Industrial Hemp (Cannabis sativa L.) Inflorescences as Novel Food: The Effect of Different Agronomical Practices on Chemical Profile
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Experimental Site and Weather Conditions
2.3. Field Experiment Description, Experimental Design, and Treatments
2.4. Field Measurements and Data Collection
2.5. UHPLC Analysis for Cannabinoid Profile
2.6. Untargeted NMR Analysis
2.7. Spectrophotometric Analysis of Total Phenolics Content and Antioxidant Activity
2.8. Statistical Analysis
3. Results and Discussion
3.1. Chemical Characterization
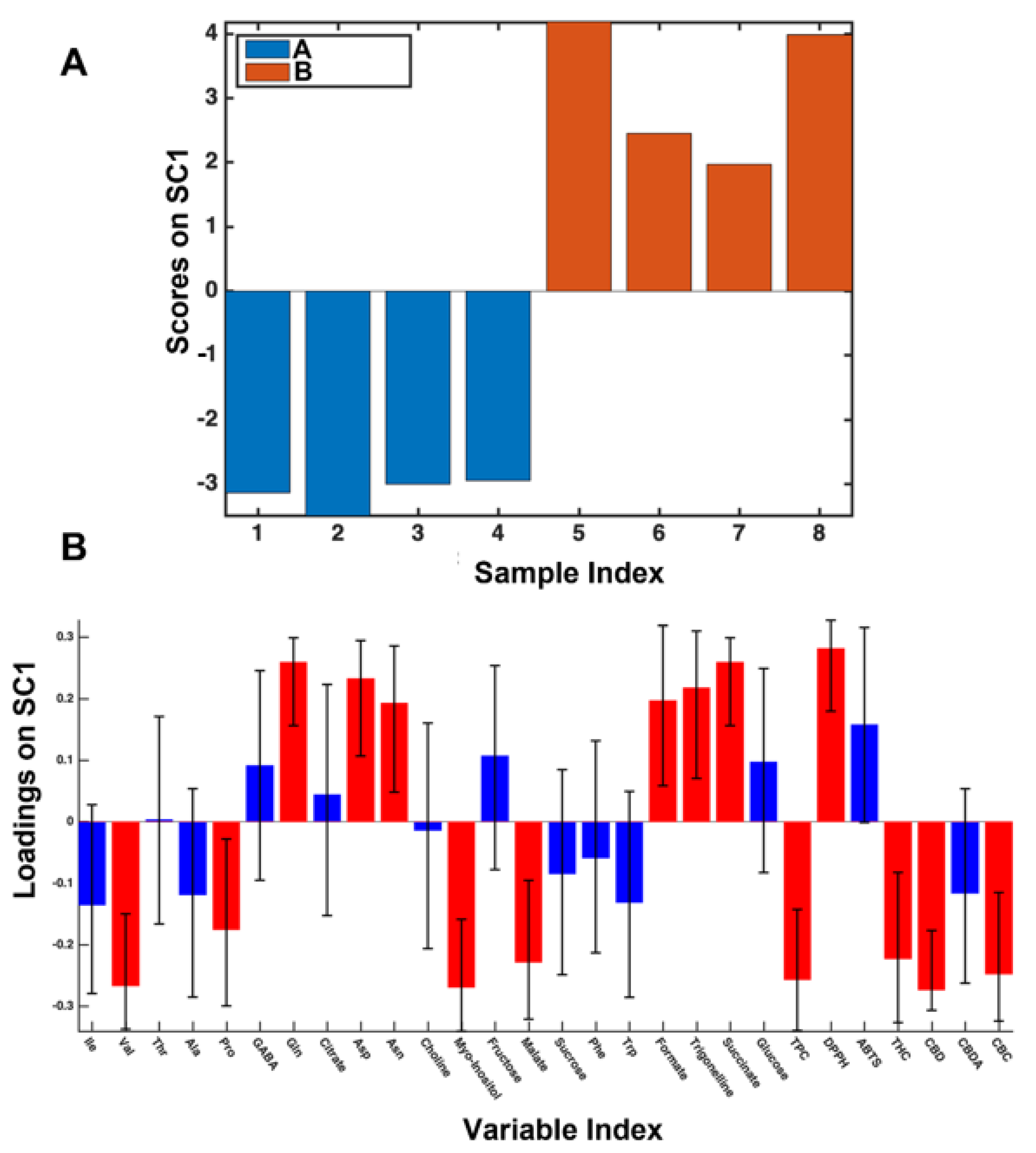
3.2. The 2017 Year-Data
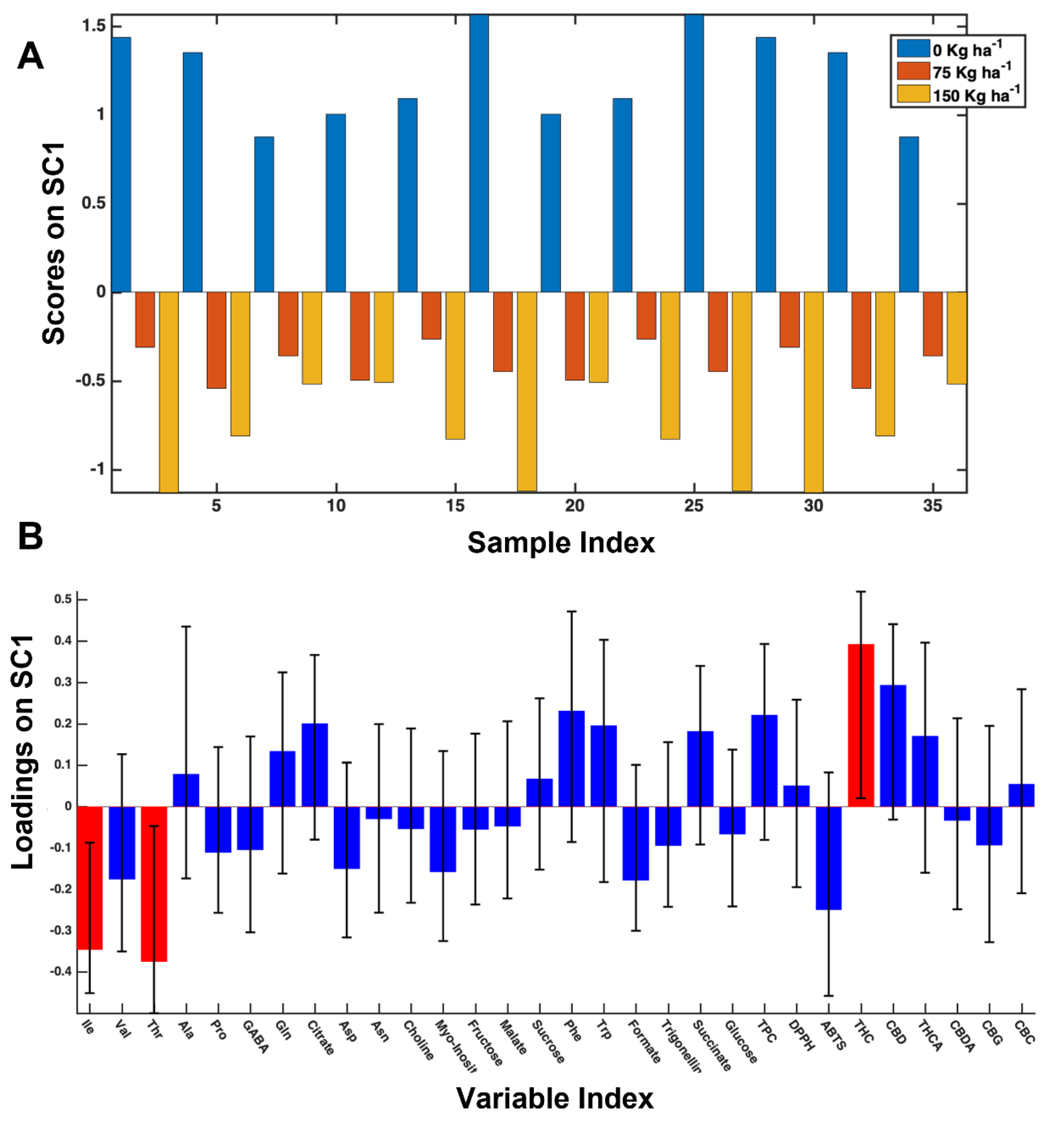
3.3. The 2018 Year-Data
3.4. 2017- and 2018-Years Data Set
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- EUR-Lex. Access to European Union Law. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:01999R1251-20040701 (accessed on 3 June 2022).
- Montserrat-De La Paz, S.; Marín-Aguilar, F.; García-Giménez, M.D.; Fernández-Arche, M.A. Hemp (Cannabis sativa L.) seed oil: Analytical and phytochemical characterization of the unsaponifiable fraction. J. Agric. Food Chem. 2014, 62, 1105–1110. [Google Scholar] [CrossRef] [PubMed]
