Influence of Low-Intensity Ultrasound on ε-Polylysine Production: Intracellular ATP and Key Biosynthesis Enzymes during Streptomyces albulus Fermentation
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganisms and Culture Media
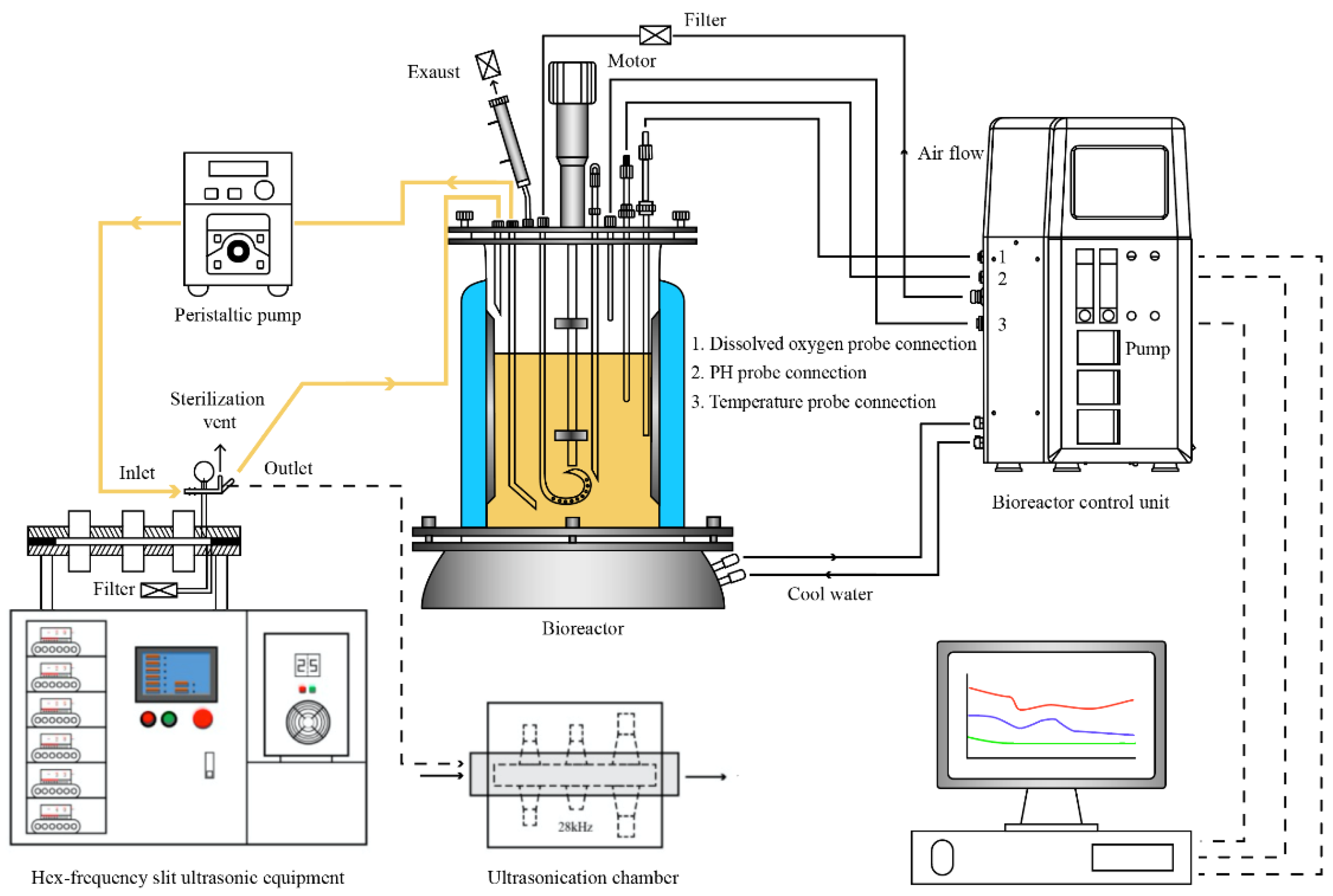
2.2. Ultrasonic Treatment of S. albulus and Fermentation of ε-Polylysine
2.3. Establishment and Assessment of Fermentation Kinetic Models
2.4. Assay of Mycelia-Viability Staining
2.5. Assay of Respiration Activity
2.6. Assay of Key Enzyme Activities in ε-PL Biosynthesis Pathway
2.7. Assay of Intracellular ATP in ε-PL Biosynthesis Pathway
2.8. qRT-PCR Assay for the Identification of the Transcription Levels of Key Enzymes in ε-PL Biosynthesis Pathway
2.9. Biomass, NH4+-N and ε-PL Concentration Analyses
2.10. Statistical Analysis
3. Results and Discussion
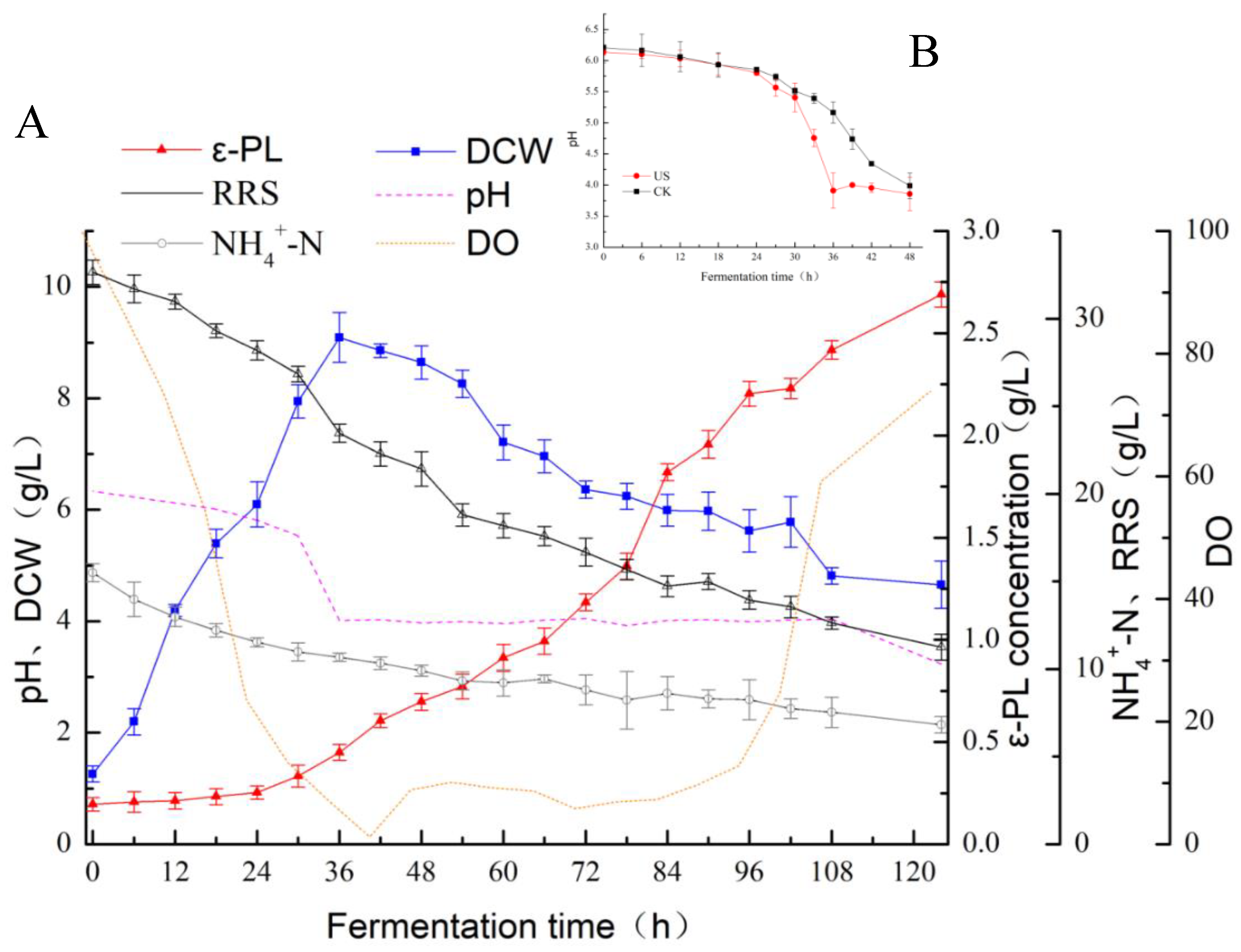
3.1. Effect of Ultrasonication on the ε-Polylysine Yield during Fermentation
3.2. Fermentation Kinetics of ε-PL
3.3. Effect of Ultrasonication on Mycelia Viability and Metabolic Activity of S. albulus
3.4. Effect of Ultrasonication on Intracellular ATP Levels of S. albulus
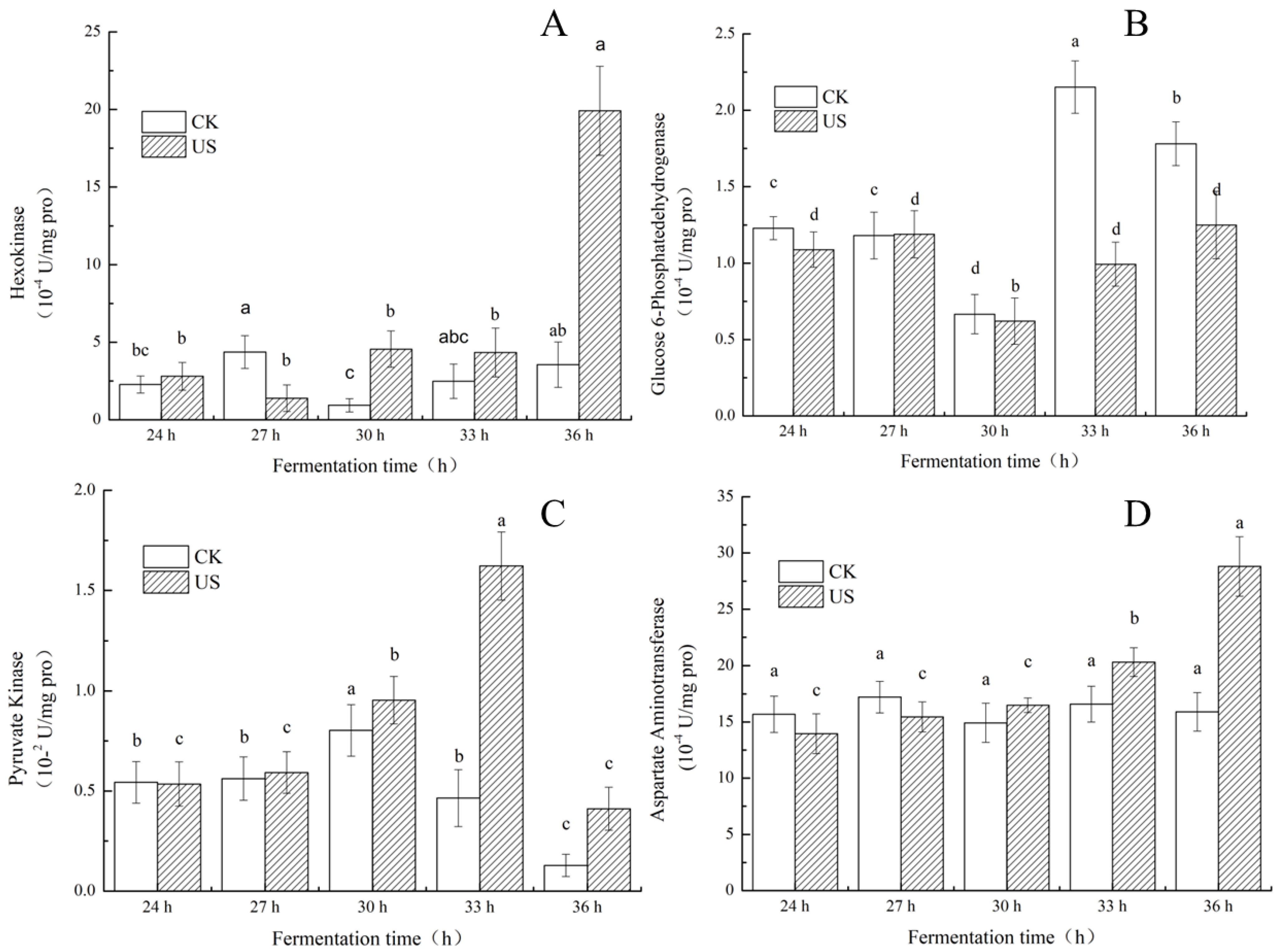
3.5. Effect of Ultrasonication on Key Enzyme Activities of S. albulus
3.6. Effect of Ultrasonication on the Expression of Key Enzymes and Genes of S. albulus
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chemat, F.; Khan, M.K. Applications of Ultrasound in Food Technology: Processing, Preservation and Extraction. Ultrason. Sonochem. 2011, 18, 813–835. [Google Scholar] [CrossRef] [PubMed]
- Awad, T.S.; Moharram, H.A.; Shaltout, O.E.; Asker, D.; Youssef, M.M. Applications of ultrasound in analysis, processing and quality control of food: A review. Food Res. Int. 2012, 48, 410–427. [Google Scholar] [CrossRef]
