The Effect of the Improvement Technology on the Quality of Midu Pork Roll
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of MPR
2.3. Determination of Physical and Chemical Indicators
2.3.1. The Cooking Loss (CL)
2.3.2. The Yield of MPR
2.3.3. pH (Acidity) Determination
2.3.4. Water Activity (Aw) Measurement
2.3.5. Moisture Determination
2.3.6. Chromaticity Assay
2.4. Texture Determination
2.5. Determination of FAAs
2.6. Volatile Compounds Analysis
2.7. Sensory Evaluation
2.8. Data Analysis
2.8.1. Statistics
2.8.2. Taste Activity Value Analysis (TAV)
2.8.3. Principal Component Analysis (PCA) and Comprehensive Evaluation
3. Results and Discussion
3.1. The Influence of Process Optimization on the Physicochemical Analysis
3.2. The Influence of Process Optimization on the Texture of MPR
3.3. The Effect of Process Improvement on the Content of FAAs of MPR
3.4. Evaluation of the TAV of FAAs
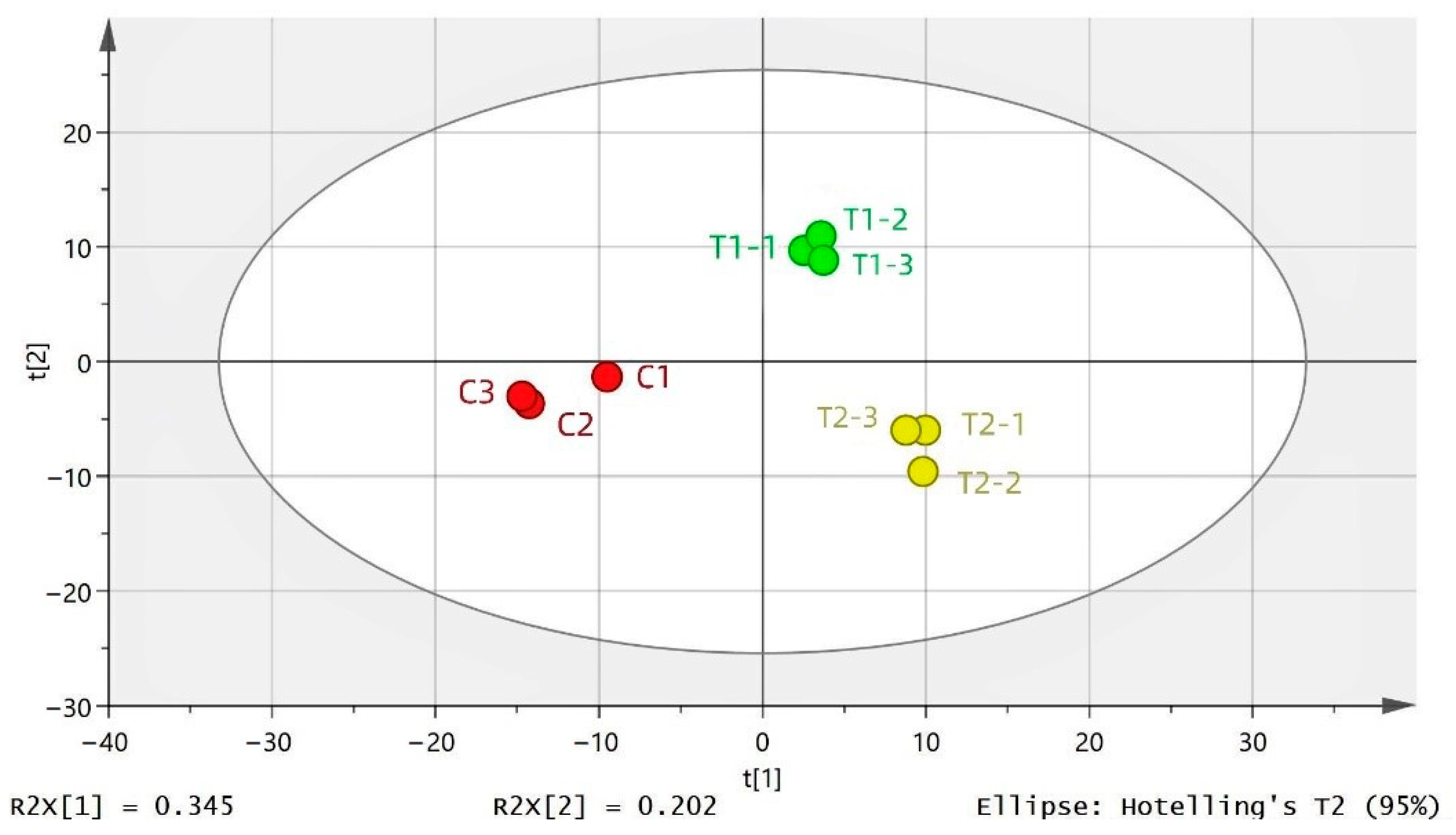
3.5. Principal Component Analysis of Free Amino Acids
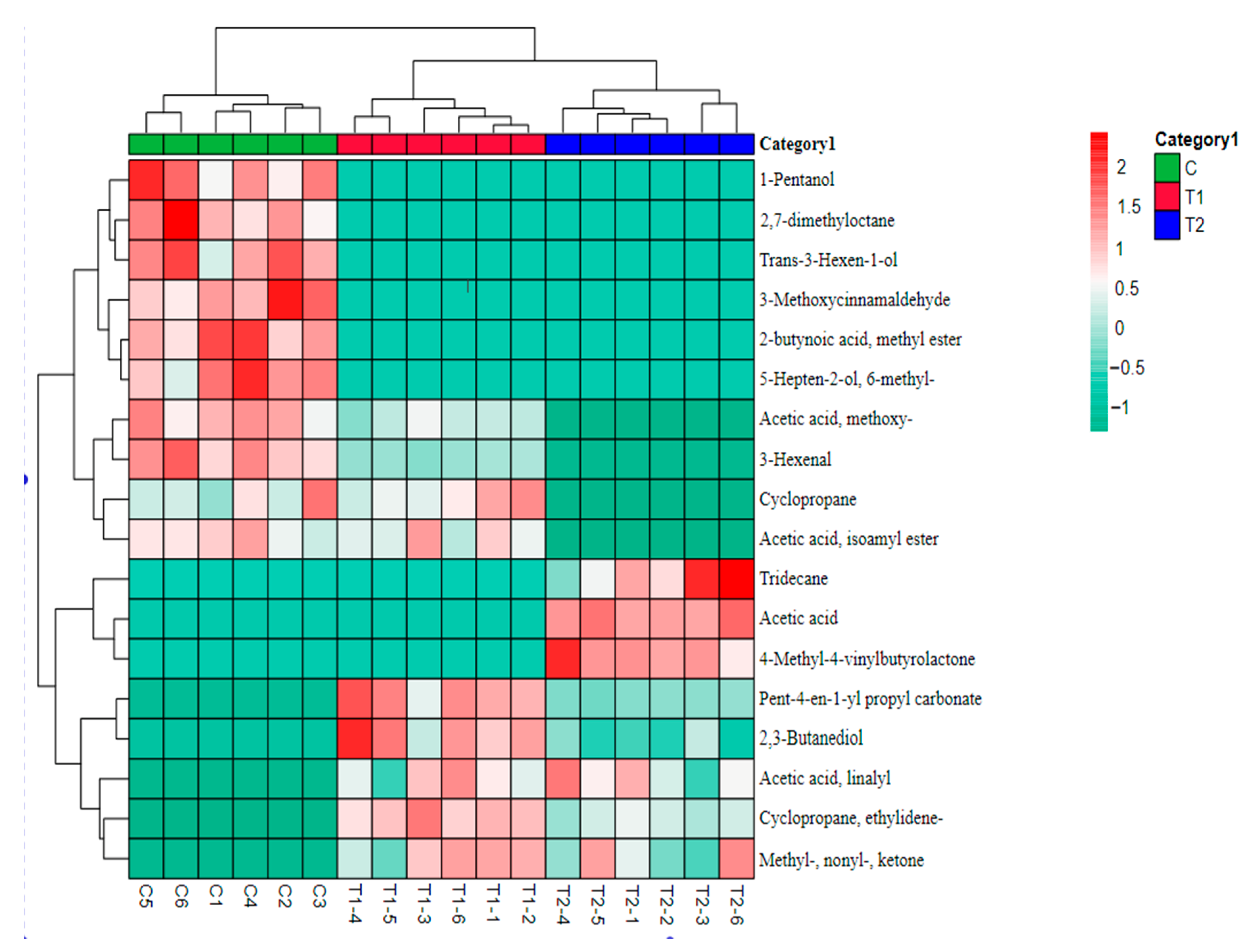
3.6. The Effect of Process Improvement in Various Volatile Compounds of MPR
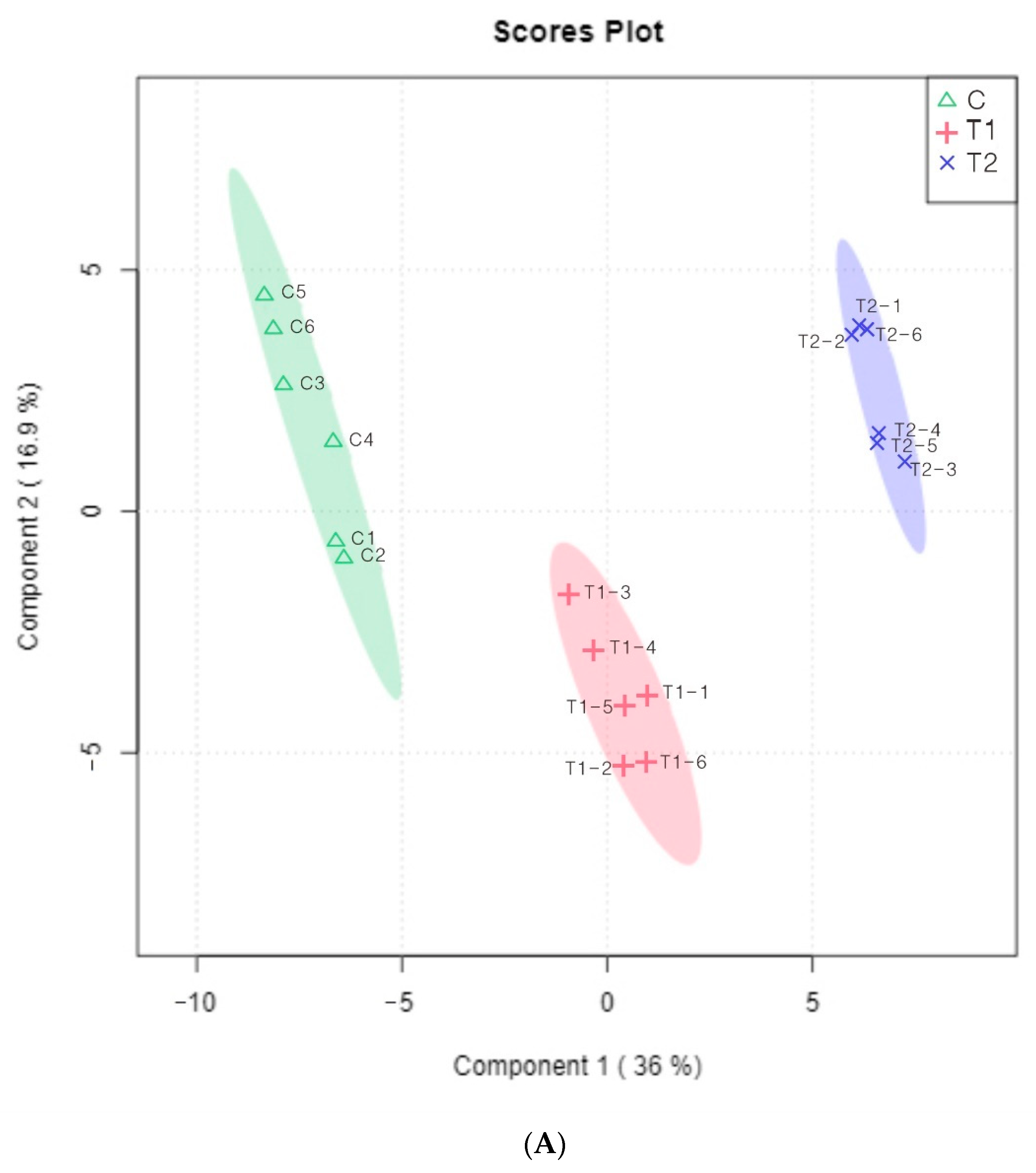
3.7. Multivariate Statistical Analysis of Volatile Components
3.8. Correlation Analysis between Processing and Volatiles
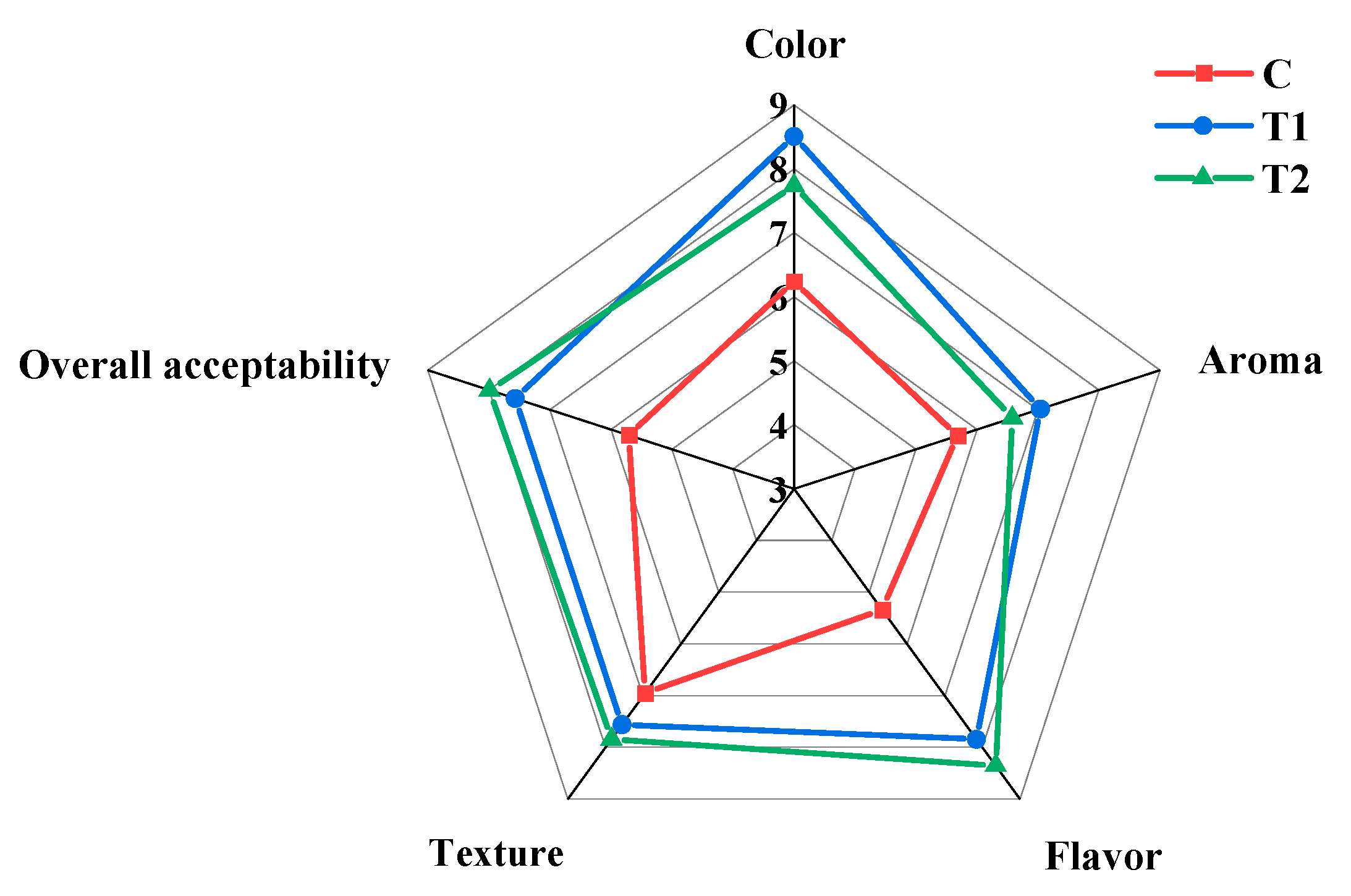
3.9. The Influence of Process Optimization on the Sensory Evaluation of MPR
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, T.; Ge, C.R.; Huang, Q.C.; Yang, H. The content change of free amino acid of Midu Juanti after fermentation. Food Sci. Technol. 2002, 8, 20–21. [Google Scholar] [CrossRef]
- Zhou, F.B.; Zhao, M.M.; Su, G.W.; Sun, W.Z. Binding of aroma compounds with myofibrillar proteins modified by a hydroxyl-radical-induced oxidative system. J. Agric. Food Chem. 2014, 62, 9544–9552. [Google Scholar] [CrossRef] [PubMed]
