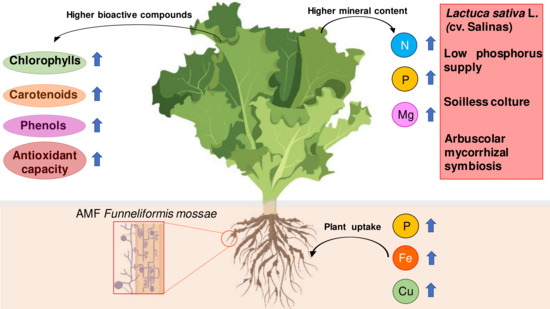
Arbuscular Mycorrhizal Fungi Increase Nutritional Quality of Soilless Grown Lettuce while Overcoming Low Phosphorus Supply
Abstract
1. Introduction
2. Materials and Methods
2.1. Biological Materials
2.2. Growing System and Nutrient Solution
2.3. Experimental Design and Plant Inoculation
2.4. Determinations
2.4.1. AMF Colonization and Growth Parameters
2.4.2. Leaf Content of Chlorophylls, Carotenoids, Total Phenols, and Antioxidant Capacity
2.4.3. Leaf and Root Mineral Content
2.4.4. Leaf Gas Exchanges
2.5. Statistical Analysis
3. Results
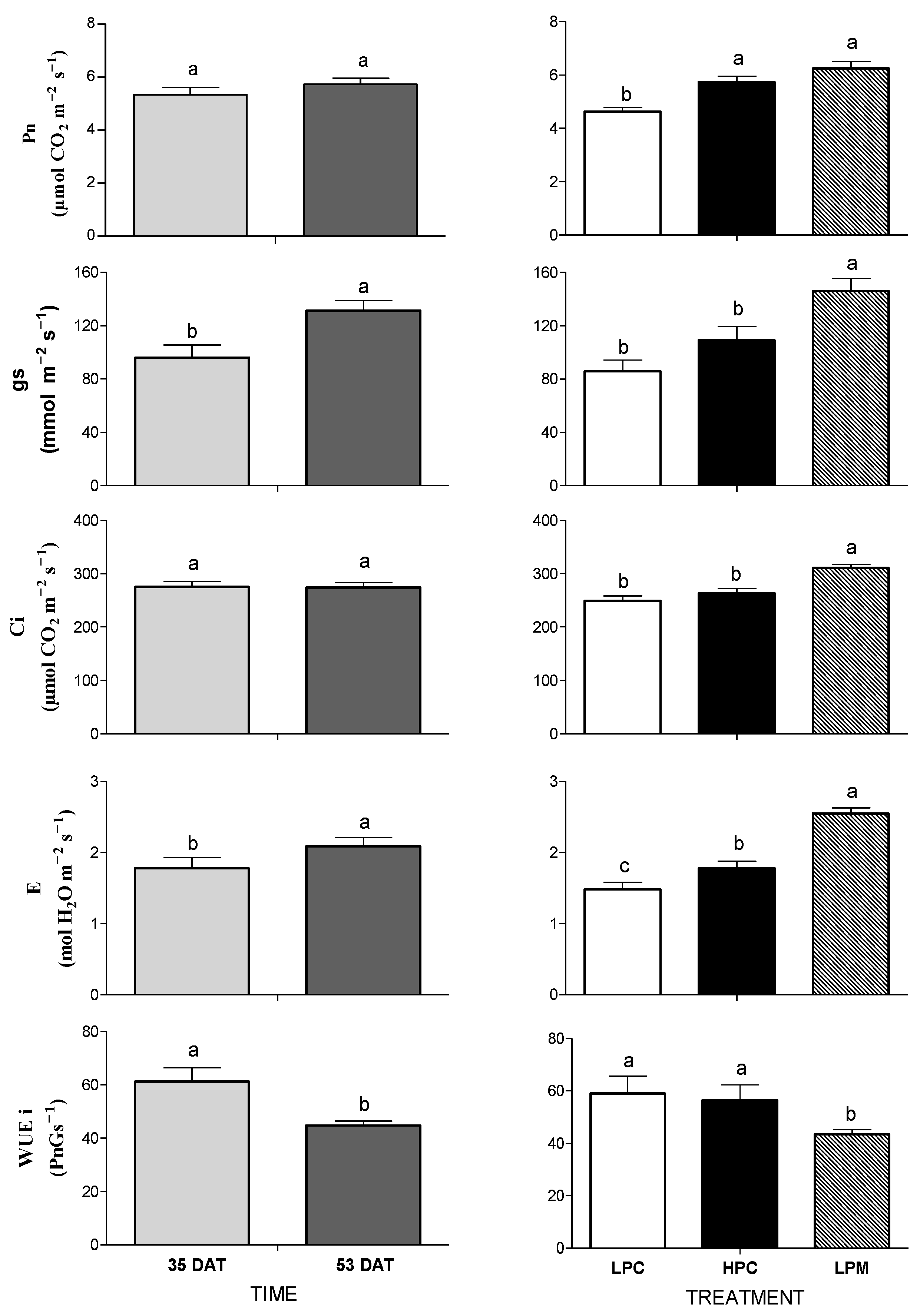
3.1. Mycorrhizal Colonization and Plant Growth, Mineral Content, and Leaf Gas Exchange
3.2. Content of Pigments, Total Phenols, Antioxidant Capacity, and Nitrates of Leaves
4. Discussion
4.1. Mycorrhizal Colonization and Plant Growth, Mineral Content, and Leaf Gas Exchange
4.2. Content of Pigments, Total Phenols, Antioxidant Capacity, and Nitrates of Leaves
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Medina-Lozano, I.; Bertolín, J.R.; Díaz, A. Nutritional Value of Commercial and Traditional Lettuce (Lactuca sativa L.) and Wild Relatives: Vitamin C and Anthocyanin Content. Food Chem. 2021, 359, 129864. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Gu, J.; Wu, H.; Rauf, A.; Emran, T.B.; Khan, Z.; Mitra, S.; Aljohani, A.S.M.; Alhumaydhi, F.A.; Al-Awthan, Y.S.; et al. Phytochemicals, Nutrition, Metabolism, Bioavailability, and Health Benefits in Lettuce—A Comprehensive Review. Antioxidants 2022, 11, 1158. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Moon, Y.; Tou, J.C.; Mou, B.; Waterland, N.L. Nutritional Value, Bioactive Compounds and Health Benefits of Lettuce (Lactuca sativa L.). J. Food Compos. Anal. 2016, 49, 19–34. [Google Scholar] [CrossRef]
- Balliu, A.; Zheng, Y.; Sallaku, G.; Fernández, J.A.; Gruda, N.S.; Tuzel, Y. Horticulturae Environmental and Cultivation Factors Affect the Morphology, Architecture and Performance of Root Systems in Soilless Grown Plants. Horticulturae 2021, 7, 243. [Google Scholar] [CrossRef]
- Savvas, D.; Gruda, N. Application of Soilless Culture Technologies in the Modern Greenhouse Industry—A Review. Eur. J. Hortic. Sci. 2018, 83, 280–293. [Google Scholar] [CrossRef]
- Sonneveld, C.; Voogt, W. Plant Nutrition in Future Greenhouse Production. In Plant Nutrition of Greenhouse Crops; Springer: Dordrecht, The Netherlands, 2009; pp. 393–403. [Google Scholar] [CrossRef]
- Han, Y.; White, P.J.; Cheng, L. Mechanisms for Improving Phosphorus Utilization Efficiency in Plants. Ann. Bot. 2022, 129, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Germano, R.P.; Melito, S.; Cacini, S.; Carmassi, G.; Leoni, F.; Maggini, R.; Montesano, F.F.; Pardossi, A.; Massa, D. Sweet Basil Can Be Grown Hydroponically at Low Phosphorus and High Sodium Chloride Concentration: Effect on Plant and Nutrient Solution Management. Sci. Hortic. 2022, 304, 111324. [Google Scholar] [CrossRef]