- Guimarães, J.T.; Balthazar, C.F.; Scudino, H.; Pimentel, T.C.; Esmerino, E.A.; Ashokkumar, M.; Freitas, M.Q.; Cruz, A.G. High-Intensity Ultrasound: A Novel Technology for the Development of Probiotic and Prebiotic Dairy Products. Ultrason. Sonochem. 2019, 57, 12–21. [Google Scholar] [CrossRef]
- Ojha, K.S.; Mason, T.J.; O’Donnell, C.P.; Kerry, J.P.; Tiwari, B.K. Ultrasound Technology for Food Fermentation Applications. Ultrason. Sonochem. 2017, 34, 410–417. [Google Scholar] [CrossRef]
- Ashokkumar, M. The characterization of acoustic cavitation bubbles—An overview. Ultrason. Sonochem. 2011, 18, 864–872. [Google Scholar] [CrossRef]
- Rastogi, N.K. Opportunities and Challenges in Application of Ultrasound in Food Processing. Crit. Rev. Food Sci. Nutr. 2011, 51, 705–722. [Google Scholar] [CrossRef]
- Golmohamadi, A.; Möller, G.; Powers, J.; Nindo, C. Effect of Ultrasound Frequency on Antioxidant Activity, Total Phenolic and Anthocyanin Content of Red Raspberry Puree. Ultrason. Sonochem. 2013, 20, 1316–1323. [Google Scholar] [CrossRef] [PubMed]
- Mota, M.J.; Lopes, R.P.; Koubaa, M.; Roohinejad, S.; Barba, F.J.; Delgadillo, I.; Saraiva, J.A. Fermentation at Non-Conventional Conditions in Food and Bio-Sciences by the Application of Advanced Processing Technologies. Crit. Rev. Biotechnol. 2018, 38, 122–140. [Google Scholar] [CrossRef]
- Raskar, H.D.; Avhad, D.N.; Rathod, V.K. Ultrasound assisted production of daunorubicin: Process intensification approach. Chem. Eng. Process. Process Intensif. 2014, 77, 7–12. [Google Scholar] [CrossRef]
- Huang, G.; Chen, S.; Dai, C.; Sun, L.; Sun, W.; Tang, Y.; Xiong, F.; He, R.; Ma, H. Effects of Ultrasound on Microbial Growth and Enzyme Activity. Ultrason. Sonochem. 2017, 37, 144–149. [Google Scholar] [CrossRef]
