The Impact of Different Withering Approaches on the Metabolism of Flavor Compounds in Oolong Tea Leaves
Abstract
1. Introduction
2. Materials and Methods
2.1. Tea Leaf Processing
2.2. Widely-Targeted Metabolomic Analysis on Non-Volatile Metabolites in Tea Samples
2.3. Volatile Compound Analysis in Tea Samples
2.4. Identification and Quantification of Proteins
2.5. The Electronic Tongue (E-Tongue) Analysis on the Tea Samples
2.6. Data Analysis
3. Results and Discussion
3.1. Changes in the Non-Volatile Metabolites during Withering Process
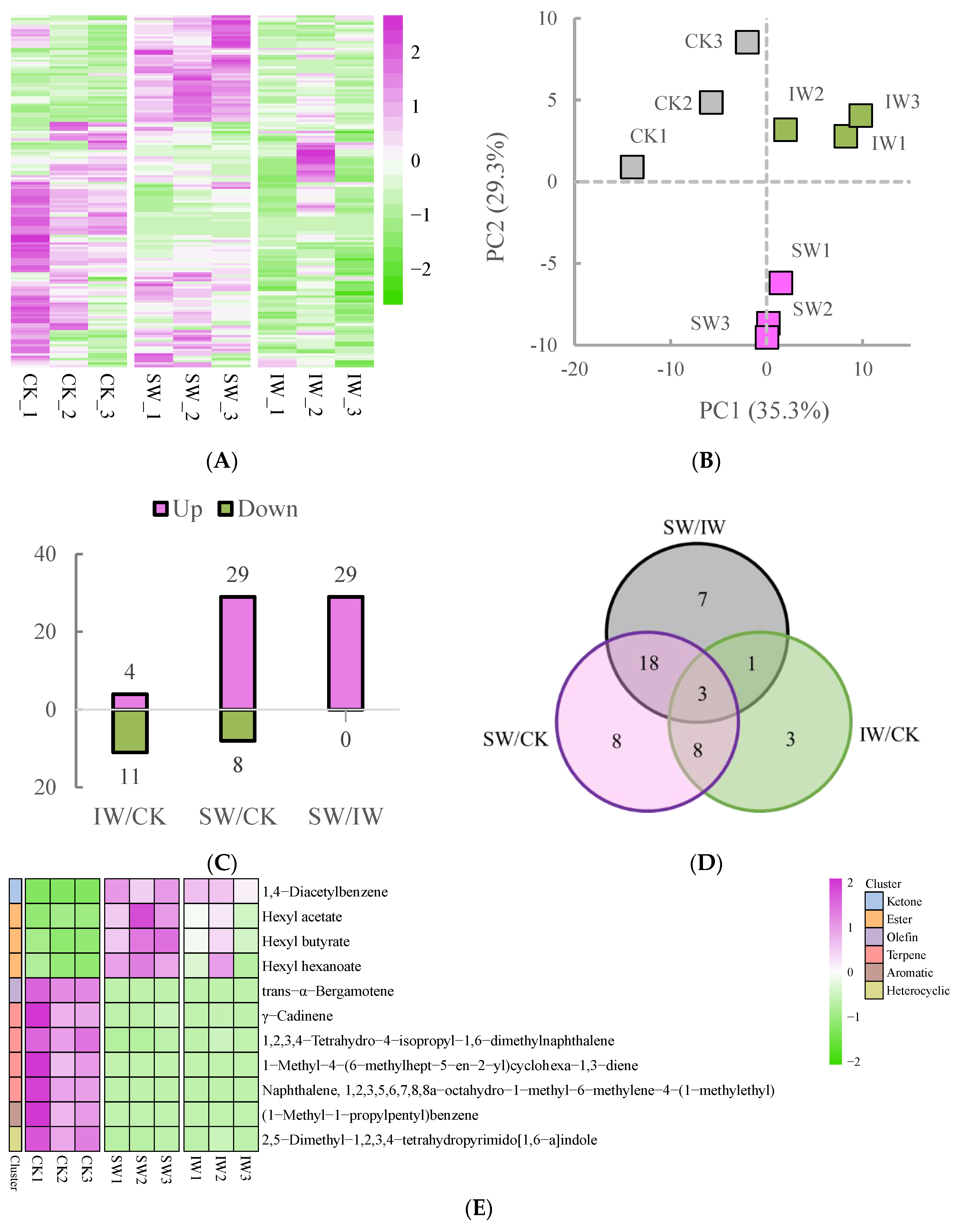
3.2. Changes in the Volatile Metabolites during Withering Process
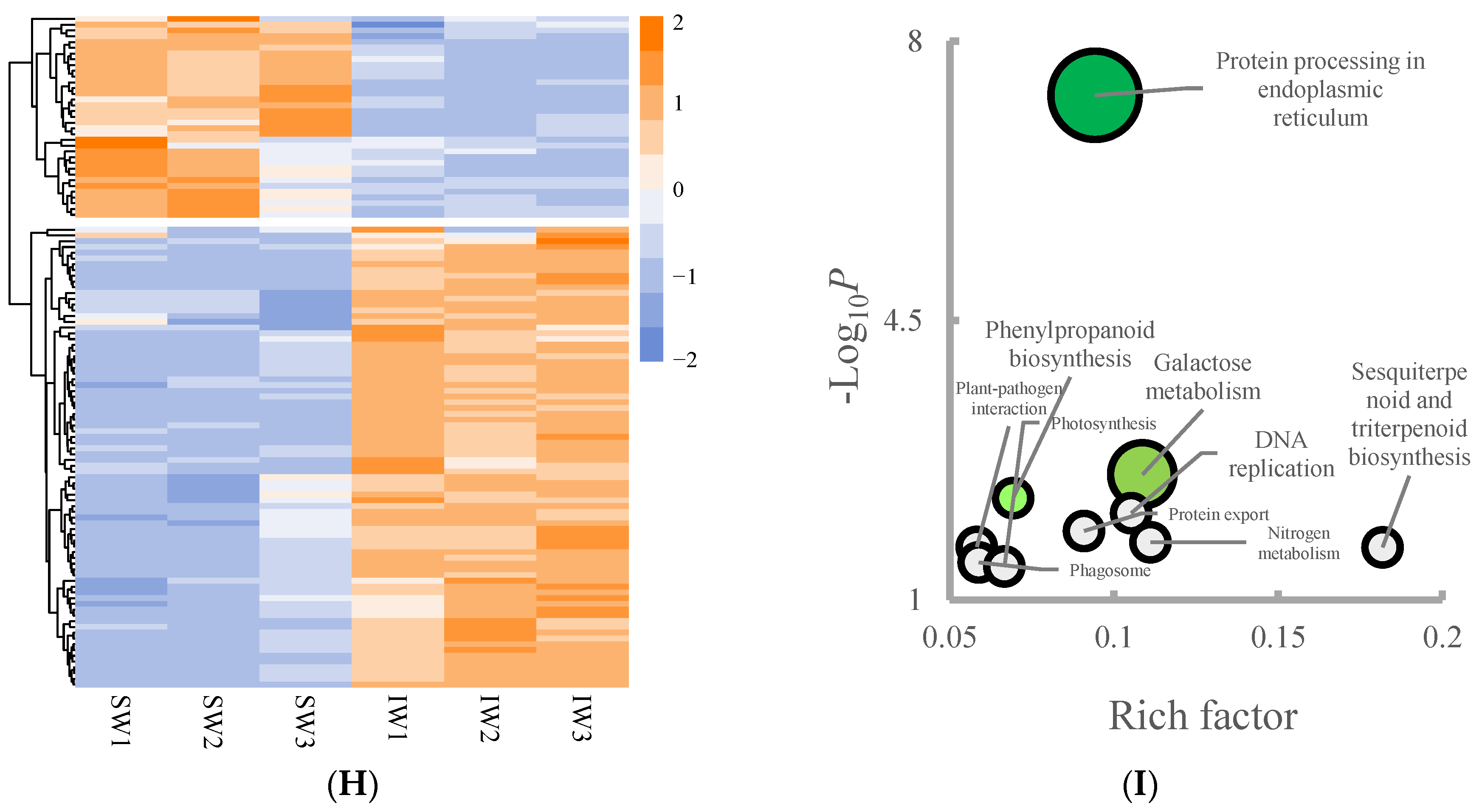
3.3. Changes in the Proteins during Withering Process
3.4. The Metabolism Cascades of Flavor Constitutes in Different-Treated Tea Leaves
3.5. The Influence of Dynamic Changes in Metabolites on the Flavor of Withered Leaves
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mei, Y.; Liang, X. Analysis of China’s Tea Production and Domestic Sales in 2021. China Tea 2022, 44, 17–22. [Google Scholar]
- Huang, X.; Cao, H.; Guo, Y.; Liu, J.; Sun, Y.; Liu, S.; Lin, J.; Wei, S.; Wu, L. The dynamic change of oolong tea constitutes during enzymatic-catalysed process of manufacturing. Int. J. Food Sci. Technol. 2020, 55, 3604–3612. [Google Scholar] [CrossRef]
- Wan, X.; Xia, T. Secondary Metabolism of Tea Plant; China Science Publishing & Media Ltd.: Beijing, China, 2015. [Google Scholar]
- Xia, T. Manufacture of Tea; China Agriculture Press: Beijing, China, 2016. [Google Scholar]
- Li, D.; Li, C.-Y.; Hu, C.-J.; Yang, Y.-S.; Lin, C.; Zhao, D.; Li, Q.-S.; Ye, J.-H.; Zheng, X.-Q.; Liang, Y.-R.; et al. Study on the Accumulation Mechanism of Amino Acids during Bruising and Withering Treatment of Oolong Tea. J. Agric. Food. Chem. 2020, 68, 14071–14080. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.T.; Watanabe, N.; Yang, Z.Y. Understanding the biosyntheses and stress response mechanisms of aroma compounds in tea (Camellia sinensis) to safely and effectively improve tea aroma. Crit. Rev. Food Sci. Nutr. 2019, 59, 2321–2334. [Google Scholar] [CrossRef]
- Ai, Z.; Zhang, B.; Chen, Y.; Yu, Z.; Chen, H.; Ni, D. Impact of light irradiation on black tea quality during withering. J. Food Sci. Technol. 2017, 54, 1212–1227. [Google Scholar] [CrossRef]