- El-Nakhel, C.; Pannico, A.; Kyriacou, M.C.; Giordano, M.; de Pascale, S.; Rouphael, Y. Macronutrient Deprivation Eustress Elicits Differential Secondary Metabolites in Red and Green-Pigmented Butterhead Lettuce Grown in a Closed Soilless System. J. Sci. Food Agric. 2019, 99, 6962–6972. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Chandra, D.; Son, J. Growth, Physicochemical, Nutritional, and Postharvest Qualities of Leaf Lettuce (Lactuca sativa L.) as Affected by Cultivar and Amount of Applied Nutrient Solution. Horticulturae 2022, 8, 436. [Google Scholar] [CrossRef]
- Ferrol, N.; Azcón-Aguilar, C.; Pérez-Tienda, J. Review: Arbuscular Mycorrhizas as Key Players in Sustainable Plant Phosphorus Acquisition: An Overview on the Mechanisms Involved. Plant Sci. 2019, 280, 441–447. [Google Scholar] [CrossRef]
- Wipf, D.; Krajinski, F.; van Tuinen, D.; Recorbet, G.; Courty, P.E. Trading on the Arbuscular Mycorrhiza Market: From Arbuscules to Common Mycorrhizal Networks. New Phytol. 2019, 223, 1127–1142. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.E.; Smith, F.A.; Jakobsen, I. Functional Diversity in Arbuscular Mycorrhizal (AM) Symbioses: The Contribution of the Mycorrhizal P Uptake Pathway Is Not Correlated with Mycorrhizal Responses in Growth or Total P Uptake. New Phytol. 2004, 162, 511–524. [Google Scholar] [CrossRef]
- Abdel-Salam, E.; Alatar, A.; El-Sheikh, M.A. Inoculation with Arbuscular Mycorrhizal Fungi Alleviates Harmful Effects of Drought Stress on Damask Rose. Saudi J. Biol. Sci. 2018, 25, 1772–1780. [Google Scholar] [CrossRef] [PubMed]
- Ahanger, M.A.; Wani, M.R.; Ahmad, P. Drought Tolerance: Role of Organic Osmolytes, Growth Regulators, and Mineral Nutrients. In Physiological Mechanisms and Adaptation Strategies in Plants Under Changing Environment: Volume 1; Springer: Berlin/Heidelberg, Germany, 2014; pp. 25–55. [Google Scholar] [CrossRef]
- Rodriguez, R.J.; Henson, J.; van Volkenburgh, E.; Hoy, M.; Wright, L.; Beckwith, F.; Kim, Y.O.; Redman, R.S. Stress Tolerance in Plants via Habitat-Adapted Symbiosis. ISME J. 2008, 2, 404–416. [Google Scholar] [CrossRef] [PubMed]
- Tigka, T.; Ipsilantis, I. Effects of Sand Dune, Desert and Field Arbuscular Mycorrhizae on Lettuce (Lactuca sativa L.) Growth in a Natural Saline Soil. Sci. Hortic. 2020, 264, 109191. [Google Scholar] [CrossRef]
- Birhane, E.; Sterck, F.J.; Fetene, M.; Bongers, F.; Kuyper, T.W. Arbuscular Mycorrhizal Fungi Enhance Photosynthesis, Water Use Efficiency, and Growth of Frankincense Seedlings under Pulsed Water Availability Conditions. Oecologia 2012, 169, 895. [Google Scholar] [CrossRef]
- Begum, N.; Qin, C.; Ahanger, M.A.; Raza, S.; Khan, M.I.; Ashraf, M.; Ahmed, N.; Zhang, L. Role of Arbuscular Mycorrhizal Fungi in Plant Growth Regulation: Implications in Abiotic Stress Tolerance. Front. Plant Sci. 2019, 10, 1068. [Google Scholar] [CrossRef]