- Dai, C.; Xiong, F.; He, R.; Zhang, W.; Ma, H. Effects of Low-Intensity Ultrasound on the Growth, Cell Membrane Permeability and Ethanol Tolerance of Saccharomyces cerevisiae. Ultrason. Sonochem. 2017, 36, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Lanchun, S.; Bochu, W.; Liancai, Z.; Jie, L.; Yanhong, Y.; Chuanren, D. The Influence of Low-Intensity Ultrasonic on Some Physiological Characteristics of Saccharomyces cerevisiae. Colloids Surf. B Biointerfaces 2003, 30, 61–66. [Google Scholar] [CrossRef]
- Zhang, Z.; Xiong, F.; Wang, Y.; Dai, C.; Xing, Z.; Dabbour, M.; Mintah, B.; He, R.; Ma, H. Fermentation of Saccharomyces Cerevisiae in a One Liter Flask Coupled with an External Circulation Ultrasonic Irradiation Slot: Influence of Ultrasonic Mode and Frequency on the Bacterial Growth and Metabolism Yield. Ultrason. Sonochem. 2019, 54, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; McLandsborough, L.; McClements, D.J. Cationic Antimicrobial (ε-Polylysine)–Anionic Polysaccharide (Pectin) Interactions: Influence of Polymer Charge on Physical Stability and Antimicrobial Efficacy. J. Agric. Food Chem. 2012, 60, 1837–1844. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Dineshkumar, R.; Dhanarajan, G.; Sen, R.; Mishra, S. Improvement of ε-Polylysine Production by Marine Bacterium Bacillus licheniformis Using Artificial Neural Network Modeling and Particle Swarm Optimization Technique. Biochem. Eng. J. 2017, 126, 8–15. [Google Scholar] [CrossRef]
- Shih, I.-L.; Shen, M.-H.; Van, Y.T. Microbial Synthesis of Poly (ε-Lysine) and Its Various Applications. Bioresour. Technol. 2006, 97, 1148–1159. [Google Scholar] [CrossRef]
- Liu, S.; Wu, Q.; Zhang, J.; Mo, S.; Yang, X.; Xiao, C. Enhanced ε-Poly-l-Lysine Production from Streptomyces ahygroscopicus by a Combination of Cell Immobilization and in Situ Adsorption. J. Microbiol. Biotechnol. 2012, 22, 1218–1223. [Google Scholar] [CrossRef]
- Chen, X.S.; Li, S.; Liao, L.J.; Ren, X.D.; Li, F.; Tang, L.; Zhang, J.H.; Mao, Z.G. Production of ε-Poly-l-Lysine Using a Novel Two-Stage PH Control Strategy by Streptomyces Sp. M-Z18 from Glycerol. Bioprocess. Biosyst. Eng. 2011, 34, 561–567. [Google Scholar] [CrossRef]