- Lewis-Bakker, M.M.; Yang, Y.; Vyawahare, R.; Kotra, L.P. Extractions of Medical Cannabis Cultivars and the Role of Decarboxylation in Optimal Receptor Responses. Cannabis Cannabinoid Res. 2019, 4, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.-S.; Mahlberg, P.G. Secretory Cavity Development in Glandular Trichomes of Cannabis sativa L. (Cannabaceae). Am. J. Bot. 1991, 78, 220. [Google Scholar] [CrossRef]
- Jin, D.; Dai, K.; Xie, Z.; Chen, J. Secondary Metabolites Profiled in Cannabis Inflorescences, Leaves, Stem Barks, and Roots for Medicinal Purposes. Sci. Rep. 2020, 10, 3309. [Google Scholar] [CrossRef] [PubMed]
- Drinić, Z.; Vladić, J.; Koren, A.; Zeremski, T.; Stojanov, N.; Kiprovski, B.; Vidović, S. Microwave-assisted extraction of cannabinoids and antioxidants from Cannabis sativa aerial parts and process modeling. J. Chem. Technol. Biotechnol. 2020, 95, 831–839. [Google Scholar] [CrossRef]
- Lanyon, V.S.; Turner, J.C.; Mahlberg, P.G. Quantitative Analysis of Cannabinoids in the Secretory Product from Capitate-Stalked Glands of Cannabis sativa L. (Cannabaceae). Bot. Gaz. 1981, 142, 316–319. [Google Scholar] [CrossRef]
- Hemphill, J.K.; Turner, J.C.; Mahlberg, P.G. Cannabinoid content of individual plant organs from different geographical strains of Cannabis sativa L. J. Nat. Prod. 1980, 43, 112–122. [Google Scholar] [CrossRef]
- European Commission EU Novel Food Catalogue. Available online: https://webgate.ec.europa.eu/fip/novel_food_catalogue/# (accessed on 27 July 2022).
