Inactivation of Clostridium perfringens C1 Spores by the Combination of Mild Heat and Lactic Acid
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Spore Preparation
2.2. LA and Heat Treatment
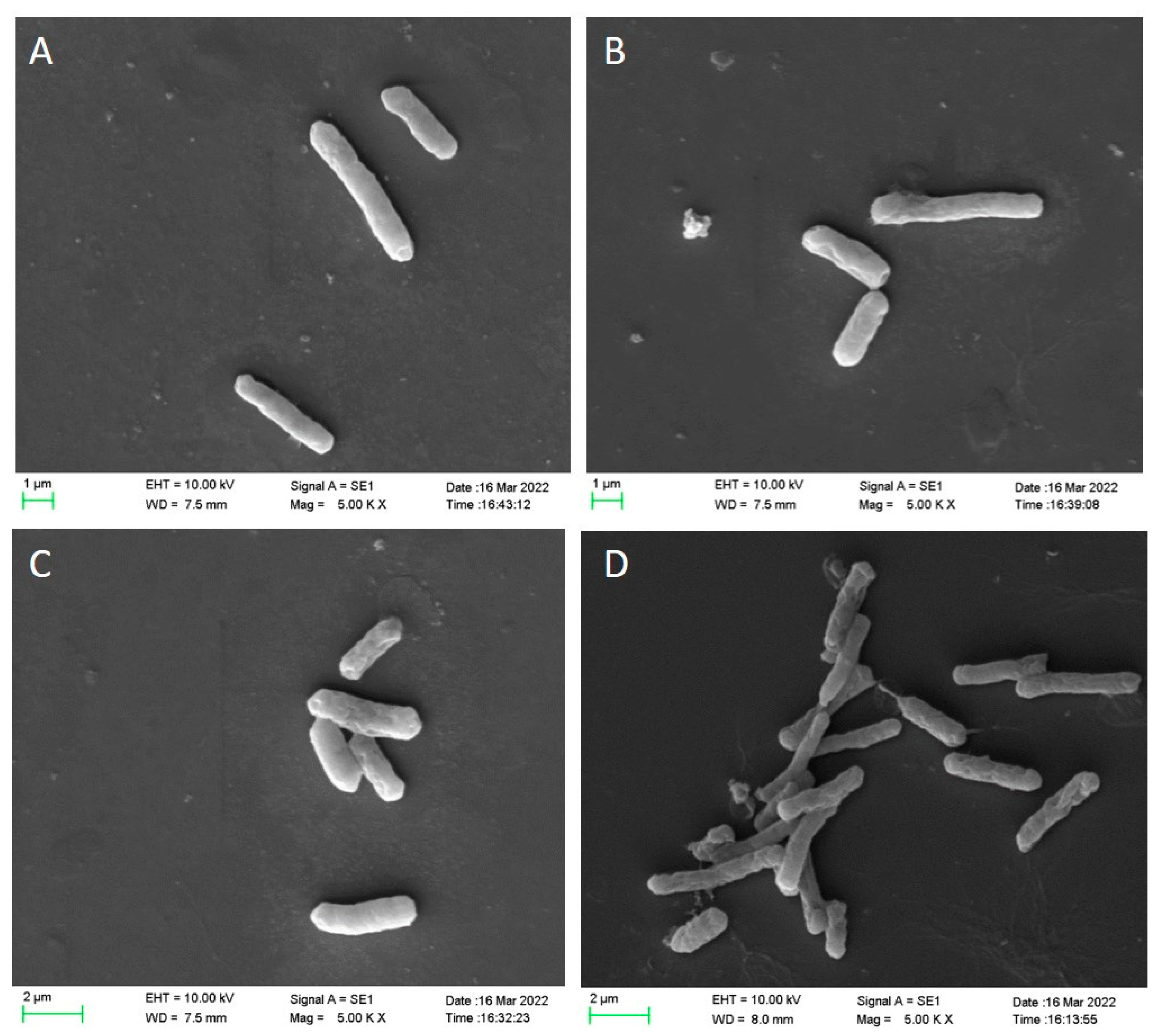
2.3. Scanning Electron Microscopy
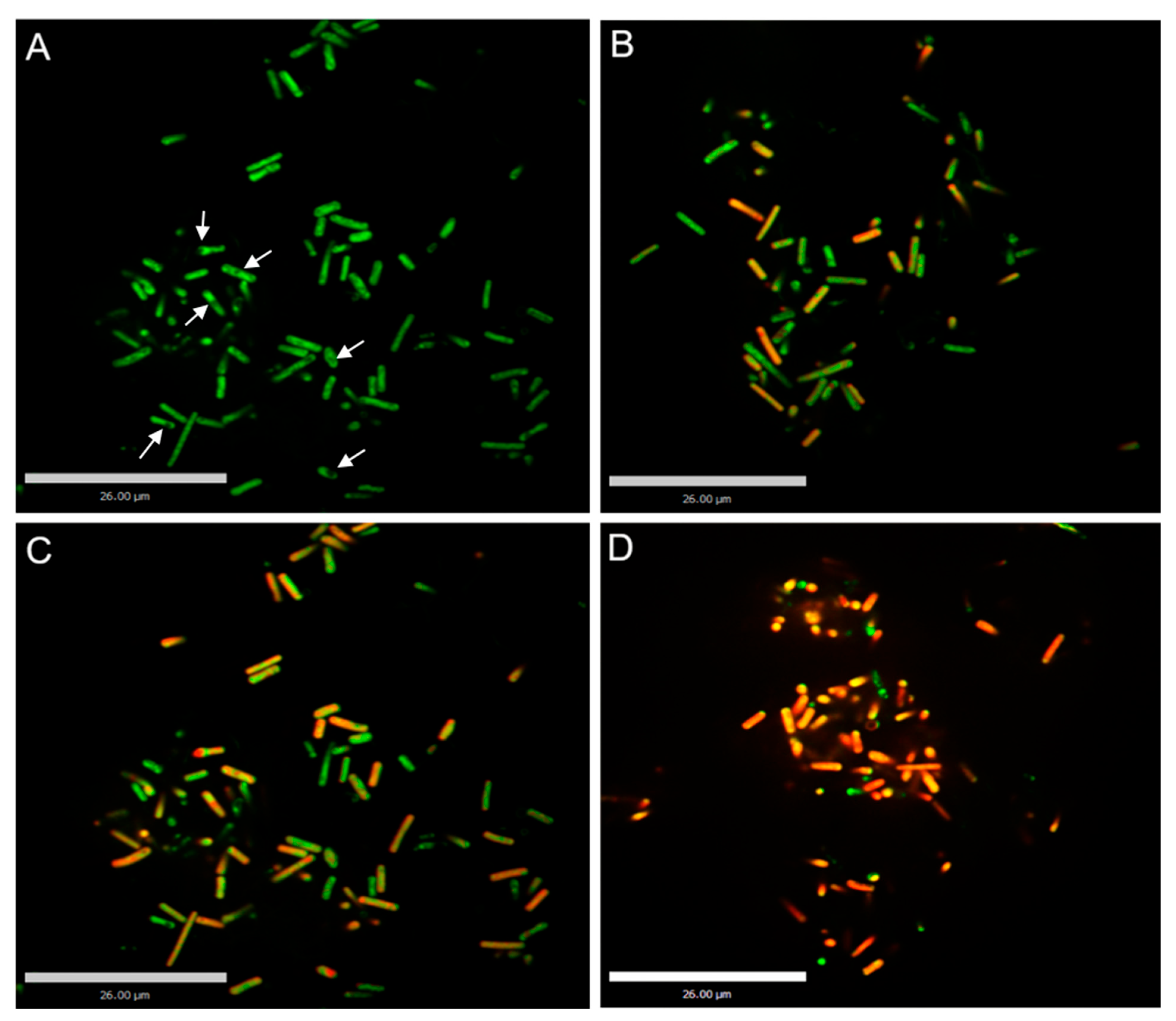
2.4. Confocal Laser Scanning Microscopy (CLSM)
2.5. Determination of the Release Rate of DPA
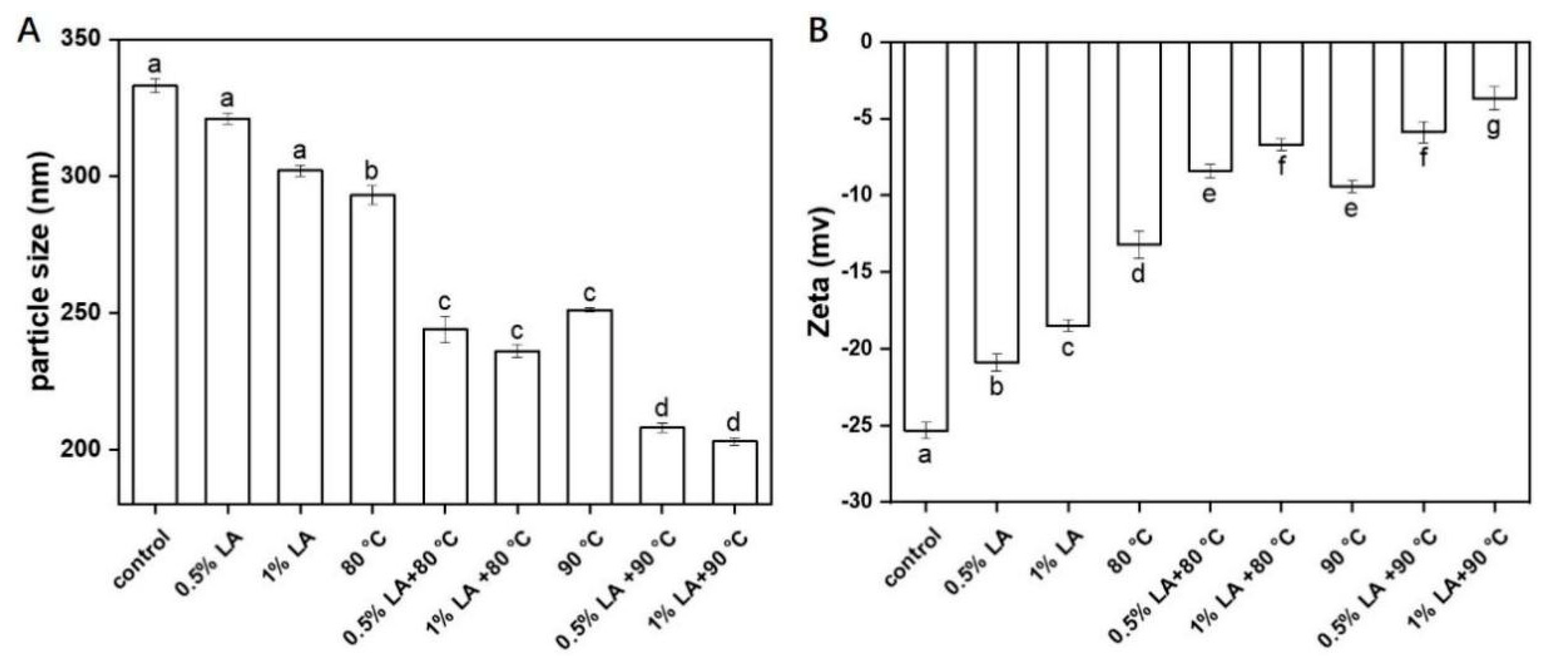
2.6. Measurement of Particle Size and Zeta Potential
2.7. Rate of Hydrophobicity Measurements
2.8. Statistical Analysis
3. Results and Discussion
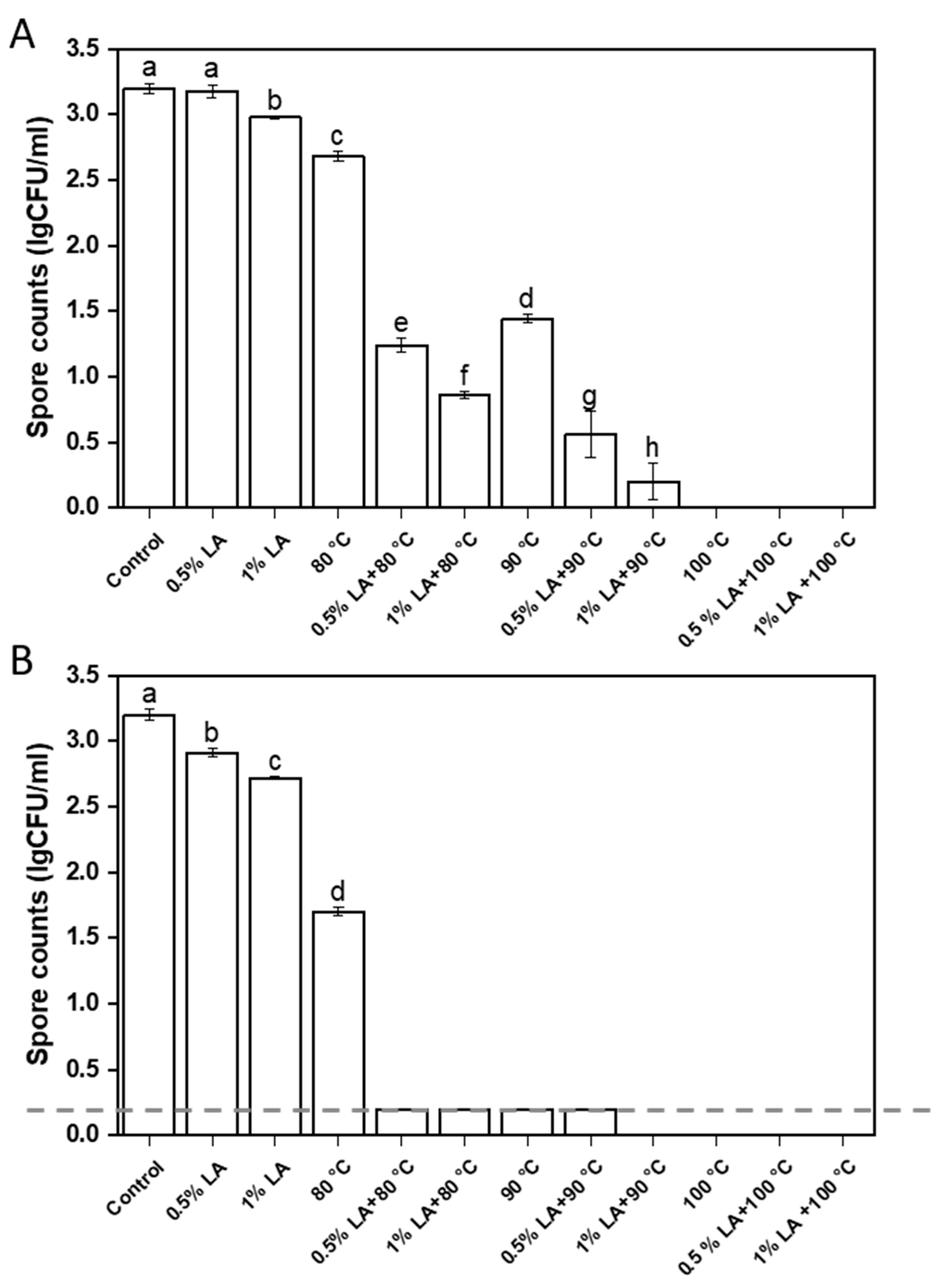
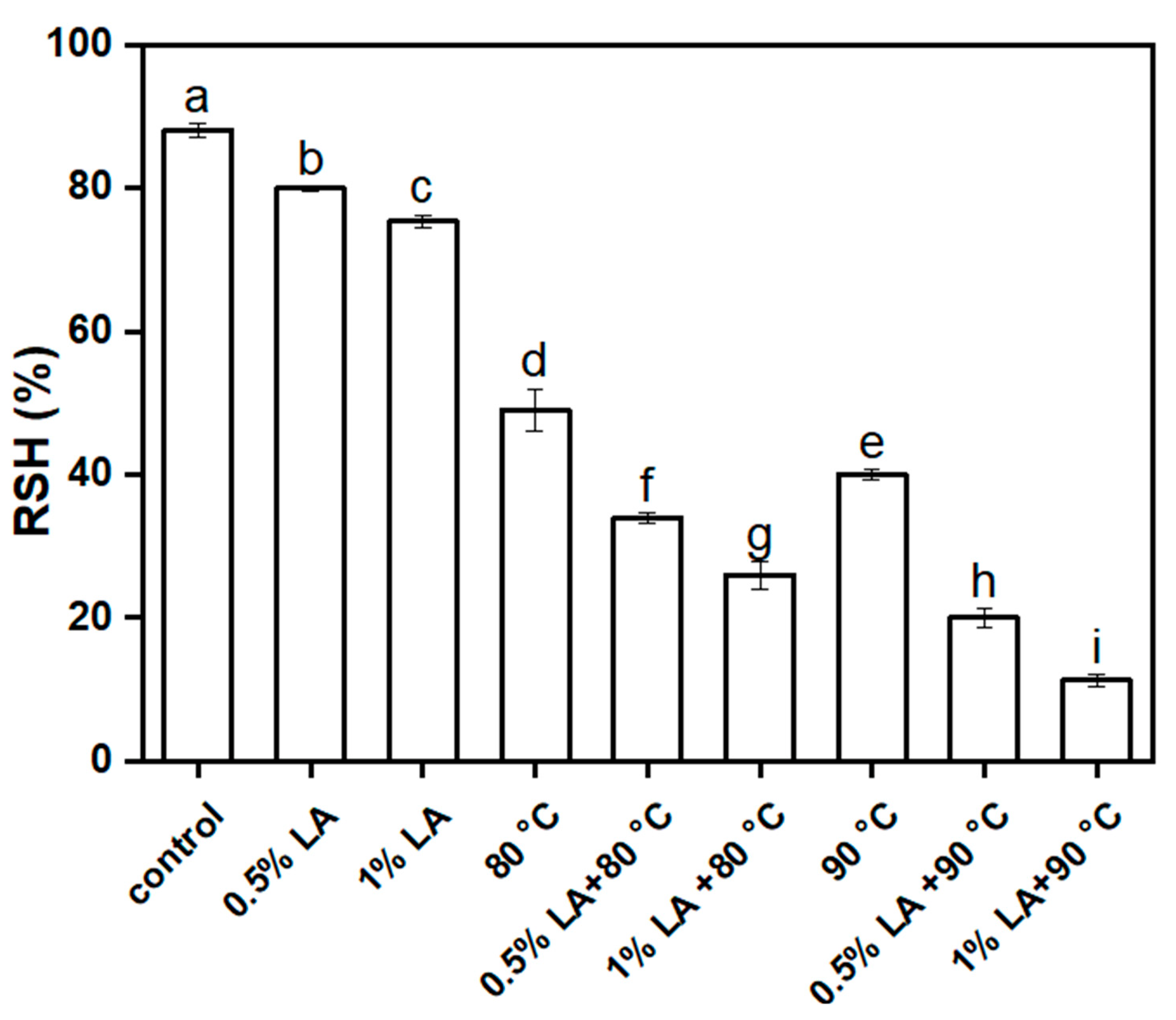
3.1. Inactivation of C. perfringens Spores
3.2. Scanning Electron Microscopy (SEM) Analysis
3.3. Confocal Laser Scanning Microscopy (CLSM) Analyses
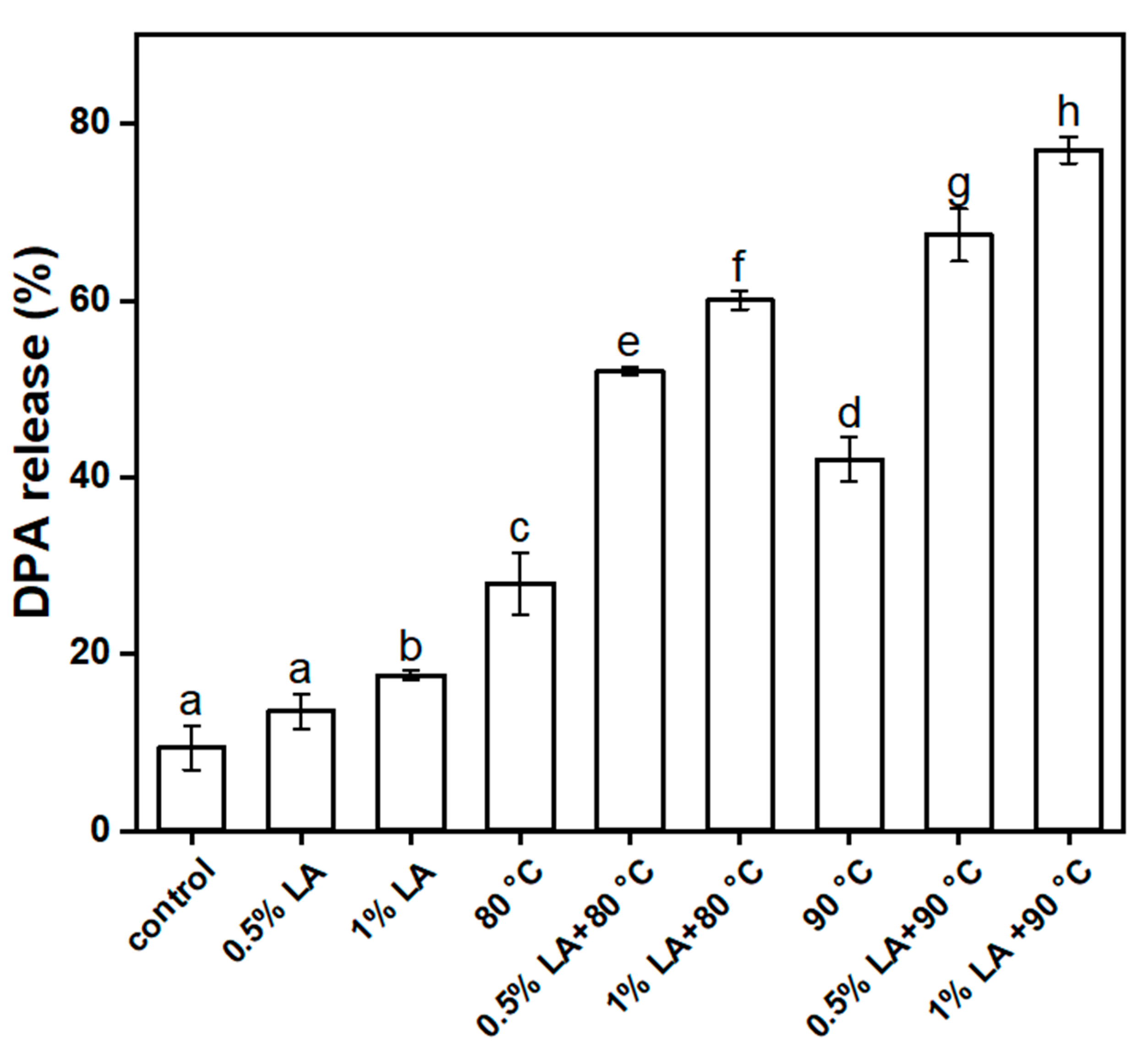
3.4. Determination of 2,6-Pyridinedicarboxylic Acid Release Rate (DPA%)
3.5. Measurement of Particle Size and Zeta-Potential
3.6. Hydrophobic Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhen, J.; Liu, Y.H.; Hwang, C.A.; Hunag, L.H. Effect of combination of oxyrase and sodium thioglycolate on growth of Clostridium perfringens from spores under aerobic incubation. Food Microbiol. 2020, 89, 103413. [Google Scholar]