- Zeng, X.; Chen, X.-S.; Ren, X.-D.; Wang, L.; Gao, Y.; Mao, Z.-G. Improved ε-poly-l-lysine productivity partly resulting from rapid cell growth in cultures using a glucose-glycerol mixed carbon source. Eng. Life Sci. 2016, 16, 443–452. [Google Scholar] [CrossRef]
- Xu, D.; Yao, H.; Xu, Z.; Wang, R.; Xu, Z.; Li, S.; Feng, X.; Liu, Y.; Xu, H. Production of ε-Poly-Lysine by Streptomyces Albulus PD-1 via Solid-State Fermentation. Bioresour. Technol. 2017, 223, 149–156. [Google Scholar] [CrossRef]
- Xu, Z.; Feng, X.; Sun, Z.; Cao, C.; Li, S.; Xu, Z.; Xu, Z.; Bo, F.; Xu, H. Economic Process to Co-Produce Poly(ε-L-Lysine) and Poly(L-Diaminopropionic Acid) by a PH and Dissolved Oxygen Control Strategy. Bioresour. Technol. 2015, 187, 70–76. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Ren, W.; Xiang, J.; Dabbour, M.; Kumah Mintah, B.; Li, Y.; Ma, H. Fermentation of Saccharomyces cerevisiae in a 7.5 Liter Ultrasound-Enhanced Fermenter: Effect of Sonication Conditions on Ethanol Production, Intracellular Ca2+ Concentration and Key Regulating Enzyme Activity in Glycolysis. Ultrason. Sonochem. 2021, 76, 105624. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Ma, H.; Wang, W.; Luo, M.; Wang, B.; Qu, W.; He, R.; Owusu, J.; Li, Y. Effects and Mechanism of Ultrasound Pretreatment on Rapeseed Protein Enzymolysis. J. Sci. Food Agric. 2016, 96, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
- Borah, A.J.; Agarwal, M.; Poudyal, M.; Goyal, A.; Moholkar, V.S. Mechanistic Investigation in Ultrasound Induced Enhancement of Enzymatic Hydrolysis of Invasive Biomass Species. Bioresour. Technol. 2016, 213, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.; Yang, Y.; Dabbour, M.; Mintah, B.K.; Zhang, Z.; Dai, C.; He, R.; Huang, G.; Ma, H. Metabolomic and Genomic Profiles of Streptomyces albulus with a Higher ε-Polylysine Production through ARTP Mutagenesis. Biochem. Eng. J. 2020, 162, 107720. [Google Scholar] [CrossRef]