- Avio, L.; Turrini, A.; Giovannetti, M.; Sbrana, C. Designing the Ideotype Mycorrhizal Symbionts for the Production of Healthy Food. Front. Plant Sci. 2018, 9, 1089. [Google Scholar] [CrossRef]
- Baslam, M.; Garmendia, I.; Goicoechea, N. Arbuscular Mycorrhizal Fungi (AMF) Improved Growth and Nutritional Quality of Greenhouse-Grown Lettuce. J. Agric. Food Chem. 2011, 59, 5504–5515. [Google Scholar] [CrossRef]
- Baslam, M.; Garmendia, I.; Goicoechea, N. The Arbuscular Mycorrhizal Symbiosis Can Overcome Reductions in Yield and Nutritional Quality in Greenhouse-Lettuces Cultivated at Inappropriate Growing Seasons. Sci. Hortic. 2013, 164, 145–154. [Google Scholar] [CrossRef]
- Aini, N.; Yamika, W.S.D.; Ulum, B. Effect of Nutrient Concentration, PGPR and AMF on Plant Growth, Yield and Nutrient Uptake of Hydroponic Lettuce. Int. J. Agric. Biol. 2019, 21, 175–183. [Google Scholar] [CrossRef]
- Kowalska, I.; Konieczny, A.; Gąstoł, M. Effect of Mycorrhiza and the Phosphorus Content in a Nutrient Solution on the Yield and Nutritional Status of Lettuce Grown on Various Substrates. J. Elem. 2015, 20, 631–642. [Google Scholar] [CrossRef]
- Jackson, L.E.; Miller, D.; Smith, S.E. Arbuscular Mycorrhizal Colonization and Growth of Wild and Cultivated Lettuce in Response to Nitrogen and Phosphorus. Sci. Hortic. 2002, 94, 205–218. [Google Scholar] [CrossRef]
- Njeru, E.M.; Bocci, G.; Avio, L.; Sbrana, C.; Turrini, A.; Giovannetti, M.; Bàrberi, P. Functional Identity Has a Stronger Effect than Diversity on Mycorrhizal Symbiosis and Productivity of Field Grown Organic Tomato. Eur. J. Agron. 2017, 86, 1–11. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. Growing Plants without Soil by the Water-Culture Method. Calif. Agric. Exp. Stn. Circular 1938, 347, 32. [Google Scholar]
- Senizza, B.; Zhang, L.; Miras-Moreno, B.; Righetti, L.; Zengin, G.; Ak, G.; Bruni, R.; Lucini, L.; Sifola, M.I.; El-Nakhel, C.; et al. The Strength of the Nutrient Solution Modulates the Functional Profile of Hydroponically Grown Lettuce in a Genotype-Dependent Manner. Foods 2020, 9, 1156. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Levkovitch, I.; Soriano, S.; Wallach, R.; Silber, A. Integrated Effect of Irrigation Frequency and Phosphorus Level on Lettuce: P Uptake, Root Growth and Yield. Plant Soil 2004, 263, 297–309. [Google Scholar] [CrossRef]
- Luo, H.Y.; Lee, S.K.; He, J. Integrated Effects of Root-Zone Temperatures and Phosphorus Levels on Aeroponically-Grown Lettuce (Lactuca sativa L.) in the Tropics. Open Hortic. J. 2009, 2, 6–12. [Google Scholar] [CrossRef]
- Porter, A.S.; Evans-Fitz Gerald, C.; McElwain, J.C.; Yiotis, C.; Elliott-Kingston, C. How Well Do You Know Your Growth Chambers? Testing for Chamber Effect Using Plant Traits. Plant Methods 2015, 11, 1–10. [Google Scholar] [CrossRef]