- Cerino, P.; Buonerba, C.; Cannazza, G.; D’Auria, J.; Ottoni, E.; Fulgione, A.; Di Stasio, A.; Pierri, B.; Gallo, A. A review of hemp as food and nutritional supplement. Cannabis Cannabinoid Res. 2021, 6, 19–27. [Google Scholar] [CrossRef]
- Ingallina, C.; Sobolev, A.P.; Circi, S.; Spano, M.; Fraschetti, C.; Filippi, A.; Di Sotto, A.; Di Giacomo, S.; Mazzoccanti, G.; Gasparrini, F.; et al. Cannabis sativa L. inflorescences from monoecious cultivars grown in central Italy: An untargeted chemical characterization from early flowering to ripening. Molecules 2020, 25, 1908. [Google Scholar] [CrossRef]
- Izzo, L.; Castaldo, L.; Narváez, A.; Graziani, G.; Gaspari, A.; Rodríguez-Carrasco, Y.; Ritieni, A. Analysis of Phenolic Compounds in Commercial Cannabis sativa L. Inflorescences using UHPLC-Q-Orbitrap HRMs. Molecules 2020, 25, 631. [Google Scholar] [CrossRef]
- Spano, M.; Di Matteo, G.; Ingallina, C.; Botta, B.; Quaglio, D.; Ghirga, F.; Balducci, S.; Cammarone, S.; Campiglia, E.; Giusti, A.M.; et al. A multimethodological characterization of Cannabis sativa L. Inflorescences from seven dioecious cultivars grown in Italy: The effect of different harvesting stages. Molecules 2021, 26, 2912. [Google Scholar] [CrossRef]
- Jankauskiene, Z.; Gruzdeviene, E.; Ivanovs, S.; Maumevicius, E. Screening hemp (Cannabis sativa L.) biomass and chemical composition as influenced by seed rate and genotype. Proc. Eng. R. Dev. 2017, 16, 317–322. [Google Scholar]
- Pieracci, Y.; Ascrizzi, R.; Terreni, V.; Pistelli, L.; Flamini, G.; Bassolino, L.; Fulvio, F.; Montanari, M.; Paris, R. Essential oil of Cannabis sativa l: Comparison of yield and chemical composition of 11 hemp genotypes. Molecules 2021, 26, 4080. [Google Scholar] [CrossRef]
- Tang, K.; Struik, P.C.; Yin, X.; Calzolari, D.; Musio, S.; Thouminot, C.; Bjelková, M.; Stramkale, V.; Magagnini, G.; Amaducci, S. A comprehensive study of planting density and nitrogen fertilization effect on dual-purpose hemp (Cannabis sativa L.) cultivation. Ind. Crops Prod. 2017, 107, 427–438. [Google Scholar] [CrossRef]
- Vera, C.L.; Malhi, S.S.; Raney, J.P.; Wang, Z.H. The effect of N and P fertilization on growth, seed yield and quality of industrial hemp in the Parkland region of Saskatchewan. Can. J. Plant Sci. 2004, 84, 939–947. [Google Scholar] [CrossRef]
- Vera, C.L.; Malhi, S.S.; Phelps, S.M.; May, W.E.; Johnson, E.N. N, P, and S fertilization effects on industrial hemp in Saskatchewan. Can. J. Plant Sci. 2010, 90, 179–184. [Google Scholar] [CrossRef]
- García-Tejero, I.F.; Durán-Zuazo, V.H.; Pérez-Álvarez, R.; Hernández, A.; Casano, S.; Morón, M.; Muriel-Fernández, J.L. Impact of plant density and irrigation on yield of hemp (Cannabis sativa L.) in a mediterranean semi-arid environment. J. Agric. Sci. Technol. 2014, 16, 887–895. [Google Scholar]
- Pejić, B.; Sikora, V.; Milić, S.; Mačkić, K.; Koren, A.; Bajić, I. Effect of drip irrigation on yield and evapotranspiration of fibre hemp (Cannabis sativa L.). Ratar. i Povrt. 2018, 55, 130–134. [Google Scholar] [CrossRef]
- Liu, M.; Fernando, D.; Daniel, G.; Madsen, B.; Meyer, A.S.; Ale, M.T.; Thygesen, A. Effect of harvest time and field retting duration on the chemical composition, morphology and mechanical properties of hemp fibers. Ind. Crops Prod. 2015, 69, 29–39. [Google Scholar] [CrossRef]
