cyclodextrin inclusion complex obtained via co-precipitation. CyTA-J. Food 2019, 17, 544–551. [Google Scholar] [CrossRef]
- Li, X.H.; Jin, Z.Y.; Wang, J. Complexation of allyl isothiocyanate by alpha- and beta-cyclodextrin and its controlled release characteristics. Food Chem. 2007, 103, 461–466. [Google Scholar] [CrossRef]
- Jacob, S.; Nair, A.B. Cyclodextrin complexes: Perspective from drug delivery and formulation. Drug Dev. Res. 2018, 79, 201–217. [Google Scholar] [CrossRef]
- Watson, M.A.; Lea, J.M.; Bett-Garber, K.L. Spray drying of pomegranate juice using maltodextrin/cyclodextrin blends as the wall material. Food Sci. Nutr. 2017, 5, 820–826. [Google Scholar] [CrossRef]
- Del Valle, E.M.M. Cyclodextrins and their uses: A review. Process Biochem. 2004, 39, 1033–1046. [Google Scholar] [CrossRef]
- Marques, H.M.C. A review on cyclodextrin encapsulation of essential oils and volatiles. Flavour Fragr. J. 2010, 25, 313–326. [Google Scholar] [CrossRef]
- Jantarat, C.; Sirathanarun, P.; Ratanapongsai, S.; Watcharakan, P.; Sunyapong, S.; Wadu, A. Curcumin-Hydroxypropyl-beta-Cyclodextrin Inclusion Complex Preparation Methods: Effect of Common Solvent Evaporation, Freeze Drying, and pH Shift on Solubility and Stability of Curcumin. Trop. J. Pharm. Res. 2014, 13, 1215–1223. [Google Scholar] [CrossRef]
- Madene, A.; Jacquot, M.; Scher, J.; Desobry, S. Flavour encapsulation and controlled release—A review. Int. J. Food Sci. Technol. 2006, 41, 1–21. [Google Scholar] [CrossRef]
- Jug, M.; Mura, P.A. Grinding as Solvent-Free Green Chemistry Approach for Cyclodextrin Inclusion Complex Preparation in the Solid State. Pharmaceutics 2018, 10, 189. [Google Scholar] [CrossRef]
- Borba, P.A.A.; Pinotti, M.; Andrade, G.R.S.; da Costa, N.B.; Olchanheski, L.R.; Fernandes, D.; de Campos, C.E.M.; Stulzer, H.K. The effect of mechanical grinding on the formation, crystalline changes and dissolution behaviour of the inclusion complex of telmisartan and beta-cyclodextrins. Carbohydr. Polym. 2015, 133, 373–383. [Google Scholar] [CrossRef]
- Lin, H.L.; Lin, S.Y.; Lin, C.C.; Hsu, C.H.; Wu, T.K.; Huang, Y.T. Mechanical grinding effect on thermodynamics and inclusion efficiency of loratadine-cyclodextrin inclusion complex formation. Carbohydr. Polym. 2012, 87, 512–517. [Google Scholar] [CrossRef]
- Su, W.; Polyakov, N.E.; Xu, W.; Su, W. Preparation of astaxanthin micelles self-assembled by a mechanochemical method from hydroxypropyl β-cyclodextrin and glyceryl monostearate with enhanced antioxidant activity. Int. J. Pharm. 2021, 605, 120799. [Google Scholar] [CrossRef]
- Zhang, W.; Xiao, P.; Lin, L.; Guo, F.; Wang, Q.; Piao, Y.; Diao, G. Study of a water-soluble supramolecular complex of curcumin and β-cyclodextrin polymer with electrochemical property and potential anti-cancer activity. Chin. Chem. Lett. 2022, 33, 4043–4047. [Google Scholar] [CrossRef]
- Rudrangi, S.R.S.; Trivedi, V.; Mitchell, J.C.; Wicks, S.R.; Alexander, B.D. Preparation of olanzapine and methyl-b-cyclodextrin complexes using a single-step, organic solvent-free supercritical fluid process: An approach to enhance the solubility and dissolution properties. Int. J. Pharm. 2015, 494, 408–416. [Google Scholar] [CrossRef]