- Gan, X.; Li, H.J.; Wang, Z.M.; Emara, A.M.; Zhang, D.; He, Z.F. Does protein oxidation affect proteolysis in low sodium Chinese traditional bacon processing? Meat Sci. 2019, 150, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Armenteros, M.; Heinonen, M.; Ollilainen, V.; Toldrá, F.; Estévez, M. Analysis of protein carbonyls in meat products by using the DNPH-method, fluorescence spectroscopy and liquid chromatography–electrospray ionisation–mass spectrometry (LC–ESI–MS). Meat Sci. 2009, 83, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Mariutti, L.R.; Bragagnolo, N. Influence of salt on lipid oxidation in meat and seafood products: A review. Food Res. Int. 2017, 94, 90–100. [Google Scholar] [CrossRef]
- Domínguez, R.; Purriños, L.; Pérez-Santaescolástica, C.; Pateiro, M.; Lorenzo, J.M. Characterization of volatile compounds of dry-cured meat products using HS-SPME-GC/MS technique. Food Anal. Methods 2019, 12, 1263–1284. [Google Scholar] [CrossRef]
- Echegaray, N.; Pateiro, M.; Zhang, W.; Domínguez, R.; Lorenzo, J.M. Influence of the Inclusion of chestnut (Castanea sativa Miller) in the finishing diet and cooking technique on the physicochemical parameters and volatile profile of biceps femoris muscle. Foods 2020, 9, 754. [Google Scholar] [CrossRef]
- Yin, X.; Lv, Y.; Wen, R.; Wang, Y.; Chen, Q.; Kong, B. Characterization of selected Harbin red sausages on the basis of their flavour profiles using HS-SPME-GC/MS combined with electronic nose and electronic tongue. Meat Sci. 2021, 172, 108345. [Google Scholar] [CrossRef]
- Cho, S.; Kang, S.; Kang, G.; Seong, P.; Park, K.; Park, B. Physicochemical meat quality, fatty acid and free amino acid composition of strip loin, chuck tender, and eye of round produced by different age groups of Hanwoo cow. Food Sci. Anim. Resour. 2013, 33, 708–714. [Google Scholar] [CrossRef][Green Version]
