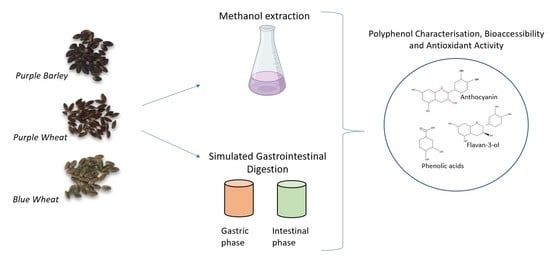
Bioaccessibility and Antioxidant Activity of Polyphenols from Pigmented Barley and Wheat
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Organic Solvent Extraction
2.3. Simulated In Vitro Gastrointestinal Digestion of Flour
2.4. Total Phenolic Content
2.5. Ferric Reducing Antioxidant Power Assay
2.6. DPPH Radical Scavenging Activity Assay
2.7. ABTS•+ Radical Scavenging Activity Assay
2.8. UHPLC Combined with Online ABTS•+
2.9. Identification of Compounds
2.10. Statistical Analysis
3. Results
3.1. Total Phenolic Content of Extracts from Digested Cereal Flours Compared with Methanol Extracts
3.2. Ferric Reducing Antioxidant Power of Extracts from Digested Cereal Flours Compared with Methanol Extracts
3.3. DPPH Radical Scavenging Activity of Extracts from Digested Cereal Flours Compared with Methanol Extracts
3.4. ABTS•+ Radical Scavenging Activity of Extracts from Digested Cereal Flours Compared with Methanol Extracts
3.5. Phenolic Characterisation and Antioxidant Activity of Pigmented Barley and Wheat Methanol Extracts
3.6. Bioaccessibility of Polyphenols during Simulated Digestion
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABTS | 2,2′-Azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| FRAP | Ferric reducing antioxidant power |
| GAE | Gallic acid equivalents |
| TE | Trolox equivalents |
| TPC | Total phenolic content |
| TPTZ | Tris(2-pyridyl)-s-triazine |
| Q-TOF LC-MS | Quad time of flight liquid chromatography mass spectra |
| UHPLC | Ultra-high performance liquid chromatography |
References
- Ed Nignpense, B.; Francis, N.; Blanchard, C.; Santhakumar, A.B. Bioaccessibility and Bioactivity of Cereal Polyphenols: A Review. Foods 2021, 10, 1595. [Google Scholar] [CrossRef]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef]
- Ed Nignpense, B.; Chinkwo, K.A.; Blanchard, C.L.; Santhakumar, A.B. Black Sorghum Phenolic Extract Modulates Platelet Activation and Platelet Microparticle Release. Nutrients 2020, 12, 1760. [Google Scholar] [CrossRef] [PubMed]
- Callcott, E.T.; Blanchard, C.L.; Snell, P.; Santhakumar, A.B. The anti-inflammatory and antioxidant effects of pigmented rice consumption in an obese cohort. Food Funct. 2019, 10, 8016–8025. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Qiu, Y.; Beta, T. Comparison of Antioxidant Activities of Different Colored Wheat Grains and Analysis of Phenolic Compounds. J. Agric. Food Chem. 2010, 58, 9235–9241. [Google Scholar] [CrossRef] [PubMed]
