Assessment of the Gut Microbiota during Juice Fasting with and without Inulin Supplementation: A Feasibility Study in Healthy Volunteers †
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design of the Pilot Intervention Study
2.2. Stool Sample Collection
2.3. Fecal Microbial DNA Isolation
2.4. 16S Ribosomal RNA (rRNA) Gene Sequencing
2.5. Bioinformatics and Statistical Analysis
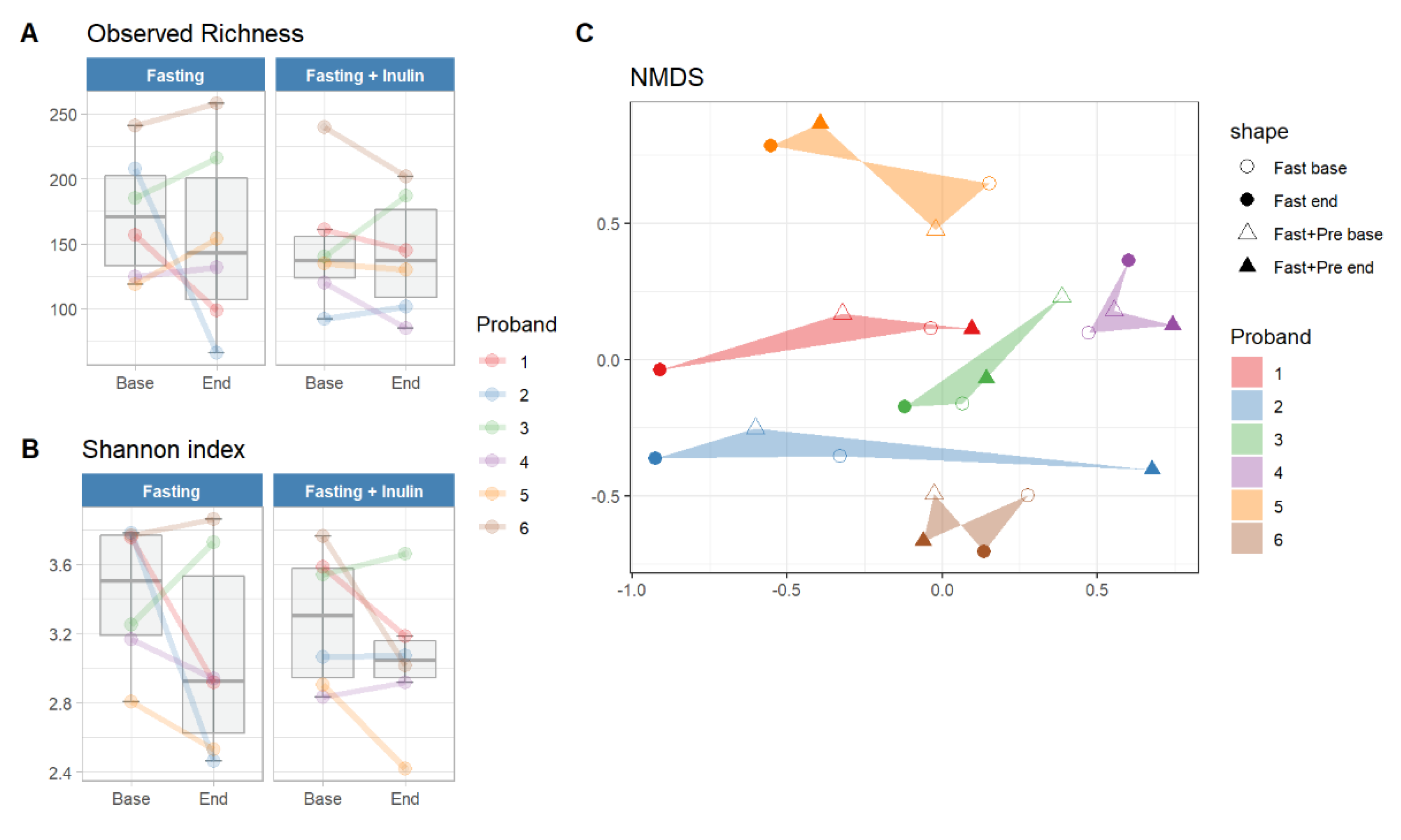
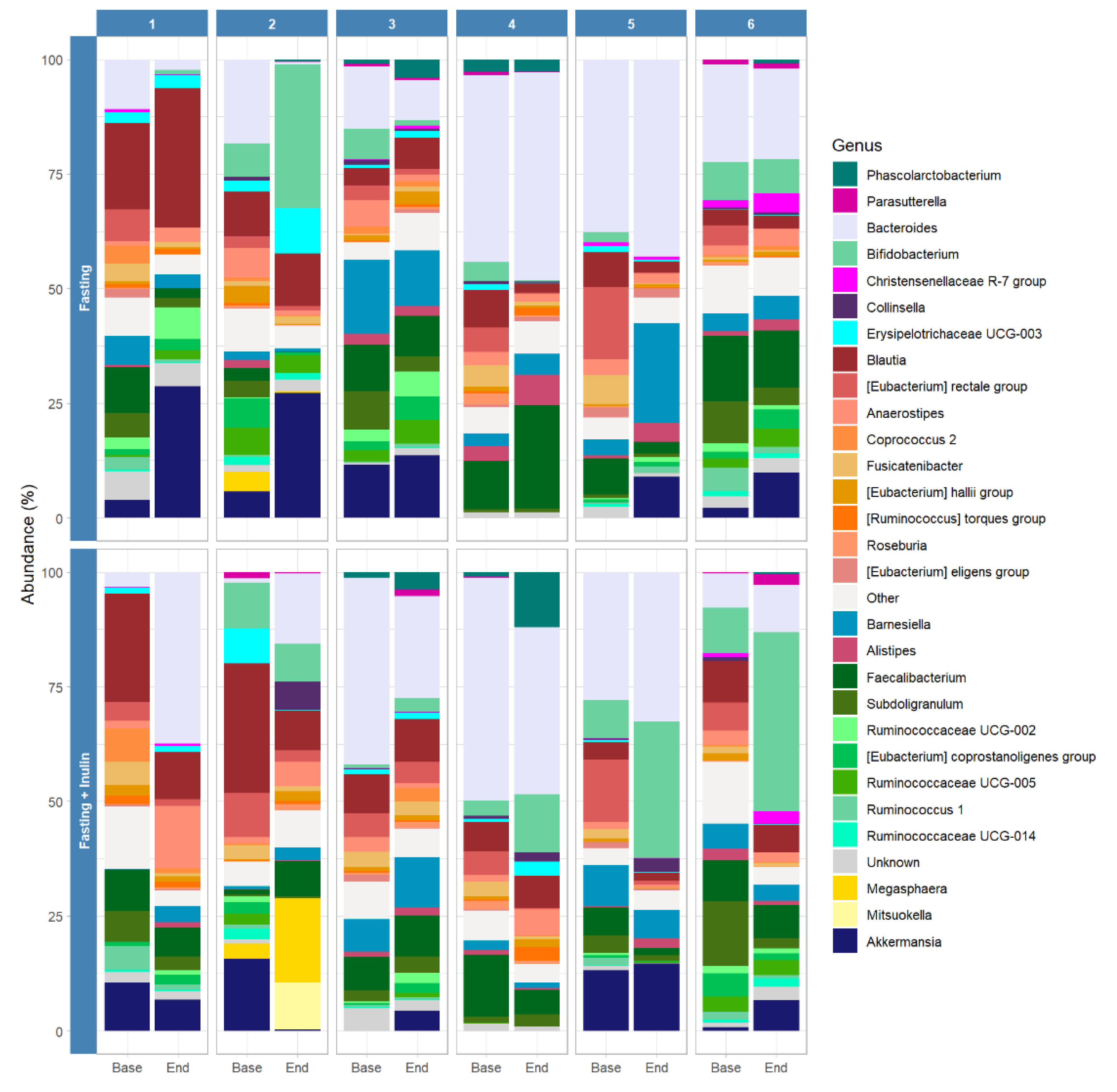
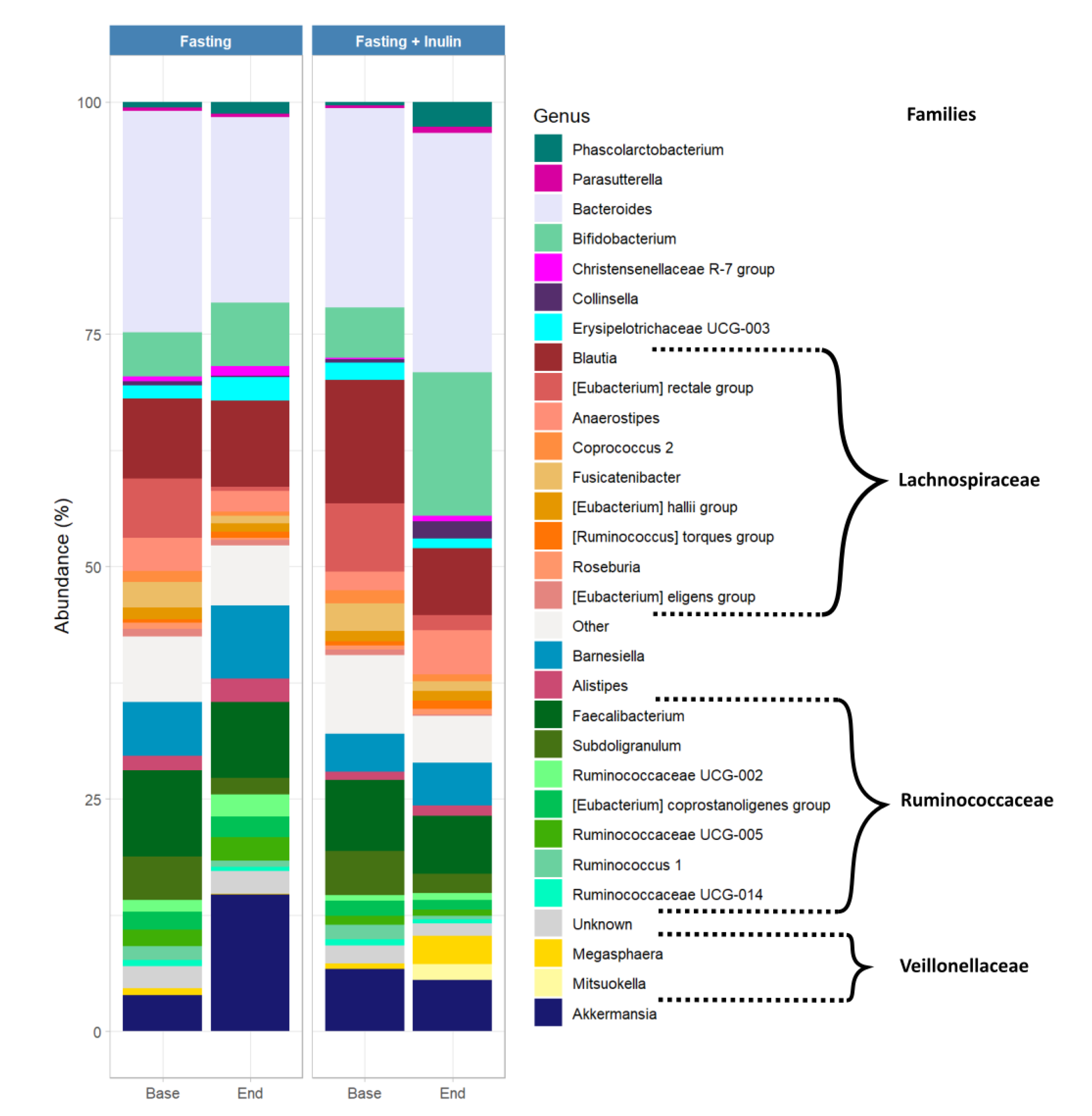
3. Results
3.1. Assessment of the Feasibility of the Study Design
3.2. Possible Associations between Inulin Consumption during Juice Fasting and the Human Gut Microbiome Composition
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Day of Intervention | Number of Calories Consumed | Diet Plan |
| Day 1 | Light, vegan diet: | |
| 800 kcal | With vegetables, fruits, cereals, bread | |
| Without meat, fish, dairy products, added sugar, sweets, alcohol, and caffeine | ||
| Day 2 | 1 pkg. carrot juice (155 kcal/500 mL) | |
| 300 kcal | 1 pkg. sauerkraut juice (50 kcal/500 mL) 1/2 pkg. beetroot juice (105 kcal/250 mL) | |
| Day 3 | 1/2 pkg. beetroot juice (105 kcal/250 mL) | |
| 300 kcal | 1 pkg. tomato juice (80 kcal/500 mL) | |
| 1 pkg. mixed vegetable juice * (95 kcal/500 mL) | ||
| 1/2 pkg. sauerkraut juice (25 kcal/500 mL) | ||
| Day 4 | 300 kcal | 1 pkg. tomato juice (80 kcal/500 mL) 1 pkg. carrot juice (155 kcal/500 mL) |
| 1 pkg. sauerkraut juice (50 kcal/500 mL) | ||
| Day 5 | 800 kcal | Light, vegan diet: With vegetables, fruits, cereals, bread |
| Without meat, fish, dairy products, added sugar, sweets, alcohol, and caffeine |
| Type of Juice | Nutrient Composition |
|---|---|
| Tomato juice | 0.50 g fat of which 0.10 g saturated fat, |
| 3.10 g carbohydrates of which 2.80 g sugar, | |
| 0.50 g fiber, 1 g protein, 0.40 g salt, 0.25 sodium | |
| Mixed vegetable juice | 0.50 g fat of which 0.10 g saturated fat, |