- Foggiaro, D.; Domínguez, R.; Pateiro, M.; Cittadini, A.; Lorenzo, J.M. Use of healthy emulsion hydrogels to improve the quality of pork burgers. Foods 2022, 11, 596. [Google Scholar] [CrossRef]
- AMSA. Research Guidelines for Cookery, Sensory Evaluation and Instrumental Tenderness Measurements of Meat, 2nd ed.; American Meat Science Association: Kearney, MO, USA, 2015. [Google Scholar]
- AOAC. Association of Official Analytical Chemists. Official Methods of Analysis; AOAC: Washington, DC, USA, 2012. [Google Scholar]
- AOAC. Association of Official Methods of Analysis Methods. Official Methods of Analysis; AOAC: Washington, DC, USA, 1995. [Google Scholar]
- Boccard, R.; Buchter, L.; Casteels, E.; Cosentino, E.; Dransfield, E.; Hood, D.E.; Joseph, R.L.; MacDougall, D.B.; Rhodes, D.N.; Schön, I.; et al. Procedures for measuring meat quality characteristics in beef production experiments. Report of a working group in the Commission of the European Communities’(CEC) beef production research programme. Livest. Prod. Sci. 1981, 8, 385–397. [Google Scholar] [CrossRef]
- Liao, R.Y.; Xia, Q.; Zhou, C.Y.; Geng, F.; Wang, Y.; Pan, D.D.; Cao, J.X. LC-MS/MS-based metabolomics and sensory evaluation characterize metabolites and texture of normal and spoiled dry-cured hams. Food Chem. 2022, 371, 754. [Google Scholar] [CrossRef]
- Luo, Q.Y.; Li, J.X.; Li, H.; Tian, X.Z.; Lu, Q. The Effects of Purple Corn Pigment on Growth Performance, Blood Biochemical Indices, Meat Quality, Muscle Amino Acids, and Fatty Acids of Growing Chickens. Foods 2022, 11, 1870. [Google Scholar] [CrossRef]
- Wen, R.X.; Hu, Y.Y.; Zhang, L.; Wang, Y.; Chen, Q.; Kong, B. Effect of NaCl substitutes on lipid and protein oxidation and flavor development of Harbin dry sausage. Meat Sci. 2019, 156, 33–43. [Google Scholar] [CrossRef]
- ISO 6658; Sensory Analysis-Methodology-General Guidance. International Organization for Standardization: Geneva, Switzerland, 2017.