- Suriano, S.; Savino, M.; Codianni, P.; Iannucci, A.; Caternolo, G.; Russo, M.; Pecchioni, N.; Troccoli, A. Anthocyanin profile and antioxidant capacity in coloured barley. Int. J. Food Sci. Technol. 2019, 54, 2478–2486. [Google Scholar] [CrossRef]
- Zhang, J.; Ding, Y.; Dong, H.; Hou, H.; Zhang, X. Distribution of Phenolic Acids and Antioxidant Activities of Different Bran Fractions from Three Pigmented Wheat Varieties. J. Chem. 2018, 2018, 6459243. [Google Scholar] [CrossRef]
- Eticha, F. Hull-Less Barley (Hordeum vulgare L.) and Pigmented Wheat (Triticum L.): Genetic Diversity for Healthy Food; University of Natural Resources and Applied Life Sciences: Vienna, Austria, 2008. [Google Scholar]
- Lee, C.; Han, D.; Kim, B.; Baek, N.; Baik, B.-K. Antioxidant and anti-hypertensive activity of anthocyanin-rich extracts from hulless pigmented barley cultivars. Int. J. Food Sci. 2013, 48, 984–991. [Google Scholar] [CrossRef]
- Galli, V.; Venturi, M.; Guerrini, S.; Blandino, M.; Luti, S.; Pazzagli, L.; Granchi, L. Antioxidant Properties of Sourdoughs Made with Whole Grain Flours of Hull-Less Barley or Conventional and Pigmented Wheat and by Selected Lactobacilli Strains. Foods 2020, 9, 640. [Google Scholar] [CrossRef]
- Garg, M.; Kaur, S.; Sharma, A.; Kumari, A.; Tiwari, V.; Sharma, S.; Kapoor, P.; Sheoran, B.; Goyal, A.; Krishania, M. Rising Demand for Healthy Foods-Anthocyanin Biofortified Colored Wheat Is a New Research Trend. Front. Nutr. 2022, 9, 878221. [Google Scholar] [CrossRef]
- Mekonnen, M.; Sharie, G.; Bayable, M.; Teshager, A.; Abebe, E.; Ferede, M.; Fentie, D.; Wale, S.; Tay, Y.; Getaneh, D.; et al. Participatory variety selection and stability analysis of Durum wheat varieties (Triticum durum Desf.) in northwest Amhara. Cogent Food Agric. 2020, 6, 1746229. [Google Scholar] [CrossRef]
- Shamanin, V.P.; Tekin-Cakmak, Z.H.; Gordeeva, E.I.; Karasu, S.; Pototskaya, I.; Chursin, A.S.; Pozherukova, V.E.; Ozulku, G.; Morgounov, A.I.; Sagdic, O.; et al. Antioxidant Capacity and Profiles of Phenolic Acids in Various Genotypes of Purple Wheat. Foods 2022, 11, 2515. [Google Scholar] [CrossRef] [PubMed]
- Geng, P.; Sun, J.; Zhang, M.; Li, X.; Harnly, J.M.; Chen, P. Comprehensive characterization of C-glycosyl flavones in wheat (Triticum aestivum L.) germ using UPLC-PDA-ESI/HRMS(n) and mass defect filtering. J. Mass Spectrom. 2016, 51, 914–930. [Google Scholar] [CrossRef] [PubMed]
- Angelino, D.; Cossu, M.; Marti, A.; Zanoletti, M.; Chiavaroli, L.; Brighenti, F.; Del Rio, D.; Martini, D. Bioaccessibility and bioavailability of phenolic compounds in bread: A review. Food Funct. 2017, 8, 2368–2393. [Google Scholar] [CrossRef]