- Maathuis, F.J. Physiological functions of mineral macronutrients. Curr. Opin. Plant Biol. 2009, 12, 250–258. [Google Scholar] [CrossRef]
- Zheng, Y. Current nutrient management practices and technologies used in North American greenhouse and nursery industries. Acta Hortic. 2018, 1227, 435–442. [Google Scholar] [CrossRef]
- Bevan, L.; Jones, M.; Zheng, Y. Optimisation of Nitrogen, Phosphorus, and Potassium for Soilless Production of Cannabis sativa in the Flowering Stage Using Response Surface Analysis. Front. Plant Sci. 2021, 12, 764103. [Google Scholar] [CrossRef] [PubMed]
- Coffman, C.B.; Gentner, W.A. Responses of Greenhouse-grown Cannabis sativa L. to Nitrogen, Phosphorus, and Potassium 1. Agron. J. 1977, 69, 832–836. [Google Scholar] [CrossRef]
- Caplan, D.; Dixon, M.; Zheng, Y. Optimal rate of organic fertilizer during the flowering stage for cannabis grown in two coir-based substrates. HortScience 2017, 52, 1796–1803. [Google Scholar] [CrossRef]
- Cosentino, S.L.; Riggi, E.; Testa, G.; Scordia, D.; Copani, V. Evaluation of European developed fibre hemp genotypes (Cannabis sativa L.) in semi-arid Mediterranean environment. Ind. Crops Prod. 2013, 50, 312–324. [Google Scholar] [CrossRef]
- Mediavilla, V.; Meier, C. Factors influencing the yield and the quality of hemp (Cannabis sativa L.) essential oil. J. Int. Hemp. Assoc. 1998, 5, 16–20. [Google Scholar]
- Caplan, D.; Dixon, M.; Zheng, Y. Increasing inflorescence dry weight and cannabinoid content in medical cannabis using controlled drought stress. HortScience 2019, 54, 964–969. [Google Scholar] [CrossRef]
- Campbell, B.J.; Berrada, A.F.; Hudalla, C.; Amaducci, S.; McKay, J.K. Genotype × Environment Interactions of Industrial Hemp Cultivars Highlight Diverse Responses to Environmental Factors. Agrosyst. Geosci. Environ. 2019, 2, 1–11. [Google Scholar] [CrossRef]
- Campiglia, E.; Radicetti, E.; Mancinelli, R. Plant density and nitrogen fertilization affect agronomic performance of industrial hemp (Cannabis sativa L.) in Mediterranean environment. Ind. Crops Prod. 2017, 100, 246–254. [Google Scholar] [CrossRef]
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Crop evapotranspiration—Guidelines for computing crop water requirements—FAO Irrigation and drainage paper 56; FAO: Rome, Italy, 1998; Volume 300, p. D05109. [Google Scholar]
- Spano, M.; Maccelli, A.; Matteo, G.D.; Ingallina, C.; Biava, M.; Crestoni, M.E.; Bardaud, J.X.; Giusti, A.M.; Mariano, A.; D’abusco, A.S.; et al. Metabolomic profiling of fresh goji (Lycium barbarum L.) berries from two cultivars grown in central italy: A multi-methodological approach. Molecules 2021, 26, 5412. [Google Scholar] [CrossRef]
- Gómez, J.; Simirgiotis, M.J.; Manrique, S.; Piñeiro, M.; Lima, B.; Bórquez, J.; Feresin, G.E.; Tapia, A. Uhplc-esi-ot-ms phenolics profiling, free radical scavenging, antibacterial and nematicidal activities of “yellow-brown resins” from Larrea spp. Antioxidants 2021, 10, 185. [Google Scholar] [CrossRef]
- Pierre, E.O.; Nicolas, N.; Pierre, F.o.D.; Martine, L.O.; Denis, O.N. Heritability of polyphenols, anthocyanins and antioxidant capacity of Cameroonian cocoa (Theobroma cacao L.) beans. Afr. J. Biotechnol. 2015, 14, 2672–2682. [Google Scholar] [CrossRef]