- Wang, L.; Li, S.; Zhao, J.; Liu, Y.; Chen, X.; Tang, L.; Mao, Z. Efficiently Activated ε-Poly-L-Lysine Production by Multiple Resistance Mutations and Acidic PH Shock Optimization in Streptomyces albulus. MicrobiologyOpen 2019, 8, e00728. [Google Scholar] [CrossRef]
- Li, S.; Li, F.; Chen, X.-S.; Wang, L.; Xu, J.; Tang, L.; Mao, Z.-G. Genome Shuffling Enhanced ε-Poly-l-Lysine Production by Improving Glucose Tolerance of Streptomyces graminearus. Appl. Biochem. Biotechnol. 2012, 166, 414–423. [Google Scholar] [CrossRef]
- Zhang, J.; Qian, H.; Chen, X. Batch Fermentation Kinetics of ε-Polylysine by Streptomyces albus KD-11. Sci. Technol. Food Ind. 2014, 12, 222–225. (In Chinese) [Google Scholar]
- Terho, P.; Lassila, O. Novel Method for Cell Debris Removal in the Flow Cytometric Cell Cycle Analysis Using Carboxy-Fluorescein Diacetate Succinimidyl Ester. Cytom. Part A. 2006, 69, 552–554. [Google Scholar] [CrossRef]
- Cui, H.; Bai, M.; Sun, Y.; Abdel-Samie; Lin, L. Antibacterial activity and mechanism of Chuzhou chrysanthemum essential oil. J. Funct. Foods 2018, 48, 159–166. [Google Scholar] [CrossRef]
- Zhang, P.; Hai, H.; Sun, D.; Yuan, W.; Liu, W.; Ding, R.; Teng, M.; Ma, L.; Tian, J.; Chen, C. A high throughput method for total alcohol determination in fermentation broths. BMC Biotechnol. 2019, 19, 30. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Tian, F.; Zhang, H.; Wei, C. Method for Quickly Determining ε-Polylysine in Fermentation Broth. Food Ferment. Ind. 2009, 35, 133–136. (In Chinese) [Google Scholar]
- Yamanaka, K.; Kito, N.; Imokawa, Y.; Maruyama, C.; Utagawa, T.; Hamano, Y. Mechanism of ε-Poly-L-Lysine Production and Accumulation Revealed by Identification and Analysis of an ε-Poly-L-Lysine-Degrading Enzyme. Appl. Environ. Microbiol. 2010, 76, 5669–5675. [Google Scholar] [CrossRef] [PubMed]