- Podio, N.S.; Baroni, M.V.; Pérez, G.T.; Wunderlin, D.A. Assessment of bioactive compounds and their in vitro bioaccessibility in whole-wheat flour pasta. Food Chem. 2019, 293, 408–417. [Google Scholar] [CrossRef]
- Thakur, N.; Raigond, P.; Singh, Y.; Mishra, T.; Singh, B.; Lal, M.K.; Dutt, S. Recent updates on bioaccessibility of phytonutrients. Trends Food Sci. Technol. 2020, 97, 366–380. [Google Scholar] [CrossRef]
- Gamel, T.H.; Abdel-Aal, E.-S.M.; Tucker, A.J.; Pare, S.M.; Faughnan, K.; O’Brien, C.D.; Dykun, A.; Rabalski, I.; Pickard, M.; Wright, A.J. Consumption of whole purple and regular wheat modestly improves metabolic markers in adults with elevated high-sensitivity C-reactive protein: A randomised, single-blind parallel-arm study. Br. J. Nutr. 2020, 124, 1179–1189. [Google Scholar] [CrossRef]
- Tomé-Sánchez, I.; Martín-Diana, A.B.; Peñas, E.; Frias, J.; Rico, D.; Jiménez-Pulido, I.; Martínez-Villaluenga, C. Bioprocessed Wheat Ingredients: Characterization, Bioaccessibility of Phenolic Compounds, and Bioactivity During in vitro Digestion. Front. Plant Sci. 2021, 12, 790898. [Google Scholar] [CrossRef]
- Bouayed, J.; Hoffmann, L.; Bohn, T. Total phenolics, flavonoids, anthocyanins and antioxidant activity following simulated gastro-intestinal digestion and dialysis of apple varieties: Bioaccessibility and potential uptake. Food Chem. 2011, 128, 14–21. [Google Scholar] [CrossRef]
- Ed Nignpense, B.; Latif, S.; Francis, N.; Blanchard, C.; Santhakumar, A.B. The impact of simulated gastrointestinal digestion on the bioaccessibility and antioxidant activity of purple rice phenolic compounds. Food Biosci. 2022, 47, 101706. [Google Scholar] [CrossRef]
- Leitao, C.; Marchioni, E.; Bergaentzlé, M.; Zhao, M.; Didierjean, L.; Miesch, L.; Holder, E.; Miesch, M.; Ennahar, S. Fate of polyphenols and antioxidant activity of barley throughout malting and brewing. J. Cereal Sci. 2012, 55, 318–322. [Google Scholar] [CrossRef]
- Rao, S.; Santhakumar, A.B.; Chinkwo, K.A.; Blanchard, C.L. Q-TOF LC/MS identification and UHPLC-Online ABTS antioxidant activity guided mapping of barley polyphenols. Food Chem. 2018, 266, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Sompong, R.; Siebenhandl-Ehn, S.; Linsberger-Martin, G.; Berghofer, E. Physicochemical and antioxidative properties of red and black rice varieties from Thailand, China and Sri Lanka. Food Chem. 2011, 124, 132–140. [Google Scholar] [CrossRef]