- Deng, H.L.; Chen, S.S.; Zhou, Z.W.; Li, X.L.; Chen, S.; Hu, J.; Lai, Z.X.; Sun, Y. Transcriptome analysis reveals the effect of short-term sunlight on aroma metabolism in postharvest leaves of oolong tea (Camellia sinensis). Food Res. Int. 2020, 137, 109347. [Google Scholar] [CrossRef]
- Zhu, C.; Zhang, S.; Zhou, C.; Chen, L.; Zaripov, T.; Zhan, D.; Weng, J.; Lin, Y.; Lai, Z.; Guo, Y. Integrated Transcriptome, microRNA, and Phytochemical Analyses Reveal Roles of Phytohormone Signal Transduction and ABC Transporters in Flavor Formation of Oolong Tea (Camellia sinensis) during Solar Withering. J. Agric. Food. Chem. 2020, 68, 12749–12767. [Google Scholar] [CrossRef]
- Xu, P.; Su, H.; Zhao, S.; Jin, R.; Cheng, H.; Xu, A.; Lai, W.; Yin, X.; Wang, Y. Transcriptome and Phytochemical Analysis Reveals the Alteration of Plant Hormones, Characteristic Metabolites, and Related Gene Expression in Tea (Camellia sinensis L.) Leaves During Withering. Plants 2020, 9, 204. [Google Scholar] [CrossRef]
- Zhou, C.-z.; Zhu, C.; Li, X.-z.; Chen, L.; Xie, S.-y.; Chen, G.-w.; Zhang, H.; Lai, Z.-x.; Lin, Y.-l.; Guo, Y.-q. Transcriptome and phytochemical analyses reveal the roles of characteristic metabolites in the taste formation of white tea during the withering process. J. Integr. Agric. 2022, 21, 862–877. [Google Scholar] [CrossRef]
- Yu, X.; Li, Y.; He, C.; Zhou, J.; Chen, Y.; Yu, Z.; Wang, P.; Ni, D. Nonvolatile metabolism in postharvest tea (Camellia sinensis L.) leaves: Effects of different withering treatments on nonvolatile metabolites, gene expression levels, and enzyme activity. Food Chem. 2020, 327, 126992. [Google Scholar] [CrossRef]
- Zhu, C.; Zhang, S.; Fu, H.; Zhou, C.; Chen, L.; Li, X.; Lin, Y.; Lai, Z.; Guo, Y. Transcriptome and Phytochemical Analyses Provide New Insights Into Long Non-Coding RNAs Modulating Characteristic Secondary Metabolites of Oolong Tea (Camellia sinensis) in Solar-Withering. Front. Plant Sci. 2019, 10, 1638. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.Y.; Huang, X.J.; Liu, S.R.; Liu, J.H.; Guo, Y.Q.; Sun, Y.; Lin, J.K.; Guo, Y.L.; Wei, S. Understanding the formation mechanism of oolong tea characteristic nonvolatile chemical constitutes during manufacturing processes by using integrated widely-targeted metabolome and DIA proteome analysis. Food Chem. 2020, 310, 125941. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.J.; Li, D.; Ma, Y.X.; Zhang, W.; Lin, C.; Zheng, X.Q.; Liang, Y.R.; Lu, J.L. Formation mechanism of the oolong tea characteristic aroma during bruising and withering treatment. Food Chem. 2018, 269, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Shi, J.; Mu, B.; Chen, Z.; Dai, W.; Lin, Z. Metabolomics combined with proteomics provides a novel interpretation of the changes in nonvolatile compounds during white tea processing. Food Chem. 2020, 332, 127412. [Google Scholar] [CrossRef]
- Ma, Y.; Ling, T.-j.; Su, X.-q.; Jiang, B.; Nian, B.; Chen, L.-j.; Liu, M.-l.; Zhang, Z.-y.; Wang, D.-p.; Mu, Y.-y.; et al. Integrated proteomics and metabolomics analysis of tea leaves fermented by Aspergillus niger, Aspergillus tamarii and Aspergillus fumigatus. Food Chem. 2021, 334, 127560. [Google Scholar] [CrossRef]
- Wu, L.; Wang, Y.; Liu, S.; Sun, Y.; Li, C.; Lin, J.; Wei, S. The stress-induced metabolites changes in the flavor formation of oolong tea during enzymatic-catalyzed process: A case study of Zhangping Shuixian tea. Food Chem. 2022, 391, 133192. [Google Scholar] [CrossRef]
- Wu, L.; Lv, Y.; Ye, Y.; Liang, Y.; Ye, J. Transcriptomic and Translatomic Analyses Reveal Insights into the Developmental Regulation of Secondary Metabolism in the Young Shoots of Tea Plants (Camellia sinensis L.). J. Agric. Food. Chem. 2020, 68, 10750–10762. [Google Scholar] [CrossRef]
- Zheng, Y.C.; Hu, Q.C.; Yang, Y.; Wu, Z.J.; Wu, L.Y.; Wang, P.J.; Deng, H.L.; Ye, N.X.; Sun, Y. Architecture and Dynamics of the Wounding-Induced GeneRegulatory Network During the Oolong Tea Manufacturing Process (Camellia sinensis). Front. Plant Sci. 2022, 1, 788469. [Google Scholar] [CrossRef]
- Noctor, G.; Gomez, L.; Vanacker, H.; Foyer, C.H. Interactions between biosynthesis, compartmentation and transport in the control of glutathione homeostasis and signalling. J. Exp. Bot. 2002, 53, 1283–1304. [Google Scholar] [CrossRef]
- Deng, Y.; Lu, S. Biosynthesis and Regulation of Phenylpropanoids in Plants. Crit. Rev. Plant Sci. 2017, 36, 257–290. [Google Scholar] [CrossRef]