- Sinisterra, J.V. Application of Ultrasound to Biotechnology: An Overview. Ultrasonics 1992, 30, 180–185. [Google Scholar] [CrossRef]
- Borah, A.J.; Roy, K.; Goyal, A.; Moholkar, V.S. Mechanistic Investigations in Biobutanol Synthesis via Ultrasound-Assisted ABE Fermentation Using Mixed Feedstock of Invasive Weeds. Bioresour. Technol. 2019, 272, 389–397. [Google Scholar] [CrossRef]
- Lu, H.; Lou, H.; Wei, T.; Liu, Z.; Jiao, Y.; Chen, Q. Ultrasound Enhanced Production of Mycelia and Exopolysaccharide by Agaricus Bitorquis (Quél.) Sacc. Chaidam. Ultrason. Sonochem. 2020, 64, 105040. [Google Scholar] [CrossRef]
- Kumar, A.; Gogate, P.R.; Pandit, A.B.; Delmas, H.; Wilhelm, A.M. Gas−Liquid Mass Transfer Studies in Sonochemical Reactors. Ind. Eng. Chem. Res. 2004, 43, 1812–1819. [Google Scholar] [CrossRef]
- Kashkooli, H.A.; Rooney, J.A.; Roxby, R. Effects of Ultrasound on Catalase and Malate Dehydrogenase. J. Acoust. Soc. Am. 1980, 67, 1798–1801. [Google Scholar] [CrossRef]
- Cabrera, R.; Ambrosio, A.L.; Garratt, R.C.; Guixé, V.; Babul, J. Crystallographic Structure of Phosphofructokinase-2 from Escherichia coli in Complex with Two ATP Molecules. Implications for Substrate Inhibition. J. Mol. Biol. 2008, 383, 588–602. [Google Scholar] [CrossRef]
- Xia, J.; Xu, Z.; Xu, H.; Feng, X.; Bo, F. The Regulatory Effect of Citric Acid on the Co-Production of Poly(ε-Lysine) and Poly(l-Diaminopropionic Acid) in Streptomyces albulus PD-1. Bioprocess Biosyst. Eng. 2014, 37, 2095–2103. [Google Scholar] [CrossRef]
- Hanke, P.D.; Fuchs, J.A. Requirement of Protein Synthesis for the Induction of Ribonucleoside Diphosphate Reductase MRNA in Escherichia coli. MGG Mol. Gen. Genet. 1984, 193, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Hillerich, B.; Westpheling, J. A New GntR Family Transcriptional Regulator in Streptomyces Coelicolor Is Required for Morphogenesis and Antibiotic Production and Controls Transcription of an ABC Transporter in Response to Carbon Source. J. Bacteriol. 2006, 188, 7477–7487. [Google Scholar] [CrossRef] [PubMed]