- Saji, N.; Schwarz, L.J.; Santhakumar, A.B.; Blanchard, C.L. Stabilization treatment of rice bran alters phenolic content and antioxidant activity. Cereal Chem. 2020, 97, 281–292. [Google Scholar] [CrossRef]
- Razgonova, M.P.; Zakharenko, A.M.; Gordeeva, E.I.; Shoeva, O.Y.; Antonova, E.V.; Pikula, K.S.; Koval, L.A.; Khlestkina, E.K.; Golokhvast, K.S. Phytochemical Analysis of Phenolics, Sterols, and Terpenes in Colored Wheat Grains by Liquid Chromatography with Tandem Mass Spectrometry. Molecules 2021, 26, 5580. [Google Scholar] [CrossRef]
- Dvorakova, M.; Moreira, M.M.; Dostalek, P.; Skulilova, Z.; Guido, L.F.; Barros, A.A. Characterization of monomeric and oligomeric flavan-3-ols from barley and malt by liquid chromatography–ultraviolet detection–electrospray ionization mass spectrometry. J. Chromatogr. A 2008, 1189, 398–405. [Google Scholar] [CrossRef]
- Abdel-Aal, E.-S.M.; Young, J.C.; Rabalski, I. Anthocyanin Composition in Black, Blue, Pink, Purple, and Red Cereal Grains. J. Agric. Food Chem. 2006, 54, 4696–4704. [Google Scholar] [CrossRef]
- Dinelli, G.; Segura Carretero, A.; Di Silvestro, R.; Marotti, I.; Fu, S.; Benedettelli, S.; Ghiselli, L.; Fernández Gutiérrez, A. Determination of phenolic compounds in modern and old varieties of durum wheat using liquid chromatography coupled with time-of-flight mass spectrometry. J. Chromatogr. A 2009, 1216, 7229–7240. [Google Scholar] [CrossRef]
- Deng, J.; Xiang, Z.; Lin, C.; Zhu, Y.; Yang, K.; Liu, T.; Xia, C.; Chen, J.; Zhang, W.; Zhang, Y.; et al. Identification and quantification of free, esterified, and insoluble-bound phenolics in grains of hulless barley varieties and their antioxidant activities. LWT 2021, 151, 112001. [Google Scholar] [CrossRef]
- Hassan, W.H.B.; Abdelaziz, S.; Al Yousef, H.M. Chemical Composition and Biological Activities of the Aqueous Fraction of Parkinsonea aculeata L. Growing in Saudi Arabia. Arab. J. Chem. 2019, 12, 377–387. [Google Scholar] [CrossRef]
- Shen, Y.; Zhang, H.; Cheng, L.; Wang, L.; Qian, H.; Qi, X. In vitro and in vivo antioxidant activity of polyphenols extracted from black highland barley. Food Chem. 2016, 194, 1003–1012. [Google Scholar] [CrossRef] [PubMed]
- Peanparkdee, M.; Borompichaichartkul, C.; Iwamoto, S. Bioaccessibility and antioxidant activity of phenolic acids, flavonoids, and anthocyanins of encapsulated Thai rice bran extracts during in vitro gastrointestinal digestion. Food Chem. 2021, 361, 130161. [Google Scholar] [CrossRef] [PubMed]
- Peanparkdee, M.; Patrawart, J.; Iwamoto, S. Physicochemical stability and in vitro bioaccessibility of phenolic compounds and anthocyanins from Thai rice bran extracts. Food Chem. 2020, 329, 127157. [Google Scholar] [CrossRef] [PubMed]