- Wang, L.H.; Qiao, K.N.; Duan, W.; Xiao, J.F.; Huang, Y. Comparison of taste components in stewed beef broth under different conditions by means of chemical analyzed. Food Sci. Nutr. 2020, 8, 55–64. [Google Scholar] [CrossRef]
- Duan, W.; Huang, Y.; Xiao, J.F.; Zhang, Y.Y.; Tang, Y.Z. Determination of free amino acids, organic acids, and nucleotides in 29 elegant spices. Food Sci. Nutr. 2020, 8, 3777–3792. [Google Scholar] [CrossRef]
- Leng, P.; Hu, H.W.; Cui, A.H.; Tang, H.J.; Liu, Y.G. HS-GC-IMS with PCA to analyze volatile flavor compounds of honey peach packaged with different preservation methods during storage. LWT-Food Sci. Technol. 2021, 149, 111963. [Google Scholar] [CrossRef]
- Gianelli, M.P.; Flores, M.; Toldrá, F. Interaction of soluble peptides and proteins from skeletal muscle with volatile compounds in model systems as affected by curing agents. J. Agric. Food Chem. 2005, 53, 1670–1677. [Google Scholar] [CrossRef]
- Shen, H.; Zhao, M.M.; Sun, W.Z. Effect of pH on the interaction of porcine myofibrillar proteins with pyrazine compounds. Food Chem. 2019, 287, 93–99. [Google Scholar] [CrossRef]
- Zhong, A.A.; Chen, W.; Duan, Y.F.; Li, K.; Tang, X.Y.; Tian, X.; Wu, Z.; Li, Z.; Wang, Y.; Wang, C. The potential correlation between microbial communities and flavors in traditional fermented sour meat. LWT-Food Sci. Technol. 2021, 149, 111873. [Google Scholar] [CrossRef]
- Patarata, L.; Fernandes, L.; Silva, J.A.; Fraqueza, M.J. The Risk of Salt Reduction in Dry-Cured Sausage Assessed by the Influence on Water Activity and the Survival of Salmonella. Foods 2022, 11, 444. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Y.; Su, W.; Mu, Y.C.; Zhao, C. Correlation between microbial diversity and volatile flavor compounds of Suan zuo rou, a fermented meat product from Guizhou, China. Front. Microbiol. 2021, 12, 736525. [Google Scholar] [CrossRef]
- Prado, N.; Sampayo, M.; González, P.; Lombó, F.; Díaz, J. Physicochemical, sensory and microbiological characterization of Asturian Chorizo, a traditional fermented sausage manufactured in Northern Spain. Meat Sci. 2019, 156, 118–124. [Google Scholar] [CrossRef]
- Benito, M.J.; Martín, A.; Aranda, E.; Pérez-Nevado, F.; Ruiz-Moyano, S.; Córdoba, M.G. Characterization and selection of autochthonous lactic acid bacteria isolated from traditional Iberian dry-fermented salchichón and chorizo sausages. J. Food Sci. 2007, 72, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Nediani, M.; García, L.; Saavedra, L.; Martínez, S.; López Alzogaray, S.; Fadda, S. Adding value to goat meat: Biochemical and technological characterization of autochthonous Lactic Acid Bacteria to achieve high-quality fermented sausages. Microorganisms 2017, 5, 26. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Su, M.B.; Jeong, J.Y. Effects of the addition levels of White Kimchi Powder and Acerola Juice Powder on the qualities of indirectly cured meat products. Food Sci. Anim. Resour. 2020, 40, 636–648. [Google Scholar] [CrossRef]
- Wu, D.; Sun, D.W. Colour measurements by computer vision for food quality control–A review. Trends Food Sci. Technol. 2013, 29, 5–20. [Google Scholar] [CrossRef]
- Kim, J.H.; Yeon, S.J.; Hong, G.E.; Park, W.; Lee, C.H. Effects of whey powder supplementation on dry-aged meat quality. Korean J. Food Sci. Anim. Resour. 2016, 36, 397–404. [Google Scholar] [CrossRef][Green Version]
- Kim, G.D.; Jeong, J.Y.; Hur, S.J.; Yang, H.S.; Jeon, J.T.; Joo, S.T. The relationship between meat color (CIE L*and a*), myoglobin content, and their influences on muscle fiber characteristics and pork quality. Food Sci. Anim. Resour. 2010, 30, 626–633. [Google Scholar] [CrossRef]
- Cheng, Y.M.; Jiang, X.F.; Xue, Y.F.; Qi, F.M.; Dai, Z.H.; Guan, D. Effect of three different proteases on horsemeat tenderness during postmortem aging. J. Food Sci. Technol. 2020, 7, 2528–2537. [Google Scholar] [CrossRef]
- White, A.; O’Sullivan, A.; Troy, D.J.; O’Neill, E.E. Effects of electrical stimulation, chilling temperature and hot-boning on the tenderness of bovine muscles. Meat Sci. 2006, 73, 196–203. [Google Scholar] [CrossRef]
- Melendo, J.A.; Beltran, J.A.; Jaime, I.; Sanacho, R.; Roncales, P. Limited proteolysis of myofibrillar protein by bromelain decreases toughness of coarse dry sausages. Food Chem. 1996, 57, 429–433. [Google Scholar] [CrossRef]
- Bozkurt, H.; Bayram, M. Colour and textural attributes of sucuk during ripening. Meat Sci. 2006, 73, 344–350. [Google Scholar] [CrossRef]
- Deniz, E.; Mora, L.; Aristoy, M.C.; Candoğan, K.; Toldrá, F. Free amino acids and bioactive peptides profile of Pastırma during its processing. Food Res. Int. 2016, 89, 194–201. [Google Scholar] [CrossRef]
- Beck, H.C.; Hansen, A.M.; Lauritsen, F.R. Catabolism of leucine to branched-chain fatty acids in Staphylococcus xylosus. J. Appl. Microbiol. 2010, 96, 1185–1193. [Google Scholar] [CrossRef]