- Monma, C.; Hatakeyama, K.; Obata, H.; Yokoyama, K.; Konishi, N.; Itoh, T.; Kai, A. Four foodborne disease outbreaks caused by a new type of enterotoxin-producing Clostridium perfringens. J. Clin. Microbiol. 2015, 53, 859–867. [Google Scholar] [CrossRef] [PubMed]
- Paredes-Sabja, D.; Raju, D.; Torres, J.A.; Sarker, M.R. Role of small, acid-soluble spore proteins in the resistance of Clostridium perfringens spores to chemicals. Int. J. Food Microbiol. 2008, 122, 333–335. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Chen, F.; Hu, X. Research progress on the spore germination. J. Chin. Inst. Food Sci. Technol. 2018, 18, 221–228. [Google Scholar]
- Cheng, Q.; Huang, S.S.; Chen, L.M. Research advances on the germination mechanism of bacillus spores. Chin. Bull. Life Sci. 2010, 22, 878–885. [Google Scholar]
- Zhu, Y.D.; Zhang, J.Y.; Li, M.Y.; Zhao, L.J.; Zhao, G.M.; Ma, Y.Y.; Ren, H.R.; Wang, W.T. Effect and prediction of peptidoglycan on spore germination rate of Clostridium perfringens in meat products. Trans. Chin. Soc. Agric. Eng. 2020, 36, 287–293. [Google Scholar]
- Coleman, W.H.; Chen, D.; Li, Y.Q.; Cowan, A.E.; Setlow, P. How moist heat kills spores of Bacillus subtilis. J. Bacteriol. 2022, 189, 8458–8466. [Google Scholar] [CrossRef]