- Dong, N.-Q.; Lin, H.-X. Contribution of phenylpropanoid metabolism to plant development and plant-environment interactions. J. Integr. Plant Biol. 2021, 63, 180–209. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Xu, Y.; Xiao, H.; Mariga, A.M.; Chen, Y.; Zhang, X.; Wang, L.; Li, D.; Li, L.; Luo, Z. Rethinking of botanical volatile organic compounds applied in food preservation: Challenges in acquisition, application, microbial inhibition and stimulation. Trends Food Sci. Technol. 2022, 125, 166–184. [Google Scholar] [CrossRef]
- Feussner, I.; Wasternack, C. The lipoxygenase pathway. Annu. Rev. Plant Biol. 2002, 53, 275–297. [Google Scholar] [CrossRef] [PubMed]
- Liu, E.-t.; Wang, G.-s.; Li, Y.-y.; Shen, X.; Chen, X.-s.; Song, F.-h.; Wu, S.-j.; Chen, Q.; Mao, Z.-q. Replanting Affects the Tree Growth and Fruit Quality of Gala Apple. J. Integr. Agric. 2014, 13, 1699–1706. [Google Scholar] [CrossRef]
- Qin, L.; Wei, Q.-P.; Kang, W.-H.; Zhang, Q.; Sun, J.; Liu, S.-Z. Comparison of Volatile Compounds in ‘Fuji’ Apples in the Different Regions in China. Food Sci. Technol. Res. 2017, 23, 79–89. [Google Scholar] [CrossRef]
- Chen, X.; Sun, H.; Qu, D.; Yan, F.; Jin, W.; Jiang, H.; Chen, C.; Zhang, Y.; Li, C.; Xu, Z. Identification and characterization of key aroma compounds in Chinese high altitude and northernmost black tea (Camellia sinensis) using distillation extraction and sensory analysis methods. Flavour Fragr. J. 2020, 35, 666–673. [Google Scholar] [CrossRef]
- Wang, J.; Wu, B.; Zhang, N.; Zhao, M.; Jing, T.; Wu, Y.; Hu, Y.; Yu, F.; Wan, X.; Schwab, W.; et al. Dehydration-Induced Carotenoid Cleavage Dioxygenase 1 Reveals a Novel Route for beta-Ionone Formation during Tea (Camellia sinensis) Withering. J. Agric. Food Chem. 2020, 68, 10815–10821. [Google Scholar] [CrossRef]
- Zhu, J.; Zhu, Y.; Wang, K.; Niu, Y.; Xiao, Z. Characterization of key aroma compounds and enantiomer distribution in Longjing tea. Food Chem. 2021, 361, 130096. [Google Scholar] [CrossRef]
- Zeng, L.; Zhou, Y.; Fu, X.; Liao, Y.; Yuan, Y.; Jia, Y.; Dong, F.; Yang, Z. Biosynthesis of Jasmine Lactone in Tea (Camellia sinensis) Leaves and Its Formation in Response to Multiple Stresses. J. Agric. Food. Chem. 2018, 66, 3899–3909. [Google Scholar] [CrossRef]
