Analysis of Isoflavones in Pueraria by UHPLC-Q-Orbitrap HRMS and Study on α-Glucosidase Inhibitory Activity
Abstract
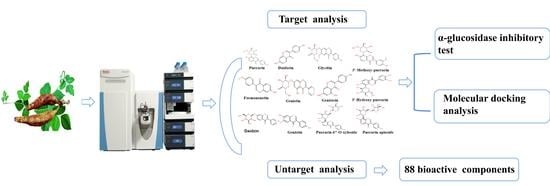
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Sample Preparation
2.3. UHPLC-Q-Exactive Orbitrap HRMS Analysis
2.3.1. Quantitative Method
2.3.2. Screening Method
2.4. Method Validation
2.5. α-Glucosidase Inhibitory Activity
2.6. Molecular Docking
3. Results and Discussion
3.1. Optimization of Chromatographic Separation and Mass Spectrometric Detection
3.2. Optimization of Extraction Conditions
3.3. Method Validation
3.4. Quantitative Analysis of Isoflavones in Pueraria
3.5. Qualitative Analysis of Bioactive Components in Pueraria
3.6. α-Glucosidase Inhibitory Activity and Molecular Docking
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wong, K.H.; Li, G.Q.; Li, K.M.; Razmovski-Naumovski, V.; Chan, K. Optimisation of Pueraria isoflavonoids by response surface methodology using ultrasonic-assisted extraction. Food Chem. 2017, 231, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Bharti, R.; Chopra, B.S.; Raut, S.; Khatri, N. Pueraria tuberosa: A Review on Traditional Uses, Pharmacology, and Phytochemistry. Front. Pharmacol. 2020, 11, 582506. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, S.; Wang, S.; Gao, P.; Dai, L. A comprehensive review on Pueraria: Insights on its chemistry and medicinal value. Biomed. Pharmacother. 2020, 131, 110734. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Luo, X.X.; Yan, F.D.; Wei, Z.B.; Tu, J. Puerariae Lobatae Radix elevated expression levels of OB-R, IRS2, GLUT1 and GLUT2 to regulate glucose metabolism in insulin-resistance HepG2 cells. Zhongguo Zhong Yao Za Zhi 2017, 42, 1939–1944. [Google Scholar]
- Lertpatipanpong, P.; Janpaijit, S.; Park, E.Y.; Kim, C.T.; Baek, S.J. Potential Anti-Diabetic Activity of Pueraria lobata Flower (Flos Puerariae) Extracts. Molecules 2020, 25, 3970. [Google Scholar] [CrossRef]
- Sun, R.; Deng, X.; Zhang, D.; Xie, F.; Wang, D.; Wang, J.; Tavallaie, M.S.; Jiang, F.; Fu, L. Anti-diabetic potential of Pueraria lobata root extract through promoting insulin signaling by PTP1B inhibition. Bioorg. Chem. 2019, 87, 12–15. [Google Scholar] [CrossRef]
- Shukla, R.; Banerjee, S.; Tripathi, Y.B. Pueraria tuberosa extract inhibits iNOS and IL-6 through suppression of PKC-alpha and NF-kB pathway in diabetes-induced nephropathy. J. Pharm. Pharmacol. 2018, 70, 1102–1112. [Google Scholar] [CrossRef]
- Liu, J.; Shi, Y.C.; Lee, D.Y. Applications of Pueraria lobata in treating diabetics and reducing alcohol drinking. Chin. Herb. Med. 2019, 11, 141–149. [Google Scholar] [CrossRef]
- Zhang, Z.T.; Guo, N.; Zhuang, G.D.; Deng, S.M.; He, W.J.; Chen, Z.Q.; Xu, Y.H.; Tang, D.; Wang, S.M. Metabolic Profiling of Carbonyl Compounds for Unveiling Protective Mechanisms of Pueraria lobata against Diabetic Nephropathy by UPLC-Q-Orbitrap HRMS/MS Analysis. J. Agric. Food Chem. 2021, 69, 10943–10951. [Google Scholar] [CrossRef]
- Wang, Q.S.; Wang, Y.L.; Zhang, W.Y.; Li, K.D.; Luo, X.F.; Cui, Y.L. Puerarin from Pueraria lobata alleviates the symptoms of irritable bowel syndrome-diarrhea. Food Funct. 2021, 12, 2211–2224. [Google Scholar] [CrossRef]