- Durante, M.; Lenucci, M.S.; Gazza, L.; Taddei, F.; Nocente, F.; Benedetto, G.E.D.; Caroli, M.D.; Piro, G.; Mita, G. Bioactive composition and sensory evaluation of innovative spaghetti supplemented with free or α-cyclodextrin chlatrated pumpkin oil extracted by supercritical CO2. Food Chem. 2019, 294, 112–122. [Google Scholar] [CrossRef]
- Jun, S.W.; Kim, M.S.; Kim, J.S.; Park, H.J.; Lee, S.; Woo, J.S.; Hwang, S.J. Preparation and characterization of simvastatin/hydroxypropyl-beta-cyclodextrin inclusion complex using supercritical antisolvent (SAS) process. Eur. J. Pharm. Biopharm. 2007, 66, 413–421. [Google Scholar] [CrossRef]
- Banchero, M. Supercritical Carbon Dioxide as a Green Alternative to Achieve Drug Complexation with Cyclodextrins. Pharmaceuticals 2021, 14, 562. [Google Scholar] [CrossRef]
- Crini, G. Review: A History of Cyclodextrins. Chem. Rev. 2014, 114, 10940–10975. [Google Scholar] [CrossRef]
- Rakmai, J.; Cheirsilp, B.; Mejuto, J.C.; Simal-Gandara, J.; Torrado-Agrasar, A. Antioxidant and antimicrobial properties of encapsulated guava leaf oil in hydroxypropyl-beta-cyclodextrin. Ind. Crops Prod. 2018, 111, 219–225. [Google Scholar] [CrossRef]
- Alonso, L.; Calvo, M.V.; Fontecha, J. A scale-up process for the manufacture of reduced-cholesterol butter using beta-cyclodextrin. J. Food Process. Eng. 2019, 42, e13009. [Google Scholar] [CrossRef]
- Petito, N.D.; Dias, D.D.; Costa, V.G.; Falcao, D.Q.; Araujo, K.G.D. Increasing solubility of red bell pepper carotenoids by complexation with 2-hydroxypropyl-beta-cyclodextrin. Food Chem. 2016, 208, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, L.B.; Martins, A.; Ribeiro, J.; Cesario, F.; Castro, F.F.E.; de Albuquerque, T.R.; Fernandes, M.N.M.; da Silva, B.A.F.; Quintans, L.J.; Araujo, A.A.D.; et al. Anti-inflammatory activity of the essential oil obtained from Ocimum basilicum complexed with beta-cyclodextrin (beta-CD) in mice. Food Chem. Toxicol. 2017, 109, 836–846. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.S.; Li, G.K.; Zhang, Z.M. Ti3C2Tx-AgNPs@beta-cyclodextrin SERS substrate for rapid and selective determination of erythrosin B in dyed food. Sens. Actuator B-Chem. 2021, 346, 130595. [Google Scholar] [CrossRef]
- Huang, Z.M.; Lei, J.H.; Ruan, H.; Gong, Y.Y.; Wang, G.; Zhou, L. One-pot synthesis of hydroxypropyl-beta-cyclodextrin capped fluorescent sulfur quantum dots for highly sensitive and selective recognition of tartrazine. Microchem. J. 2021, 164, 106031. [Google Scholar] [CrossRef]
- Tang, J.; Zheng, S.B.; Jiang, S.X.; Li, J.; Guo, T.; Guo, J.H. Metal organic framework (ZIF-67)-derived Co nanoparticles/N-doped carbon nanotubes composites for electrochemical detecting of tert-butyl hydroquinone. Rare Met. 2021, 40, 478–488. [Google Scholar] [CrossRef]
- Sebastian, N.; Yu, W.C.; Balram, D.; Al-Mubaddel, F.S.; Noman, M.T. Nanomolar detection of food additive tert-butylhydroquinone in edible oils based on novel ternary metal oxide embedded beta-cyclodextrin functionalized carbon black. Food Chem. 2022, 377, 131867. [Google Scholar] [CrossRef]