| 3.50 g carbohydrates of which 3.30 g sugar, 0.60 g fiber, 0.90 g protein, 0 g salt | |
| Beetroot juice | 0.50 g fat of which 0.10 g saturated fat, |
| 9.20 g carbohydrates of which 9.20 g sugar, | |
| 0.50 g fiber, 1 g protein, 0.13 g salt, 0.05 sodium | |
| Sauerkraut juice | 0.50 g fat of which 0.10 g saturated fat, |
| 1.50 g carbohydrates of which 1 g sugar, 0.50 g fiber, 1 g protein, 0.60 g salt, 0.60 sodium | |
| 0.50 g fat of which 0.10 g saturated fat, | |
| Carrot juice | 6.80 g carbohydrates of which 6.80 g sugar, |
| 0.50 g fiber, 1 g protein, 0.13 g salt, 0.05 sodium |
References
- Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature 2012, 486, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Martino, C.; Dilmore, A.H.; Burcham, Z.M.; Metcalf, J.L.; Jeste, D.; Knight, R. Microbiota succession throughout life from the cradle to the grave. Nat. Rev. Genet. 2022, 20, 707–720. [Google Scholar] [CrossRef] [PubMed]
- De Vos, W.M.; Tilg, H.; Hul, M.V.; Cani, P.D. Gut microbiome and health: Mechanistic insights. Gut 2022, 71, 1020–1032. [Google Scholar] [CrossRef]
- Hills, R.D., Jr.; Pontefract, B.A.; Mishcon, H.R.; Black, C.A.; Sutton, S.C.; Theberge, C.R. Gut Microbiome: Profound Implications for Diet and Disease. Nutrients 2019, 11, 1613. [Google Scholar] [CrossRef] [PubMed]
- Valdes, A.; Walter, J.; Segal, E.; Spector, T.D. Role of the gut microbiota in nutrition and health. BMJ 2018, 361, k2179. [Google Scholar] [CrossRef]
- D’Argenio, V.; Salvatore, F. The role of the gut microbiome in the healthy adult status. Clin. Chim. Acta 2015, 451, 97–102. [Google Scholar] [CrossRef]
- Lloyd-Price, J.; Arze, C.; Ananthakrishnan, A.N.; Schirmer, M.; Avila-Pacheco, J.; Poon, T.W.; Andrews, E.; Ajami, N.J.; Bonham, K.S.; Brislawn, C.J.; et al. Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature 2019, 569, 655–662. [Google Scholar] [CrossRef]
- Mars, R.A.; Yang, Y.; Ward, T.; Houtti, M.; Priya, S.; Lekatz, H.R.; Tang, X.; Sun, Z.; Kalari, K.R.; Korem, T.; et al. Longitudinal Multi-omics Reveals Subset-Specific Mechanisms Underlying Irri-table Bowel Syndrome. Cell 2020, 183, 1137–1140. [Google Scholar] [CrossRef]