- Smilde, A.K.; Jansen, J.J.; Hoefsloot, H.C.J.; Lamers, R.J.A.N.; van der Greef, J.; Timmerman, M.E. ANOVA-simultaneous component analysis (ASCA): A new tool for analyzing designed metabolomics data. Bioinformatics 2005, 21, 3043–3048. [Google Scholar] [CrossRef]
- Zwanenburg, G.; Hoefsloot, H.C.J.; Westerhuis, J.A.; Jansen, J.J.; Smilde, A.K. ANOVA–principal component analysis and ANOVA–simultaneous component analysis: A comparison. J. Chemom. 2011, 25, 561–567. [Google Scholar] [CrossRef]
- Glivar, T.; Eržen, J.; Kreft, S.; Zagožen, M.; Čerenak, A.; Čeh, B.; Tavčar Benković, E. Cannabinoid content in industrial hemp (Cannabis sativa L.) varieties grown in Slovenia. Ind. Crops Prod. 2020, 145, 112082. [Google Scholar] [CrossRef]
- Palmieri, S.; Fanti, F.; Oliva, E.; Viteritti, E.; Sergi, M.; Pepe, A.; Compagnone, D. Chemical characterization and evaluation of antioxidant activity from different cultivars of Cannabis sativa L. of Abruzzo’s region. Nat. Prod. Res. 2022, 2022, 1–15. [Google Scholar] [CrossRef]
- Anas, M.; Liao, F.; Verma, K.K.; Sarwar, M.A.; Mahmood, A.; Chen, Z.L.; Li, Q.; Zeng, X.P.; Liu, Y.; Li, Y.R. Fate of nitrogen in agriculture and environment: Agronomic, eco-physiological and molecular approaches to improve nitrogen use efficiency. Biol. Res. 2020, 53, 47. [Google Scholar] [CrossRef]

| Harvesting Period | Fertilization | Δ9-THC % w/w | CBD % w/w | Δ9-THCA % w/w | CBDA % w/w | CBG % w/w | CBC % w/w |
|---|---|---|---|---|---|---|---|
| 2017 year | |||||||
| Stage A | P0 N0 I | 0.08 ± 0.01 a | 1.74 ± 0.14 a | / | 0.77 ± 0.10 a | / | 0.08 ± 0.01 a |
| P0 N150 I | 0.06 ± 0.01 | 1.58 ± 0.09 | / | 0.60 ± 0.14 | / | 0.07 ± 0.01 | |
| P90 N0 I | 0.06 ± 0.01 | 1.54 ± 0.07 a | / | 0.84 ± 0.03 a | / | 0.06 ± 0.01 | |
| P90 N150 I | 0.07 ± 0.01 | 1.71 ± 0.17 a | / | 0.64 ± 0.04 | / | 0.08 ± 0.01 a | |
| Stage B | P0 N0 I | 0.05 ± 0.01 | 0.83 ± 0.01 | / | 0.52 ± 0.01 | / | 0.05 ± 0.01 |
| P0 N150 I | 0.06 ± 0.01 | 1.28 ± 0.20 | / | 0.67 ± 0.05 | / | 0.06 ± 0.01 | |
| P90 N0 I | 0.05 ± 0.01 | 1.03 ± 0.08 | / | 0.71 ± 0.03 | / | 0.05 ± 0.01 | |
| P90 N150 I | 0.05 ± 0.01 | 0.97 ± 0.03 | / | 0.66 ± 0.03 | / | 0.05 ± 0.01 | |
| 2018 year | |||||||
| Stage A | P0 N0 D | 0.08 ± 0.01 | 0.94 ± 0.04 a | 0.07 ± 0.01 a,b | 0.47 ± 0.02 a,b | 0.07 ± 0.01 b | 0.15 ± 0.04 a |
| P0 N0 I | 0.09 ± 0.01 a | 0.60 ± 0.10 a,b | / | 0.27 ± 0.03 | /a | 0.19 ± 0.01 a | |
| P0 N75 D | 0.06 ± 0.01 | 0.70 ± 0.10 a | / | 0.39 ± 0.02 a,b | 0.04 ± 0.01 b | 0.12 ± 0.01 a,b | |
| P0 N75 I | 0.08 ± 0.01 a | 0.76 ± 0.02 | / | 0.29 ± 0.03 a | 0.07 ± 0.01 a | 0.19 ± 0.01 a | |
| P0 N150 D | 0.06 ± 0.01 | 1.30 ± 0.02 b | / | 0.39 ± 0.03 a,b | 0.05 ± 0.01 a | 0.12 ± 0.01 a,b | |
| P0 N150 I | 0.07 ± 0.02 | 0.87 ± 0.02 a | / | 0.16 ± 0.01 a | 0.07 ± 0.01 a | 0.19 ± 0.01 a | |
| P45 N0 D | 0.08 ± 0.01 | 1.30 ± 0.20 b | 0.08 ± 0.01 a,b | 0.43 ± 0.01 a | 0.05 ± 0.01 a | 0.12 ± 0.01 a,b | |
| P45 N0 I | 0.09 ± 0.01 | 0.45 ± 0.01 a | / | 0.45 ± 0.01 a | 0.07 ± 0.01 a | 0.20 ± 0.01 a | |
| P45 N75 D | 0.06 ± 0.01 | 0.60 ± 0.10 a | 0.07 ± 0.01 a,b | 0.34 ± 0.05 b | 0.04 ± 0.01 a | 0.11 ± 0.01 a,b | |
| P45 N75 I | 0.08 ± 0.01 | 0.60 ± 0.10 a | / | 0.10 ± 0.01 a | 0.06 ± 0.02 | 0.19 ± 0.01 a | |
| P45 N150 D | 0.06 ± 0.01 b | 1.00 ± 0.10 a,b | 0.08 ± 0.01 a,b | 0.17 ± 0.01 a,b | 0.05 ± 0.01 a | 0.12 ± 0.01 a,b | |
| P45 N 150 I | 0.09 ± 0.01 | 0.80 ± 0.04 a | / | 0.48 ± 0.04 | 0.07 ± 0.01 a | 0.20 ± 0.01 a | |