- Giovannetti, M.; Mosse, B. An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. N. Phytol. 1980, 84, 489–500. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and Carotenoids: Measurement and Characterization by UV-VIS Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Kang, H.-M.; Saltveit, M.E. Antioxidant Capacity of Lettuce Leaf Tissue Increases after Wounding. J. Agric. Food Chem. 2002, 50, 7536–7541. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Page, A.L. Methods of Soil Analysis-Part 2: Chemical and Microbiological Properties, 2nd ed.; American Society of Agronomy Inc. Publisher: Madison, WI, USA, 1982; Volume 9. [Google Scholar]
- Kacar B Chemical Analysis of Plant and Soil: II. Plant Analysis. Available online: https://scholar.google.com/scholar_lookup?title=Chemical%20Analysis%20of%20Plant%20and%20Soil.%20II.%20Plant%20Analysis&publication_year=1972&author=B.%20Kacar (accessed on 26 January 2022).
- Cataldo, D.A.; Haroon, M.H.; Schrader, L.E.; Youngs, V.L. Rapid Colorimetric Determination of Nitrate in Plant Tissue by Nitration of Salicylic Acid. Commun. Soil Sci. Plant Anal. 1975, 6, 71–80. [Google Scholar] [CrossRef]
- Kowalska, I.; Konieczny, A. Effect of Mycorrhiza on Yield and Quality of Lettuce Grown on Medium with Different Levels of Phosphorus and Selenium. Agric. Food Sci. 2019, 28, 84–92-84–92. [Google Scholar] [CrossRef]
- Avio, L.; Sbrana, C.; Giovannetti, M.; Frassinetti, S. Arbuscular Mycorrhizal Fungi Affect Total Phenolics Content and Antioxidant Activity in Leaves of Oak Leaf Lettuce Varieties. Sci. Hortic. 2017, 224, 265–271. [Google Scholar] [CrossRef]
- Santander, C.; Ruiz, A.; García, S.; Aroca, R.; Cumming, J.; Cornejo, P. Efficiency of Two Arbuscular Mycorrhizal Fungal Inocula to Improve Saline Stress Tolerance in Lettuce Plants by Changes of Antioxidant Defense Mechanisms. J. Sci. Food Agric. 2020, 100, 1577–1587. [Google Scholar] [CrossRef]
- Gavito, M.E.; Azcón-Aguilar, C. Temperature Stress in Arbuscular Mycorrhizal Fungi: A Test for Adaptation to Soil Temperature in Three Isolates of Funneliformis Mosseae from Different Climates. Agric. Food Sci. 2012, 21, 2–11. [Google Scholar] [CrossRef]
- Ganugi, P.; Masoni, A.; Sbrana, C.; Dell’acqua, M.; Pietramellara, G.; Benedettelli, S.; Avio, L. Genetic Variability Assessment of 127 Triticum Turgidum L. Accessions for Mycorrhizal Susceptibility-Related Traits Detection. Sci. Rep. 2021, 11, 13426. [Google Scholar] [CrossRef]
- Pawlowski, M.L.; Vuong, T.D.; Valliyodan, B.; Nguyen, H.T.; Hartman, G.L. Whole-Genome Resequencing Identifies Quantitative Trait Loci Associated with Mycorrhizal Colonization of Soybean. Theor. Appl. Genet. 2020, 133, 409–417. [Google Scholar] [CrossRef]
- Amijee, F.; Tinker, P.B.; Stribley, D.P. The Development of Endomycorrhizal Root Systems. New Phytol. 1989, 111, 435–446. [Google Scholar] [CrossRef]