- Leonard, M.M.; Valitutti, F.; Karathia, H.; Pujolassos, M.; Kenyon, V.; Fanelli, B.; Troisi, J.; Subramanian, P.; Camhi, S.; Colucci, A.; et al. Microbiome signatures of progression toward celiac disease onset in at-risk children in a longitudinal prospective cohort study. Proc. Natl. Acad. Sci. USA 2021, 118, e2020322118. [Google Scholar] [CrossRef]
- Cheng, Y.; Ling, Z.; Li, L. The Intestinal Microbiota and Colorectal Cancer. Front. Immunol. 2020, 11, 3100. [Google Scholar] [CrossRef]
- Tilg, H.; Adolph, T.E.; Gerner, R.R.; Moschen, A.R. The Intestinal Microbiota in Colorectal Cancer. Cancer Cell 2018, 33, 954–964. [Google Scholar] [CrossRef] [PubMed]
- Marco, M.L.; Sanders, M.E.; Gänzle, M.; Arrieta, M.C.; Cotter, P.D.; De Vuyst, L.; Hill, C.; Holzapfel, W.; Lebeer, S.; Merenstein, D.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on fermented foods. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 196–208. [Google Scholar] [CrossRef] [PubMed]
- Akutko, K.; Stawarski, A. Probiotics, Prebiotics and Synbiotics in Inflammatory Bowel Diseases. J. Clin. Med. 2021, 10, 2466. [Google Scholar] [CrossRef] [PubMed]
- Sanders, M.E.; Merenstein, D.J.; Reid, G.; Gibson, G.R.; Rastall, R.A. Probiotics and prebiotics in intestinal health and disease: From biology to the clinic. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Naseer, M.; Poola, S.; Ali, S.; Samiullah, S.; Tahan, V. Prebiotics and Probiotics in Inflammatory Bowel Disease: Where are we now and where are we going? Curr. Clin. Pharmacol. 2020, 15, 216–233. [Google Scholar] [PubMed]
- Tsurumaki, M.; Kotake, M.; Iwasaki, M.; Saito, M.; Tanaka, K.; Aw, W.; Fukuda, S.; Tomita, M. The application of omics technologies in the functional evaluation of inulin and inulin-containing prebiotics dietary supplementation. Nutr. Diabetes 2015, 5, e185. [Google Scholar] [CrossRef]