- Pigni, N.B.; Aranibar, C.; Lucini Mas, A.; Aguirre, A.; Borneo, R.; Wunderlin, D.; Baroni, M.V. Chemical profile and bioaccessibility of polyphenols from wheat pasta supplemented with partially-deoiled chia flour. LWT 2020, 124, 109134. [Google Scholar] [CrossRef]
- Abdel-Aal, E.-S.M.; Hucl, P.; Shipp, J.; Rabalski, I. Compositional Differences in Anthocyanins from Blue- and Purple-Grained Spring Wheat Grown in Four Environments in Central Saskatchewan. Cereal Chem. 2016, 93, 32–38. [Google Scholar] [CrossRef]
- Tian, W.; Hu, R.; Chen, G.; Zhang, Y.; Wang, W.; Li, Y. Potential bioaccessibility of phenolic acids in whole wheat products during in vitro gastrointestinal digestion and probiotic fermentation. Food Chem. 2021, 362, 130135. [Google Scholar] [CrossRef]
- Drawbridge, P.C.; Apea-Bah, F.; Silveira Hornung, P.; Beta, T. Bioaccessibility of phenolic acids in Canadian hulless barley varieties. Food Chem. 2021, 358, 129905. [Google Scholar] [CrossRef]
- Mateo Anson, N.; van den Berg, R.; Havenaar, R.; Bast, A.; Haenen, G.R.M.M. Bioavailability of ferulic acid is determined by its bioaccessibility. J. Cereal Sci. 2009, 49, 296–300. [Google Scholar] [CrossRef]
- Fernandes, I.; de Freitas, V.; Mateus, N. Anthocyanins and human health: How gastric absorption may influence acute human physiology. Nutr. Aging 2014, 2, 1–14. [Google Scholar] [CrossRef]
- Awika, J.M.; Rose, D.J.; Simsek, S. Complementary effects of cereal and pulse polyphenols and dietary fiber on chronic inflammation and gut health. Food Funct. 2018, 9, 1389–1409. [Google Scholar] [CrossRef]
- Riedl, K.M.; Hagerman, A.E. Tannin-protein complexes as radical scavengers and radical sinks. J. Agri. Food Chem. 2001, 49, 4917–4923. [Google Scholar] [CrossRef] [PubMed]




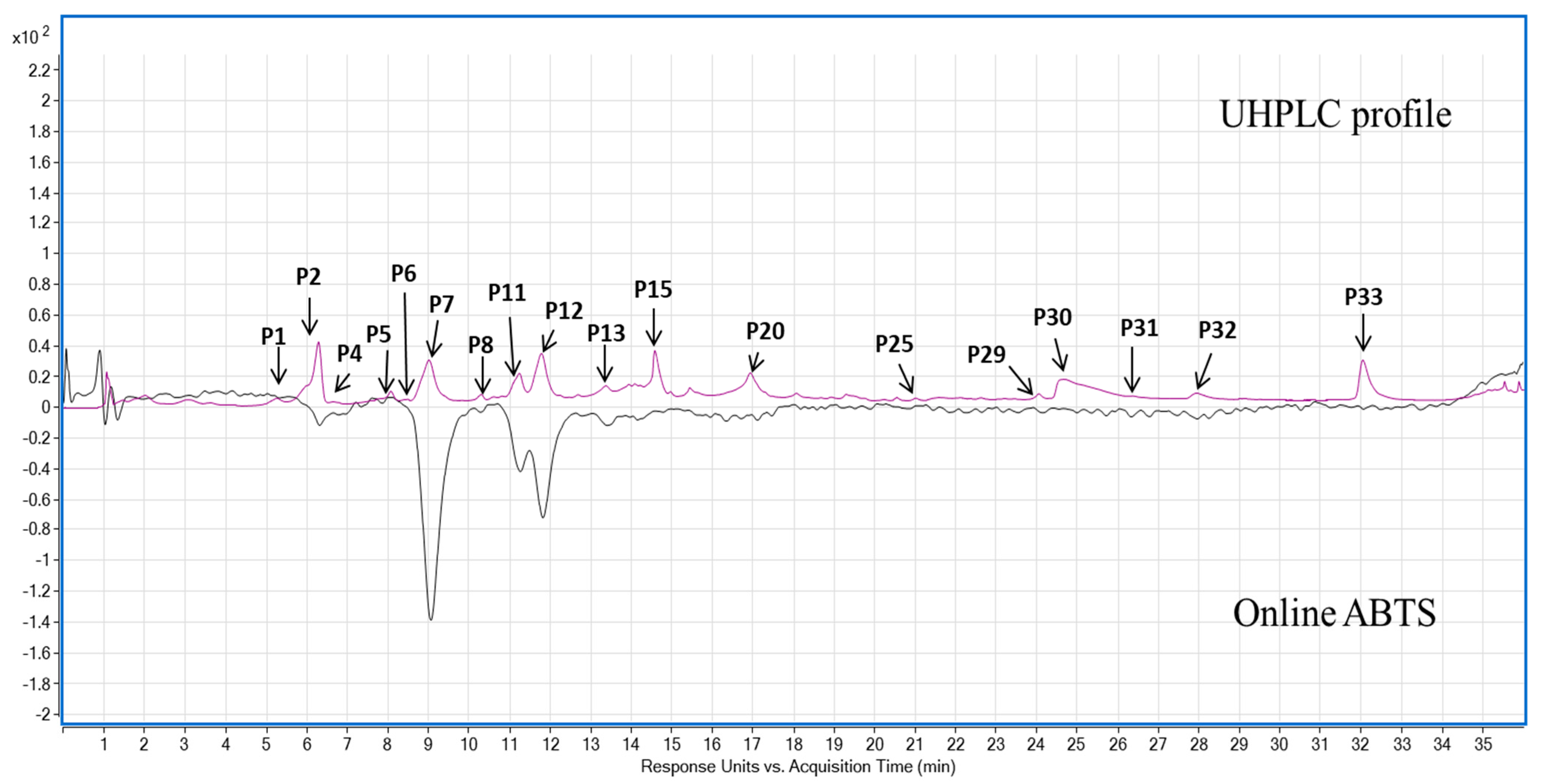


| Peak | RT (min) | Λmax (nm) | m/z | Tentative Identification | Phenolic Quantification (mg GAE/100 g dw) | Class | Reference | ||
|---|---|---|---|---|---|---|---|---|---|
| Purple Barley | Purple Wheat | Blue Wheat | |||||||
| P1 | 5.7 | 280 | 153.0208 | Protocatechuic acid | 0.06 ± 0.02 a | 0.10 ± 0.04 a | 0.10 ± 0.03 a | Phenolic acid | Standard |
| P4 | 7.2 | 280 | 305.0667 | Gallocatechin | 0.07 ± 0.01 | – | – | Flavan-3-ol | [26] |
| P7 | 9.5 | 280 | 593.1287 | Prodelphinidin B3 | 0.91 ± 0.13 | – | – | Flavan-3-ol | [27] |
| P11 | 11.8 | 280 | 289.0723 | Catechin | 0.58 ± 0.17 | – | – | Flavan-3-ol | Standard |