- Leuschner, R.G.K.; Lillford, P.J. Thermal properties of bacterial spores and biopolymers. Int. J. Food Microbiol. 2002, 80, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Degeer, S.; Wang, L.; Singh, M.; Bilgili, S.; Bratcher, C. Evaluation of lactic acid and sodium metasilicate against pathogens of concern on fresh beef, pork, and deli meats. Meat Sci. 2015, 101, 155. [Google Scholar] [CrossRef]
- Palop, A.; Marco, A.; Raso, J.; Sala, F.J.; Condón, S. Survival of heated Bacillus coagulans spores in a medium acidified with lactic or citric acid. Int. J. Food Microbiol. 1997, 38, 25–30. [Google Scholar] [CrossRef]
- Su, R.X.; Vermeulen, A.; Devlieghere, F. Modeling the combined effect of temperature, pH, acetic and lactic acid concentrations on the growth/no growth interface of acid-tolerant Bacillus spores. Int. J. Food Microbiol. 2021, 360, 109419. [Google Scholar]
- Velugoti, P.R.; Kumar, S.; Bohra, L.K.; Juneja, V.K.; Thippareddi, H. Inhibition of germination and outgrowth of Clostridium perfringens spores by buffered calcium, potassium and sodium citrates in cured and non-cured injected pork during cooling. LWT 2020, 123, 109074. [Google Scholar] [CrossRef]
- Akhtar, S.; Paredes-Sabja, D.; Torres, J.A.; Sarker, M.R. Strategy to inactivate Clostridium perfringens spores in meat products. Food Microbiol. 2009, 26, 272–277. [Google Scholar] [CrossRef]