- Le Bastard, Q.; Chapelet, G.; Javaudin, F.; Lepelletier, D.; Batard, E.; Montassier, E. The effects of inulin on gut microbial composition: A systematic re-view of evidence from human studies. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 403–413. [Google Scholar] [CrossRef]
- Bonnema, A.L.; Kolberg, L.W.; Thomas, W.; Slavin, J.L. Gastrointestinal Tolerance of Chicory Inulin Products. J. Am. Diet. Assoc. 2010, 110, 865–868. [Google Scholar] [CrossRef]
- Healey, G.; Murphy, R.; Butts, C.; Brough, L.; Whelan, K.; Coad, J. Habitual dietary fibre intake influences gut microbiota response to an inu-lin-type fructan prebiotic: A randomised, double-blind, placebo-controlled, cross-over, human intervention study. Br. J. Nutr. 2018, 119, 176–189. [Google Scholar] [CrossRef]
- Zmora, N.; Suez, J.; Elinav, E. You are what you eat: Diet, health and the gut microbiota. Nat. Rev. Gastroen Terol. Hepatol. 2019, 16, 35–56. [Google Scholar] [CrossRef]
- Muegge, B.D.; Kuczynski, J.; Knights, D.; Clemente, J.C.; González, A.; Fontana, L.; Henrissat, B.; Knight, R.; Gordon, J.I. Diet Drives Convergence in Gut Microbiome Functions Across Mam-malian Phylogeny and Within Humans. Science 2011, 332, 970–974. [Google Scholar] [CrossRef] [PubMed]
- Van Zyl, K.N.; Matukane, S.R.; Hamman, B.L.; Whitelaw, A.C.; Newton-Foot, M. Effect of antibiotics on the human microbiome: A systematic review. Int. J. Antimicrob. Agents 2022, 59, 106502. [Google Scholar] [CrossRef] [PubMed]
- Patangia, D.V.; Anthony Ryan, C.; Dempsey, E.; Paul Ross, R.; Stanton, C. Impact of antibiotics on the human microbiome and conse-quences for host health. MicrobiologyOpen 2022, 11, e1260. [Google Scholar] [CrossRef] [PubMed]
- Angoorani, P.; Ejtahed, H.S.; Hasani-Ranjbar, S.; Siadat, S.D.; Soroush, A.R.; Larijani, B. Gut Microbiota Modulation as a Possible Mediating Mecha-nism for Fasting-Induced Alleviation of Metabolic Complications: A Systematic Review. Nutr. Metab. 2021, 18, 105. [Google Scholar] [CrossRef] [PubMed]