- Wu, Z.-J.; Ma, H.-Y.; Zhuang, J. iTRAQ-based proteomics monitors the withering dynamics in postharvest leaves of tea plant (Camellia sinensis). Mol. Genet. Genom. 2018, 293, 45–59. [Google Scholar] [CrossRef]
- Lurie, S.; Pedreschi, R. Fundamental aspects of postharvest heat treatments. Hortic. Res. 2014, 1, 14030. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Zhang, S.T.; Zhou, C.Z.; Xie, S.Y.; Chen, G.W.; Tian, C.Y.; Xu, K.; Lin, Y.L.; Lai, Z.X.; Guo, Y.Q. Genome-Wide Investigation of N6-Methyladenosine Regulatory Genes and Their Roles in Tea (Camellia sinensis) Leaves during Withering Process. Front. Plant Sci. 2021, 12, 702303. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Yang, Z. Understanding different regulatory mechanisms of proteinaceous and non-proteinaceous amino acid formation in tea (Camellia sinensis) provides new insights into the safe and effective alteration of tea flavor and function. Crit. Rev. Food Sci. Nutr. 2020, 60, 844–858. [Google Scholar] [CrossRef] [PubMed]
- Lanot, A.; Hodge, D.; Lim, E.K.; Vaistij, F.E.; Bowles, D.J. Redirection of flux through the phenylpropanoid pathway by increased glucosylation of soluble intermediates. Planta 2008, 228, 609–616. [Google Scholar] [CrossRef]
- Liang, J.; Chen, X.; Deng, G.; Pan, Z.; Zhang, H.; Li, Q.; Yang, K.; Long, H.; Yu, M. Dehydration induced transcriptomic responses in two Tibetan hulless barley (Hordeum vulgare var. nudum) accessions distinguished by drought tolerance. BMC Genom. 2017, 18, 775. [Google Scholar] [CrossRef]
- Barua, P.; Lande, N.V.; Subba, P.; Gayen, D.; Pinto, S.; Prasad, T.S.K.; Chakraborty, S.; Chakraborty, N. Dehydration-responsive nuclear proteome landscape of chickpea (Cicer arietinum L.) reveals phosphorylation-mediated regulation of stress response. Plant Cell Environ. 2019, 42, 230–244. [Google Scholar] [CrossRef]
- Ye, J.H.; Ye, Y.; Yin, J.F.; Jin, J.; Liang, Y.R.; Liu, R.Y.; Tang, P.; Xu, Y.Q. Bitterness and astringency of tea leaves and products: Formation mechanism and reducing strategies. Trends Food Sci. Technol. 2022, 123, 130–143. [Google Scholar] [CrossRef]
- Zeng, L.; Jin, S.; Xu, Y.Q.; Granato, D.; Fu, Y.Q.; Sun, W.J.; Yin, J.F.; Xu, Y.Q. Exogenous stimulation-induced biosynthesis of volatile compounds: Aroma formation of oolong tea at postharvest stage. Crit. Rev. Food Sci. Nutr. 2022, 8, 1–16. [Google Scholar] [CrossRef]