- Juneja, V.K.; Call, J.E.; Miller, A.J. Evaluation of methylxanthines and related compounds to enhance Clostridium perfringens sporulation using a modified Duncan and strong medium. J. Rapid Methods Autom. Microbiol. 1993, 2, 203–218. [Google Scholar] [CrossRef]
- Mauricio, R.A.; Carol, V.M.; Vijay, K.J.; Dennis, E.B.; Harshavardhan, T. Control of Clostridium perfringens spore germination and outgrowth by potassium lactate and sodium diacetate in ham containing reduced sodium chloride. LWT-Food Sci. Technol. 2020, 137, 110395. [Google Scholar]
- Fan, L.H.; Hou, F.; Muhammad, A.I.; Ruiling, L.V.; Watharkar, R.B.; Guo, M.; Ding, T.; Liu, D. Synergistic inactivation and mechanism of thermal and ultrasound treatments against Bacillus subtilis spores. Food Res. Int. 2019, 116, 1094–1102. [Google Scholar] [CrossRef]
- Liu, F.; Wang, F.T.; Du, L.H.; Zhao, T.; Doyle, M.P.; Wang, D.Y.; Zhang, X.X.; Sun, Z.L.; Xu, W.M. Antibacterial and antibiofilm activity of phenyllactic acid against Enterobacter cloacae. Food Control 2018, 84, 442–448. [Google Scholar] [CrossRef]
- Liu, F.; Du, L.H.; Zhao, T.; Zhao, P.; Michael, P.D. Effects of phenyllactic acid as sanitizing agent for inactivation of Listeria monocytogenes biofilms. Food Control 2017, 78, 72–78. [Google Scholar] [CrossRef]
- Liu, F.; Sun, Z.L.; Wang, F.T.; Liu, Y.W.; Zhu, Y.Z.; Du, L.H.; Wang, D.Y.; Xu, W.M. Inhibition of biofilm formation and exopolysaccharide synthesis of Enterococcus faecalis by phenyllactic acid. Food Microbiol. 2020, 86, 103344. [Google Scholar] [CrossRef] [PubMed]