- Rao, S.R.; Ravishankar, G.A. Vanilla flavour: Production by conventional and biotechnological routes. J. Sci. Food Agric. 2000, 80, 289–304. [Google Scholar]
- Duran, G.M.; Contento, A.M.; Rios, A. beta-Cyclodextrin coated CdSe/ZnS quantum dots for vanillin sensoring in food samples. Talanta 2015, 131, 286–291. [Google Scholar] [CrossRef]
- Zhu, X.X.; Xiao, Y.; Jiang, X.Y.; Li, J.H.; Qin, H.L.; Huang, H.M.; Zhang, Y.Y.; He, X.X.; Wang, K.M. A ratiometric nanosensor based on conjugated polyelectrolyte-stabilized AgNPs for ultrasensitive fluorescent and colorimetric sensing of Melamine. Talanta 2016, 151, 68–74. [Google Scholar] [CrossRef]
- Faraji, M.; Adeli, M. Sensitive determination of melamine in milk and powdered infant formula samples by high-performance liquid chromatography using dabsyl chloride derivatization followed by dispersive liquid-liquid microextraction. Food Chem. 2017, 221, 139–146. [Google Scholar] [CrossRef]
- Xavier, S.S.J.; Karthikeyan, C.; Kumar, G.G.; Kim, A.R.; Yoo, D.J. Colorimetric detection of melamine using beta-cyclodextrin-functionalized silver nanoparticles. Anal. Methods 2014, 6, 8165–8172. [Google Scholar] [CrossRef]
- Liao, X.F.; Chen, C.J.; Shi, P.P.; Yue, L.Z. Determination of melamine in milk based on beta-cyclodextrin modified carbon nanoparticles via host-guest recognition. Food Chem. 2021, 338, 127769. [Google Scholar] [CrossRef]
- Singh, V.R.; Pandey, S.P.; Singh, P.K. A unique supramolecular assembly between sulfated cyclodextrin, silver and melamine: Towards a fluorescence based dual wavelength detection approach for melamine. J. Photochem. Photobiol. A-Chem. 2022, 428, 113862. [Google Scholar] [CrossRef]
- Umesha, S.; Manukumar, H.M. Advanced molecular diagnostic techniques for detection of food-borne pathogens: Current applications and future challenges. Crit. Rev. Food Sci. Nutr. 2018, 58, 84–104. [Google Scholar] [CrossRef]
- Law, J.W.F.; Ab Mutalib, N.S.; Chan, K.G.; Lee, L.H. Rapid methods for the detection of foodborne bacterial pathogens: Principles, applications, advantages and limitations. Front. Microbiol. 2015, 5, 770. [Google Scholar] [CrossRef]
- Matencio, A.; Navarro-Orcajada, S.; Garcia-Carmona, F.; Lopez-Nicolas, J.M. Applications of cyclodextrins in food science. A review. Trends Food Sci. Technol. 2020, 104, 132–143. [Google Scholar] [CrossRef]
- Moura, A.; Criscuolo, A.; Pouseele, H.; Maury, M.M.; Leclercq, A.; Tarr, C.; Bjorkman, J.T.; Dallman, T.; Reimer, A.; Enouf, V.; et al. Whole genome-based population biology and epidemiological surveillance of Listeria monocytogenes. Nat. Microbiol. 2017, 2, 16185. [Google Scholar] [CrossRef]
- Li, X.R.; Zhang, R.H.; Wang, C.; Wang, X.F.; Yang, Y.; Cui, S.H.; Guo, Y.C. Use of beta-cyclodextrin and milk protein-coated activated charcoal for rapid detection of Listeria monocytogenes in leafy greens by PCR without pre-enrichment. Food Control 2022, 140, 109118. [Google Scholar] [CrossRef]