- Noctor, G.; Mhamdi, A.; Chaouch, S.; Han, Y.; Neukermans, J.; Marquez-Garcia, B.; Queval, G.; Foyer, C.H. Glutathione in plants: An integrated overview. Plant Cell Environ. 2012, 35, 454–484. [Google Scholar] [CrossRef]
- Liao, H.S.; Chung, Y.H.; Hsieh, M.H. Glutamate: A multifunctional amino acid in plants. Plant Sci. 2022, 318, 111238. [Google Scholar] [CrossRef]
- Tsai, S.Y.; Huang, S.J.; Lo, S.H.; Wu, T.P.; Lian, P.Y.; Mau, J.L. Flavour components and antioxidant properties of several cultivated mushrooms. Food Chem. 2009, 113, 578–584. [Google Scholar] [CrossRef]
- Ishimaru, M.; Haraoka, M.; Hatate, H.; Tanaka, R. Simultaneous Analysis of Purine and Pyrimidine Compounds Associated with the Freshness and Taste of Marine Foods. Food Anal. Methods 2016, 9, 1606–1615. [Google Scholar] [CrossRef]
- Zhang, L.; Cao, Q.-Q.; Granato, D.; Xu, Y.-Q.; Ho, C.-T. Association between chemistry and taste of tea: A review. Trends Food Sci. Technol. 2020, 101, 139–149. [Google Scholar] [CrossRef]
- Deng, W.W.; Ogita, S.; Ashihara, H. Distribution and biosynthesis of theanine in Theaceae plants. Plant Physiol. Biochem. 2010, 48, 70–72. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.-W.; Fei, Y.; Wang, S.; Wan, X.-C.; Zhang, Z.-Z.; Hu, X.-Y. Effect of shade treatment on theanine biosynthesis in Camellia sinensis seedlings. Plant Growth Regul. 2013, 71, 295–299. [Google Scholar] [CrossRef]
- Seifikalhor, M.; Aliniaeifard, S.; Hassani, B.; Niknam, V.; Lastochkina, O. Diverse role of gamma-aminobutyric acid in dynamic plant cell responses. Plant Cell Rep. 2019, 38, 847–867. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, P.C.; Liu, P.P.; Song, X.W.; Guo, F.; Li, Y.Y.; Ni, D.J.; Jiang, C.J. Novel insight into the role of withering process in characteristic flavor formation of teas using transcriptome analysis and metabolite profiling. Food Chem. 2019, 272, 313–322. [Google Scholar] [CrossRef]
- Garcia-Caparros, P.; De Filippis, L.; Gul, A.; Hasanuzzaman, M.; Ozturk, M.; Altay, V.; Lao, M.T. Oxidative Stress and Antioxidant Metabolism under Adverse Environmental Conditions: A Review. Bot. Rev. 2021, 87, 421–466. [Google Scholar] [CrossRef]