| P90 N0 D | 0.07 ± 0.01 | 0.98 ± 0.01 a | / b | 0.46 ± 0.03 a,b | / b | 0.12 ± 0.01 a,b | |
| P90 N0 I | 0.11 ± 0.03 a | 1.30 ± 0.50 | 0.13 ± 0.01 a | 0.35 ± 0.03 | 0.08 ± 0.01 a | 0.22 ± 0.03 a | |
| P90 N75 D | 0.06 ± 0.01 b | 0.55 ± 0.05 a,b | 0.07 ± 0.01 b | 0.55 ± 0.04 a,b | / b | 0.11 ± 0.01 b | |
| P90 N75 I | 0.09 ± 0.01 | 0.93 ± 0.01 a | / | 0.31 ± 0.01 a | 0.07 ± 0.01 a | 0.20 ± 0.01 a | |
| P90 N150 D | 0.07 ± 0.01 | 0.93 ± 0.08 | / | 0.18 ± 0.02 a,b | 0.05 ± 0.01 a,b | 0.12 ± 0.01 a,b | |
| P90 N150 I | 0.09 ± 0.01 | 0.92 ± 0.07 | / | 0.46 ± 0.03 a | 0.08 ± 0.01 a | 0.20 ± 0.01 a | |
| Stage B | P0 N0 D | 0.07 ± 0.01 | 1.72 ± 0.09 | / | 0.30 ± 0.01 | 0.05 ± 0.01 | 0.07 ± 0.01 |
| P0 N0 I | 0.06 ± 0.01 | 1.60 ± 0.27 | / | 0.30 ± 0.01 | 0.04 ± 0.01 | 0.09 ± 0.03 | |
| P0 N75 D | 0.07 ± 0.01 | 1.40 ± 0.01 b | / | 0.72 ± 0.01 b | 0.05 ± 0.01 b | 0.07 ± 0.01 b | |
| P0 N75 I | 0.05 ± 0.01 | 0.80 ± 0.10 | / | 0.36 ± 0.02 | / | / | |
| P0 N150 D | 0.08 ± 0.01 | 1.38 ± 0.06 | / | 0.74 ± 0.01 b | / b | 0.07 ± 0.01 b | |
| P0 N150 I | 0.07 ± 0.01 | 1.30 ± 0.10 | / | 0.33 ± 0.03 | 0.04 ± 0.01 | 0.13 ± 0.01 | |
| P45 N0 D | 0.08 ± 0.01 | 1.43 ± 0.01 | / | 0.67 ± 0.02 | / | 0.07 ± 0.01 b | |
| P45 N0 I | 0.08 ± 0.01 | 1.60 ± 0.27 | / | 0.66 ± 0.03 | / | 0.12 ± 0.01 | |
| P45 N75 D | 0.06 ± 0.01 | 1.33 ± 0.05 b | / | 0.32 ± 0.01 b | / b | 0.07 ± 0.01 b | |
| P45 N75 I | 0.08 ± 0.01 | 1.10 ± 0.10 | / | 0.55 ± 0.01 | 0.04 ± 0.01 | 0.12 ± 0.01 | |
| P45 N150 D | 0.06 ± 0.01 | 1.36 ± 0.01 b | / | 0.30 ± 0.01 b | / b | 0.07 ± 0.01 b | |
| P45 N 150 I | 0.08 ± 0.01 | 1.25 ± 0.01 | / | 0.56 ± 0.03 | 0.04 ± 0.01 | 0.13 ± 0.01 | |
| P90 N0 D | 0.08 ± 0.01 | 1.67 ± 0.01 b | / | 0.29 ± 0.01 b | / | 0.08 ± 0.01 b | |
| P90 N0 I | 0.08 ± 0.01 | 1.13 ± 0.05 | / | 0.39 ± 0.01 | / | 0.11 ± 0.01 | |
| P90 N75 D | 0.07 ± 0.01 | 0.99 ± 0.13 b | 0.05 ± 0.01 b | 0.77 ± 0.02 | / | 0.09 ± 0.04 | |
| P90 N75 I | 0.08 ± 0.01 | 1.35 ± 0.13 | / | 0.80 ± 0.10 | / | 0.12 ± 0.01 | |
| P90 N150 D | 0.07 ± 0.01 | 1.01 ± 0.06 | 0.05 ± 0.01 b | 0.53 ± 0.07 b | / | 0.06 ± 0.01 b | |
| P90 N150 I | 0.07 ± 0.01 | 1.01 ± 0.05 | / | 0.72 ± 0.03 | / | 0.12 ± 0.01 |
| Harvesting Period | Fertilization | TPC (mg kg−1 Fresh Hemp) | DPPH (EC50 in mg Fresh Hemp mL−1) | ABTS (mg TE g−1 Fresh Hemp) |
|---|---|---|---|---|
| 2017 year | ||||
| Stage A | P0 N0 I | 3788.15 ± 198.83 a | 0.84 ± 0.32 a | 0.75 ± 0.34 |
| P0 N150 I | 3911.21 ± 158.83 a | 0.82 ± 0.46 a | 0.75 ± 0.42 | |
| P90 N0 I | 3859.71 ± 311.84 a | 0.82 ± 0.14 | 0.75 ± 0.45 | |
| P90 N150 I | 3607.90 ± 278.35 | 0.88 ± 0.52 | 0.75 ± 0.46 | |
| Stage B | P0 N0 I | 3201.61 ± 175.29 | 0.93 ± 0.01 | 0.75 ± 0.14 |
| P0 N150 I | 3515.79 ± 88.44 | 0.97 ± 0.29 | 0.75 ± 0.19 | |
| P90 N0 I | 3031.07 ± 198.78 | 0.95 ± 0.37 | 0.75 ± 0.27 | |
| P90 N150 I | 3351.50 ± 33.45 | 0.96 ± 0.62 | 0.75 ± 0.24 | |
| 2018 year | ||||
| Stage A | P0 N0 D | 1686.54 ± 88.95 a | 0.72 ± 0.26 a | 0.76 ± 0.38 |
| P0 N0 I | 1811.72 ± 190.37 a | 0.74 ± 0.57 | 0.75 ± 0.43 | |
| P0 N75 D | 2958.75 ± 455.53 a,b | 0.90 ± 0.52 | 0.76 ± 0.17 | |
| P0 N75 I | 1712.82 ± 4.04 a | 0.68 ± 0.30 | 0.74 ± 0.29 | |
| P0 N150 D | 1610.49 ± 10.75 a | 0.70 ± 0.23 | 0.75 ± 0.01 | |
| P0 N 150 I | 1605.26 ± 7.18 a | 0.72 ± 0.19 | 0.76 ± 0.66 | |
| P45 N0 D | 1650.77 ± 206.59 a | 0.75 ± 0.36 | 0.76 ± 0.15 | |