- Liu, Y.; Qiu, Y.; Chen, Q.; Han, X.; Cai, M.; Hao, L. Puerarin suppresses the hepatic gluconeogenesis via activation of PI3K/Akt signaling pathway in diabetic rats and HepG(2) cells. Biomed. Pharmacother. 2021, 137, 111325. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.H.; Razmovski-Naumovski, V.; Li, K.M.; Li, G.Q.; Chan, K. Comparing morphological, chemical and anti-diabetic characteristics of Puerariae Lobatae Radix and Puerariae Thomsonii Radix. J. Ethnopharmacol. 2015, 164, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.F.; Li, J.L.; Meng, X.W.; Zhang, P.Z.; Wu, W.T.; Liu, R.H. Research advances in chemical constituents and pharmacological activities of Pueraria genus. Zhongguo Zhong Yao Za Zhi 2021, 46, 1311–1331. [Google Scholar]
- Papoutsis, K.; Zhang, J.; Bowyer, M.C.; Brunton, N.; Gibney, E.R.; Lyng, J. Fruit, vegetables, and mushrooms for the preparation of extracts with alpha-amylase and alpha-glucosidase inhibition properties: A review. Food Chem. 2021, 338, 128119. [Google Scholar] [CrossRef] [PubMed]
- Hou, Z.W.; Chen, C.H.; Ke, J.P.; Zhang, Y.Y.; Qi, Y.; Liu, S.Y.; Yang, Z.; Ning, J.M.; Bao, G.H. alpha-Glucosidase Inhibitory Activities and the Interaction Mechanism of Novel Spiro-Flavoalkaloids from YingDe Green Tea. J. Agric. Food Chem. 2022, 70, 136–148. [Google Scholar] [CrossRef]
- Assefa, S.T.; Yang, E.Y.; Chae, S.Y.; Song, M.; Lee, J.; Cho, M.C.; Jang, S. Alpha Glucosidase Inhibitory Activities of Plants with Focus on Common Vegetables. Plants 2019, 9, 2. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, C.; Zhang, B.; Huang, Q. The inhibitory effects of flavonoids on alpha-amylase and alpha-glucosidase. Crit. Rev. Food Sci. Nutr. 2020, 60, 695–708. [Google Scholar] [CrossRef]
- Kumar, S.; Narwal, S.; Kumar, V.; Prakash, O. alpha-glucosidase inhibitors from plants: A natural approach to treat diabetes. Pharmacogn. Rev. 2011, 5, 19–29. [Google Scholar] [CrossRef]
- Juengsanguanpornsuk, W.; Yusakul, G.; Kitisripanya, T.; Krittanai, S.; Juengwatanatrakul, T.; Sakamoto, S.; Putalun, W. Quantification of methylisomiroestrol, a phytoestrogen of Pueraria candollei, by enzyme-linked immunosorbent assay in comparison with high-performance liquid chromatography. J. Pharm. Biomed. Anal. 2021, 192, 113674. [Google Scholar] [CrossRef]
- Krittanai, S.; Kitisripanya, T.; Udomsin, O.; Tanaka, H.; Sakamoto, S.; Juengwatanatrakul, T.; Putalun, W. Development of a colloidal gold nanoparticle-based immunochromatographic strip for the one-step detection of miroestrol and puerarin. Biomed. Chromatogr. 2018, 32, e4330. [Google Scholar] [CrossRef]
- Huang, Z.Y.; Shen, Q.N.; Li, P.; Su, L.L.; Chen, L.H.; Lu, T.L.; Mao, C.Q. Quality research of Puerariae Lobatae Radix from different habitats with UPLC fingerprint and determination of multi-component content. Zhongguo Zhong Yao Za Zhi 2019, 44, 2051–2058. [Google Scholar] [PubMed]
- Qu, L.; Song, K.; Zhang, Q.; Guo, J.; Huang, J. Simultaneous Determination of Six Isoflavones from Puerariae Lobatae Radix by CPE-HPLC and Effect of Puerarin on Tyrosinase Activity. Molecules 2020, 25, 344. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.J.; Kim, H.J.; Lee, A.; Min, S.S.; In, S.; Kim, E. Determination of 12 herbal compounds for estimating the presence of Angelica Gigas Root, Cornus Fruit, Licorice Root, Pueraria Root, and Schisandra Fruit in foods by LC-MS/MS. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess 2020, 37, 1437–1448. [Google Scholar] [CrossRef] [PubMed]