- Abudureheman, B.; Yu, X.; Fang, D.; Zhang, H. Enzymatic Oxidation of Tea Catechins and Its Mechanism. Molecules 2022, 27, 942. [Google Scholar] [CrossRef]
- Lin, J.Z.; Tu, Z.; Zhu, H.K.; Chen, L.; Wang, Y.W.; Yang, Y.F.; Lv, H.W.; Zhu, Y.; Yu, L.Y.; Ye, Y. Effects of Shaking and Withering Processes on the Aroma Qualities of Black Tea. Horticulturae 2022, 8, 549. [Google Scholar] [CrossRef]
- Liu, P.; Zheng, P.; Feng, L.; Gong, Z.; Zheng, L.; Gao, S.; Wang, X.; Ye, F.; Huang, J.; Liu, Z. Dynamic changes in the aroma profile of Qingzhuan tea during its manufacture. Food Chem. 2022, 375, 131847. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Cao, J.; Li, Z.; Li, Q.; Lai, X.; Sun, L.; Chen, R.; Wen, S.; Sun, S.; Lai, Z. HS-SPME and GC/MS volatile component analysis of Yinghong No. 9 dark tea during the pile fermentation process. Food Chem. 2021, 357, 129654. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.; Yang, Y.C.; Zhang, G.H.; Chen, H.; Lu, J.L.; Du, Y.Y.; Ye, J.H.; Ye, Q.; Borthakur, D.; Zheng, X.Q.; et al. Effect of ultra-violent B on release of volatiles in tea leaf. Int. J. Food Prop. 2010, 13, 608–617. [Google Scholar] [CrossRef]
- Lin, S.J.; Chen, Z.P.; Chen, T.T.; Deng, W.W.; Wan, X.C.; Zhang, Z.L. Theanine metabolism and transport in tea plants (Camellia sinensis L.): Advances and perspectives. Crit. Rev. Biotechnol. 2022, 10, 1–15. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Li, C.; Lin, J.; Sun, Y.; Wei, S.; Wu, L. The Impact of Different Withering Approaches on the Metabolism of Flavor Compounds in Oolong Tea Leaves. Foods 2022, 11, 3601. https://doi.org/10.3390/foods11223601
Wang Y, Li C, Lin J, Sun Y, Wei S, Wu L. The Impact of Different Withering Approaches on the Metabolism of Flavor Compounds in Oolong Tea Leaves. Foods. 2022; 11(22):3601. https://doi.org/10.3390/foods11223601
Chicago/Turabian StyleWang, Yahui, Chenxue Li, Jiaqi Lin, Yun Sun, Shu Wei, and Liangyu Wu. 2022. "The Impact of Different Withering Approaches on the Metabolism of Flavor Compounds in Oolong Tea Leaves" Foods 11, no. 22: 3601. https://doi.org/10.3390/foods11223601
APA StyleWang, Y., Li, C., Lin, J., Sun, Y., Wei, S., & Wu, L. (2022). The Impact of Different Withering Approaches on the Metabolism of Flavor Compounds in Oolong Tea Leaves. Foods, 11(22), 3601. https://doi.org/10.3390/foods11223601