- Chambers, H.F.; Deleo, F.R. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat. Rev. Microbiol. 2009, 7, 629–641. [Google Scholar] [CrossRef]
- Mukdasai, S.; Poosittisak, S.; Ngeontae, W.; Srijaranai, S. A highly sensitive electrochemical determination of L-tryptophan in the presence of ascorbic acid and uric acid using in situ addition of tetrabutylammonium bromide on the beta-cyclodextrin incorporated multi-walled carbon nanotubes modified electrode. Sens. Actuator B-Chem. 2018, 272, 518–525. [Google Scholar] [CrossRef]
- Kubendhiran, S.; Sakthivel, R.; Chen, S.M.; Mutharani, B.; Chen, T.W. Innovative Strategy Based on a Novel Carbon-Black-beta-Cyclodextrin Nanocomposite for the Simultaneous Determination of the Anticancer Drug Flutamide and the Environmental Pollutant 4-Nitrophenol. Anal. Chem. 2018, 90, 6283–6291. [Google Scholar] [CrossRef] [PubMed]
- Gill, A.A.S.; Singh, S.; Nate, Z.; Chauhan, R.; Thapliyal, N.B.; Karpoormath, R.; Maru, S.M.; Reddy, T.M. A novel copper-based 3D porous nanocomposite for electrochemical detection and inactivation of pathogenic bacteria. Sens. Actuators B-Chem. 2020, 321, 128499. [Google Scholar] [CrossRef]
- Liang, W.L.; Wang, B.; Cheng, J.L.; Xiao, D.X.; Xie, Z.G.; Zhao, J.H. 3D, eco-friendly metal-organic frameworks@carbon nanotube aerogels composite materials for removal of pesticides in water. J. Hazard. Mater. 2021, 401, 123718. [Google Scholar] [CrossRef] [PubMed]
- Aktar, M.W.; Sengupta, D.; Chowdhury, A. Impact of pesticides use in agriculture: Their benefits and hazards. Interdiscip. Toxicol. 2009, 2, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.Z.; Lee, H.K. Micro-solid-phase extraction of organochlorine pesticides using porous metal-organic framework MIL-101 as sorbent. J. Chromatogr. A 2015, 1401, 9–16. [Google Scholar] [CrossRef]
- Zhu, W.X.; Liu, W.; Li, T.B.; Yue, X.Y.; Liu, T.; Zhang, W.T.; Yu, S.X.; Zhang, D.H.; Wang, J.L. Facile green synthesis of graphene-Au nanorod nanoassembly for online extraction and sensitive stripping analysis of methyl parathion. Electrochim. Acta 2014, 146, 419–428. [Google Scholar] [CrossRef]
- Yi, Y.H.; Zeng, W.; Zhu, G.B. beta-Cyclodextrin functionalized molybdenum disulfide quantum dots as nanoprobe for sensitive fluorescent detection of parathion-methyl. Talanta 2021, 222, 121703. [Google Scholar] [CrossRef]
- Li, F.; Liu, R.Q.; Dubovyk, V.; Ran, Q.W.; Zhao, H.Y.; Komarneni, S. Rapid determination of methyl parathion in vegetables using electrochemical sensor fabricated from biomass-derived and beta-cyclodextrin functionalized porous carbon spheres. Food Chem. 2022, 384, 132643. [Google Scholar] [CrossRef]
- Chadha, R.; Das, A.; Lobo, J.; Meenu, V.O.; Paul, A.; Ballal, A.; Maiti, N. gamma-Cyclodextrin capped silver and gold nanoparticles as colorimetric and Raman sensor for detecting traces of pesticide “Chlorpyrifos” in fruits and vegetables. Colloid Surf. A-Physicochem. Eng. Asp. 2022, 641, 128558. [Google Scholar] [CrossRef]