- Masada, S.; Hosoe, J.; Arai, R.; Demizu, Y.; Hakamatsuka, T.; Goda, Y.; Uchiyama, N. Miroestrol Quantification in Pueraria mirifica Crude Drugs and Products by Single-Reference UPLC/PDA/MS Using Relative Molar Sensitivities to Kwakhurin. Chem. Pharm. Bull. 2021, 69, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Fu, Q.; Mei, Q.; Tu, Z.; Zhang, L. Extraction optimization and screening of angiotensin-converting enzyme inhibitory peptides from Channa striatus through bioaffinity ultrafiltration coupled with LC-Orbitrap-MS/MS and molecular docking. Food Chem. 2021, 354, 129589. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Ma, S.; Li, K.; Xiong, P.; Qin, S.; Cai, W. Systematic Screening of Chemical Constituents in the Traditional Chinese Medicine Arnebiae Radix by UHPLC-Q-Exactive Orbitrap Mass Spectrometry. Molecules 2022, 27, 2631. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Yao, C.; Guo, D.A. Insight into chemical basis of traditional Chinese medicine based on the state-of-the-art techniques of liquid chromatography-mass spectrometry. Acta Pharm. Sin. B 2021, 11, 1469–1492. [Google Scholar] [CrossRef]
- Ricciutelli, M.; Moretti, S.; Galarini, R.; Sagratini, G.; Mari, M.; Lucarini, S.; Vittori, S.; Caprioli, G. Identification and quantification of new isomers of isopropyl-malic acid in wine by LC-IT and LC-Q-Orbitrap. Food Chem. 2019, 294, 390–396. [Google Scholar] [CrossRef]
- AOAC International. Guidelines for validation of botanical identification methods. J. AOAC Int. 2012, 95, 268–272. [Google Scholar] [CrossRef]
- Hu, G.; Peng, X.; Dong, D.; Nian, Y.; Gao, Y.; Wang, X.; Hong, D.; Qiu, M. New ent-kaurane diterpenes from the roasted arabica coffee beans and molecular docking to alpha-glucosidase. Food Chem. 2021, 345, 128823. [Google Scholar] [CrossRef]
- Wu, W.; Zhang, Y.; Zhang, F.; Liu, J.; Ren, Z.; Xu, Y.; Liu, T.; Zhou, W.; Li, H.; Zhang, C. An analytical strategy for accurate, rapid and sensitive quantitative analysis of isoflavones in traditional Chinese medicines using ultra-high performance supercritical fluid chromatography: Take Radix Puerariae as an example. J. Chromatogr. A 2019, 1606, 460385. [Google Scholar] [CrossRef] [PubMed]
- Du, G.; Zhao, H.; Song, Y.; Zhang, Q.; Wang, Y. Rapid simultaneous determination of isoflavones in Radix puerariae using high-performance liquid chromatography-triple quadrupole mass spectrometry with novel shell-type column. J. Sep. Sci. 2011, 34, 2576–2585. [Google Scholar] [CrossRef] [PubMed]
- di Stefano, E.; Oliviero, T.; Udenigwe, C.C. Functional significance and structure–activity relationship of food-derived α-glucosidase inhibitors. Curr. Opin. Food Sci. 2018, 20, 7–12. [Google Scholar] [CrossRef]
- Liu, B.; Kongstad, K.T.; Qinglei, S.; Nyberg, N.T.; Jager, A.K.; Staerk, D. Dual high-resolution alpha-glucosidase and radical scavenging profiling combined with HPLC-HRMS-SPE-NMR for identification of minor and major constituents directly from the crude extract of Pueraria lobata. J. Nat. Prod. 2015, 78, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.; Shangguan, X.; Yin, Z.; Wu, S.; Zhang, Q.; Peng, W.; Li, J.; Zhang, L.; Chen, J. Inhibitory Effect of Fisetin on alpha-Glucosidase Activity: Kinetic and Molecular Docking Studies. Molecules 2021, 26, 5306. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.L.; Kong, Y.C.; Miao, J.Y.; Mei, X.Y.; Wu, S.Y.; Yan, Y.C.; Cao, X.Y. Spectroscopy and molecular docking analysis reveal structural specificity of flavonoids in the inhibition of alpha-glucosidase activity. Int. J. Biol. Macromol. 2020, 152, 981–989. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Pandey, H.; Singh, S.K.; Tripathi, Y.B. Anti-oxidant, anti-apoptotic, anti-hypoxic and anti-inflammatory conditions induced by PTY-2 against STZ-induced stress in islets. Biosci. Trends 2019, 13, 382–393. [Google Scholar] [CrossRef]
