Treatment of Fresh Meat, Fish and Products Thereof with Cold Atmospheric Plasma to Inactivate Microbial Pathogens and Extend Shelf Life
Abstract
1. Introduction
2. What Is ‘Cold Atmospheric Plasma’ and How Is It Generated?
2.1. Generation of Plasma
2.2. Low Pressure Plasma vs. Atmospheric-Pressure Plasma
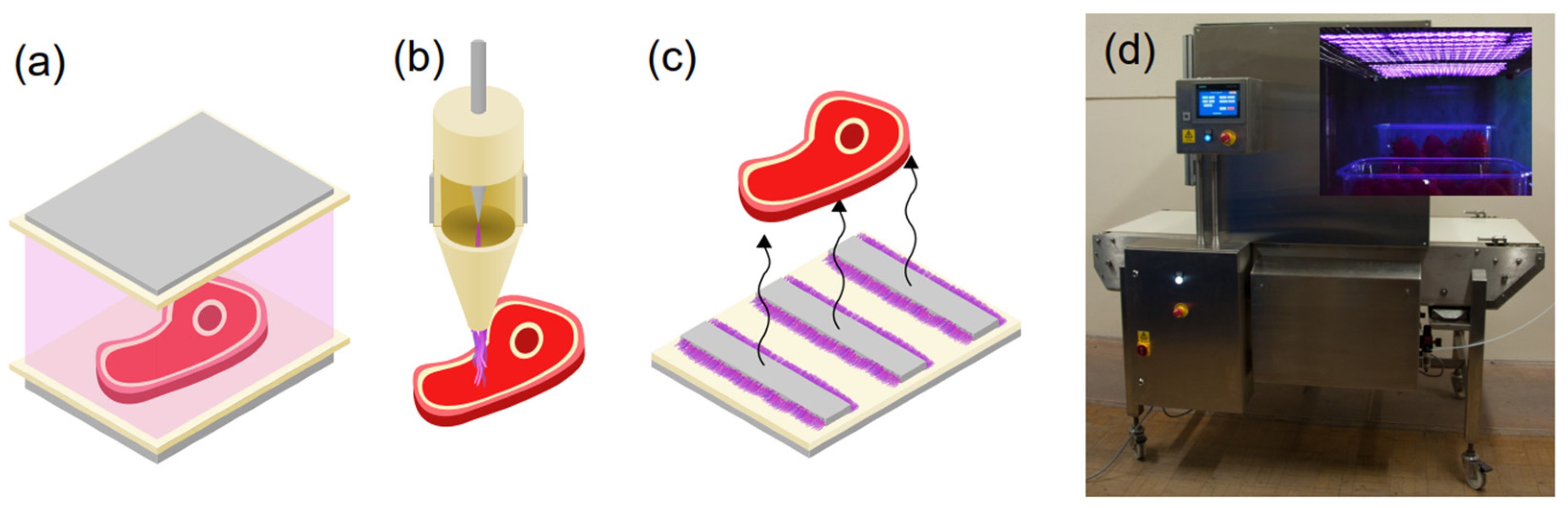
2.3. Application of Atmospheric-Pressure Plasma to Tissues, Foods and Food Contact Surfaces
2.4. In-Package Cold Plasma Treatment
3. Effects of CAP Treatment of Fresh Meat and Meat Products
3.1. Fresh Meat
3.2. Meat Products
4. Effects of CAP Treatment of Aquatic Foods of Animal Origin: Review of Recent Model Experiments
4.1. Oily Fish
4.1.1. Atlantic Mackerel
4.1.2. Tuna
4.1.3. Herring
4.2. Whitefish
4.2.1. Alaska Pollock
4.2.2. Hairtail Fish
4.3. Shrimps
4.3.1. Pacific White Shrimp and Greasyback Shrimp
4.3.2. A Short Note on Freshwater Shrimps’ Role in Spreading Antibiotics Resistance
4.4. Squid
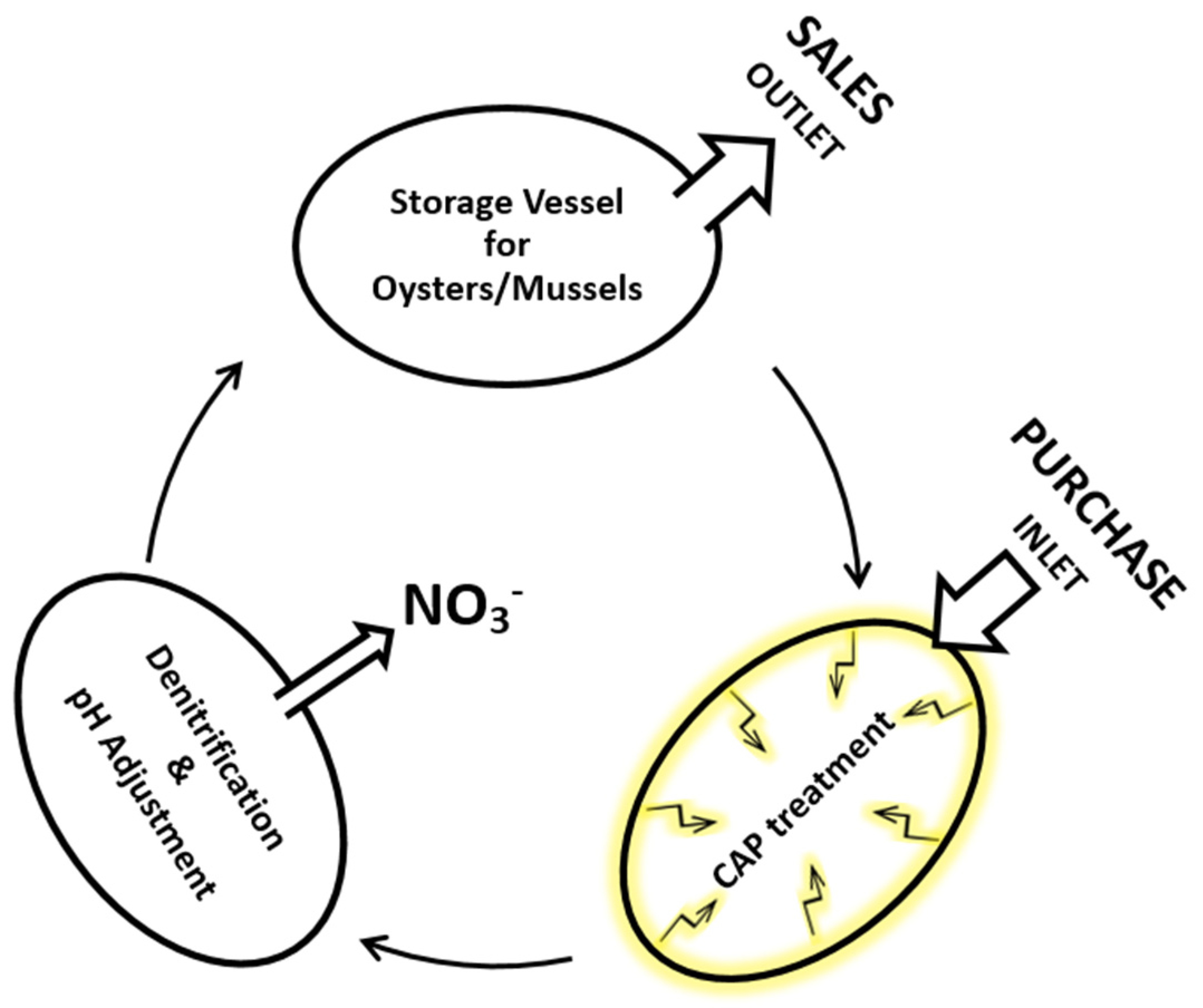
4.5. Molluscs (Mussels and Oysters)
5. A Note on Food-Contaminating Viruses; Why Surrogate Viruses Are Used for Studying the Virucidal Effect of Cold Atmospheric Plasma
6. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lipinski, B. Why do animal-based food loss and waste matter? Anim. Front. 2020, 10, 48–52. [Google Scholar] [CrossRef] [PubMed]
- FAO. Food and Agriculture Organization of the United Nations. Food Wastage Footprint and Climate Change, Rome Italy, 2015. Available online: https://www.fao.org/3/bb144e/bb144e.pdf (accessed on 19 August 2022).