- Chiu, C.H.; Paszkowski, U. Mechanisms and Impact of Symbiotic Phosphate Acquisition. Cold Spring Harb. Perspect. Biol. 2019, 11, a034603. [Google Scholar] [CrossRef] [PubMed]
- Nouri, E.; Breuillin-Sessoms, F.; Feller, U.; Reinhardt, D.; Dutilh, B.E. Phosphorus and Nitrogen Regulate Arbuscular Mycorrhizal Symbiosis in Petunia Hybrida. PLoS ONE 2014, 9, e90841. [Google Scholar] [CrossRef] [PubMed]
- Sah, S.; Reed, S.; Jayachandran, K.; Dunn, C.; Fisher, J.B. The Effect of Repeated Short-Term Flooding on Mycorrhizal Survival in Snap Bean Roots. HortScience 2006, 41, 598–602. [Google Scholar] [CrossRef]
- Tajini, F.; Suriyakup, P.; Vailhe, H.; Jansa, J.; Drevon, J.J. Assess Suitability of Hydroaeroponic Culture to Establish Tripartite Symbiosis between Different AMF Species, Beans, and Rhizobia. BMC Plant Biol. 2009, 9, 73. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; George, E. Development of a Nutrient Film Technique Culture System for Arbuscular Mycorrhizal Plants. HortScience 2005, 40, 378–380. [Google Scholar] [CrossRef]
- Hochmuth, G.; Maynard, D.; Vavrina, C.; Hanlon, E.; Simonne, E. Plant Tissue Analysis and Interpretation for Vegetable Crops in Florida. Fla. Coop. Ext. Serv. Spec. Ser. SS-VEC-42 1991. [Google Scholar]
- Sallaku, G.; Sandén, H.; Babaj, I.; Kaciu, S.; Balliu, A.; Rewald, B. Specific Nutrient Absorption Rates of Transplanted Cucumber Seedlings Are Highly Related to RGR and Influenced by Grafting Method, AMF Inoculation and Salinity. Sci. Hortic. 2019, 243, 177–188. [Google Scholar] [CrossRef]
- Balliu, A.; Sallaku, G.; Rewald, B. AMF Inoculation Enhances Growth and Improves the Nutrient Uptake Rates of Transplanted, Salt-Stressed Tomato Seedlings. Sustainability 2015, 7, 15967–15981. [Google Scholar] [CrossRef]
- Aroca, R.; Ruiz-Lozano, J.M.; Zamarreño, Á.M.; Paz, J.A.; García-Mina, J.M.; Pozo, M.J.; López-Ráez, J.A. Arbuscular Mycorrhizal Symbiosis Influences Strigolactone Production under Salinity and Alleviates Salt Stress in Lettuce Plants. J. Plant Physiol. 2013, 170, 47–55. [Google Scholar] [CrossRef]
- Vicente-Sánchez, J.; Nicolás, E.; Pedrero, F.; Alarcón, J.J.; Maestre-Valero, J.F.; Fernández, F. Arbuscular Mycorrhizal Symbiosis Alleviates Detrimental Effects of Saline Reclaimed Water in Lettuce Plants. Mycorrhiza 2014, 24, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Saia, S.; Colla, G.; Raimondi, G.; di Stasio, E.; Cardarelli, M.; Bonini, P.; Vitaglione, P.; de Pascale, S.; Rouphael, Y. An Endophytic Fungi-Based Biostimulant Modulated Lettuce Yield, Physiological and Functional Quality Responses to Both Moderate and Severe Water Limitation. Sci. Hortic. 2019, 256, 108595. [Google Scholar] [CrossRef]
- Chen, S.; Zhao, H.; Zou, C.; Li, Y.; Chen, Y.; Wang, Z.; Jiang, Y.; Liu, A.; Zhao, P.; Wang, M.; et al. Combined Inoculation with Multiple Arbuscular Mycorrhizal Fungi Improves Growth, Nutrient Uptake and Photosynthesis in Cucumber Seedlings. Front. Microbiol. 2017, 8, 2516. [Google Scholar] [CrossRef]