- Luo, X.X.; Xu, G.L.; Li, Y.; Li, B.T.; Tu, J. Kudzu root (Ge-Gen) regulates on glucose and lipid metabolism to ameliorating insulin resistance on 3T3-L1 adipocytes. Zhongguo Zhong Yao Za Zhi 2016, 41, 2687–2694. [Google Scholar]




| No. | Compound | CAS | Retention Time | Adduct Ion | Precursor Ion | Delta | Product Ion |
|---|---|---|---|---|---|---|---|
| (min) | (m/z) | (ppm) | (m/z) | ||||
| 1 | 3′-Hydroxy puerarin | 117076-54-5 | 5.50 | [M + H]+ | 433.11365 | 0.169 | 313.07080, 283.06024, 415.10297 |
| 2 | Puerarin | 3681-99-0 | 6.50 | [M + H]+ | 417.11819 | 0.043 | 297.07581, 321.07520, 399.10709 |
| 3 | 3′-Methoxy puerarin | 117047-07-1 | 6.88 | [M + H]+ | 447.12888 | 0.069 | 327.08643, 429.11844, 297.07590 |
| 4 | Daidzin | 552-66-9 | 7.53 | [M + H]+ | 417.11811 | 0.024 | 255.06538, 199.07590, 227.07002 |
| 5 | Glycitin | 40246-10-4 | 7.83 | [M + H]+ | 447.12851 | −0.013 | 285.07599, 270.05240, 229.08578 |
| 6 | Glycitein | 40957-83-3 | 10.15 | [M + H]+ | 285.07571 | −0.014 | 270.05215, 242.05724, 225.05472 |
| 7 | Genistin | 529-59-9 | 9.09 | [M + H]+ | 433.11301 | 0.021 | 271.06015, 128.06224, 153.01804 |
| 8 | Daidzein | 486-66-8 | 10.07 | [M + H]+ | 255.06535 | 0.063 | 227.07027, 199.07556, 137.02327 |
| 9 | Genistein | 446-72-0 | 10.51 | [M + H]+ | 271.06033 | 0.085 | 243.06522, 215.07027, 153.01839 |
| 10 | Formononetin | 485-72-3 | 10.89 | [M + H]+ | 269.08060 | −0.089 | 254.05652, 213.09102, 197.05974 |
| 11 | Puerarin 6″-O-xyloside | 114240-18-5 | 6.86 | [M + H]+ | 549.16083 | 0.102 | 297.07562, 417.11765, 399.10779 |
| 12 | Puerarin apioside | 103654-50-8 | 6.63 | [M + H]+ | 549.16052 | 0.046 | 297.07556, 417.11746, 399.10742 |
| Compounds | Linear Regression Data | LOD | LOQ | Recovery Test | Precision (RSD, %, n = 6) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Equation | Linearity (r2) | (μg/kg) | (μg/kg) | Originals (μg) | Spiked (μg) | Found (μg) | Recovery (%) | Intra-Day | Inter-Day | |
| 3′-Hydroxy puerarin | Y = 48,674.5X + 2400.93 | 0.9994 | 1.83 | 6.10 | 130.1 | 65.0 | 193.4 | 97.4 | 2.2 | 3.6 |
| 130.0 | 259.3 | 99.4 | 2.8 | 5.2 | ||||||
| 195.0 | 349.7 | 102.6 | 1.4 | 4.9 | ||||||
| Puerarin | Y = 118,793X + 339,229 | 0.9991 | 0.57 | 1.89 | 402.8 | 200.0 | 555.4 | 96.3 | 1.7 | 5.3 |
| 400.0 | 741.7 | 104.7 | 2.8 | 5.1 | ||||||
| 600.0 | 904.6 | 93.6 | 1.9 | 4.8 | ||||||
| 3′-Methoxy puerarin | Y = 110,435X + 194,781 | 0.9989 | 0.95 | 3.16 | 158.4 | 80.0 | 223.6 | 91.5 | 2.9 | 4.1 |
| 160.0 | 281.1 | 96.7 | 3.0 | 5.6 | ||||||
| 240.0 | 358.8 | 103.5 | 2.2 | 5.3 | ||||||
| Daidzin | Y = 127,342X + 344,625 | 0.9987 | 0.63 | 2.08 | 108.9 | 54.0 | 150.0 | 90.1 | 2.3 | 5.9 |
| 108.0 | 190.0 | 95.1 | 2.7 | 4.2 | ||||||
| 162.0 | 242.0 | 92.2 | 1.8 | 5.4 | ||||||
| Glycitin | Y = 182,859X + 539,463 | 0.9990 | 0.57 | 1.89 | 12.4 | 6.0 | 17.0 | 86.2 | 3.2 | 7.3 |
| 12.0 | 21.4 | 85.2 | 3.8 | 5.1 | ||||||
| 18.0 | 32.4 | 91.0 | 2.6 | 5.5 | ||||||
| Glycitein | Y = 384,176X + 499,669 | 0.9995 | 0.12 | 0.40 | 2.0 | 1.0 | 3.0 | 88.6 | 5.8 | 7.5 |
| 2.0 | 4.3 | 112.2 | 4.2 | 5.9 | ||||||
| 3.0 | 5.4 | 114.8 | 3.5 | 5.4 | ||||||
| Genistin | Y = 30,610.1X – 34,832.6 | 0.9989 | 2.78 | 9.26 | 16.6 | 8.0 | 23.0 | 92.9 | 3.4 | 6.0 |
| 16.0 | 29.1 | 88.1 | 3.8 | 7.3 | ||||||
| 24.0 | 35.5 | 95.8 | 3.2 | 6.7 | ||||||
| Daidzein | Y = 246,022X + 456,106 | 0.9992 | 0.18 | 0.61 | 20.0 | 10.0 | 27.7 | 87.0 | 3.4 | 5.6 |
| 20.0 | 36.4 | 89.9 | 3.7 | 4.8 | ||||||