- Warner, R.D.; Greenwood, P.L.; Pethick, D.W.; Ferguson, D.M. Genetic and environmental effects on meat quality. Meat Sci. 2010, 86, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Jan, A.T.; Lee, E.J.; Ahmad, S.; Choi, I. Meeting the meat: Delineating the molecular machinery of muscle development. J. Anim. Sci. Technol. 2016, 58, 18. [Google Scholar] [CrossRef] [PubMed]
- Mohammadabadi, M.; Bordbar, F.; Jensen, J.; Du, M.; Guo, W. Key genes regulating skeletal muscle development and growth in farm animals. Animals 2021, 11, 835. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, A.; Nassiry, M.R.; Mosafer, J.; Mohammadabadi, M.R. Distribution of BoLA-DRB3 allelic frequencies and identification of a new allele in the Iranian cattle breed Sistani (Bos indicus). Russ. J. Genet. 2009, 45, 198–202. [Google Scholar] [CrossRef]
- Mossel, D.A.A. Adequate protection of the public against food-transmitted diseases of microbial aetiology. Achievements and challenges half a century after the introduction of the Prescott-Meyer-Wilson strategy of active intervention. Int. J. Food Microbiol. 1989, 9, 271–294. [Google Scholar] [CrossRef]
- FAO. Food and Agriculture Organization of the United Nations. Rome Declaration on World Food Security. World Food Summit, 13–17 November 1996, Rome, Italy. 1996. Available online: https://www.fao.org/3/w3613e/w3613e00.htm (accessed on 2 September 2022).
- UN World Commission on Environment and Development. Report of the World Commission on Environment and Development: Our Common Future. 1987. Available online: https://sustainabledevelopment.un.org/content/documents/5987our-common-future.pdf (accessed on 21 August 2022).
- European Commission. Farm to Fork Strategy; for a Fair, Healthy and Environmentally-Friendly System. Available online: https://food.ec.europa.eu (accessed on 22 September 2020).
- Warne, G.R.; Williams, P.M.; Pho, H.Q.; Tran, N.N.; Hessel, V.; Fisk, I.D. Impact of cold plasma on the biomolecules and organoleptic properties of foods: A review. Food Sci. 2021, 86, 3762–3777. [Google Scholar] [CrossRef]
- Jadhav, H.B.; Annapure, U. Consequences of non-thermal cold plasma treatment on meat and dairy lipids—A review. Future Foods 2021, 4, 100095. [Google Scholar] [CrossRef]
- Olatunde, O.O.; Singh, A.; Shiekh, K.A.; Nuthong, P.; Benjakul, S. Effect of High Voltage Cold Plasma on Oxidation, Physiochemical, and Gelling Properties of Myofibrillar Protein Isolate from Asian Sea Bass (Lates calcarifer). Foods 2021, 10, 326. [Google Scholar] [CrossRef]
- Domínguez, R.; Pateiro, M.; Munekata, P.E.S.; Zhang, W.; Garcia-Oliveira, P.; Carpena, M.; Prieto, M.A.; Bohrer, B.; Lorenzo, J.M. Protein Oxidation in Muscle Foods: A Comprehensive Review. Antioxidants 2022, 11, 60. [Google Scholar] [CrossRef]
- Nawaz, A.; Irshad, S.; Ali Khan, I.; Khalifa, I.; Walayat, N.; Aadil, R.M.; Kumar, M.; Wang, M.; Chen, F.; Cheng, K.-W.; et al. Protein oxidation in muscle-based products: Effects on physicochemical properties, quality concerns, and challenges to food industry. Food Res. Int. 2022, 157, 111322. [Google Scholar] [CrossRef] [PubMed]
- Raizer, Y.P. Gas Discharge Physics; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1997. [Google Scholar]
- Lieberman, M.A.; Lichtenberg, A.J. Principles of Plasma Discharges and Materials Processing, 2nd ed.; Wiley & Sons: Hoboken, NJ, USA, 2005. [Google Scholar]
- Bourke, P.; Ziuzina, D.; Han, L.; Cullen, P.J.; Gilmore, B.F. Microbiological Interactions with Cold Plasma. J. Appl. Microbiol. 2017, 123, 308–324. [Google Scholar] [CrossRef] [PubMed]
- Martines, E. 2020. Interaction of Cold Atmospheric Plasmas with Cell Membranes in Plasma Medicine Studies. Jpn. J. Appl. Phys. 2020, 59, SA0803. [Google Scholar] [CrossRef]
- Mendis, D.A.; Rosenberg, M.; Azam, F. A Note on the Possible Electrostatic Disruption of Bacteria. IEEE Trans. Plasma Sci. 2000, 28, 1304–1306. [Google Scholar] [CrossRef]
- Lackmann, J.-W.; Schneider, S.; Edengeiser, E.; Jarzina, F.; Brinckmann, S.; Steinborn, E.; Havenith, M.; Benedikt, J.; Bandow, J.E. Photons and Particles Emitted from Cold Atmospheric-Pressure Plasma Inactivate Bacteria and Biomolecules Independently and Synergistically. J. R. Soc. Interface. 2013, 10, 20130591. [Google Scholar] [CrossRef] [PubMed]
- Bruggeman, P.J.; Iza, F.; Brandenburg, R. Foundations of Atmospheric Pressure Non-Equilibrium Plasmas. Plasma Sources Sci. Technol. 2017, 26, 123002. [Google Scholar] [CrossRef]
- Brandenburg, R. Dielectric Barrier Discharges: Progress on Plasma Sources and on the Understanding of Regimes and Single Filaments. Plasma Sources Sci. Technol. 2017, 26, 053001. [Google Scholar] [CrossRef]