| P12 | 11.9 | 280 | 577.1345 | Procyanidin B3 | 0.75 ± 0.16 | – | – | Flavan-3-ol | Standard; [27] |
| P13 | 13.6 | 280, 520 | 447.0925 | Cyanidin 3-glucoside | 0.7 ± 0.15 | trace | – | Anthocyanin | [6,28] |
| P19 | 17.1 | 270, 340 | 563.1422 | Apigenin 6-C-arabinoside-8-C-hexoside | – | 0.49 ± 0 a | 0.50 ± 0.01 a | Flavone glycoside | [29] |
| P20 | 17.5 | 320, 520 | 533.0932 | Malvidin 3-(6″-acetylglucoside) | 1.06 ± 0.07 | – | – | Anthocyanin | [6,28] |
| P22 | 17.8 | 270, 240 | 563.1422 | Apigenin-6-C-arabinoside-8-C-hexoside isomer 1 | – | 0.22 ± 0 a | 0.22 ± 0 a | Flavone glycoside | [29] |
| P23 | 18.2 | 270, 340 | 563.1422 | Apigenin-6-C-arabinoside-8-C-hexoside isomer 2 | – | 0.99 ± 0 a | 0.81 ± 0.01 b | Flavone glycoside | [29] |
| P25 | 21.5 | 270, 350 | 461.1077 | Chrysoeriol-7-O-glucoside | 0.39 ± 0.03 | – | – | Flavone glycoside | [30] |
| P26 | 22.4 | 330 | 769.1973 | Apigenin-8-C-sinapoylpentoside-6-C-hexoside | – | 0.27 ± 0 a | 0.1 ± 0 b | Flavone glycoside | [5] |
| P27 | 22.8 | 330 | 769.1973 | Apigenin-8-C-sinapoylpentoside-6-C-hexoside isomer | – | 0.43 ± 0 a | 0.18 ± 0 b | Flavone glycoside | [5] |
| P28 | 23.2 | 330 | 739.1866 | Chrysoeriol 4′-O-pentoside-7′ O-rutinoside | – | – | 0.07 ± 0 | Flavone glycoside | [31] |
| P29 | 24.4 | 270, 350 | 461.1077 | Chrysoeriol-7-O-glucoside isomer 1 | 0.24 ± 0 | – | – | Flavone glycoside | [30] |
| P30 | 24.7 | 270, 350 | 475.0874 | Chrysoeriol-7-O-glucuronide | 2.7 ± 0.04 | – | – | Flavone glycoside | [30] |
| P31 | 24.9 | 350 | 461.1077 | Chrysoeriol-7-O-glucoside isomer 2 | 0.09 ± 0 | – | – | Flavone glycoside | [30] |
| P32 | 28.2 | 270, 350 | 285.0391 | Luteolin | 0.34 ± 0.01 | – | – | Flavone | Standard |
| P33 | 32.5 | 270, 350 | 299.0558 | Chrysoeriol | 1.37 ± 0.05 a | 0.08 ± 0 b | – | Flavone | [14] |
| Peak | Tentative Identification | ABTS•+ Antioxidant Activity (mg TE/100 g dw) | ||
|---|---|---|---|---|
| Purple Barley | Purple Wheat | Blue Wheat | ||
| P3 | Unknown | – | 5.86 ± 1.04 a | 2.52 ± 0.52 b |
| P4 | Gallocatechin | 4.94 ± 1.01 | – | – |
| P7 | Prodelphinidin B3 | 49.05 ± 0.00 | – | – |
| P11 | Catechin | 8.72 ± 2.10 | – | – |
| P12 | Procyanidin B3 | 8.26 ± 1.24 | – | – |
| Peak | Tentative Identification | Purple Barley mg GAE/100 g dw | Purple Wheat mg GAE/100 g dw | Blue Wheat mg GAE/100 g dw | |||
|---|---|---|---|---|---|---|---|
| Gastric | Intestinal | Gastric | Intestinal | Gastric | Intestinal | ||
| P1 | Protocatechuic acid | 1.22 ± 0.66 a | 0.92 ± 0.45 a | 0.691 ± 0.164 | – | 0.469 ± 0.104 | – |
| P4 | Gallocatechin | 0.13 ± 0.07 | – | – | – | – | – |