- Ruiz-Lozano, J.M.; Aroca, R. Host Response to Osmotic Stresses: Stomatal Behaviour and Water Use Efficiency of Arbuscular Mycorrhizal Plants. In Arbuscular Mycorrhizas: Physiology and Function; Springer: Berlin/Heidelberg, Germany, 2010; pp. 239–256. [Google Scholar] [CrossRef]
- Eroğlu, G.; Cabral, C.; Ravnskov, S.; Topbjerg, H.B.; Wollenweber, B. Arbuscular Mycorrhiza Influences Carbon-Use Efficiency and Grain Yield of Wheat Grown under Pre-and Post-Anthesis Salinity Stress. Plant Biol. 2020, 22, 863–871. [Google Scholar] [CrossRef] [PubMed]
- Augé, R.M. Water Relations, Drought and Vesicular-Arbuscular Mycorrhizal Symbiosis. Mycorrhiza 2001, 11, 3–42. [Google Scholar] [CrossRef]
- Bowles, T.M.; Jackson, L.E.; Cavagnaro, T.R. Mycorrhizal Fungi Enhance Plant Nutrient Acquisition and Modulate Nitrogen Loss with Variable Water Regimes. Glob. Chang. Biol. 2018, 24, e171–e182. [Google Scholar] [CrossRef]
- Nurbaity, A.; Istifadah, N.; Haryantini, B.A.; Ilhami, M.F.; Habibullah, M.I.; Arifin, M. Optimization of Hydroponic Technology for Production of Mycorrhiza Biofertilizer. IOP Conf. Ser. Earth Environ. Sci. 2019, 347, 012017. [Google Scholar] [CrossRef]
- Shtark, O.; Puzanskiy, R.; Avdeeva, G.; Yemelyanov, V.; Shavarda, A.; Romanyuk, D.; Kliukova, M.; Kirpichnikova, A.; Tikhonovich, I.; Zhukov, V.; et al. Metabolic Alterations in Pisum Sativum Roots during Plant Growth and Arbuscular Mycorrhiza Development. Plants 2021, 10, 1033. [Google Scholar] [CrossRef]
- Pepe, A.; di Baccio, D.; Magnani, E.; Giovannetti, M.; Sbrana, C. Zinc and Iron Biofortification and Accumulation of Health-Promoting Compounds in Mycorrhizal Cichorium intybus L. J. Soil Sci. Plant Nutr. 2022. [Google Scholar] [CrossRef]
- Hooks, T.; Masabni, J.; Sun, L.; Niu, G.; Hooks, T.; Masabni, J.; Sun, L.; Niu, G. Effects of Organic Fertilizer with or without a Microbial Inoculant on the Growth and Quality of Lettuce in an NFT Hydroponic System. Technol. Hortic. 2022, 2, 1–8. [Google Scholar] [CrossRef]
- Colla, G.; Kim, H.J.; Kyriacou, M.C.; Rouphael, Y. Nitrate in Fruits and Vegetables. Sci. Hortic. 2018, 237, 221–238. [Google Scholar] [CrossRef]
- European Commission (EU). Commission Regulation (EC) No 1258/2011 of 2 December 2011 Amending Regulation (EC) No 1881/2006 as Regards Maximum Levels for Nitrates in Foodstuffs Setting. Off. J. Eur. Union 2011, 320, 15–17. [Google Scholar]
- Buwalda, F.; Warmenhoven, M. Growth-Limiting Phosphate Nutrition Suppresses Nitrate Accumulation in Greenhouse Lettuce. J. Exp. Bot. 1999, 50, 813–821. [Google Scholar] [CrossRef][Green Version]
- Guffanti, D.; Cocetta, G.; Franchetti, B.M.; Ferrante, A. The Effect of Flushing on the Nitrate Content and Postharvest Quality of Lettuce (Lactuca sativa L. Var. Acephala) and Rocket (Eruca sativa Mill.) Grown in a Vertical Farm. Horticulturae 2022, 8, 604. [Google Scholar] [CrossRef]