- Laroussi, M. Nonthermal Decontamination of Biological Media by Atmospheric-Pressure Plasmas: Review, Analysis, and Prospects. IEEE Trans. Plasma Sci. 2002, 30, 1409–1415. [Google Scholar] [CrossRef]
- Laroussi, M.; Bekeschus, S.; Bogaerts, A.; Fridman, A.; Lu, X.; Miller, V.; Laux, C.; Walsh, J.; Jiang, C.; Liu, D.; et al. Low-Temperature Plasma for Biology, Hygiene, and Medicine: Perspective and Roadmap. IEEE Trans. Radiat. Plasma Med. Sci. 2022, 6, 127–157. [Google Scholar] [CrossRef]
- Brun, P.; Vono, M.; Venier, P.; Tarricone, E.; Deligianni, V.; Martines, E.; Zuin, M.; Spagnolo, S.; Cavazzana, R.; Cardin, R.; et al. Disinfection of Ocular Cells and Tissues by Atmospheric-Pressure Cold Plasma. PLoS ONE 2012, 7, e33245. [Google Scholar] [CrossRef]
- Diwan, R.; Debta, F.M.; Deoghare, A.; Ghom, S.; Khandelwhal, A.; Sikdar, S.; Ghom, A. Plasma therapy; An overview. J. Indian Acad. Oral Med. Radiol. 2011, 23, 120–123. [Google Scholar] [CrossRef]
- Lackmann, J.-W.; Bandow, J.E. Inactivation of microbes and macromolecules by atmospheric-pressure plasma jets. Appl. Microbiol. Biotechnol. 2014, 98, 6205–6213. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, J. Cold Atmospheric Plasma in Medicine—From Basic Research to Application, Max Planck Institute of Pathology, Technical University Munich, Habilitation Treatise; 2013. Available online: http://mediatum.ub.tum.de/?id=1219297 (accessed on 2 September 2022).
- Bekeschus, S.; von Woedtke, T.; Emmert, S.; Schmidt, A. Medical Gas Plasma-Stimulated Wound Healing: Evidence and Mechanisms. Redox Biol. 2021, 46, 102116. [Google Scholar] [CrossRef] [PubMed]
- Melotti, L.; Martinello, T.; Perazzi, A.; Martines, E.; Zuin, M.; Modenese, D.; Cordaro, L.; Ferro, S.; Maccatrozzo, L.; Iacopetti, I.; et al. Could cold plasma act synergistically with allogeneic mesenchymal stem cells to improve wound skin regeneration in a large size animal model? Res. Vet. Sci. 2021, 136, 97–110. [Google Scholar] [CrossRef]
- Scholtz, V.; Pazlarova, J.; Souskova, H.; Julak, J. Nonthermal Plasma—A Tool for Decontamination and Disinfection. Biotechnol. Adv. 2015, 33, 1108–1119. [Google Scholar] [CrossRef]
- Misra, N.N.; Schlüter, O.; Cullen, P.J. Chapter 1—Plasma in Food and Agriculture. In Cold Plasma in Food and Agriculture; Misra, N.N., Schlüter, O., Cullen, P.J., Eds.; Academic Press: San Diego, CA, USA, 2016; pp. 1–16. [Google Scholar]
- Csadek, I.; Paulsen, P.; Bak, K.H.; Smulders, F.J.M. Application of atmospheric pressure cold plasma (ACP) on meat and meat products. Part 1. Effects on bacterial surface flora. Fleischwirtschaft 2021, 101, 96–104. [Google Scholar]
- Shimizu, T.; Sakiyama, Y.; Graves, D.; Zimmerman, J.; Morfill, G. The dynamics of ozone generation and mode transition in air surface micro-discharge plasma at atmospheric pressure. New J. Phys. 2012, 14, 103028. [Google Scholar] [CrossRef]
- Reuter, S.; Winter, J.; Iseni, S.; Schmidt-Bleker, A.; Dünnbier, M.; Masur, K.; Wende, K.; Weltmann, K.-D. The Influence of Feed Gas Humidity Versus Ambient Humidity on Atmospheric Pressure Plasma Jet-Effluent Chemistry and Skin Cell Viability. IEEE Trans. Plasma Sci. 2015, 43, 3185–3192. [Google Scholar] [CrossRef]
- Lis, K.A.; Boulaaba, A.; Binder, S.; Li, Y.; Kehrenberg, C.; Zimmermann, J.L.; Klein, G.; Ahlfeld, B. Inactivation of Salmonella Typhimurium and Listeria monocytogenes on Ham with Nonthermal Atmospheric Pressure Plasma. PLoS ONE 2018, 13, e0197773. [Google Scholar] [CrossRef]
- Katsigiannis, A.S.; Bayliss, D.L.; Walsh, J.L. Cold Plasma for the Disinfection of Industrial Food-contact Surfaces: An Overview of Current Status and Opportunities. Comp. Rev. Food Sci. Food Saf. 2022, 21, 1026–1124. [Google Scholar] [CrossRef]
- López, M.; Calvo, T.; Prieto, M.; Múgica-Vidal, R.; Muro-Fraguas, I.; Alba-Elías, F.; Alvarez-Ordóñez, A. A Review on Non-Thermal Atmospheric Plasma for Food Preservation: Mode of Action, Determinants of Effectiveness, and Applications. Front. Microbiol. 2019, 10, 622. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.I.; Walsh, J.L. Influence of Gas Flow Velocity on the Transport of Chemical Species in an Atmospheric Pressure Air Plasma Discharge. Appl. Phys. Lett. 2017, 110, 134102. [Google Scholar] [CrossRef]
- Misra, N.N.; Yepez, X.; Xu, L.; Keener, K. In-Package Cold Plasma Technologies. J. Food Eng. 2019, 244, 21–31. [Google Scholar] [CrossRef]
- Ziuzina, D.; Patil, S.; Cullen, P.J.; Keener, K.M.; Bourke, P. Atmospheric Cold Plasma Inactivation of Escherichia coli in Liquid Media inside a Sealed Package. J. Appl. Microbiol. 2013, 114, 778–787. [Google Scholar] [CrossRef]