- Wilhelmi de Toledo, F.; Grundler, F.; Sirtori, C.R.; Ruscica, M. Unravelling the Health Effects of Fasting: A Long Road from Obesity Treatment to Healthy Life Span Increase and Improved Cognition. Ann. Med. 2020, 52, 147–461. [Google Scholar] [CrossRef]
- Di Francesco, A.; Di Germanio, C.; Bernier, M.; De Cabo, R. A time to fast. Science 2018, 362, 770–775. [Google Scholar] [CrossRef]
- Longo, V.D.; Mattson, M.P. Fasting: Molecular Mechanisms and Clinical Applications. Cell Metab. 2014, 19, 181–192. [Google Scholar] [CrossRef]
- Dirks, A.J.; Leeuwenburgh, C. Caloric restriction in humans: Potential pitfalls and health concerns. Mech. Ageing Dev. 2006, 127, 1–7. [Google Scholar] [CrossRef]
- Sonnenburg, E.D.; Sonnenburg, J.L. Starving our Microbial Self: The Deleterious Consequences of a Diet Deficient in Microbiota-Accessible Carbohydrates. Cell Metab. 2014, 20, 779–786. [Google Scholar] [CrossRef]
- Johnstone, A. Fasting for weight loss: An effective strategy or latest dieting trend? Int. J. Obes. 2015, 39, 727–733. [Google Scholar] [CrossRef]
- Schmidt, N.S.; Lorentz, A. Dietary restrictions modulate the gut microbiota: Implications for health and disease. Nutr. Res. 2021, 89, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Huber, R.; Nauck, M.; Lüdtke, R.; Scharnagl, H. Effects of one week juice fasting on lipid metabolism: A cohort study in healthy subjects. Forsch. Komplement. Und Klass. Nat. Res. Complement. Nat. Class. Med. 2003, 10, 7–10. [Google Scholar] [CrossRef] [PubMed]
- De Toledo, F.W.; Buchinger, A.; Burggrabe, H.; Hölz, G.; Kuhn, C.; Lischka, E.; Lischka, N.; Lützner, H.; May, W.; Ritzmann-Widderich, M.; et al. Fasting Therapy—An Expert Panel Update of the 2002 Consensus Guidelines. Complement. Med. Res. 2013, 20, 434–443. [Google Scholar] [CrossRef] [PubMed]
- De Toledo, F.W.; Grundler, F.; Bergouignan, A.; Drinda, S.; Michalsen, A. Safety, health improvement and well-being during a 4 to 21-day fasting period in an observational study including 1422 subjects. PLoS ONE 2019, 14, e0209353. [Google Scholar]