- Reyes-Chin-Wo, S.; Wang, Z.; Yang, X.; Kozik, A.; Arikit, S.; Song, C.; Xia, L.; Froenicke, L.; Lavelle, D.O.; Truco, M.-J.; et al. ARTICLE Genome Assembly with in Vitro Proximity Ligation Data and Whole-Genome Triplication in Lettuce. Nat. Commun. 2017, 8, 14953. [Google Scholar] [CrossRef] [PubMed]

| Sampling Time (DAT) | Treatment | Leaf FW (g Plant−1) | Leaf DW (g Plant−1) | Root DW (g Plant−1) | Root/Shoot DW Ratio | Leaf Area (cm2 Plant−1) |
|---|---|---|---|---|---|---|
| 35 | LPC | 66.6 ± 6.9 | 2.94 ± 0.36 | 0.48 ± 0.17 | 0.17 ± 0.02 | 719.1 ± 80.9 |
| 35 | HPC | 104.8 ± 3.5 | 4.93 ± 0.39 | 0. 65 ± 0.07 | 0.13 ± 0.01 | 1233.3 ± 142.6 |
| 35 | LPM | 155.8 ± 14.4 | 7.32 ± 0.58 | 0.88 ± 0.05 | 0.12 ± 0.01 | 1674.5 ± 172.7 |
| 53 | LPC | 135.6 ± 10.3 | 5.46 ± 0.36 | 0. 93 ± 0.07 | 0.17 ± 0.01 | 1524.9 ± 120.4 |
| 53 | HPC | 221.4 ± 38.7 | 9.34 ± 1.28 | 1.00 ± 0.05 | 0.11 ± 0.01 | 2471.0 ± 418.6 |
| 53 | LPM | 263.9 ± 29.1 | 10.86 ± 0.90 | 1.79 ± 0.24 | 0.16 ± 0.01 | 2968.7 ± 328.7 |
| MAIN EFFECTS | ||||||
| 35 | 109.1 ± 12.1 b | 5.06 ± 0.59 b | 0.67 ± 0.06 b | 0.14 ± 0.01 a | 1208.9 ± 137.9 b | |
| 53 | 206.9 ± 21.9 a | 8.55 ± 0.84 a | 1.24 ± 0.14 a | 0.15 ± 0.01 a | 2321.6 ± 244.3 a | |
| LPC | 101.1 ± 14.2 b | 4.20 ± 0.53 b | 0.71 ± 0.09 b | 0.17 ± 0.01 a | 1122.0 ± 166.5 b | |
| HPC | 163.1 ± 28.4 a | 7.13 ± 1.04 a | 0.82 ± 0.08 b | 0.12 ± 0.01 b | 1852.1 ± 310.8 ab | |
| LPM | 209.9 ± 25.4 a | 9.09 ± 0.83 a | 1.33 ± 0.21 a | 0.14 ± 0.01 ab | 2321.6 ± 298.9 a | |
| ANOVA (ns, not significant; **, significant at 1%; ***, significant at 0.1%) | ||||||
| Time | *** | *** | *** | ns | *** | |
| Treatment | *** | *** | *** | ** | *** | |
| Interaction | ns | ns | ns | ns | ns | |
| Treatment | N | P | K | Na | Ca | Mg | Fe | Cu | Mn | Zn |
|---|---|---|---|---|---|---|---|---|---|---|
| (g kg−1 DW) | (mg kg−1 DW) | |||||||||
| LPC | 35.7 ± 3.0 b | 3.2 ± 0.2 b | 79.7 ± 5.9 a | 4.8 ± 0.8 a | 16.3 ± 1.4 a | 3.1 ± 0.3 b | 119.6 ± 19.7 a | 10.7 ± 0.6 a | 111.5 ± 10.2 a | 48.3 ± 2.2 a |
| HPC | 41.5 ± 0.8 a | 6.5 ± 0.3 a | 70.5 ± 4.7 a | 6.0 ± 0.9 a | 15.2 ± 1.2 a | 2.4 ± 0.3 b | 174.5 ± 58.0 a | 9.1 ± 0.5 a | 111.9 ± 4.9 a | 49.1 ± 2.1 a |
| LPM | 42.7 ± 0.5 a | 6.4 ± 0.1 a | 71.2 ± 6.0 a | 4.9 ± 0.4 a | 16.9 ± 2.0 a | 4.2 ± 0.2 a | 127.3 ± 7.8 a | 10.7 ± 0.5 a | 129.6 ± 11.8 a | 76.5 ± 28.0 a |
| ANOVA (ns, not significant; **, significant at 1%; ***, significant at 0.1%) | ||||||||||
| Treatment | ** | *** | ns | ns | ns | ** | ns | ns | ns | ns |
| Treatment | N | P | K | Na | Ca | Mg | Fe | Cu | Mn | Zn |
|---|---|---|---|---|---|---|---|---|---|---|
| (g kg−1 DW) | (mg kg−1 DW) | |||||||||
| LPC | 19.3 ± 5.0 a | 3.3 ± 0.2 b | 27.9 ± 2.0 a | 8.4 ± 2.3 a | 12.3 ± 1.0 a | 12.9 ± 0.6 a | 1616.2 ± 199.2 b | 12.5 ± 1.2 a | 267.9 ± 33.7 a | 77.6 ± 6.9 a |
| HPC | 17.7 ± 1.6 a | 3.3 ± 0.4 b | 26.1 ± 1.7 a | 11.7 ± 1.5 a | 10.9 ± 0.9 a | 15.0 ± 2.9 a | 1276.4 ± 173.8 b | 7.7 ± 1.1 b | 247.3 ± 29.2 a | 56.5 ± 4.5 a |