| 30.0 | 50.0 | 100.1 | 2.2 | 4.2 | ||||||
| Genistein | Y = 49,834.2X – 66,499.2 | 0.9991 | 0.95 | 3.16 | 1.4 | 0.7 | 2.0 | 81.5 | 5.6 | 8.3 |
| 1.4 | 3.0 | 90.8 | 5.3 | 6.6 | ||||||
| 2.1 | 3.9 | 89.9 | 3.5 | 3.2 | ||||||
| Formononetin | Y = 614,878X + 47,444.8 | 0.9993 | 0.10 | 0.30 | 1.1 | 0.6 | 1.7 | 89.4 | 6.0 | 6.4 |
| 1.2 | 2.2 | 94.4 | 3.7 | 7.0 | ||||||
| 1.8 | 2.8 | 90.6 | 5.5 | 5.9 | ||||||
| Puerarin 6″-O-xyloside | Y = 42,977.4X + 57,239.4 | 0.9996 | 1.88 | 6.25 | 226.4 | 110.0 | 309.2 | 95.3 | 2.1 | 4.6 |
| 220.0 | 398.9 | 103.4 | 2.6 | 5.2 | ||||||
| 330.0 | 497.0 | 92.0 | 1.8 | 3.8 | ||||||
| Puerarin apioside | Y = 45,781.5X + 38,439.8 | 0.9994 | 1.63 | 5.43 | 60.0 | 30.0 | 89.1 | 97.0 | 4.0 | 5.8 |
| 60.0 | 116.1 | 93.5 | 2.8 | 6.2 | ||||||
| 90.0 | 138.4 | 87.1 | 3.3 | 7.0 | ||||||
| No. | Compound | Sample 1 | Sample 2 | Sample 3 | Sample 4 | Sample 5 | Sample 6 |
|---|---|---|---|---|---|---|---|
| 1 | 3′-Hydroxy puerarin | 10.56 ± 1.21 | 7.70 ± 2.03 | 8.52 ± 0.94 | 7.66 ± 1.79 | 10.81 ± 0.84 | 8.67 ± 2.02 |
| 2 | Puerarin | 27.6 ± 2.80 | 17.68 ± 1.52 | 22.42 ± 0.80 | 16.70 ± 3.80 | 19.00 ± 0.92 | 25.20 ± 1.61 |
| 3 | 3′-Methoxy-puerarin | 9.83 ± 0.83 | 10.43 ± 2.12 | 8.10 ± 1.25 | 6.90 ± 1.45 | 9.91 ± 2.32 | 10.25 ± 3.10 |
| 4 | Daidzin | 6.53 ± 1.00 | 6.59 ± 0.34 | 5.31 ± 0.67 | 5.39 ± 1.21 | 5.82 ± 0.53 | 7.05 ± 0.63 |
| 5 | Glycitin | 0.95 ± 0.23 | 1.04 ± 0.08 | 0.83 ± 0.18 | 0.73 ± 0.04 | 1.02 ± 0.32 | 0.83 ± 0.33 |
| 6 | Glycitein | 0.23 ± 0.05 | 0.09 ± 0.04 | 0.30 ± 0.10 | 0.12 ± 0.04 | 0.14 ± 0.02 | 0.09 ± 0.01 |
| 7 | Genistin | 1.15 ± 0.03 | 1.04 ± 0.17 | 0.86 ± 0.32 | 0.92 ± 0.09 | 1.07 ± 0.04 | 1.17 ± 0.05 |
| 8 | Daidzein | 0.94 ± 0.26 | 0.64 ± 0.09 | 1.47 ± 0.15 | 0.80 ± 0.32 | 0.79 ± 0.08 | 1.40 ± 0.10 |
| 9 | Genistein | 0.05 ± 0.02 | 0.03 ± 0.01 | 0.09 ± 0.03 | 0.05 ± 0.03 | 0.05 ± 0.02 | 0.09 ± 0.04 |
| 10 | Formononetin | 0.03 ± 0.01 | 0.04 ± 0.02 | 0.06 ± 0.02 | 0.04 ± 0.03 | 0.06 ± 0.02 | 0.07 ± 0.02 |
| 11 | Puerarin 6″-O-xyloside | 9.07 ± 0.73 | 8.15 ± 1.54 | 7.62 ± 0.98 | 8.70 ± 2.01 | 7.47 ± 0.83 | 13.18 ± 2.39 |
| 12 | Puerarin apioside | 1.23 ± 0.05 | 1.04 ± 0.13 | 0.93 ± 0.28 | 1.28 ± 0.16 | 0.93 ± 0.21 | 2.78 ± 0.06 |
| No. | Compound | Formula | RT | Adduct Ion | Precursor Ion | Delta | Product Ion |
|---|---|---|---|---|---|---|---|
| (min) | (m/z) | (ppm) | (m/z) | ||||
| 1 | Guanine | C5H5N5O | 0.76 | [M + H]+ | 152.0567 | 0.077 | 110.03531, 135.03018 |
| 2 | L-Tyrosine | C9H11NO3 | 0.87 | [M + H]+ | 182.08121 | 0.205 | 136.07578, 123.04443 |
| 3 | 5-Hydroxymethylfurfural | C6H6O3 | 0.87 | [M + H]+ | 127.03912 | 1.149 | 81.0342, 109.02873 |
| 4 | L-Phenylalanine | C9H11NO2 | 0.92 | [M + H]+ | 166.08615 | −0.656 | 120.08102, 149.05966 |
| 5 | Diglycolic acid | C4H6O5 | 0.94 | [M − H]− | 133.01241 | −0.74 | 72.99128, 89.02254 |
| 6 | Adenosine | C10H13N5O4 | 1.15 | [M + H]+ | 268.10385 | −0.666 | 136.06184, 85.02911 |
| 7 | 5-Hydroxymethylfurfural | C6H6O3 | 1.18 | [M + H]+ | 127.03904 | 0.548 | 81.03403, 109.02885 |
| 8 | Vitexin7-O-sulfate | C24H15O13 | 2.35 | [M − H]− | 511.05307 | 2.353 | 421.02237, 391.01184, 283.06030, 262.00897 |
| 9 | Diethyl tartrate | C8H14O6 | 4.26 | [M − H]− | 205.07042 | −0.245 | 72.99133, 115.07466, 129.05391, 143.06963 |
| 10 | 3,5,7-Trihydroxyflavone 3-glucoside−8-sulfate | C24H15O13 | 4.73 | [M − H]− | 511.05325 | 2.533 | 311.05515, 391.01157, 283.06018, 341.06555 |
| 11 | 3′-Hydroxy−4′-O-β-D-glucosyl-Puerarin | C27H30O15 | 5.01 | [M + H]+ | 595.16559 | −0.157 | 313.07053, 283.06000, 433.11307 |