- Wang, M.; Su, K.; Cao, J.; She, Y.X.; Abd El-Aty, A.M.; Hacimuftuoglu, A.; Wang, J.; Yan, M.M.; Hong, S.H.; Lao, S.B.; et al. “Off-On” non-enzymatic sensor for malathion detection based on fluorescence resonance energy transfer between beta-cyclodextrin@Ag and fluorescent probe. Talanta 2019, 192, 295–300. [Google Scholar] [CrossRef]
- Madrigal, J.M.; Sargis, R.M.; Persky, V.; Turyk, M.E. Multiple organochlorine pesticide exposures and measures of sex steroid hormones in adult males: Cross-sectional findings from the 1999–2004 National Health and Nutrition Examination Survey. Int. J. Hyg. Environ. Health 2021, 231, 113609. [Google Scholar] [CrossRef]
- Yamazaki, K.; Itoh, S.; Araki, A.; Miyashita, C.; Minatoya, M.; Ikeno, T.; Kato, S.; Fujikura, K.; Mizutani, F.; Chisaki, Y.; et al. Associations between prenatal exposure to organochlorine pesticides and thyroid hormone levels in mothers and infants: The Hokkaido study on environment and children’s health. Environ. Res. 2020, 189, 109840. [Google Scholar] [CrossRef]
- Sun, X.Z.; Fu, Z.B.; Jiang, T.Y.; Ning, F.H.; Cheng, Y.Z.; Fu, T.H.; Zhu, M.S.; Zhang, H.Y.; Zhang, M.; Hu, P. Application of beta-Cyclodextrin metal-organic framework/titanium dioxide hybrid nanocomposite as dispersive solid-phase extraction adsorbent to organochlorine pesticide residues in honey samples. J. Chromatogr. A 2022, 1663, 462750. [Google Scholar] [CrossRef]
- DiScenza, D.J.; Lynch, J.; Miller, J.; Verderame, M.; Levine, M. Detection of Organochlorine Pesticides in Contaminated Marine Environments via Cyclodextrin-Promoted Fluorescence Modulation. ACS Omega 2017, 2, 8591–8599. [Google Scholar] [CrossRef]
- Zhao, Y.J.; Zheng, X.H.; Wang, Q.Z.; Zhe, T.T.; Bai, Y.W.; Bu, T.; Zhang, M.; Wang, L. Electrochemical behavior of reduced graphene oxide/cyclodextrins sensors for ultrasensitive detection of imidacloprid in brown rice. Food Chem. 2020, 333, 127495. [Google Scholar] [CrossRef]
- Junqueira, V.B.; Muller, C.; Rodrigues, A.A.; Amaral, T.S.; Batista, P.F.; Silva, A.A.; Costa, A.C. Do fungicides affect the physiology, reproductive development and productivity of healthy soybean plants? Pestic. Biochem. Physiol. 2021, 172, 104754. [Google Scholar] [CrossRef]
- Jing, X.; Huang, X.; Zhang, Y.M.; Wang, M.; Xue, H.Y.; Wang, X.W.; Jia, L.Y. Cyclodextrin-based dispersive liquid-liquid microextraction for the determination of fungicides in water, juice, and vinegar samples via HPLC. Food Chem. 2022, 367, 130664. [Google Scholar] [CrossRef]
- Sebastian, N.; Yu, W.C.; Balram, D.; Al-Mubaddel, F.S.; Noman, M.T. Functionalization of CNFs surface with beta-cyclodextrin and decoration of hematite nanoparticles for detection and degradation of toxic fungicide carbendazim. Appl. Surf. Sci. 2022, 586, 152666. [Google Scholar] [CrossRef]
- Liu, R.Q.; Li, B.; Li, F.; Dubovyk, V.; Chang, Y.Q.; Li, D.D.; Ding, K.J.; Ran, Q.W.; Wang, G.F.; Zhao, H.Y. A novel electrochemical sensor based on beta-cyclodextrin functionalized carbon nanosheets@carbon nanotubes for sensitive detection of bactericide carbendazim in apple juice. Food Chem. 2022, 384, 132573. [Google Scholar] [CrossRef]