- Huang, M.; Wang, J.; Zhuang, H.; Yan, W.; Zhao, J.; Zhang, J. Effect of In-Package High Voltage Dielectric Barrier Discharge on Microbiological, Color and Oxidation Properties of Pork in Modified Atmosphere Packaging during Storage. Meat Sci. 2019, 149, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Patange, A.; Boehm, D.; Bueno-Ferrer, C.; Cullen, P.J.; Bourke, P. Controlling Brochothrix thermosphacta as a Spoilage Risk Using In-Package Atmospheric Cold Plasma. Food Microbiol. 2017, 66, 48–54. [Google Scholar] [CrossRef]
- Wang, J.; Zhuang, H.; Hinton, A.; Zhang, J. Influence of in-package cold plasma treatment on microbiological shelf life and appearance of fresh chicken breast fillets. Food Microbiol. 2016, 60, 142–146. [Google Scholar] [CrossRef]
- Levif, P.; Séguin, J.; Moisan, M.; Soum-Glaude, A.; Barbeau, J. Packaging materials for plasma sterilization with the flowing afterglow of an N2—O2 discharge: Damage assessment and inactivation efficiency of enclosed bacterial spores. J. Phys. D Appl. Phys. 2011, 44, 405201. [Google Scholar] [CrossRef]
- Brayfield, R.S.; Jassem, A.; Lauria, M.V.; Fairbanks, A.J.; Keener, K.M.; Garner, A.L. Characterization of High Voltage Cold Atmospheric Plasma Generation in Sealed Packages as a Function of Container Material and Fill Gas. Plasma Chem. Plasma Process 2018, 38, 379–395. [Google Scholar] [CrossRef]
- Bauer, A.; Ni, Y.; Bauer, S.; Paulsen, P.; Modic, M.; Walsh, J.L.; Smulders, F.J.M. The effects of atmospheric pressure cold plasma treatment on microbiological, physical-chemical and sensory characteristics of vacuum packaged beef loin. Meat Sci. 2017, 128, 77–87. [Google Scholar] [CrossRef]
- Rød, S.K.; Hansen, F.; Leipold, F.; Knøchel, S. Cold atmospheric pressure plasma treatment of ready-to-eat meat: Inactivation of Listeria innocua and changes in product quality. Food Microbiol. 2012, 30, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Roh, S.; Oh, Y.; Le, S.; Kann, J.; Min, S. Inactivation of Escherichia coli O157:H7, Salmonella, Listeria monocytogenes and Tulane virus in processed chicken breast via atmospheric in-package cold plasma treatment. LWT Food Sci. Technol. 2020, 127, 109429. [Google Scholar] [CrossRef]
- Jayasena, D.; Kim, H.; Yong, H.; Park, S.; Kim, K.; Choe, W.; Jo, C. Flexible thin-layer dielectric barrier discharge plasma treatment of pork butt and beef loin: Effects on pathogen inactivation and meat-quality attributes. Food Microbiol. 2015, 46, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Harrison, W.A.; Griffith, C.J.; Tennant, D.; Peters, A.C. Incidence of Campylobacter and Salmonella isolated from retail chicken and associated packaging in South Wales. Lett. Appl. Microbiol. 2001, 33, 450–454. [Google Scholar] [CrossRef] [PubMed]
- Regulation (EU) 2015/2283 of the European Parliament and of the Council of 25 November 2015 on novel foods, amending Regulation (EU) No 1169/2011 of the European Parliament and of the Council and repealing Regulation (EC) No 258/97 of the European Parliament and of the Council and Commission Regulation (EC) No 1852/2001. Off. J. Eur. Union 2015, L 327, 1–22. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32015R2283 (accessed on 13 November 2022).
- Cooperation in Science and Technology (COST 013/20) Decision 24 March; Memorandum of Understanding for the Implementation of the COST Action “Plasma Application for Smart and Sustainable Agriculture (P/Agri) CA 19110”, Brussels, 16 pp., COST, Avenue Boulevard 21, Brussels, Belgium, 2020. Available online: https://plagri.eu/wp-content/uploads/2021/02/CA19110-e.pdf (accessed on 2 September 2022).
- 55. German Research Community (Deutsche Forschungsgemeinschaft). Stellungnahme Zum Einsatz von Plasmaverfahren zur Behandlung von Lebensmitteln [Opinion on the Application of Plasma-technology for Treatment of Foods], 2012. Available online: www.dfg.de/sklm (accessed on 19 August 2022).
- Ekezie, C.; Suan, D.; Cheng, J. Review on recent advances in cold plasma technology for the food industry: Current applications and future trends. Trends Food Sci. Technol. 2017, 69, 46–58. [Google Scholar] [CrossRef]
- Nørrung, B.; Buncic, S. Microbial safety of meat in the European Union. Meat Sci. 2008, 78, 14–24. [Google Scholar] [CrossRef]
- Das, A.K.; Nanda, P.K.; Das, A.; Biswas, S. Hazards and Safety Issues of Meat and Meat Products. In Food Safety and Human Health; Singh, R.L., Mondal, S., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 145–168. [Google Scholar]
- Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety. Off. J. Eur. Union 2002, L 31, 1–24. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex%3A32002R0178 (accessed on 13 November 2022).