- Hu, Y.Y.; Chen, Q.; Wen, R.X.; Wang, Y.; Qin, L.G.; Kong, B.H. Quality characteristics and flavor profile of Harbin dry sausages inoculated with lactic acid bacteria and Staphylococcus xylosus. LWT-Food Sci. Technol. 2019, 114, 108392. [Google Scholar] [CrossRef]
- Han, D.; Zhang, C.H.; Fauconnier, M.L. Effect of Seasoning Addition on Volatile Composition and Sensory Properties of Stewed Pork. Foods 2021, 10, 10083. [Google Scholar] [CrossRef]
- Cardoso, M.T.; Manuel, L.J.; Erick, S.; Iliani, P.; Cristina, O.A.; Schmidt, M.B. Relationship between volatile organic compounds, free amino acids, and sensory profile of smoked bacon. Meat Sci. 2021, 181, 108596. [Google Scholar] [CrossRef]
- Xiao, Z.C.; Ge, C.R.; Zhou, G.H.; Zhang, W.G.; Liao, G.Z. 1H NMR-based metabolic characterization of Chinese Wuding Chicken meat. Food Chem. 2018, 274, 574–582. [Google Scholar] [CrossRef]
- Kato, H.; Rhue, M.R.; Nishimura, T. Role of Free Amino Acids and Peptides in Food Taste; ACS Symposium Series-American Chemical Society (USA): Washington, DC, USA, 1989; pp. 158–174. [Google Scholar] [CrossRef]
- Ju, M.G.; Kim, J.H.; Jang, H.J.; Yeon, S.J.; Hong, G.E.; Park, W.J.; Seo, H.G.; Lee, C.H. Changes of physicochemical and sensory properties of fermented sausage from sulfur-fed pork. Korean J. Food Sci. Anim. Resour. 2016, 36, 729–736. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Yoshikawa, T.; Ikeda, S.; Ninomiya, T. Measurement of the relative taste intensity of some l-α-amino acids and 5′-nucleotides. J. Food Sci. 1971, 36, 846–849. [Google Scholar] [CrossRef]
- Suami, T.; Hough, L. Molecular mechanisms of sweet taste 1: Sweet and non-sweet tasting amino acids. J. Carbohydr. Chem. 1991, 10, 851–860. [Google Scholar] [CrossRef]
- Chen, Q.; Kong, B.H.; Han, Q.; Xia, X.F.; Xu, L. The role of bacterial fermentation in lipolysis and lipid oxidation in Harbin dry sausages and its flavour development. LWT-Food Sci. Technol. 2017, 77, 389–396. [Google Scholar] [CrossRef]
- Ravyts, F.; Vuyst, L.D.; Leroy, F. Bacterial diversity and functionalities in food fermentations. Eng. Life Sci. 2012, 12, 356–367. [Google Scholar] [CrossRef]
- García, L.C.; Jiménez, S.R.; Belloch, C.; Flores, M. Generation of aroma compounds in a fermented sausage meat model system by Debaryomyces hansenii strains. Food Chem. 2014, 151, 364–373. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, Y.-T.; Cao, J.-X.; Chen, Y.-J.; Sun, Y.-Y.; Zeng, X.-Q.; Pan, D.-D.; Ou, C.-R.; Gan, N. Study on lipolysis-oxidation and volatile flavour compounds of dry-cured goose with different curing salt content during production. Food Chem. 2016, 190, 33–40. [Google Scholar] [CrossRef]
- Ansorena, D.; Gimeno, O.; Astiasaran, I.; Bello, I. Analysis of volatile compounds by GC–MS of a dry fermented sausage: Chorizo de Pamplona. Food Res. Int. 2001, 34, 67–75. [Google Scholar] [CrossRef]
- Herranz, B.; Fernández, M.; de la Hoz, L.; Ordóñez, J.A. Use of bacterial extracts to enhance amino acid breakdown in dry fermented sausages. Meat Sci. 2006, 72, 318–325. [Google Scholar] [CrossRef]
- Papamanoli, E.; Kotzekidou, P.; Tzanetakis, N.; Litopoulou-Tzanetaki, E. Characterization of Micrococcaceae isolated from dry fermented sausage. Food Microbiol. 2002, 19, 441–449. [Google Scholar] [CrossRef]
- Purriños, L.; Franco, D.; Carballo, J.; Lorenzo, J.M. Influence of the salting time on volatile compounds during the manufacture of dry-cured pork shoulder “lacón”. Meat Sci. 2012, 92, 627–634. [Google Scholar] [CrossRef]
- Flores, M.; Durá, M.A.; Marco, A.; Toldrá, F. Effect of Debaryomyces spp. on aroma formation and sensory quality of dry-fermented sausages. Meat Sci. 2004, 68, 439–446. [Google Scholar] [CrossRef]
- Stahnke, L. Aroma components from dried sausages fermented with Staphylococcus xylosus. Meat Sci. 1994, 38, 39–53. [Google Scholar] [CrossRef]
- Carroll, A.L.; Desai, S.H.; Atsumi, S. Microbial production of scent and flavor compounds. Curr. Opin. Biotechnol. 2016, 37, 8–15. [Google Scholar] [CrossRef]
- Wang, X.Y.; Xu, R.; Tong, X.; Cai, S.F.; Chen, Y.S.; Mo, D.L. Characterization of different meat flavor compounds in Guangdong small-ear spotted and Yorkshire pork using two-dimensional gas chromatography–time-of-flight mass spectrometry and multi-omics. LWT Food Sci. Technol. 2022, 169, 114010. [Google Scholar] [CrossRef]


| Formula | Groups | ||
|---|---|---|---|
| C | T1 | T2 | |
| Salt | 4.00% | 2.00% | 2.00% |
| Rice wine | 0.75% | 0.75% | 0.75% |
| Red Yeast Rice | 0.30% | 0.44% | 0.44% |
| Isolated Soy Protein | N. | 1.50% | 1.50% |
| Potato modified starch | N. | 3.00% | 3.00% |
| Sodium Alginate | N. | 0.18% | 0.18% |
| Hexametaphosphate | N. | 0.21% | 0.21% |
| Sodium Nitrite | 0.01% | N. | N. |
| Spice addition amount | 0.40% | 0.15% | 0.15% |
| Chili | N. | 0.60% | N. |
| Ginger | N. | 0.50% | 0.50% |
| Star anise | 0.60% | 0.10% | 0.35% |
| Cinnamon | 0.20% | N. | 0.015% |