- Adachi, F.; Yamamoto, A.; Takakura, K.I.; Kawahara, R. Occurrence of fluoroquinolones and fluoroquinolone-resistance genes in the aquatic environment. Sci. Total Environ. 2013, 444, 508–514. [Google Scholar] [CrossRef]
- Qiu, X.Q.; Gu, J.; Yang, T.Q.; Ma, C.Q.; Li, L.; Wu, Y.M.; Zhu, C.; Gao, H.; Yang, Z.C.; Wang, Z.R.; et al. Sensitive determination of Norfloxacin in milk based on beta-cyclodextrin functionalized silver nanoparticles SERS substrate. Spectroc. Acta Pt. A-Molec. Biomolec. Spectr. 2022, 276, 121212. [Google Scholar] [CrossRef] [PubMed]
- Belenguer-Sapina, C.; Pellicer-Castell, E.; El Haskouri, J.; Simo-Alfonso, E.F.; Amoros, P.; Mauri-Aucejo, A.R. A type UVM-7 mesoporous silica with gamma-cyclodextrin for the isolation of three veterinary antibiotics (ofloxacin, norfloxacin, and ciprofloxacin) from different fat-rate milk samples. J. Food Compos. Anal. 2022, 109, 104463. [Google Scholar] [CrossRef]
- Verma, M.; Lee, I.; Sharma, S.; Kumar, R.; Kumar, V.; Kim, H. Simultaneous Removal of Heavy Metals and Ciprofloxacin Micropollutants from Wastewater Using Ethylenediaminetetraacetic Acid-Functionalized β-Cyclodextrin-Chitosan Adsorbent. ACS Omega 2021, 6, 34624–34634. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.; Naidu, R.; Du, J.H.; Qi, F.J.; Ahsan, M.A.; Liu, Y.J. Magnetic responsive mesoporous alginate/beta-cyclodextrin polymer beads enhance selectivity and adsorption of heavy metal ions. Int. J. Biol. Macromol. 2022, 207, 826–840. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.C.; Pan, Y.F.; Li, B.; Wang, L.D.; Xiao, H.N. Dual-Functional Redox-Responsive Nanocarriers for Loading Phytohormone and Complexation with Heavy Metal Ions. J. Agric. Food Chem. 2020, 68, 5076–5085. [Google Scholar] [CrossRef]
- Mahato, P.; Saha, S.; Suresh, E.; Di Liddo, R.; Parnigotto, P.P.; Conconi, M.T.; Kesharwani, M.K.; Ganguly, B.; Das, A. Ratiometric Detection of Cr3+ and Hg2+ by a Naphthalimide-Rhodamine Based Fluorescent Probe. Inorg. Chem. 2012, 51, 1769–1777. [Google Scholar] [CrossRef]
- Cui, L.; Wu, J.; Li, M.Q.; Ju, H.X. Highly sensitive electrochemical detection of mercury (II) via single ion-induced three-way junction of DNA. Electrochem. Commun. 2015, 59, 77–80. [Google Scholar] [CrossRef]
- Prabu, S.; Mohamad, S. Curcumin/beta-cyclodextrin inclusion complex as a new “turn-off’ fluorescent sensor system for sensitive recognition of mercury ion. J. Mol. Struct. 2020, 1204, 127528. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, Y.; Jin, Y.; Yang, Q.B.; Du, J.S.; Li, Y.X. Fluorescent magnetic nanosensors for Zn2+ and CN- in aqueous solution prepared from adamantane-modified fluorescein and beta-cyclodextrin-modified Fe3O4@SiO2 via host-guest interactions. RSC Adv. 2015, 5, 68815–68821. [Google Scholar] [CrossRef]
- Celebioglu, A.; Topuz, F.; Yildiz, Z.I.; Uyar, T. Efficient Removal of Polycyclic Aromatic Hydrocarbons and Heavy Metals from Water by Electrospun Nanofibrous Polycyclodextrin Membranes. ACS Omega 2019, 4, 7850–7860. [Google Scholar] [CrossRef]
- Guo, Y.L.; Jian, F.F.; Kang, X.F. Nanopore sensor for copper ion detection using a polyamine decorated beta- cyclodextrin as the recognition element. RSC Adv. 2017, 7, 15315–15320. [Google Scholar] [CrossRef]