| 12 | Puerarin−4′-O-β-D-glucopyra noside | C27H30O14 | 5.42 | [M + H]+ | 579.17059 | −0.237 | 267.06497, 297.07559, 417.11777 |
| 13 | Hypaphorine | C14H18N2O2 | 5.6 | [M + H]+ | 247.144 | 0.063 | 188.07057, 118.06542, 146.06004 |
| 14 | 7,8-Dihydroxyflavone | C15H10O4 | 6.24 | [M + H]+ | 255.06511 | −0.075 | 227.07043, 237.05447, 199.07541 |
| 15 | 3′-Hydroxy Puerarin * | C21H20O10 | 6.42 | [M + H]+ | 433.1127 | −0.223 | 313.07047, 283.05994, 433.11270 |
| 16 | Apigenin-6-C-glucoside-7-O-glucoside | C27H30O15 | 6.64 | [M − H]− | 593.14929 | −0.806 | 473.10712, 310.04730, 282.05231 |
| 17 | 3′-Methoxy-4′-O-glucosyl-Puerarin | C28H32O15 | 6.71 | [M + H]+ | 609.18152 | 0.123 | 285.07562, 270.05203 |
| 18 | 3′-Hydroxy puerarin xyloside | C26H28O14 | 7.06 | [M + H]+ | 565.15515 | −0.032 | 313.07043, 283.05981, 433.11282 |
| 19 | Puerarin xyloside | C26H28O13 | 7.29 | [M − H]− | 547.1438 | −0.817 | 267.06537, 295.06003, 275.07001 |
| 20 | Puerarin-6″-O-glucoside | C27H30O14 | 7.39 | [M + H]+ | 579.17053 | −0.302 | 267.06500, 297.07562, 399.10739 |
| 21 | Puerarin * | C21H20O9 | 7.45 | [M + H]+ | 417.11768 | −0.329 | 297.07559, 267.06503, 399.10727 |
| 22 | Puerarin-6″-O-apioside * | C26H28O13 | 7.97 | [M + H]+ | 549.15997 | −0.297 | 417.11771, 267.06500, 297.07559 |
| 23 | Puerarin apioside * | C26H28O13 | 8.03 | [M + H]+ | 549.15985 | −0.763 | 297.07568, 381.09705 |
| 24 | Daidzein-6-C-(6″-glucosyl)glucoside | C27H30O14 | 8.15 | [M + H]+ | 579.17059 | −0.242 | 327.04984, 299.05505 |
| 25 | 3′-Methoxy puerarin * | C22H22O10 | 8.6 | [M + H]+ | 447.12842 | −0.153 | 327.08609, 297.07550, 429.11783 |
| 26 | Daidzin * | C21H20O9 | 8.62 | [M + H]+ | 417.1174 | −0.609 | 255.06500, 199.07533, 227.07014 |
| 27 | Calycosin | C16H12O5 | 8.66 | [M − H]− | 283.06033 | −3.062 | 268.03683, 211.03873 |
| 28 | 3,2′-Dihydroxyflavone | C15H10O4 | 8.67 | [M − H]− | 253.04967 | 0.135 | 224.04665, 133.02780 |
| 29 | Genistein-4′-O-glucoside | C21H20O10 | 9.01 | [M + H]+ | 433.11108 | −1.843 | 313.07007, 283.06003, 415.10181 |
| 30 | Genistein-8-C-glucoside | C21H20O10 | 9.09 | [M + H]+ | 433.11276 | −0.163 | 313.07047, 283.05994, 415.10199 |
| 31 | Glycitin * | C22H22O10 | 9.12 | [M + H]+ | 447.12952 | 0.947 | 285.07562, 270.05206, 253.04936 |
| 32 | 3′-Methoxydaidzein | C16H12O5 | 9.14 | [M + H]+ | 285.07556 | −0.19 | 285.07556, 270.05200, 253.04945 |
| 33 | Genistein-8-C-(6″-O-apioside)-glucoside | C26H28O14 | 9.21 | [M + H]+ | 565.1543 | −0.877 | 433.11264, 313.07028, 283.05978 |
| 34 | Apigenin | C15H10O5 | 9.4 | [M + H]+ | 271.05966 | −1.616 | 215.07030, 153.01831, 243.06546 |
| 35 | Thermopsoside | C22H22O11 | 9.62 | [M − H]− | 461.1076 | −0.198 | 298.04742, 341.06580, 326.04242 |
| 36 | Oroxin B | C27H30O15 | 9.62 | [M − H]− | 593.14978 | −0.866 | 269.04340, 341.06537, 298.04712 |
| 37 | Daidzein-4′-O-glycoside | C21H20O9 | 9.64 | [M + H]+ | 417.11743 | −0.579 | 255.06497, 199.07524, 255.06497 |
| 38 | Pueroside A | C29H34O14 | 9.67 | [M + H]+ | 607.2017 | −0.21 | 107.04955, 299.0919, 253.08572 |
| 39 | Genistein-8-C-apiosyl (1-6)- glucoside | C26H28O14 | 10.05 | [M + H]+ | 565.15411 | 1.072 | 271.05994, 433.11292 |
| 40 | Genistin | C21H20O10 | 10.25 | [M + H]+ | 433.1123 | −0.623 | 215.07022, 153.01828, 271.06003 |
| 41 | Emodin | C15H10O5 | 10.25 | [M-H]- | 269.04468 | 0.23 | 224.04639, 133.02776 |
| 42 | Isoembigenin | C23H24O10 | 10.37 | [M + H]+ | 461.144 | −0.388 | 107.04961, 299.09137, 253.08554 |