- Pearce, R.A.; Bolton, D.J.; Sheridan, J.J.; McDowell, D.A.; Blair, I.S.; Harrington, D. Studies to determine the critical control points in pork slaughter hazard analysis and critical control point systems. Int. J. Microbiol. 2004, 90, 331–339. [Google Scholar] [CrossRef]
- Antic, D.; Houf, K.; Michalopoulou, E.; Blagojevic, B. Beef abattoir interventions in a risk-based meat safety assurance system. Meat Sci. 2021, 182, 108622. [Google Scholar] [CrossRef]
- Paulsen, P.; Smulders, F.J.M. Combining natural antimicrobial systems with other preservation techniques: The case of meat. In Food Preservation Techniques; Zeuthen, P., Bogh-Sorensen, L., Eds.; Woodhead Publishing: Abington, UK, 2003; pp. 71–89. [Google Scholar]
- Aymerich, T.; Picouet, T.A.; Monfort, J.M. Decontamination technologies for meat products. Meat Sci. 2008, 78, 114–129. [Google Scholar] [CrossRef] [PubMed]
- Loretz, M.; Stephan, R.; Zweifel, C. Antibacterial activity of decontamination treatments for cattle hides and beef carcasses. Food Control 2011, 22, 347–359. [Google Scholar] [CrossRef]
- Buncic, S.; Sofos, J. Interventions to Control Salmonella Contamination during Poultry, Cattle and Pig Slaughter. Food Res. Int. 2012, 45, 641–655. [Google Scholar] [CrossRef]
- Buncic, S.; Nychas, G.-J.; Lee, M.R.F.; Koutsoumanis, K.; Hébraud, M.; Desvaux, M.; Chorianopoulos, N.; Bolton, D.; Blagojevic, B.; Antic, D. Microbial pathogen control in the beef chain: Recent research advances. Meat Sci. 2014, 97, 288–297. [Google Scholar] [CrossRef]
- Albert, T.; Braun, P.G.; Saffaf, J.; Wiacek, C. Physical Methods for the Decontamination of Meat Surfaces. Curr. Clin. Microbiol. Rep. 2021, 8, 9–20. [Google Scholar] [CrossRef]
- Zdolec, N.; Kotsiri, A.; Houf, K.; Alvarez-Ordóñez, A.; Blagojevic, B.; Karabasil, N.; Salines, M.; Antic, D. Systematic Review and Meta-Analysis of the Efficacy of Interventions Applied during Primary Processing to Reduce Microbial Contamination on Pig Carcasses. Foods 2022, 11, 2110. [Google Scholar] [CrossRef]
- Gill, C.O.; Bedard, D.; Jones, T. The decontaminating performance of a commercial apparatus for pasteurizing polished pig carcasses. Food Microbiol. 1997, 14, 71–79. [Google Scholar] [CrossRef]
- Gill, C.O.; Jones, T.; Badoni, M. The effects of hot water pasteurizing treatments on the microbiological conditions and appearances of pig and sheep carcasses. Food Res. Int. 1998, 31, 273–278. [Google Scholar] [CrossRef]
- Alban, L.; Sørensen, L.L. Hot-water decontamination is an effective way of reducing risk of Salmonella in pork. Fleischwirtschaft 2010, 90, 109–113. [Google Scholar]
- Paulsen, P.; Smulders, F.J.M. Reduction of the microbial contamination of carcasses and meat cuts with particular reference to the application of organic acids. In Food Safety and Veterinary Public Health, Volume 2: Safety Assurance during Food Processing; Smulders, F.J.M., Collins, J.D., Eds.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2004; pp. 177–199. [Google Scholar]
- Smulders, F.J.M.; Wellm, G.; Hiesberger, J.; Rohrbacher, I.; Bauer, A.; Paulsen, P. Microbiological and sensory effects of the combined application of hot-cold organic acid sprays and steam condensation at subatmospheric pressure for decontamination of inoculated pig tissue surfaces. J. Food Prot. 2011, 74, 1338–1344. [Google Scholar] [CrossRef]
- Smulders, F.J.M.; Wellm, G.; Hiesberger, J.; Bauer, A.; Paulsen, P. The potential of the combined application of hot water sprays and steam condensation at subatmospheric pressure for decontaminating inoculated pig skin and muscle surfaces. Food Control 2012, 24, 154–159. [Google Scholar] [CrossRef]
- EFSA Panel on Biological Hazards (BIOHAZ). Scientific Opinion on the evaluation of the safety and efficacy of lactic acid for the removal of microbial surface contamination of beef carcasses, cuts and trimmings. EFSA J. 2011, 9, 2317. [Google Scholar]
- EFSA Panel on Food Contact Materials, Enzymes and Processing Aids (CEP); Silano, V.; Barat Baviera, J.M.; Bolognesi, C.; Brüschweiler, B.J.; Chesson, A.; Cocconcelli, P.S.; Crebelli, R.; Gott, D.M.; Grob, K.; et al. Evaluation of the safety and efficacy of the organic acids lactic and acetic acids to reduce microbiological surface contamination on pork carcasses and pork cuts. EFSA J. 2018, 16, e05482. [Google Scholar]
- Commission Regulation (EU) No 101/2013 of 4 February 2013 concerning the use of lactic acid to reduce microbiological surface contamination on bovine carcasses. Off. J. Eur. Union 2013, L34, 1–3. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2013:034:0001:0003:EN:PDF (accessed on 13 November 2022).