- Hindle, A.A.; Hall, E.A.H. Dipicolinic acid (DPA) assay revisited and appraised for spore detection. Analyst 1999, 124, 1599–1604. [Google Scholar] [CrossRef]
- Lv, R.; Zou, M.; Chen, W.; Zhou, J.; Liu, D. Ultrasound: Enhance the detachment of exosporium and decrease the hydrophobicity of bacillus cereus spores. LWT-Food Sci. Technol. 2019, 116, 108473. [Google Scholar] [CrossRef]
- Noma, S.; Kiyohara, K.; Hirokado, R.; Yamashita, N.; Migita, Y.; Tanaka, M.; Furukawa, S.; Ogihara, H.; Morinaga, Y.; Igura, N. Increase in hydrophobicity of bacillus subtilis spores by heat, hydrostatic pressure, and pressurized carbon dioxide treatments. J. Biosci. Bioeng. 2018, 125, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Setlow, B.; Atluri, S.; Kitchel, R.; Koziol-Dube, K.; Setlow, P. Role of dipicolinic acid in resistance and stability of spores of Bacillus subtilis with or without DNA-protective α/β-type small acid-soluble proteins. J. Bacteriol. 2006, 188, 3740–3747. [Google Scholar] [CrossRef] [PubMed]
- Raju, D.; Setlow, P.; Sarker, M.R. Antisense-RNA-mediated decreased synthesis of small, acid-soluble spore proteins leads to decreased resistance of Clostridium perfringens spores to moist heat and UV radiation. Appl. Environ. Microbiol. 2007, 73, 2048–2053. [Google Scholar] [CrossRef]