- Zheng, J.; Zhou, Y.; Li, S.; Zhang, P.; Zhou, T.; Xu, D.-P.; Li, H.-B. Effects and Mechanisms of Fruit and Vegetable Juices on Cardiovascular Diseases. Int. J. Mol. Sci. 2017, 18, 555. [Google Scholar] [CrossRef] [PubMed]
- Lamport, D.J.; Saunders, C.; Butler, L.T.; Spencer, J.P. Fruits, vegetables, 100% juices, and cognitive function. Nutr. Rev. 2014, 72, 774–789. [Google Scholar] [CrossRef] [PubMed]
- Byrne, C.S.; Chambers, E.S.; Morrison, D.J.; Frost, G. The role of short chain fatty acids in appetite regulation and energy homeostasis. Int. J. Obes. 2015, 39, 1331–1338. [Google Scholar] [CrossRef] [PubMed]
- Haring, E.; Andrieux, G.; Uhl, F.M.; Krausz, M.; Proietti, M.; Sauer, B.; Esser, P.R.; Martin, S.F.; Pfeifer, D.; Schmitt-Graeff, A.; et al. Therapeutic targeting of endoplasmic reticulum stress in acute graft-versus-host disease. Haematologica 2021, 107, 1538–1554. [Google Scholar] [CrossRef] [PubMed]
- Norona, J.; Apostolova, P.; Schmidt, D.; Ihlemann, R.; Reischmann, N.; Taylor, G.; Köhler, N.; De Heer, J.; Heeg, S.; Andrieux, G.; et al. Glucagon-like peptide 2 for intestinal stem cell and Paneth cell repair during graft-versus-host disease in mice and humans. Blood 2020, 136, 1442–1455. [Google Scholar] [CrossRef]
- Haring, E.; Uhl, F.M.; Andrieux, G.; Proietti, M.; Bulashevska, A.; Sauer, B.; Braun, L.M.; Gomez, E.D.V.; Esser, P.R.; Martin, S.F.; et al. Bile acids regulate intestinal antigen presentation and reduce graft-versus-host disease without impairing the graft-versus-leukemia effect. Haematologica 2020, 106, 2131–2146. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Lozupone, C.A.; Turnbaugh, P.J.; Fierer, N.; Knight, R. Global patterns of 16S rRNA diversity at a depth of millions of se-quences per sample. Proc. Natl. Acad. Sci. USA 2011, 108, 4516–4522. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Gonzalez Peña, A.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naïve Bayesian Classifier for Rapid Assignment of rRNA Sequences into the New Bacterial Taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [PubMed]