- Choi, S.; Puligundla, P.; Mok, C. Corona discharge plasma jet for inactivation of Escherichia coli O157:H157 and Listeria monocytogenes on inoculated pork and its impact on meat quality attributes. Ann. Microbiol. 2016, 66, 685–694. [Google Scholar] [CrossRef]
- Yong, J.; Kim, H.; Park, S.; Choe, W.; Oh, M.; Jo, C. Evaluation of the Treatment of Both Sides of Raw Chicken Breasts with an Atmospheric Pressure Plasma Jet for the Inactivation of Escherichia Coli. Foodborne Pathog. Dis. 2014, 11, 652–657. [Google Scholar] [CrossRef]
- Misra, N.N.; Jo, C. Applications of cold plasma technology for microbiological safety in meat industry. Trends Food Sci. Tech. 2017, 64, 74–86. [Google Scholar] [CrossRef]
- Lawrie, R.A.; Ledward, D.A. Lawrie’s Meat Science, 7th ed.; Woodhead: Cambridge, UK, 2006. [Google Scholar]
- Pérez-Andrés, J.M.; Cropotova, J.; Harrison, S.M.; Brunton, N.P.; Cullen, P.J.; Rustad, T.; Tiwari, B.K. Effect of Cold Plasma on Meat Cholesterol and Lipid Oxidation. Foods 2020, 9, 1786. [Google Scholar] [CrossRef]
- Bak, K.H.; Csadek, I.; Paulsen, P.; Smulders, F.J.M. Application of atmospheric pressure cold plasma (ACP) on meat and meat products. Part 2. Effects on the sensory quality with special focus on meat colour and lipid oxidation. Fleischwirtschaft 2021, 101, 100–105. [Google Scholar]
- Kim, J.-S.; Lee, E.-J.; Choi, E.H.; Kim, Y.-J. Inactivation of Staphylococcus aureus on the beef jerky by radio-frequency atmospheric pressure plasma discharge treatment. Innov. Food Sci. Emerg. Technol. 2014, 22, 124–130. [Google Scholar] [CrossRef]
- Yong, H.I.; Lee, H.; Park, S.; Park, J.; Choe, W.; Jung, S.; Jo, C. Flexible thin-layer plasma inactivation of bacteria and mold survival in beef jerky packaging and its effects on the meat’s physicochemical properties. Meat Sci. 2017, 123, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Yong, H.I.; Lee, S.H.; Kim, S.Y.; Park, S.; Park, J.; Choe, W.; Jo, C. Color development, physiochemical properties, and microbiological safety of pork jerky processed with atmospheric pressure plasma. Innov. Food Sci. Emerg. Technol. 2019, 53, 78–84. [Google Scholar] [CrossRef]
- Jung, S.; Kim, H.J.; Park, S.; Yong, H.I.; Choe, J.H.; Jeon, H.-J.; Choe, W.; Jo, C. The use of atmospheric pressure plasma-treated water as a source of nitrite for emulsion-type sausage. Meat Sci. 2015, 108, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Kim, H.J.; Park, S.; Yong, H.I.; Choe, J.H.; Jeon, H.J.; Choe, W.; Jo, C. Color Developing Capacity of Plasma-treated Water as a Source of Nitrite for Meat Curing. Korean J. Food Sci. Anim. Resour. 2015, 35, 703–706. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Jo, K.; Lim, Y.; Jeon, H.J.; Choe, J.H.; Jo, C.; Jung, S. The use of atmospheric pressure plasma as a curing process for canned ground ham. Food Chem. 2018, 240, 430–436. [Google Scholar] [CrossRef]
- Yong, H.I.; Park, J.; Kim, H.-J.; Jung, S.; Park, S.; Lee, H.J.; Choe, W.; Jo, C. An innovative curing process with plasma-treated water for production of loin ham and for its quality and safety. Plasma Process. Polym. 2018, 15, 1700050. [Google Scholar] [CrossRef]
- Jo, K.; Lee, S.; Yong, H.I.; Choi, Y.-S.; Jung, S. Nitrite sources for cured meat products. LWT-Food Sci. Technol. 2020, 129, 109583. [Google Scholar] [CrossRef]
- Alahakoon, A.U.; Jayasena, D.D.; Ramachandra, S.; Jo, C. Alternatives to nitrite in processed meat: Up to date. Trends Food Sci. Technol. 2015, 45, 37–49. [Google Scholar] [CrossRef]
- Guerrero-Legarreta, I. Meat and poultry—Spoilage of Cooked Meat and Meat Products. In Encyclopedia of Food Microbiology, 2nd ed.; Batt, C.A., Tortorello, M.L., Eds.; Academic Press: Oxford, UK, 2014; pp. 508–513. [Google Scholar]
- Ganan, M.; Hierro, E.; Hospital, X.F.; Barroso, E.; Fernández, M. Use of pulsed light to increase the safety of ready-to-eat cured meat products. Food Control 2013, 32, 512–517. [Google Scholar] [CrossRef]
- Possas, A.; Valdramidis, V.; García-Gimeno, R.M.; Pérez-Rodríguez, F. High hydrostatic pressure processing of sliced fermented sausages: A quantitative exposure assessment for Listeria monocytogenes. Innov. Food Sci. Emerg. Technol. 2019, 52, 406–419. [Google Scholar] [CrossRef]
- Hadjicharalambous, C.; Grispoldi, L.; Goga, B.C. Quantitative risk assessment of Listeria monocytogenes in a traditional RTE product. EFSA J. 2019, 17 (Suppl. S2), e170906. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Commission Regulation (EC) No 2073/2005 of 15 November 2005 on microbiological criteria for foodstuffs. Off. J. Eur. Union 2005, L338, 1–26. [Google Scholar]
- Awaiwanont, N.; Smulders, F.J.M.; Paulsen, P. Growth potential of Listeria monocytogenes in traditional Austrian cooked-cured meat products. Food Control 2017, 50, 150–156. [Google Scholar] [CrossRef]
- Csadek, I.; Vankat, U.; Schrei, J.; Graf, M.; Bauer, S.; Pilz, B.; Schwaiger, K.; Smulders, F.J.M.; Paulsen, P. Treatment of Ready-to-Eat Cooked Meat Products with Cold Atmospheric Plasma to Inactivate Listeria and Escherichia coli. Foods. submitted.
- Wikipedia. Seafood. Available online: https://en.wikipedia.org/wiki/Seafood (accessed on 19 August 2022).
- FSA (Food Standards Agency). What Is an Oily Fish? Available online: https://webarchive.nationalarchives.gov.uk/ukgwa/20101210005807/http://www.food.gov.uk/news/newsarchive/2004/jun/oilyfishdefinition (accessed on 19 August 2022).
- NHS (National Health Services). 2018. Available online: https://www.nhs.uk/live-well/eat-well/food-types/fish-and-shellfish-nutrition (accessed on 19 August 2022).
- Rathod, N.B.; Chudaman, R.; Bhagwat, P.K.; Ozugul, F.; Benjakul, S.; Sottawat, B.; Pilai, S.; Annapure, U.S. Cold Plasma for the preservation of aquatic food products: An overview. Compr. Rev. Food Sci. Food Saf. 2021, 20, 4407–4425. [Google Scholar] [CrossRef] [PubMed]
- Albertos, I.; Martin Diana, A.B.; Cullen, P.J.; Tiwari, B.K.; Ojha, S.K.; Bourke, P.; Alvarez, C.; Rico, D. Effects of dielectric barrier discharge (DBD) generated plasma on microbial reduction and quality parameters of fresh mackerel (Scomber scombrus) fillets. Innov. Food Sci. Emerg. Technol. 2017, 44, 117–122. [Google Scholar] [CrossRef]
- Trevisani, M.; Cavoli, C.; Ragni, L.; Cecchini, M.; Berardinelli, A. Effect of non-thermal atmospheric plasma on viability and histamine-producing activity of psychrotrophic bacteria in mackerel fillets. Front. Microbiol. 2021, 12, 653597. [Google Scholar] [CrossRef]
- Kulawik, P.; Kumar Tiwari, B. Recent advancements in the application of non-thermal plasma technology for the seafood industry. Crit. Rev. Food Sci. Nutr. 2018, 59, 3199–3210. [Google Scholar] [CrossRef]
- Pan, W.; Benjakul, S.; Sanmartin, C.; Guidi, A.; Ying, X.; Ma, L.; Weng, X.; Yu, J.; Deng, S. Characterization of the Flavor Profile of Bigeye Tuna Slices Treated by Cold Plasma Using E-Nose and GC-IMS. Fishes 2022, 7, 13. [Google Scholar] [CrossRef]
- Albertos, I.; Martin Diana, A.B.; Cullen, P.J.; Tiwari, B.K.; Ojha, S.K.; Bourke, P.; Rico, D. Shelf life extension of herring (Clupea harengus) using in-package atmospheric plasma technology. Innov. Food Sci. Emerg. Technol. 2019, 53, 85–91. [Google Scholar] [CrossRef]
- Choi, S.; Puligundla, P.; Mok, C. Microbial decontamination of dried Alaska pollock shreds using corona discharge plasma jet: Effects on physico-chemical and sensory characteristics. J. Food Sci. 2016, 81, M952. [Google Scholar] [CrossRef]
- Tiwari, B.K.; Muthukumarappan, K.; O’Donnell, C.P.; Cullen, P.J. Effects of sonication on the kinetics of orange juice quality parameters. J. Agric. Food Chem. 2008, 56, 2423–2428. [Google Scholar] [CrossRef]
- CIE (Commission Internationale de L’Éclairage (International Commission on Illumination). Recommendations on Uniform Color Spaces, Color-Difference Equations, Psychometric Color Terms; Commission Internationale de l’Eclairage: Paris, France, 1978. [Google Scholar]
- Upton, S. Delta E: The Color Difference. Delta E, CHROMIX Colornews, 17. Available online: http://www.chromix.com/ColorNews/ (accessed on 13 November 2022).