- Mohandoss, S.; Palanisamy, S.; You, S.; Lee, Y.R. Synthesis of cyclodextrin functionalized photoluminescent metal nanoclusters for chemoselective Fe3+ ion detection in aqueous medium and its applications of paper sensors and cell imaging. J. Mol. Liq. 2022, 356, 118999. [Google Scholar] [CrossRef]
- King, T.; Cole, M.; Farber, J.M.; Eisenbrand, G.; Zabaras, D.; Fox, E.M.; Hill, J.P. Food safety for food security: Relationship between global megatrends and developments in food safety. Trends Food Sci. Technol. 2017, 68, 160–175. [Google Scholar] [CrossRef]
- Chen, Y.L.; Lu, Z.C.; Huang, S.M.; Li, G.K.; Hu, Y.L.; Zhong, Q.S. Simultaneous enrichment of bisphenols and polyfluoroalkyl substances by cyclodextrin-fluorinated covalent organic frameworks membrane in food packaging samples. J. Chromatogr. A 2022, 1666, 462864. [Google Scholar] [CrossRef]
- Belenguer-Sapina, C.; Pellicer-Castell, E.; El Haskouri, J.; Simo-Alfonso, E.F.; Amoros, P.; Mauri-Aucejo, A.R. Assessment of migrating endocrine-disrupting chemicals in bottled acidic juice using type UVM-7 mesoporous silica modified with cyclodextrin. Food Chem. 2022, 380, 132207. [Google Scholar] [CrossRef]
- Cromwell, B.; Dubnicka, M.; Dubrawski, S.; Levine, M. Identification of 15 Phthalate Esters in Commercial Cheese Powder via Cyclodextrin-Promoted Fluorescence Detection. ACS Omega 2019, 4, 17009–17015. [Google Scholar] [CrossRef]
- Boon, Y.H.; Zain, N.N.M.; Mohamad, S.; Osman, H.; Raoov, M. Magnetic poly(beta-cyclodextrin-ionic liquid) nanocomposites for micro-solid phase extraction of selected polycyclic aromatic hydrocarbons in rice samples prior to GC-FID analysis. Food Chem. 2019, 278, 322–332. [Google Scholar] [CrossRef]



| Methods | Advantages | Disadvantages | References |
|---|---|---|---|
| Saturated aqueous solution |
|
| [41] |
| Kneading method |
|
| [8,10] |
| Spray drying |
|
| [9,42] |
| Freeze drying |
|
| [43,44] |
| Colloid grinding |
|
| [45,46] |
| Supercritical fluid |
|
| [40] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; Jia, J.; He, J.; Li, J.; Cai, J. Cyclodextrin Inclusion Complexes and Their Application in Food Safety Analysis: Recent Developments and Future Prospects. Foods 2022, 11, 3871. https://doi.org/10.3390/foods11233871
Zhou J, Jia J, He J, Li J, Cai J. Cyclodextrin Inclusion Complexes and Their Application in Food Safety Analysis: Recent Developments and Future Prospects. Foods. 2022; 11(23):3871. https://doi.org/10.3390/foods11233871
Chicago/Turabian StyleZhou, Jiaojiao, Jilai Jia, Jiangling He, Jinjie Li, and Jie Cai. 2022. "Cyclodextrin Inclusion Complexes and Their Application in Food Safety Analysis: Recent Developments and Future Prospects" Foods 11, no. 23: 3871. https://doi.org/10.3390/foods11233871
APA StyleZhou, J., Jia, J., He, J., Li, J., & Cai, J. (2022). Cyclodextrin Inclusion Complexes and Their Application in Food Safety Analysis: Recent Developments and Future Prospects. Foods, 11(23), 3871. https://doi.org/10.3390/foods11233871