- Sarker, M.R.; Shivers, R.P.; Sparks, S.G.; Juneja, V.K.; Mcclane, B.A. Comparative experiments to examine the effects of heating on vegetative cells and spores of Clostridium perfringens isolates carrying plasmid enterotoxin genes versus chromosomal enterotoxin genes. Appl. Environ. Microbiol. 2000, 66, 3234–3240. [Google Scholar] [CrossRef]
- Wang, G.; Paredes-Sabja, D.; Sarker, M.R.; Green, C.; Setlow, P.; Li, Y.Q. Effects of wet heat treatment on the germination of individual spores of Clostridium perfringens. J. Appl. Microbiol. 2012, 113, 824–836. [Google Scholar] [CrossRef]
- Liang, D.; Wang, X.; Wu, X.M.; Liao, X.J.; Chen, F.; Hu, X.S. The effect of high pressure combined with moderate temperature and peptidoglycan fragments on spore inactivation. Food Res. Int. 2021, 148, 110615. [Google Scholar] [CrossRef]
- Filipa, E.V.M. Use of power ultrasound to enhance the thermal inactivation of Clostridium perfringens spores in beef slurry. Int. J. Food Microbiol. 2015, 206, 17–23. [Google Scholar]
- Chen, L.; Liu, Q.; Zhao, X.; Zhang, H.F.; Pang, X.Y.; Yang, H.S. Inactivation efficacies of lactic acid and mild heat treatments against Escherichia coli strains in organic broccoli sprouts. Food Control 2022, 133, 108577. [Google Scholar] [CrossRef]