- Koddy, J.K.; Miao, W.; Hatab, S.; Tang, L.; Xu, H.; Nyaisaba, B.M.; Chen, M.; Deng, S. Understanding the role of atmospheric cold plasma (CAP) in maintaining the quality of hairtail (Trichiurus lepturus). Food Chem. 2020, 343, 128418. [Google Scholar] [CrossRef]
- de Souza Silva, D.A.; da Silva Campelo, M.C.; de Oliviera Soares Rebouças, L.; de Oliveira Vitoriano, J.; Alves, C., Jr.; Alves da Silva, J.B.; de Oliveira Lima, P. Use of cold atmospheric plasma to preserve the quality of white shrimp (Litopenaeus vannamei). J. Food Prot. 2019, 82, 1217–1223. [Google Scholar] [CrossRef]
- Elliot, M.; Chen, J.; Chen, D.-Z.; Ekaterina, N.; Deng, S.G. Effect of cold plasma-assisted shrimp processing chain in biochemical and sensory quality alterations in Pacific White Shrimps (Penaeus vannamei). Food Sci. Techn. Internat. 2022, 28, 683–693. [Google Scholar] [CrossRef]
- Liao, X.; Su, V.; Liu, D.; Chen, S.; Hu, Y.; Ye, X.; Wang, J.; Ding, T. Application of atmospheric cold plasma-activated water (PAW) ice for preservation of shrimps (Metapenaeus ensis). Food Control 2018, 94, 307–314. [Google Scholar] [CrossRef]
- Kahn, M.; Lively, J.A. Determination of sulphite and antimicrobial residues in important shrimps to the USA. Aquac. Rep. 2020, 18, 100529. [Google Scholar] [CrossRef]
- Rahman, M. Are Ghost Shrimps Edible? Available online: https://acuariopets.com/are-ghost-shrimps-edible/ (accessed on 21 August 2022).
- Roberts, J. Ghost Shrimp (Complete Care, Diet, Set-up and Breeding Guide). Available online: http://www.buildyouraquarium.com/ghost-shrimp (accessed on 21 August 2022).
- Choi, S.; Puligundla, P.; Mok, C. Impact of corona discharge plasma treatment on microbial load and physicochemical and sensory characteristics of semi-dried squid (Todarodes pacificus). Food Sci. Biotechnol. 2017, 26, 1137–1144. [Google Scholar] [CrossRef]
- Choi, S.; Puligundla, P.; Mok, C. Effect of corona discharge plasma on microbial decontamination of dried squid shreds including physico-chemical and sensory evaluation. LWT-Food Sci. Technol. 2017, 75, 323–328. [Google Scholar] [CrossRef]
- Dumen, E.; Ekici, G.; Ergin, S.; Bayrakal, G.M. Presence of Foodborne Pathogens in Seafood and Risk Ranking for Pathogens. Foodborne Pathog. Dis. 2020, 17, 541–546. [Google Scholar] [CrossRef]
- Hall, A.J.; Wikswo, M.E.; Pringle, K.; Gould, L.H.; Parashar, U.D. Vital Signs: Foodborne Norovirus Outbreaks—United States, 2009–2012. Morb. Mortal. Wkly. Rep. 2014, 63, 491–495. [Google Scholar]
- Koopmans, M. Foodborne Viruses and Seafood Safety in an Environmental Health Perspective. Epidemiology 2009, 20, S233. [Google Scholar] [CrossRef]
- Mizan, F.R.; Jahid, I.K.; Ha, S.D. Microbial biofilms in seafood: A food-hygiene challenge. Food Microbiol. 2015, 49, 41–55. [Google Scholar] [CrossRef]
- Sala, M.R.; Arias, C.; Dominguez, A.; Bartolomé, R.; Muntada, J.M. Foodborne outbreak of gastroenteritis due to Norovirus and Vibrio parahaemolyticus. Epidemol. Infect. 2008, 137, 626–629. [Google Scholar] [CrossRef]
- Elbashir, S.; Parveen, S.; Schwarz, J.; Rippen, T.; Jahncke, M.; DePaola, A. Seafood pathogens and information on antimicrobial resistance: A review. Food Microbiol. 2018, 70, 85–93. [Google Scholar] [CrossRef]
- Alfano-Sobsey, M.; Davies, M.; Ledford, S.I. Norovirus Outbreak associated with undercooked oysters and secondary household transmission. Epidemiol. Infect. 2012, 140, 276–282. [Google Scholar] [CrossRef]
- Brucker, R.; Bui, T.; Kwan-Gett, T.; Stewart, L. Centers for Disease Control and Prevention, Notes from the Field: Norovirus Infections Associated with Frozen Raw Oysters, Washington. Morb. Mortal. Wkly. Rep. 2011, 307, 1480. Available online: http://www.cdc.gov/mmwr/preview/mmwrhtmL/mm6106a3.htm (accessed on 29 September 2021).