| P7 | Prodelphinidin B3 | 0.188 ± 0.00 | – | – | – | – | – |
| P11 | Catechin | 0.196 ± 0.07 | – | – | – | – | – |
| P12 | Procyanidin B3 | 0.192 ± 0.04 | – | – | – | – | – |
| P13 | Cyanidin 3-glucoside | 0.158 ± 0.01 | – | – | – | – | – |
| P19 | Apigenin 6-C-arabinoside-8-C-hexoside | – | – | 0.529 ± 0.05 b | 0.805 ± 0.09 a | 0.905 ± 0.25 | trace |
| P20 | Malvidin 3-(6″-acetylglucoside) | – | – | – | – | – | – |
| P22 | Apigenin-6-C-arabinoside-8-C-hexoside isomer 1 | – | – | 0.687 ± 0.27 a | 0.877 ± 0.15 a | 0.409 ± 0.17 a | 0.470 ± 0.07 a |
| P23 | Apigenin-6-C-arabinoside-8-C-hexoside isomer 2 | – | – | 1.50 ± 0.17 a | 1.67 ± 0.15 a | 1.975 ± 0.29 a | 1.59 ± 0.30 a |
| P25 | Chrysoeriol-7-O-glucoside | 0.47 ± 0.30 a | 0.72 ± 0.17 a | – | – | – | – |
| P26 | Apigenin-8-C-sinapoylpentoside-6-C-hexoside | – | – | 1.26 ± 0.32 a | 0.483 ± 0.04 b | 0.903 ± 0.11 | trace |
| P27 | Apigenin-8-C-sinapoylpentoside-6-C-hexoside isomer | – | – | 0.703 ± 0.09 | – | trace | – |
| P28 | Chrysieriol 4′-O-pentoside-7′ O-rutinoside | – | – | – | – | trace | trace |
| P29 | Chrysoeriol-7-O-glucoside isomer 1 | 0.26 ± 0.09 b | 0.56 ± 0.12 a | – | – | – | – |
| P30 | Chrysoeriol-7-O-glucuronide | 4.64 ± 1.81 b | 9.93 ± 1.74 a | – | – | – | – |
| P31 | Chrysoeriol-7-O-glucoside isomer 2 | 0.30 ± 0.18 | trace | – | – | – | – |
| P32 | Luteolin | 0.33 ± 0.16 a | 0.30 ± 0.03 a | – | – | – | – |
| P33 | Chrysoeriol | 0.36 ± 0.09 b | 0.58 ± 0.10 a | trace | – | – | – |
| No. of compounds detected | 13 | 7 | 7 | 4 | 7 | 5 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ed Nignpense, B.; Latif, S.; Francis, N.; Blanchard, C.; Santhakumar, A.B. Bioaccessibility and Antioxidant Activity of Polyphenols from Pigmented Barley and Wheat. Foods 2022, 11, 3697. https://doi.org/10.3390/foods11223697
Ed Nignpense B, Latif S, Francis N, Blanchard C, Santhakumar AB. Bioaccessibility and Antioxidant Activity of Polyphenols from Pigmented Barley and Wheat. Foods. 2022; 11(22):3697. https://doi.org/10.3390/foods11223697
Chicago/Turabian StyleEd Nignpense, Borkwei, Sajid Latif, Nidhish Francis, Christopher Blanchard, and Abishek Bommannan Santhakumar. 2022. "Bioaccessibility and Antioxidant Activity of Polyphenols from Pigmented Barley and Wheat" Foods 11, no. 22: 3697. https://doi.org/10.3390/foods11223697
APA StyleEd Nignpense, B., Latif, S., Francis, N., Blanchard, C., & Santhakumar, A. B. (2022). Bioaccessibility and Antioxidant Activity of Polyphenols from Pigmented Barley and Wheat. Foods, 11(22), 3697. https://doi.org/10.3390/foods11223697