| LPM | 22.9 ± 2.7 a | 4.5 ± 0.3 a | 32.2 ± 2.9 a | 13.4 ± 1.5 a | 12.9 ± 1.3 a | 16.3 ± 1.2 a | 2993.5 ± 554.5 a | 13.6 ± 1.4 a | 301.7 ± 32.6 a | 70.8 ± 4.3 a |
| ANOVA (ns, not significant; *, significant at 5%) | ||||||||||
| Treatment | ns | * | ns | ns | ns | ns | * | * | ns | ns |
| Sampling Time (DAT) | Treatment | Chlorophylls (μg g−1 FW) | Carotenoids (μg g−1 FW) | Total Phenols (mg GAE g−1 FW) | FRAP (μmol Fe(II) g−1 FW) |
|---|---|---|---|---|---|
| 35 | LPC | 627.25 ± 14.60 | 95.14 ± 5.58 | 2.82 ± 0.23 | 15.95 ± 0.96 |
| 35 | HPC | 608.80 ± 38.77 | 95.18 ± 7.80 | 2.65 ± 0.19 | 16.07 ± 1.25 |
| 35 | LPM | 845.55 ± 40.26 | 131.32 ± 2.89 | 3.49 ± 0.09 | 21.32 ± 1.51 |
| 53 | LPC | 593.57 ± 46.04 | 91.95 ± 4.81 | 2.16 ± 0.08 | 16.90 ± 0.69 |
| 53 | HPC | 663.04 ± 24.06 | 93.68 ± 4.37 | 2.08 ± 0.26 | 14.15 ± 1.55 |
| 53 | LPM | 888.93 ± 49.48 | 126.00 ± 6.59 | 2.98 ± 0.17 | 20.97 ± 1.20 |
| MAIN EFFECT | |||||
| 35 | 693.87 ± 36.80 a | 107.21 ± 5.96 a | 2.99 ± 0.14 a | 17.78 ± 1.00 a | |
| 53 | 715.18 ± 43.74 a | 103.88 ± 5.48 a | 2.41 ± 0.14 b | 17.34 ± 1.05 a | |
| LPC | 610.41 ± 23.25 b | 93.55 ± 3.46 b | 2.49 ± 0.17 b | 16.42 ± 0.58 b | |
| HPC | 635.92 ± 23.48 b | 94.43 ± 4.15 b | 2.36 ± 0.15 b | 15.11 ± 0.99 b | |
| LPM | 867.24 ± 30.65 a | 128.66 ± 3.48 a | 3.24 ± 0.13 a | 21.14 ± 0.89 a | |
| ANOVA (ns, not significant; **, significant at 1%; ***, significant at 0.1%) | |||||
| Time | ns | ns | *** | ns | |
| Treatment | *** | *** | *** | *** | |
| Interaction | ns | ns | ns | ns | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cela, F.; Avio, L.; Giordani, T.; Vangelisti, A.; Cavallini, A.; Turrini, A.; Sbrana, C.; Pardossi, A.; Incrocci, L. Arbuscular Mycorrhizal Fungi Increase Nutritional Quality of Soilless Grown Lettuce while Overcoming Low Phosphorus Supply. Foods 2022, 11, 3612. https://doi.org/10.3390/foods11223612
Cela F, Avio L, Giordani T, Vangelisti A, Cavallini A, Turrini A, Sbrana C, Pardossi A, Incrocci L. Arbuscular Mycorrhizal Fungi Increase Nutritional Quality of Soilless Grown Lettuce while Overcoming Low Phosphorus Supply. Foods. 2022; 11(22):3612. https://doi.org/10.3390/foods11223612
Chicago/Turabian StyleCela, Fatjon, Luciano Avio, Tommaso Giordani, Alberto Vangelisti, Andrea Cavallini, Alessandra Turrini, Cristiana Sbrana, Alberto Pardossi, and Luca Incrocci. 2022. "Arbuscular Mycorrhizal Fungi Increase Nutritional Quality of Soilless Grown Lettuce while Overcoming Low Phosphorus Supply" Foods 11, no. 22: 3612. https://doi.org/10.3390/foods11223612
APA StyleCela, F., Avio, L., Giordani, T., Vangelisti, A., Cavallini, A., Turrini, A., Sbrana, C., Pardossi, A., & Incrocci, L. (2022). Arbuscular Mycorrhizal Fungi Increase Nutritional Quality of Soilless Grown Lettuce while Overcoming Low Phosphorus Supply. Foods, 11(22), 3612. https://doi.org/10.3390/foods11223612