- Chironna, M.; Germinario, C.; De Medici, D.; Fiore, A.; Di Pasquale, S.; Quartoa, M.; Barbuti, S. Detection of hepatitis A virus in mussels from different sources marketed in Puglia region (South Italy). Int. J. Food Microbiol. 2002, 75, 11–18. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Increase in Vibrio parahaemolyticus Illnesses Associated with Consumption of Shellfish from Several Atlantic Coast Harvest Areas, United States. 2013. Available online: https://www.cdc.gov/vibrio/investigations/vibriop-09-13/index.html (accessed on 12 October 2021).
- Zuber, S.C.; Butot, S.; Baert, L. Effects of treatments used in food processing on viruses. In Food Safety Assurance and Veterinary Public Health, Volume 6: Foodborne Viruses and Prions and Their Significance for Public Health; ECVPH (European College of Veterinary Public Health/European Food Safety Authority); Smulders, F.J.M., Nørrung, B., Budka, H., Eds.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2013; pp. 113–136. [Google Scholar]
- Choi, M.S.; Jeon, E.B.; Kim, J.; Chou, E.H.; Lim, J.S.; Choi, J.; Ha, K.S.; Kwon, J.Y.; Jeong, S.H.; Park, S.Y. Virucidal Effects of Dielectric Barrier Discharge Plasma on Human Norovirus Infectivity in Fresh Oysters (Crassostrea gigas). Foods 2020, 9, 1731. [Google Scholar] [CrossRef]
- Csadek, I.; Paulsen, P.; Weidinger, P.; Bak, K.H.; Bauer, S.; Pilz, B.; Nowotny, N.; Smulders, F.J.M. Nitrogen Accumulation in Oyster (Crassostrea gigas) Slurry Exposed to Virucidal Cold Atmospheric Plasma Treatment. Life 2021, 11, 1333. [Google Scholar] [CrossRef]
- van Holde, K.E.; Miller, K.I.; Decker, H. Hemocyanins and Invertebrate Evolution. J. Biol. Chem. 2001, 19, 15563–15566. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Xu, R.; Gou, L.; Liu, Z.; Zhao, Y.; Liu, D.; Zhang, L.; Chen, H. Mechanism of Virus Inactivation by Cold Atmospheric-Pressure Plasma and Plasma-Activated Water. Appl. Environ. Microbiol. 2018, 84, e00726-18. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, Y.; Wang, J.; Xu, S.; Yu, L.; Corvini, P.; Wintgens, T. Nitrate removal from water by new polymeric adsorbent modified with amino and quaternary ammonium groups: Batch and column adsorption study. J. Taiwan Inst. Chem. Eng. 2016, 66, 191–199. [Google Scholar] [CrossRef]
- El-Hanache, L.; Sundermann, L.; Lebeau, B.; Toufaily, J.; Hamieh, T.; Daou, T.J. Surfactant-modified MFI-type nanozeolites: Super-adsorbents for nitrate removal from contaminated water. Microporous Mesoporous Mater. 2019, 283, 1–13. [Google Scholar] [CrossRef]
- Filipić, A.; Gutierrez-Aguirre, I.; Primc, G.; Mozetič, M.; Dobnik, D. Cold Plasma, a New Hope in the Field of Virus Inactivation. Trends Biotechnol. 2020, 38, 1278–1291. [Google Scholar] [CrossRef]
- McMinn, B.R.; Ashbolt, N.J.; Korajkic, A. Bacteriophages as indicators of faecal pollution and enteric virus removal. Lett. Appl. Microbiol. 2017, 65, 11–26. [Google Scholar] [CrossRef]
- Cromeans, T.; Park, G.W.; Costantini, V.; Lee, D.; Wang, Q.; Farkas, T.; Lee, A.; Vinjé, J. Comprehensive comparison of cultivable norovirus surrogates in response to different inactivation and disinfection treatments. Appl. Environ. Microbiol. 2014, 80, 5743–5751. [Google Scholar] [CrossRef]
- Mohamed, H.; Nayak, G.; Rendine, N.; Wigdahl, B.; Krebs, F.C.; Bruggeman, P.J.; Miller, V. Non-thermal plasma as a novel strategy for treating or preventing viral infection and associated disease. Front. Phys. 2021, 9, 683118. [Google Scholar] [CrossRef]
- Weiss, M.; Daeschlein, G.; Kramer, A.; Burchardt, M.; Brucker, S.; Wallwiener, D.; Stope, M.B. Virucide properties of cold atmospheric plasma for future clinical applications. J. Med. Virol. 2017, 89, 952–959. [Google Scholar] [CrossRef]
- Mormann, S.; Dabisch, M.; Becker, B. Effects of technological processes on the tenacity and inactivation of norovirus genogroup II in experimentally contaminated foods. Appl. Environ. Microbiol. 2010, 76, 536–545. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paulsen, P.; Csadek, I.; Bauer, A.; Bak, K.H.; Weidinger, P.; Schwaiger, K.; Nowotny, N.; Walsh, J.; Martines, E.; Smulders, F.J.M. Treatment of Fresh Meat, Fish and Products Thereof with Cold Atmospheric Plasma to Inactivate Microbial Pathogens and Extend Shelf Life. Foods 2022, 11, 3865. https://doi.org/10.3390/foods11233865
Paulsen P, Csadek I, Bauer A, Bak KH, Weidinger P, Schwaiger K, Nowotny N, Walsh J, Martines E, Smulders FJM. Treatment of Fresh Meat, Fish and Products Thereof with Cold Atmospheric Plasma to Inactivate Microbial Pathogens and Extend Shelf Life. Foods. 2022; 11(23):3865. https://doi.org/10.3390/foods11233865
Chicago/Turabian StylePaulsen, Peter, Isabella Csadek, Alexandra Bauer, Kathrine H. Bak, Pia Weidinger, Karin Schwaiger, Norbert Nowotny, James Walsh, Emilio Martines, and Frans J. M. Smulders. 2022. "Treatment of Fresh Meat, Fish and Products Thereof with Cold Atmospheric Plasma to Inactivate Microbial Pathogens and Extend Shelf Life" Foods 11, no. 23: 3865. https://doi.org/10.3390/foods11233865
APA StylePaulsen, P., Csadek, I., Bauer, A., Bak, K. H., Weidinger, P., Schwaiger, K., Nowotny, N., Walsh, J., Martines, E., & Smulders, F. J. M. (2022). Treatment of Fresh Meat, Fish and Products Thereof with Cold Atmospheric Plasma to Inactivate Microbial Pathogens and Extend Shelf Life. Foods, 11(23), 3865. https://doi.org/10.3390/foods11233865