- Price, M.N.; Dehal, P.S.; Arkin, A.P. FastTree 2—Approximately Maximum-Likelihood Trees for Large Alignments. PLoS ONE 2010, 5, e9490. [Google Scholar] [CrossRef] [PubMed]
- McMurdie, P.J.; Holmes, S. phyloseq: An R package for reproducible interactive analysis and graphics of microbi-ome census data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef]
- He, Y.; Yin, J.; Lei, J.; Liu, F.; Zheng, H.; Wang, S.; Wu, S.; Sheng, H.; McGovern, E.; Zhou, H. Fasting challenges human gut microbiome resilience and reduces Fusobacterium. Med. Microecol. 2019, 1–2, 100003. [Google Scholar] [CrossRef]
- Michalsen, A.; Li, C. Fasting Therapy for Treating and Preventing Disease—Current State of Evidence. Complement. Med. Res. 2013, 20, 444–453. [Google Scholar] [CrossRef]
- Gabel, K.; Marcell, J.; Cares, K.; Kalam, F.; Cienfuegos, S.; Ezpeleta, M.; Varady, K.A. Effect of time restricted feeding on the gut microbiome in adults with obesity: A pilot study. Nutr. Health 2020, 26, 79–85. [Google Scholar] [CrossRef]
- Vacca, M.; Celano, G.; Calabrese, F.M.; Portincasa, P.; Gobbetti, M.; De Angelis, M. The Controversial Role of Human Gut Lachnospiraceae. Microorganisms 2020, 8, 573. [Google Scholar] [CrossRef]
- Vazquez-Moreno, M.; Perez-Herrera, A.; Locia-Morales, D.; Dizzel, S.; Meyre, D.; Stearns, J.C.; Cruz, M. Association of gut microbiome with fasting tri-glycerides, fasting insulin and obesity status in Mexican children. Pediatr. Obes. 2021, 16, e12748. [Google Scholar] [CrossRef] [PubMed]

| P1 | P2 | P3 | P4 | P5 | P6 | |
|---|---|---|---|---|---|---|
| Feasibility of the study design | ||||||
| Did you find it difficult to give up solid food completely for three days? | no | no | yes | yes | yes | no |
| Was the daily amount (300 kcal) of vegetable juices sufficient for you to keep up the fast? | yes | yes | no | yes | yes | yes |