| Pepper | 0.4% | 0.3% | 0.22% |
| Tsaoko | 0.60% | 0.10% | 0.04% |
| Clove | 0.20% | N. | 0.04% |
| Angelica dahurica | N. | 0.10% | 0.22% |
| Bay leaf | N. | N. | 0.01% |
| Cumin | N. | N. | 0.02% |
| Amomum | N. | 0.10% | 0.15% |
| Nutmeg | N. | 0.10% | 0.18% |
| Physical and Chemical Indicators | C | T1 | T2 | Sig. |
|---|---|---|---|---|
| pH | 4.26 ± 0.01 c | 5.54 ± 0.03 b | 5.79 ± 0.02 a | ** |
| Aw | 0.96 ± 0.01 a | 0.95 ± 0.01 a | 0.96 ± 0.01 a | ns |
| Moisture content (%) | 56.05 ± 0.49 c | 60.32 ± 1.13 a | 59.18 ± 0.83 b | ** |
| L* | 58.70 ± 1.68 a | 47.42 ± 0.47 c | 53.28 ± 0.41 b | ** |
| a* | 14.49 ± 0.25 c | 24.11 ± 0.86 a | 21.57 ± 0.45 b | ** |
| b* | 13.99 ± 0.13 c | 20.85 ± 1.28 a | 15.80 ± 0.30 b | ** |
| Cooking loss rate (100%) | 18.21% ± 1.76 a | 8.79% ± 0.40 c | 9.80% ± 0.73 b | ** |
| Yield (100%) | 75.23% ± 1.37 b | 85.91% ± 0.25 a | 86.03% ± 0.47 a | ** |
| Texture Index | C | T1 | T2 | Sig. |
|---|---|---|---|---|
| Hardness (g) | 31,729.96 ± 1798.13 a | 9053.36 ± 522.11 b | 9096.49 ± 486.25 b | ** |
| Elasticity | 0.93 ± 0.04 a | 0.62 ± 0.02 b | 0.67 ± 0.03 b | * |
| Cohesion | 0.49 ± 0.00 a | 0.29 ± 0.01 c | 0.38 ± 0.01 b | ** |
| Chewiness | 6906.15 ± 463.92 a | 2341.48 ± 153.38 b | 2653.33 ± 229.63 b | ** |
| Resilience | 0.17 ± 0.01 a | 0.16 ± 0.01 a | 0.17 ± 0.02 a | ns |
| Shear force (N) | 8771.12 ± 643.97 a | 4826.65 ± 592.92 b | 4962.39 ± 663.91 b | ** |
| Taste Characteristics | FAAs | C | T1 | T2 | Sig. |
|---|---|---|---|---|---|
| Umami Amino Acids | Glutamic acid (Glu) | 0.74 ± 0.03 c | 1.49 ± 0.06 b | 1.66 ± 0.19 a | ** |
| Aspartic acid (Asp) | 0.09 ± 0.01 c | 0.13 ± 0.01 b | 0.15 ± 0.01 a | ** | |
| Sweet amino acid | Alanine (Ala) | 0.01 ± 0.01 c | 0.03 ± 0.01 a | 0.02 ± 0.01 b | ** |
| Glycine (Gly) | 0.07 ± 0.01 a | 0.04 ± 0.01 c | 0.06 ± 0.01 b | ** | |
| Serine (Ser) | 0.12 ± 0.01 a | 0.03 ± 0.01 c | 0.08 ± 0.01 b | ** | |
| Threonine (Thr) | 0.12 ± 0.02 a | 0.03 ± 0.01 c | 0.09 ± 0.01 b | ** | |
| Bitter amino acid | Arginine (Arg) | 0.14 ± 0.02 a | 0.05 ± 0.01 c | 0.08 ± 0.02 b | ** |
| Isoleucine (Ile) | 0.08 ± 0.01 a | 0.03 ± 0.01 c | 0.04 ± 0.01 b | ** | |
| Leucine (Leu) | 0.07 ± 0.01 a | 0.03 ± 0.01 b | 0.04 ± 0.01 b | ** | |
| Methionine (Met) | 0.17 ± 0.01 a | 0.06 ± 0.01 b | 0.06 ± 0.01 b | ** | |
| Phenylalanine (Phe) | 0.13 ± 0.01 a | 0.05 ± 0.03 b | 0.11 ± 0.01 a | ** | |
| Tyrosine (Tyr) | 0.01 ± 0.03 a | 0.02 ± 0.01 a | 0.02 ± 0.01 a | ns | |
| Valine (Val) | 0.12 ± 0.02 a | 0.08 ± 0.02 b | 0.08 ± 0.02 b | ** | |
| Odorless amino acids | Lysine (Lys) | 0.02 ± 0.01 c | 0.08 ± 0.01 b | 0.16 ± 0.03 a | ** |
| Proline (Pro) | 0.06 ± 0.02 a | 0.06 ± 0.01 a | 0.07 ± 0.01 a | ns | |
| Total content | Total FAAs | 1.96 ± 0.05 c | 2.23 ± 0.08 b | 2.74 ± 0.19 a | ** |
| Umami Amino Acids | 0.83 ± 0.02 c | 1.63 ± 0.06 b | 1.81 ± 0.09 a | ** | |
| Sweet amino acid | 0.32 ± 0.02 a | 0.13 ± 0.01 c | 0.25 ± 0.01 b | ** | |
| Bitter amino acid | 0.72 ± 0.03 a | 0.33 ± 0.03 c | 0.44 ± 0.13 b | ** | |
| Odorless amino acids | 0.09 ± 0.01 c | 0.14 ± 0.01 b | 0.24 ± 0.03 a | ** |
| Taste Characteristics | FAAs | Threshold (mg/g) | TAV | Sig. | ||
|---|---|---|---|---|---|---|
| C | T1 | T2 | ||||
| Umami Amino Acids | Glu | 0.3 | 2.47 ± 0.03 c | 4.97 ± 0.06 b | 5.53 ± 0.19 a | ** |
| Asp | 1 | 0.09 ± 0.01 c | 0.13 ± 0.01 b | 0.15 ± 0.01 a | ** | |
| Total | — | 2.56 ± 0.02 c | 5.10 ± 0.06 b | 5.68 ± 0.09 a | ** | |
| Sweet amino acid | Ala | 0.6 | 0.02 ± 0.01 c | 0.05 ± 0.01 a | 0.03 ± 0.03 b | ** |
| Gly | 1.3 | 0.05 ± 0.02 a | 0.03 ± 0.01 b | 0.05 ± 0.01 a | ** | |
| Ser | 1.5 | 0.08 ± 0.01 a | 0.02 ± 0.03 c | 0.05 ± 0.01 b | ** | |
| Thr | 2.6 | 0.05 ± 0.01 a | 0.01 ± 0.02 c | 0.03 ± 0.01 b | ** | |
| Total | — | 0.2 ± 0.01 a | 0.11 ± 0.02 c | 0.17 ± 0.02 b | ** | |
| Bitter amino acid | Arg | 0.5 | 0.28 ± 0.01 a | 0.10 ± 0.03 c | 0.16 ± 0.01 b | ** |
| Ile | 0.9 | 0.09 ± 0.01 a | 0.03 ± 0.02 b | 0.04 ± 0.02 b | ** | |
| Leu | 1.9 | 0.04 ± 0.02 a | 0.02 ± 0.01 b | 0.02 ± 0.01 b | ** | |
| Met | 0.3 | 0.37 ± 0.01 a | 0.20 ± 0.01 b | 0.20 ± 0.01 b | ** | |
| Phe | 0.9 | 0.14 ± 0.02 a | 0.06 ± 0.01 b | 0.12 ± 0.01 a | ** | |
| Val | 0.4 | 0.30 ± 0.02 a | 0.20 ± 0.01 b | 0.20 ± 0.01 b | ** | |
| Total | — | 1.22 ± 0.02 a | 0.61 ± 0.01 c | 0.74 ± 0.02 b | ** | |
| Odorless amino acids | Lys | 0.5 | 0.04 ± 0.03 c | 0.16 ± 0.02 b | 0.32 ± 0.01 a | ** |
| Pro | 3 | 0.02 ± 0.01 a | 0.02 ± 0.02 a | 0.02 ± 0.01 a | ns | |
| Total | — | 0.06 ± 0.02 c | 0.18 ± 0.02 b | 0.34 ± 0.01 a | ** | |
| NO. | Volatile Compounds | C | T1 | T2 | Sig. |
|---|---|---|---|---|---|
| Alkanes | 5.83 ± 0.18 b | 10.14 ± 1.55 a | 7.00 ± 0.54 b | ** | |
| 1 | Cyclopropane, ethylidene- | ND | 4.18 ± 1.03 a | 2.74 ± 0.32 b | ** |
| 2 | Cyclopropane | 2.20 ± 0.21 a | 2.41 ± 0.40 a | ND | ** |
| 3 | Dodecane, 4-methyl- | 0.18 ± 0.23 a | 0.02 ± 0.01 a | ND | ns |