| 43 | Salicylic acid | C7H6O3 | 10.65 | [M − H]− | 137.02267 | −0.651 | 137.02267, 93.03277 |
| 44 | 6″-O-Malonyl daidzin | C24H22O12 | 10.66 | [M + H]+ | 503.11694 | −1.462 | 255.06517, 199.07542 |
| 45 | Kaempferide | C16H12O6 | 10.93 | [M − H]− | 299.05505 | 0.035 | 284.03162, 255.02873, 299.05505 |
| 46 | Formononetin-8-C-glucosid e-O-xyloside | C27H30O13 | 11 | [M + H]+ | 563.17535 | −0.567 | 431.13345, 311.09109, 281.08060 |
| 47 | Biochanin A * | C16H12O5 | 11.1 | [M − H]− | 283.06015 | 0.05 | 268.03677, 239.03368, 211.03868 |
| 48 | 5-Hydroxy genistein-4′-O-(6″-malonyl) glucoside | C25H24O13 | 11.12 | [M + H]+ | 533.12976 | 0.793 | 285.07574, 270.05219 |
| 49 | Azelaic acid | C9H16O4 | 11.63 | [M − H]− | 187.09612 | −0.385 | 126.09541, 187.09610 |
| 50 | 6″-O-Acetyl Daidzin | C23H22O10 | 11.85 | [M + H]+ | 459.12704 | −1.533 | 255.06511, 199.07536 |
| 51 | 13-HODE | C18H32O3 | 11.93 | [M − H]− | 295.22656 | −0.211 | 277.21674, 195.13684, 224.59799 |
| 52 | Ferulic Acid | C10H10O4 | 11.93 | [M − H]− | 193.04945 | −0.085 | 134.03557, 178.02600 |
| 53 | Genistein-4′-O-(6″-malonyl)glucoside | C24H22O13 | 12.14 | [M + H]+ | 519.11395 | 0.633 | 215.07027, 153.01833, 271.06012 |
| 54 | Curcumenol | C15H22O2 | 12.57 | [M − H]− | 233.15355 | −0.056 | 214.91223, 119.00460 |
| 55 | Chrysin | C15H10O4 | 12.88 | [M − H]− | 253.04951 | −0.025 | 224.04666, 209.05945 |
| 56 | Daidzein * | C15H10O4 | 12.93 | [M + H]+ | 255.06516 | −0.025 | 199.07542, 227.07018, 137.02342 |
| 57 | Glycitein | C16H12O5 | 13.45 | [M + H]+ | 285.07574 | −0.01 | 285.07574, 270.05222, 253.04958 |
| 58 | Ononin | C16H12O5 | 13.68 | [M + H]+ | 431.1364 | −0.019 | 269.08075, 293.08151 |
| 59 | 2″,6″-Di-O-Acetyl isovitexin | C22H22O9 | 14.54 | [M + H]+ | 517.13501 | 0.957 | 268.08087, 254.05725 |
| 60 | Adenine | C20H16O4 | 14.54 | [M + H]+ | 136.0618 | 0.028 | 119.03558, 91.05481 |
| 61 | Tournefolal | C5H5N5 | 15.17 | [M − H]− | 269.04456 | 0.11 | 133.02759, 224.04640 |
| 62 | Genistein | C15H10O5 | 15.25 | [M + H]+ | 271.05997 | −0.13 | 153.01831, 215.07030, 243.06546 |
| 63 | Fraxetin | C15H10O5 | 15.25 | [M + H]+ | 209.19009 | 0.098 | 167.14314, 153.12741, 111.08086 |
| 64 | D-Gluconic acid | C10H8O5 | 15.35 | [M − H]− | 195.0495 | −0.429 | 159.02824, 129.01747 |
| 65 | Guanine | C6H12O7 | 15.6 | [M + H]+ | 152.05678 | 0.094 | 128.04552, 110.03531, 135.03018 |
| 66 | Formononetin-7-O-(6″-acetylglucoside) | C5H5N5O | 16.08 | [M + H]+ | 473.14249 | −1.733 | 269.08066, 254.05722 |
| 67 | Schaftoside | C24H24O10 | 16.64 | [M − H]− | 563.13873 | −0.802 | 311.05511, 283.06024, 133.02768 |
| 68 | Isoferulic acid | C26H28O14 | 17.09 | [M − H]− | 193.04945 | −0.085 | 134.03557, 149.05949 |
| 69 | Formononetin * | C10H10O4 | 17.62 | [M + H]+ | 269.08072 | −0.79 | 213.09091, 118.04166 |
| 70 | Pterolactam | C16H12O4 | 17.63 | [M + H]+ | 116.07082 | 0.215 | 70.06588, 99.01923 |
| 71 | Dimethyl-1-Phenylazulene-4,5-dicarboxylate | C5H9NO2 | 19.92 | [M + H]+ | 321.11185 | −0.286 | 147.04407, 159.08043, 306.08713 |
| 72 | 4-Hydroxybenzaldehyde | C20H16O4 | 19.93 | [M + H]+ | 123.04082 | −3.236 | 118.03519, 100.02482, 82.01437 |
| 73 | Licoflavone A | C7H6O2 | 21.06 | [M + H]+ | 323.12753 | −0.256 | 267.06509, 239.07011, 199.07509 |
| 74 | 5-Hydroxy-6,7-dimethoxyflavone | C20H18O4 | 21.09 | [M − H]− | 297.07553 | −0.215 | 282.05240, 267.02899, 239.03381 |
| 75 | Neobavaisoflavone | C17H14O5 | 21.1 | [M − H]− | 321.11197 | −0.166 | 237.05444, 265.04950, 277.04938 |