| Did you find it difficult to fast twice? | no | no | no | no | yes | no |
| Did you find the time between the two fasting periods sufficient? | yes | yes | yes | yes | yes | yes |
| Side effects | ||||||
| Did you have side effects during the fasting periods? | no | yes | yes | yes | yes | yes |
| If yes, how much did these side effects interfere with your daily life? (Not at all 1, Very much 10) | 1 | 1 | 4 | 10 | 7 | 5 |
| Did you tolerate the inulin well? | yes | no | no | no | yes | yes |
| Adaptations for future studies | ||||||
| Could you imagine giving up solid food completely for five days? | yes | yes | - | yes | no | yes |
| With tea only | yes | yes | - | no | no | - |
| With vegetable broth and tea only | yes | yes | yes | yes | yes | yes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thriene, K.; Stanislas, V.; Amend, L.; Strowig, T.; Michels, K.B. Assessment of the Gut Microbiota during Juice Fasting with and without Inulin Supplementation: A Feasibility Study in Healthy Volunteers. Foods 2022, 11, 3673. https://doi.org/10.3390/foods11223673
Thriene K, Stanislas V, Amend L, Strowig T, Michels KB. Assessment of the Gut Microbiota during Juice Fasting with and without Inulin Supplementation: A Feasibility Study in Healthy Volunteers. Foods. 2022; 11(22):3673. https://doi.org/10.3390/foods11223673
Chicago/Turabian StyleThriene, Kerstin, Virginie Stanislas, Lena Amend, Till Strowig, and Karin B. Michels. 2022. "Assessment of the Gut Microbiota during Juice Fasting with and without Inulin Supplementation: A Feasibility Study in Healthy Volunteers" Foods 11, no. 22: 3673. https://doi.org/10.3390/foods11223673
APA StyleThriene, K., Stanislas, V., Amend, L., Strowig, T., & Michels, K. B. (2022). Assessment of the Gut Microbiota during Juice Fasting with and without Inulin Supplementation: A Feasibility Study in Healthy Volunteers. Foods, 11(22), 3673. https://doi.org/10.3390/foods11223673