| P45 N0 I | 1766.50 ± 36.08 | 0.74 ± 0.43 | 0.74 ± 0.49 | |
| P45 N75 D | 1884.70 ± 103.77 a | 0.70 ± 0.20 | 0.75 ± 0.11 | |
| P45 N75 I | 1752.48 ± 62.43 a | 0.75 ± 0.18 | 0.76 ± 0.44 | |
| P45 N150 D | 1806.23 ± 3.89 a,b | 0.69 ± 0.34 | 0.76 ± 0.51 | |
| P45 N 150 I | 1496.75 ± 5.26 a | 0.79 ± 0.14 | 0.76 ± 0.58 | |
| P90 N0 D | 2806.45 ± 198.60 a,b | 0.78 ± 0.11 | 0.76 ± 0.50 | |
| P90 N0 I | 1603.93 ± 8.84 a | 0.71 ± 0.30 | 0.75 ± 0.16 | |
| P90 N75 D | 1598.98 ± 1.20 a | 0.70 ± 0.17 | 0.76 ± 0.38 | |
| P90 N75 I | 1758.90 ± 118.94 a | 0.86 ± 0.16 | 0.76 ± 0.18 | |
| P90 N150 D | 1478.74 ± 52.86 a,b | 0.77 ± 0.15 | 0.75 ± 0.31 | |
| P90 N150 I | 2074.89 ± 144.55 | 0.87 ± 0.01 | 0.76 ± 0.65 | |
| Stage B | P0 N0 D | 2114.52 ± 206.76 | 0.83 ± 0.06 | 0.74 ± 0.35 |
| P0 N0 I | 2517.61 ± 158.69 | 0.79 ± 0.51 | 0.76 ± 0.66 | |
| P0 N75 D | 1827.67 ± 183.84 b | 0.64 ± 0.60 | 0.76 ± 0.01 | |
| P0 N75 I | 2441.43 ± 69.45 | 0.83 ± 0.04 | 0.74 ± 0.25 | |
| P0 N150 D | 1235.93 ± 37.86 b | 0.69 ± 0.42 | 0.76 ± 0.33 | |
| P0 N150 I | 1408.64 ± 37.68 | 0.91 ± 0.45 | 0.75 ± 0.19 | |
| P45 N0 D | 2176.90 ± 192.05 | 0.77 ± 0.03 | 0.76 ± 0.09 | |
| P45 N0 I | 2339.09 ± 490.98 | 0.86 ± 0.24 | 0.75 ± 0.17 | |
| P45 N75 D | 2514.91 ± 7.71 b | 0.77 ± 0.46 | 0.76 ± 0.22 | |
| P45 N75 I | 2480.07 ± 0.35 | 0.83 ± 0.14 | 0.75 ± 0.07 | |
| P45 N150 D | 2089.19 ± 25.88 b | 0.71 ± 0.38 | 0.75 ± 0.18 | |
| P45 N 150 I | 2535.18 ± 42.93 | 0.79 ± 0.29 | 0.76 ± 0.01 | |
| P90 N0 D | 2183.52 ± 104.90 b | 0.68 ± 0.17 | 0.75 ± 0.17 | |
| P90 N0 I | 2618.61 ± 10.21 | 0.86 ± 0.43 | 0.75 ± 0.22 | |
| P90 N75 D | 2283.75 ± 67.69 | 0.66 ± 0.24 | 0.75 ± 0.22 | |
| P90 N75 I | 2462.76 ± 234.60 | 0.91 ± 0.67 | 0.76 ± 0.21 | |
| P90 N150 D | 2335.18 ± 60.61 | 0.67 ± 0.05 | 0.75 ± 0.02 | |
| P90 N150 I | 2177.69 ± 191.41 | 0.91 ± 0.23 | 0.76 ± 0.48 |
| Harvesting Period | Fertilization | Biomass (kg ha−1) | Inflorescences (kg ha−1) | Inf/Bio |
|---|---|---|---|---|
| 2017 year | ||||
| Stage A | P0 N0 I | 4540.11 ± 346.75 a | 616.61 ± 34.16 a | 0.14 ± 0.01 a |
| P0 N150 I | 6476.78 ± 293.35 a | 840.66 ± 38.91 a | 0.13 ± 0.01 a | |
| P90 N0 I | 4932.39 ± 380.13 a | 616.09 ± 60.50 a | 0.12 ± 0.01 a | |
| P90 N150 I | 6717.79 ± 173.38 a | 862.28 ± 45.66 a | 0.13 ± 0.01 a | |
| Stage B | P0 N0 I | 5618.85 ± 258.66 | 967.38 ± 112.69 | 0.17 ± 0.01 |
| P0 N150 I | 8115.37 ± 133.85 | 1281.79 ± 89.10 | 0.16 ± 0.01 | |
| P90 N0 I | 5967.57 ± 288.33 | 1032.14 ± 79.09 | 0.17 ± 0.01 | |
| P90 N150 I | 8429.39 ± 478.14 | 1446.22 ± 99.63 | 0.17 ± 0.01 | |
| 2018 year | ||||
| Stage A | P0 N0 D | 1567.65 ± 161.07 b | 217.15 ± 27.87 a,b | 0.14 ± 0.01 a |
| P0 N0 I | 4839.72 ± 346.76 a | 679.69 ± 24.19 a | 0.14 ± 0.01 a | |
| P0 N75 D | 1245.49 ± 80.32 a,b | 170.37 ± 4.56 a,b | 0.14 ± 0.01 a | |
| P0 N75 I | 5636.54 ± 682.71 a | 776.25 ± 72.03 a | 0.14 ± 0.01 a | |
| P0 N150 D | 1061.00 ± 106.68 a,b | 143.97 ± 19.53 a,b | 0.14 ± 0.01 a | |
| P0 N 150 I | 6877.93 ± 221.06 a | 976.34 ± 56.00 a | 0.14 ± 0.01 a | |
| P45 N0 D | 1533.95 ± 49.27 a,b | 210.27 ± 18.05 a,b | 0.14 ± 0.01 a | |
| P45 N0 I | 5215.94 ± 579.36 a | 718.69 ± 100.28 a | 0.14 ± 0.01 a | |
| P45 N75 D | 1373.71 ± 131.17 b | 174.38 ± 29.37 a,b | 0.13 ± 0.01 a | |
| P45 N75 I | 5990.38 ± 199.68 a | 824.92 ± 25.75 a | 0.14 ± 0.01 a | |