| 4 | Dodecane | 0.80 ± 0.07 a | 0.29 ± 0.03 b | 0.27 ± 0.02 b | ** |
| 5 | 2,7-dimethyloctane | 0.24 ± 0.03 | ND | ND | ** |
| 6 | Tridecane, 5-methyl- | 0.02 ± 0.01 ab | 0.01 ± 0.01 b | 0.12 ± 0.09 a | ns |
| 7 | Tridecane, 3-methyl- | 0.09 ± 0.01 a | 0.04 ± 0.01 b | 0.04 ± 0.01 b | ** |
| 8 | Tetradecane | 0.10 ± 0.01 a | 0.07 ± 0.01 b | 0.05 ± 0.01 b | ** |
| 9 | n-Hexadecane | 0.04 ± 0.01 a | 0.07 ± 0.04 a | 0.05 ± 0.01 a | ns |
| 10 | n-Pentadecane | 0.03 ± 0.01 b | 0.06 ± 0.01 a | 0.05 ± 0.02 ab | ns |
| 11 | Pentadecane, 3-methyl- | 0.01 ± 0.01 | ND | ND | ns |
| 12 | Nonane, 3-methyl- | 0.13 ± 0.04 a | 0.05 ± 0.01 b | 0.13 ± 0.03 a | * |
| 13 | Decane | 1.97 ± 0.04 a | 1.00 ± 0.14 c | 1.19 ± 0.09 b | ** |
| 14 | 2,2,7,7-Tetramethyloctane | ND | 1.95 ± 0.22 a | 2.11 ± 0.11 a | ** |
| 15 | Tridecane | ND | ND | 0.20 ± 0.01 | ** |
| 16 | Bicyclo[3.1.1]heptane, 6,6-dimethyl-2-methylene-, (1S)- | 0.03 ± 0.01 b | ND | 0.06 ± 0.01 a | ** |
| Alcohols | 34.43 ± 2.02 a | 36.80 ± 2.23 a | 35.30 ± 2.26 a | ns | |
| 17 | Ethanol | 7.11 ± 0.63 a | 5.07 ± 0.51 b | 4.56 ± 0.60 b | ** |
| 18 | Propanol | 0.20 ± 0.05 | ND | ND | ** |
| 19 | 1,5-hexadiene-3-ol | 0.06 ± 0.01 | ND | ND | ** |
| 20 | (+)-3-Methyl-2-butanol | 0.22 ± 0.16 a | 0.22 ± 0.02 a | ND | * |
| 21 | Pentanol | 4.78 ± 0.37 a | 5.05 ± 0.59 a | 4.23 ± 0.63 a | ns |
| 22 | 1-Pentanol | 0.14 ± 0.01 | ND | ND | ** |
| 23 | 1-Pentanol, 4-methyl- | ND | 0.01 ± 0.01 | ND | ns |
| 24 | Trans-3-Hexen-1-ol | 0.16 ± 0.01 | ND | ND | ** |
| 25 | 4-Hexen-1-ol, (E)- | 8.93 ± 0.67 b | 13.01 ± 3.27 a | 12.90 ± 1.48 a | ns |
| 26 | 2-Heptanol | 0.34 ± 0.05 a | 0.17 ± 0.02 b | 0.21 ± 0.01 b | ** |
| 27 | 1-Penten-3-ol | 0.06 ± 0.01 b | 0.15 ± 0.13 ab | 0.34 ± 0.16 a | ns |
| 28 | (R)-(-)-3-Methyl-2-butanol | ND | 0.24 ± 0.02 | ND | ** |
| 29 | 5-Hepten-2-ol, 6-methyl- | 2.10 ± 0.14 | ND | ND | ** |
| 30 | 2-phenyl-2-butanol | 0.02 ± 0.01 | ND | ND | ** |
| 31 | Linalool | 7.48 ± 0.88 b | 9.49 ± 0.42 a | 9.91 ± 0.63 a | ** |
| 32 | Terpinen-4-ol | 0.54 ± 0.07 b | 0.35 ± 0.04 c | 0.91 ± 0.02 a | ** |
| 33 | Bicyclo[2.2.1]heptan-2-ol, 2,3,3-trimethyl- | 0.02 ± 0.01 b | 0.03 ± 0.01 ab | 0.05 ± 0.01 a | * |
| 34 | α-Methyl-α-[4-methyl-3-pentenyl]oxiranemethanol | 0.95 ± 0.14 a | 0.48 ± 0.09 b | 0.47 ± 0.03 b | ** |
| 35 | Nerol | 0.44 ± 0.08 a | 0.27 ± 0.02 b | 0.03 ± 0.01 c | ** |
| 36 | Nerolidol | 0.02 ± 0.01 a | 0.01 ± 0.01 a | 0.02 ± 0.01 a | ns |
| 37 | 2,3-Butanediol | ND | 0.30 ± 0.07 a | 0.07 ± 0.01 b | ** |
| 38 | 2,3-Butanediol, [S-(R*,R*)]- | ND | 0.57 ± 0.04 | ND | ** |
| 39 | 2-Cyclohexen-1-ol, 3-methyl-6-(1-methylethyl)-, trans- | 0.17 ± 0.03 b | 0.32 ± 0.05 a | ND | ** |
| 40 | 2-Cyclohexen-1-ol, 1-methyl-4-(1-methylethyl)-, trans- | 0.30 ± 0.01 b | 0.45 ± 0.09 b | 1.21 ± 0.14 a | ** |
| 41 | 2-Isopropyl-1,3-propanediol | ND | 0.06 ± 0.01 | ND | ** |
| 42 | 5-Isopropyl-2-methylbicyclo[3.1.0]hexan-2-ol | 0.26 ± 0.02 b | 0.34 ± 0.01 a | 0.31 ± 0.07 a | ns |
| 43 | Phenylethyl Alcohol | 0.14 ± 0.01 ab | 0.20 ± 0.01 a | 0.10 ± 0.07 b | ns |
| Aldehydes | 2.56 ± 0.21 a | 2.86 ± 0.46 a | 2.65 ± 0.62 a | ns | |
| 44 | n-Hexanal | 0.34 ± 0.01 a | 0.44 ± 0.16 a | 0.46 ± 0.14 a | ns |
| 45 | 3-Hexenal | 0.62 ± 0.01 a | 0.29 ± 0.04 b | ND | ** |
| 46 | 2,4-Hexadienal, (E,E)- | 0.44 ± 0.22 b | 1.55 ± 0.55 a | 1.49 ± 0.60 ab | ns |
| 47 | 2-Hexadienal, (E)- | 0.05 ± 0.01 | ND | ND | ** |
| 48 | Benzaldehyde | 0.07 ± 0.04 a | 0.04 ± 0.01 a | 0.03 ± 0.01 a | ns |
| 49 | Benzeneacetaldehyde | ND | 0.01 ± 0.01 a | 0.01 ± 0.01 a | ns |
| 50 | 2,6-Octadienal, 3,7-dimethyl-, (E)- | 0.05 ± 0.01 a | 0.02 ± 0.01 a | 0.03 ± 0.01 a | * |
| 51 | Cinnamaldehyde | 0.04 ± 0.01 a | 0.06 ± 0.01 a | 0.08 ± 0.01 a | ** |
| 52 | 3-Methoxycinnamaldehyde | 0.86 ± 0.04 | ND | ND | ** |
| 53 | Hexadedehyde | 0.02 ± 0.01 | ND | ND | ** |
| 54 | Benzaldehyde, 4-(1-methylethyl)- | 0.02 ± 0.01 b | 0.22 ± 0.01 a | 0.19 ± 0.02 a | ** |
| 55 | 3-Hexenal, (Z)- | ND | 0.19 ± 0.01 a | 0.16 ± 0.05 a | ** |
| 56 | 2-heptanal | 0.05 ± 0.01 a | 0.05 ± 0.01 a | 0.06 ± 0.01 a | ns |
| 57 | Benzaldehyde, 4-methoxy- | ND | ND | 0.14 ± 0.01 | ** |
| Ketones | 1.69 ± 0.21 b | 2.95 ± 0.37 a | 1.71 ± 0.03 b | ** | |
| 58 | 2,5-Hexanedione | 0.02 ± 0.01 | ND | ND | * |
| 59 | Bicyclo[3.1.1]heptan-2-one, 6,6-dimethyl-, (1 R)- | 0.69 ± 0.08 a | 0.72 ± 0.02 a | ND | ** |
| 60 | Thujone | 0.28 ± 0.02 b | 0.51 ± 0.03 a | 0.43 ± 0.03 a | ** |
| 61 | Bicyclo[3.1.0]hexan-3-one, 4-methyl-1-(1-methylethyl)-, [1S-(1α,4β,5α)]- | 0.20 ± 0.01 a | 0.22 ± 0.01 a | 0.13 ± 0.01 b | ** |
| 62 | Piperitone | 0.42 ± 0.09 a | 0.24 ± 0.04 b | 0.14 ± 0.01 b | ** |
| 63 | 1-Propanone, 1-(4-methoxyphenyl)- | 0.04 ± 0.01 | ND | ND | ** |
| 64 | 4’,6’-Dimethoxy-2’,3’-dimethylacetophenone | 0.04 ± 0.01 a | 0.02 ± 0.01 ab | 0.01 ± 0.01 b | * |
| 65 | Methyl-, nonyl-, ketone | ND | 1.24 ± 0.37 a | 1.01 ± 0.03 b | ** |
| Esters | 25.27 ± 0.50 a | 14.56 ± 1.69 b | 18.92 ± 0.79 b | * | |