- Wang, C.J.; Chang, T.; Yang, H.; Cui, M. Antibacterial mechanism of lactic acid on physiological and morphological properties of Salmonella enteritidis, Escherichia coli and Listeria monocytogenes. Food Control 2015, 47, 231–236. [Google Scholar] [CrossRef]
- Ning, Y.W.; Fu, Y.N.; He, J.Z.; Su, D.; Hou, L.L.; Wang, Z.X.; Jia, Y.M. Synergistic antibacterial mechanism of phenyllactic acid combined with acetic acid against Listeria monocytogenes. Food Sci. 2020, 41, 70–76. [Google Scholar]
- Bevilacqua, A.; Ciuffreda, E.; Sinigaglia, M.; Corbo, M.R. Spore inactivation and DPA release in Alicyclobacillus acidoterrestris under different stress conditions. Food Microbiol. 2015, 46, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Pizarro-Guajardo, M.; Calderón-Romero, P.; Paredes-Sabja, D. Ultrastructure variability of the exosporium layer of clostridium difficile spores from sporulating cultures and biofilms. Appl. Environ. Microbiol. 2016, 82, 5892–5898. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Ma, X.; Wang, W.; Lv, R.; Guo, M.; Ding, T.; Ye, X.; Miao, S.; Liu, D. Preparation of modified whey protein isolate with gum acacia by ultrasound maillard reaction. Food Hydrocoll. 2019, 95, 298–307. [Google Scholar] [CrossRef]