- Horinouchi, S. AfsR as an Integrator of Signals That Are Sensed by Multiple Serine/Threonine Kinases in Streptomyces coelicolor A3(2). J. Ind. Microbiol. Biotechnol. 2003, 30, 462–467. [Google Scholar] [CrossRef] [PubMed]





| Time (min) | Ri (mmol/g·h) |
|---|---|
| 0 | 5.38 ± 0.23 b |
| 30 | 6.06 ± 0.46 a |
| 60 | 5.41 ± 0.31 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiang, J.; Dabbour, M.; Gao, X.; Mintah, B.K.; Yang, Y.; Ren, W.; He, R.; Dai, C.; Ma, H. Influence of Low-Intensity Ultrasound on ε-Polylysine Production: Intracellular ATP and Key Biosynthesis Enzymes during Streptomyces albulus Fermentation. Foods 2022, 11, 3525. https://doi.org/10.3390/foods11213525
Xiang J, Dabbour M, Gao X, Mintah BK, Yang Y, Ren W, He R, Dai C, Ma H. Influence of Low-Intensity Ultrasound on ε-Polylysine Production: Intracellular ATP and Key Biosynthesis Enzymes during Streptomyces albulus Fermentation. Foods. 2022; 11(21):3525. https://doi.org/10.3390/foods11213525
Chicago/Turabian StyleXiang, Jiahui, Mokhtar Dabbour, Xianli Gao, Benjamin Kumah Mintah, Yao Yang, Wenbin Ren, Ronghai He, Chunhua Dai, and Haile Ma. 2022. "Influence of Low-Intensity Ultrasound on ε-Polylysine Production: Intracellular ATP and Key Biosynthesis Enzymes during Streptomyces albulus Fermentation" Foods 11, no. 21: 3525. https://doi.org/10.3390/foods11213525
APA StyleXiang, J., Dabbour, M., Gao, X., Mintah, B. K., Yang, Y., Ren, W., He, R., Dai, C., & Ma, H. (2022). Influence of Low-Intensity Ultrasound on ε-Polylysine Production: Intracellular ATP and Key Biosynthesis Enzymes during Streptomyces albulus Fermentation. Foods, 11(21), 3525. https://doi.org/10.3390/foods11213525