| 66 | Acrylic acid, Isoamyl ester | 0.16 ± 0.12 b | 0.48 ± 0.14 a | 0.25 ± 0.12 ab | ns |
| 67 | Acetic acid, propyl ester | 0.15 ± 0.03 a | 0.09 ± 0.03 b | 0.01 ± 0.01 c | ** |
| 68 | Butyric acid, methyl ester | 0.01 ± 0.01 a | 0.01 ± 0.01 a | ND | ns |
| 69 | Butyric acid, ethyl ester | 0.32 ± 0.02 a | 0.24 ± 0.03 b | 0.31 ± 0.01 a | ** |
| 70 | 2-butynoic acid, methyl ester | 14.94 ± 1.89 | ND | ND | ** |
| 71 | Ethyl acetate | 6.96 ± 1.24 b | 7.37 ± 1.52 a | 7.41 ± 0.68 a | ns |
| 72 | 2,4-Pentadienoic acid, 1-cyclopenten-3-on-1-yl ester | ND | ND | 1.69 ± 0.28 | ** |
| 73 | Propanedioic acid, oxo-, bis(1-methylethyl) ester | ND | 3.08 ± 0.81 | ND | ** |
| 74 | Diisopropyl 2-oxomalonate | 0.04 ± 0.01 | ND | ND | ** |
| 75 | Hexanoic acid, methyl ester | 0.04 ± 0.02 a | 0.08 ± 0.01 a | 0.12 ± 0.01 a | ** |
| 76 | Isocaproic acid, ethyl ester | 0.02 ± 0.01 | ND | ND | ** |
| 77 | n-Hexanoic acid, ethyl ester | 0.30 ± 0.02 a | 0.19 ± 0.01 b | 0.17 ± 0.01 b | ** |
| 78 | Acetic acid, hexyl ester | 0.01 ± 0.01 a | 0.01 ± 0.01 a | ND | ns |
| 79 | Sorbic acid, methyl ester | 0.08 ± 0.11 a | ND | 0.01 ± 0.01 a | ns |
| 80 | Octanoic acid, methyl ester | 0.06 ± 0.01 a | 0.04 ± 0.02 ab | 0.02 ± 0.01 b | * |
| 81 | Benzoic acid, ethyl ester | 0.07 ± 0.04 b | 0.07 ± 0.01 b | 0.25 ± 0.02 a | ** |
| 82 | Octanoic acid, ethyl ester | 0.94 ± 0.14 a | 0.48 ± 0.09 b | 0.47 ± 0.07 b | ** |
| 83 | Acetic acid, geranyl ester | 0.13 ± 0.02 a | 0.10 ± 0.02 a | 0.13 ± 0.01 a | ns |
| 84 | Decanoic acid, ethyl ester | 0.32 ± 0.06 a | 0.17 ± 0.06 b | 0.10 ± 0.01 b | ** |
| 85 | Tetradecanoic acid, ethyl ester | 0.02 ± 0.01 a | 0.02 ± 0.01 a | 0.01 ± 0.01 a | ns |
| 86 | 9-Octadecenoic acid, ethyl ester | 0.02 ± 0.01 a | 0.01 ± 0.01 a | 0.01 ± 0.01 a | ns |
| 87 | Glycidyl palmitoleate | 0.01 ± 0.01 b | 0.04 ± 0.01 a | 0.02 ± 0.01 b | ** |
| 88 | Hydroxymethyl 2-hydroxy-2-methylpropionate | ND | 0.37 ± 0.08 a | 0.25 ± 0.04 a | ** |
| 89 | Propionic acid, ethyl ester | 0.17 ± 0.03 a | 0.11 ± 0.01 a | ND | ** |
| 90 | Acetic acid, isoamyl ester | 0.48 ± 0.06 a | 0.46 ± 0.04 a | ND | ** |
| 91 | Butanoic acid, 3-hydroxy-, ethyl ester | ND | 0.07 ± 0.01 | ND | ** |
| 92 | Pent-4-en-1-yl propyl carbonate | ND | 0.33 ± 0.06 a | 0.13 ± 0.02 b | ** |
| 93 | Acetic acid, 4-terpineol, ester | ND | 0.17 ± 0.02 | ND | |
| 94 | 4-Methyl-4-vinylbutyrolactone | ND | ND | 2.16 ± 0.04 | ** |
| 95 | Lactic acid, ethyl ester, (L)- | ND | 0.20 ± 0.01 a | 0.10 ± 0.01 b | ** |
| 96 | 5-Oxotetrahydrofuran-2-carboxylic acid, ethyl ester | ND | ND | 2.12 ± 0.02 | ** |
| 97 | 3,3-dimethyl-, 1-butyl acid, ester | ND | ND | 2.11 ± 0.01 | ** |
| 98 | Acetic acid, linalyl, ester | ND | 0.40 ± 0.04 a | 0.41 ± 0.03 a | ** |
| Acids | 7.12 ± 0.71 a | 4.71 ± 0.19 b | 3.48 ± 0.05 b | ** | |
| 99 | Acetic acid, methoxy- | 3.97 ± 0.21 a | 2.45 ± 0.11 b | ND | ** |
| 100 | Butanoic acid | 0.45 ± 0.12 a | 0.45 ± 0.15 a | 0.16 ± 0.08 b | * |
| 101 | Isovaleric acid | 0.04 ± 0.01 b | 0.09 ± 0.02 a | 0.03 ± 0.01 b | ** |
| 102 | Sorbic acid | 1.43 ± 0.47 a | 1.04 ± 0.10 a | 0.41 ± 0.02 b | * |
| 103 | Octanoic acid | 0.58 ± 0.04 | ND | ND | ** |
| 104 | n-Decanoic acid | 0.12 ± 0.03 ab | 0.10 ± 0.01 b | 0.15 ± 0.01 a | ns |
| 105 | Myristic acid | 0.01 ± 0.01 | ND | ND | ns |
| 106 | Palmitic acid | 0.01 ± 0.01 a | 0.01 ± 0.01 a | 0.01 ± 0.01 a | ns |
| 107 | Oleic Acid | 0.34 ± 0.46 a | 0.34 ± 0.02 a | 0.17 ± 0.03 a | ns |
| 108 | Acetic acid, (acetyloxy)- | ND | 0.10 ± 0.04 | ND | ** |
| 109 | L-Lactic acid | 0.15 ± 0.01 a | 0.15 ± 0.01 a | ND | ** |
| 110 | Acetic acid | ND | ND | 2.55 ± 0.12 | ** |
| Other volatile compounds | 3.31 ± 0.06 a | 3.12 ± 0.25 a | 3.26 ± 0.18 a | ns | |
| 111 | Methyl mercaptan | 0.02 ± 0.01 | ND | ND | ** |
| 112 | Eucalyptol | 3.21 ± 0.27 a | 3.11 ± 0.76 a | 2.03 ± 0.25 b | * |
| 113 | 2-Furanmethanol, 5-ethenyltetrahydro-α,α,5-trimethyl-, cis- | ND | ND | 1.16 ± 0.05 | ** |
| 114 | Trans-α-bergamotene | 0.08 ± 0.05 a | 0.01 ± 0.01 b | 0.07 ± 0.01 ab | ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, X.; Wang, B.; Zhao, P.; Ge, C.; Li, S.; Xiao, Z. The Effect of the Improvement Technology on the Quality of Midu Pork Roll. Foods 2022, 11, 3684. https://doi.org/10.3390/foods11223684
Xiao X, Wang B, Zhao P, Ge C, Li S, Xiao Z. The Effect of the Improvement Technology on the Quality of Midu Pork Roll. Foods. 2022; 11(22):3684. https://doi.org/10.3390/foods11223684
Chicago/Turabian StyleXiao, Xue, Bowen Wang, Ping Zhao, Changrong Ge, Shijun Li, and Zhichao Xiao. 2022. "The Effect of the Improvement Technology on the Quality of Midu Pork Roll" Foods 11, no. 22: 3684. https://doi.org/10.3390/foods11223684
APA StyleXiao, X., Wang, B., Zhao, P., Ge, C., Li, S., & Xiao, Z. (2022). The Effect of the Improvement Technology on the Quality of Midu Pork Roll. Foods, 11(22), 3684. https://doi.org/10.3390/foods11223684