- Shuster, B.; Khemmani, M.; Abe, K.; Huang, X.; Nakaya, Y.; Maryn, N.; Buttar, S.; Gonzalez, A.N.; Driks, A.; Sato, T.; et al. Contributions of crust proteins to spore surface properties in Bacillus subtilis. Mol. Microbiol. 2019, 111, 825–843. [Google Scholar] [CrossRef]
- Kutima, P.M.; Foegeding, P.M. Involvement of the spore coat in germination of bacillus cereus t spores. Appl. Environ. Microbiol. 1987, 53, 47–52. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, T.; Bian, H.; Sun, Z.; Wang, X.; Liu, F.; Wang, D. Inactivation of Clostridium perfringens C1 Spores by the Combination of Mild Heat and Lactic Acid. Foods 2022, 11, 3771. https://doi.org/10.3390/foods11233771
Lin T, Bian H, Sun Z, Wang X, Liu F, Wang D. Inactivation of Clostridium perfringens C1 Spores by the Combination of Mild Heat and Lactic Acid. Foods. 2022; 11(23):3771. https://doi.org/10.3390/foods11233771
Chicago/Turabian StyleLin, Tingting, Huan Bian, Zhilan Sun, Xinxia Wang, Fang Liu, and Daoying Wang. 2022. "Inactivation of Clostridium perfringens C1 Spores by the Combination of Mild Heat and Lactic Acid" Foods 11, no. 23: 3771. https://doi.org/10.3390/foods11233771
APA StyleLin, T., Bian, H., Sun, Z., Wang, X., Liu, F., & Wang, D. (2022). Inactivation of Clostridium perfringens C1 Spores by the Combination of Mild Heat and Lactic Acid. Foods, 11(23), 3771. https://doi.org/10.3390/foods11233771