| 76 | Isoliquiritigenin | C20H18O4 | 21.1 | [M + H]+ | 257.08066 | −0.175 | 137.02339, 147.04402, 119.04937 |
| 77 | Glabrone | C15H12O4 | 23.5 | [M − H]− | 335.09191 | 0.21 | 307.09659, 291.06564, 277.04761 |
| 78 | Oleamide | C20H16O5 | 23.63 | [M + H]+ | 282.27893 | −0.211 | 265.25244, 247.24191, 69.07063, 83.08614 |
| 79 | Stigmasterol | C18H35NO | 23.72 | [M + H]+ | 413.37805 | 0.257 | 109.06524,97.06529, 395.36667 |
| 80 | Physcion | C29H48O | 23.73 | [M − H]− | 283.06024 | 0.14 | 268.03671, 239.03365, 211.03847 |
| 81 | β-Sitosterol | C16H12O5 | 26.98 | [M + H]+ | 415.72112 | 4.522 | 91.05481, 384.09723 |
| 82 | Isoliquiritigenin | C10H14O | 26.99 | [M − H]− | 255.06522 | 0.035 | 119.04842, 135.00705, 153.01749 |
| 83 | Proline | C5H9NO2 | 27.56 | [M + H]+ | 116.07082 | 0.215 | 70.06588, 99.01923, 73.04050 |
| 84 | Allantoin | C4H6N4O3 | 27.69 | [M − H]− | 157.03476 | −0.857 | 114.02900, 111.95873 |
| 85 | Glyinflanin G | C25H24O5 | 29.59 | [M + H]+ | 405.16962 | −0.03 | 281.0444, 319.09567, 293.04517 |
| 86 | Psoralidin | C20H16O5 | 29.59 | [M + H]+ | 337.10654 | −0.51 | 309.11124, 281.11731, 253.05052, 223.07600 |
| 87 | Curcumanolide A | C15H22O2 | 35.42 | [M + H]+ | 235.16924 | −0.016 | 179.10669, 123.04434 |
| 88 | 7-Methoxycoumarin | C10H8O3 | 38.04 | [M + H]+ | 177.05463 | 0.009 | 63.03896, 149.05975, 135.04413, 117.03380 |
| No. | Compound | IC50 | Docking Energy | Active Site Residues | Hydrogen Bond Number |
|---|---|---|---|---|---|
| (mg/L) | (kcal/mol) | ||||
| 1 | 3′-Hydroxy puerarin | 203.6 ± 4.2 | −8.6 | GLU-767, ILE-734, ARG-653 | 4 |
| 2 | Puerarin | 154.2 ± 1.7 | −8.1 | GLU-658, ILE-734, ARG-653, TYR-733 | 4 |
| 3 | 3′-Methoxy-puerarin | 95.9 ± 3.8 | −7.9 | GLU-767, GLU-658, ARG-643, ARG-647, ARG-653, TYR-660, GLU-788 | 9 |
| 4 | Daidzin | 72.94 ± 2.5 | −8.3 | GLU-658, GLN-272, ARG-653, TYR-660 | 5 |
| 5 | Glycitin | 139.7 ± 5.1 | −9.0 | GLU-658, GLU-767, TYR-660 | 3 |
| 6 | Glycitein | 246.0 ± 2.6 | −8.1 | LEU-754, LYS-817, HIS-625 | 3 |
| 7 | Genistin | 101.3 ± 6.3 | −8.7 | GLN-275, ASP-649, GLN-272, ARG-653, GLU-767, ILE-734 | 8 |
| 8 | Daidzein | 114.8 ± 1.9 | −8.1 | GLU-767 | 2 |
| 9 | Genistein | / | −8.1 | / | / |
| 10 | Formononetin | 128.3 ± 2.0 | −8.1 | GLU-767 | 2 |
| 11 | Puerarin 6″-O-xyloside | 169.6 ± 3.4 | −8.7 | LYS-776,ARG-283,ALA-644 | 4 |
| 12 | puerarin apioside | 96.55 ± 2.6 | −9.0 | GLU-767,TYR-660,AGR-730 | 3 |
| 13 | Acarbose | 58.6 ± 4.4 | −7.9 | GLN-275,ASP-759,GLN-272,ARG-730,GLU-662,TYR-660,GLY-731 | 10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Zhao, H.; Zhu, F.; Liu, X.; Liu, Y.; Zeng, F.; Liu, B. Analysis of Isoflavones in Pueraria by UHPLC-Q-Orbitrap HRMS and Study on α-Glucosidase Inhibitory Activity. Foods 2022, 11, 3523. https://doi.org/10.3390/foods11213523
Yang Y, Zhao H, Zhu F, Liu X, Liu Y, Zeng F, Liu B. Analysis of Isoflavones in Pueraria by UHPLC-Q-Orbitrap HRMS and Study on α-Glucosidase Inhibitory Activity. Foods. 2022; 11(21):3523. https://doi.org/10.3390/foods11213523
Chicago/Turabian StyleYang, Yan, Hui Zhao, Furong Zhu, Xiaoyan Liu, Yu Liu, Feng Zeng, and Bin Liu. 2022. "Analysis of Isoflavones in Pueraria by UHPLC-Q-Orbitrap HRMS and Study on α-Glucosidase Inhibitory Activity" Foods 11, no. 21: 3523. https://doi.org/10.3390/foods11213523
APA StyleYang, Y., Zhao, H., Zhu, F., Liu, X., Liu, Y., Zeng, F., & Liu, B. (2022). Analysis of Isoflavones in Pueraria by UHPLC-Q-Orbitrap HRMS and Study on α-Glucosidase Inhibitory Activity. Foods, 11(21), 3523. https://doi.org/10.3390/foods11213523