| P45 N150 D | 1085.79 ± 12.56 b | 143.32 ± 17.78 a,b | 0.13 ± 0.01 a | |
| P45 N 150 I | 7181.05 ± 380.83 a | 993.08 ± 47.33 a | 0.14 ± 0.01 a | |
| P90 N0 D | 1534.55 ± 93.93 a,b | 209.62 ± 20.90 a,b | 0.14 ± 0.01 a | |
| P90 N0 I | 5356.79 ± 475.80 a | 700.44 ± 50.03 a | 0.13 ± 0.01 a | |
| P90 N75 D | 1292.04 ± 187.33 b | 177.43 ± 14.05 a,b | 0.14 ± 0.01 a | |
| P90 N75 I | 6046.85 ± 366.29 a | 807.35 ± 68.69 a | 0.13 ± 0.01 a | |
| P90 N150 D | 1154.10 ± 231.08 b | 170.97 ± 18.02 a,b | 0.15 ± 0.01 a | |
| P90 N150 I | 7125.98 ± 313.94 a | 984.88 ± 51.93 a | 0.14 ± 0.01 a | |
| Stage B | P0 N0 D | 1883.45 ± 154.60 b | 341.47 ± 37.53 b | 0.18 ± 0.01 |
| P0 N0 I | 6265.84 ± 422.05 | 1100.76 ± 41.47 | 0.18 ± 0.01 | |
| P0 N75 D | 1548.50 ± 88.45 b | 261.39 ± 31.92 b | 0.17 ± 0.01 | |
| P0 N75 I | 7213.44 ± 565.81 | 1284.05 ± 96.02 | 0.18 ± 0.0 | |
| P0 N150 D | 1363.78 ± 78.17 b | 226.66 ± 29.39 b | 0.17 ± 0.01 | |
| P0 N150 I | 8377.37 ± 223.66 | 1510.85 ± 85.60 | 0.18 ± 0.01 | |
| P45 N0 D | 1945.90 ± 142.73 b | 329.57 ± 50.37 b | 0.17 ± 0.01 | |
| P45 N0 I | 6489.49 ± 468.28 | 1180.99 ± 177.02 | 0.18 ± 0.01 | |
| P45 N75 D | 1558.84 ± 92.52 b | 285.11 ± 17.12 b | 0.18 ± 0.01 | |
| P45 N75 I | 7451.66 ± 364.56 | 1308.29 ± 90.31 | 0.18 ± 0.01 | |
| P45 N150 D | 1204.93 ± 144.89 b | 233.02 ± 24.49 b | 0.19 ± 0.01 | |
| P45 N 150 I | 8655.74 ± 514.37 | 1629.76 ± 102.02 | 0.19 ± 0.01 | |
| P90 N0 D | 1945.84 ± 168.74 b | 334.18 ± 54.75 b | 0.17 ± 0.01 | |
| P90 N0 I | 6528.28 ± 410.66 | 1215.83 ± 135.20 | 0.19 ± 0.01 | |
| P90 N75 D | 1536.66 ± 129.23 b | 274.72 ± 28.83 b | 0.18 ± 0.01 | |
| P90 N75 I | 7408.61 ± 105.87 | 1363.41 ± 60.00 | 0.18 ± 0.01 | |
| P90 N150 D | 1177.53 ± 109.00 b | 250.68 ± 31.20 b | 0.21 ± 0.01 b | |
| P90 N150 I | 8635.76 ± 396.31 | 1590.19 ± 97.10 | 0.18 ± 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spano, M.; Di Matteo, G.; Ingallina, C.; Sobolev, A.P.; Giusti, A.M.; Vinci, G.; Cammarone, S.; Tortora, C.; Lamelza, L.; Prencipe, S.A.; et al. Industrial Hemp (Cannabis sativa L.) Inflorescences as Novel Food: The Effect of Different Agronomical Practices on Chemical Profile. Foods 2022, 11, 3658. https://doi.org/10.3390/foods11223658
Spano M, Di Matteo G, Ingallina C, Sobolev AP, Giusti AM, Vinci G, Cammarone S, Tortora C, Lamelza L, Prencipe SA, et al. Industrial Hemp (Cannabis sativa L.) Inflorescences as Novel Food: The Effect of Different Agronomical Practices on Chemical Profile. Foods. 2022; 11(22):3658. https://doi.org/10.3390/foods11223658
Chicago/Turabian StyleSpano, Mattia, Giacomo Di Matteo, Cinzia Ingallina, Anatoly Petrovich Sobolev, Anna Maria Giusti, Giuliana Vinci, Silvia Cammarone, Carola Tortora, Lara Lamelza, Sabrina Antonia Prencipe, and et al. 2022. "Industrial Hemp (Cannabis sativa L.) Inflorescences as Novel Food: The Effect of Different Agronomical Practices on Chemical Profile" Foods 11, no. 22: 3658. https://doi.org/10.3390/foods11223658
APA StyleSpano, M., Di Matteo, G., Ingallina, C., Sobolev, A. P., Giusti, A. M., Vinci, G., Cammarone, S., Tortora, C., Lamelza, L., Prencipe, S. A., Gobbi, L., Botta, B., Marini, F., Campiglia, E., & Mannina, L. (2022). Industrial Hemp (Cannabis sativa L.) Inflorescences as Novel Food: The Effect of Different Agronomical Practices on Chemical Profile. Foods, 11(22), 3658. https://doi.org/10.3390/foods11223658