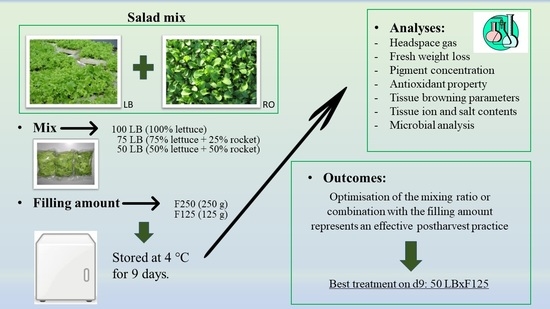
The Mixing Ratio and Filling-Amount Affect the Tissue Browning and Antioxidant Properties of Fresh-Cut Baby Leaf Lettuce (Lactuca sativa L.) and Rocket (Eruca sativa Mill.) Grown in Floating Growing Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growing Conditions
2.2. Processing, Packaging, and Storage Conditions
2.3. Headspace Gas and Fresh Weight Loss Assay
2.4. Pigment Content Analysis
2.5. Antioxidant Property Parameter Assay
2.6. Tissue Browning Parameter Assay
2.7. Tissue Ion and Salt Content Assay
2.8. Microbial Analysis
2.9. Statistical Analysis
3. Results
3.1. The Headspace Gas and Fresh Weight Loss
3.2. Pigment Content
3.3. Antioxidant Property Parameters
3.3.1. Antioxidant Capacity
3.3.2. Total Phenolic Content
3.3.3. Ascorbic Acid and Dehydroascorbic Acid Contents
3.4. Tissue Browning Parameters
3.4.1. Browning Potential and So-Q Content
3.4.2. Enzyme Activity
3.5. Ion and Salt Contents of the Tissue
3.6. Microbial Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- D’Imperio, M.; Renna, M.; Cardinali, A.; Buttaro, D.; Serio, F.; Santamaria, P. Calcium biofortification and bio accessibility in soilless “baby leaf” vegetable production. Food Chem. 2016, 213, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Lenzi, A.; Orlandini, A.; Bulgari, R.; Ferrante, A.; Bruschi, P. Antioxidant and Mineral Composition of Three Wild Leafy Species: A Comparison Between Microgreens and Baby Greens. Foods 2019, 8, 487. [Google Scholar] [CrossRef] [PubMed]
- Di Gioia, F.; Avato, P.; Serio, F.; Argentieri, M.P. Glucosinolate profile of Eruca sativa, Diplotaxis tenuifolia and Diplotaxis erucoides grown in soil and soilless systems. J. Food Compos. Anal. 2018, 69, 197–204. [Google Scholar] [CrossRef]
- Conversa, G.; Bonasia, A.; Lazzizera, C.; La Rotonda, P.; Elia, A. Reduction of Nitrate Content in Baby-Leaf Lettuce and Cichorium endivia through the Soilless Cultivation System, Electrical Conductivity and Management of Nutrient Solution. Front. Plant Sci. 2021, 12, 645671. [Google Scholar] [CrossRef]
- Ayhan, Z. Effect of Packaging on the Quality and Shelf-life of Minimally Processed/Ready to Eat Foods. Akademik Gıda 2011, 9, 36–41. [Google Scholar]
- Pignata, G.; Ertani, A.; Casale, M.; Piano, S.; Nicola, S. Mixing fresh-cut baby green and red leaf lettuce from soilless cultivation preserves phytochemical content and safety. Agric. Food Sci. 2020, 29, 55–65. [Google Scholar] [CrossRef]
- Kumar, D.; Kumar, S.; Shekhar, C. Nutritional components in green leafy vegetables: A review. J. Pharmacogn. Phytochem. 2020, 9, 2498–2502. [Google Scholar]
- Keiner, R.; Frosch, T.; Massad, T.; Trumbore, S.; Popp, J. Enhanced Raman multigas sensing—A novel tool for control and analysis of13CO2labeling experiments in environmental research. Analyst 2014, 139, 3879–3884. [Google Scholar] [CrossRef]
- Mudau, A.R.; Araya, H.T.; Mudau, F.N. The quality of baby spinach as affected by developmental stage as well as postharvest storage conditions. Acta Agric. Scand. Sect. B—Soil Plant Sci. 2018, 69, 26–35. [Google Scholar] [CrossRef]
- De Corato, U. Improving the shelf-life and quality of fresh and minimally-processed fruits and vegetables for a modern food industry: A comprehensive critical review from the traditional technologies into the most promising advancements. Crit. Rev. Food Sci. Nutr. 2019, 60, 940–975. [Google Scholar] [CrossRef]
- Zhan, L. The Qualitative and Physiological Responses of Minimally Processed Leafy Vegetables to Pre/Postharvest Factors. Ph.D. Dissertation, Università degli Studi di Torino, Torino, Italy, 2009. [Google Scholar]
- Islam, M.Z.; Lee, Y.; Mele, M.A.; Choi, I.; Kang, H. Physicochemical Properties and Antioxidants Capacity of Baby Leaf Lettuce Influence by Modified Atmosphere Packaging. Europe PMC 2019. [Google Scholar] [CrossRef]
- Batziakas, K.G.; Singh, S.; Ayub, K.; Kang, Q.; Brecht, J.K.; Rivard, C.L.; Pliakoni, E.D. Reducing postharvest losses of spinach stored at non optimum temperatures with the implementation of passive modified atmosphere packaging. HortScience 2020, 55, 326–335. [Google Scholar] [CrossRef]
- Carvalho, P.D.T.; Clemente, E. The influence of the broccoli (Brassica oleracea var. itálica)fill weigth on postharvest quality. Food Sci. Technol. 2004, 24, 646–651. [Google Scholar] [CrossRef][Green Version]
- Nicola, S.; Fontana, E.; Tibaldi, G.; Zhan, L. Qualitative and physiological response of minimally processed rocket (Eruca sativa mill.) to package filling amount and shelf-life temperature. Acta Hortic. 2010, 877, 611–618. [Google Scholar] [CrossRef]
- Nicola, S.; Fontana, E. Fresh-cut produce quality: Implications for a systems approach. In Postharvest Handling: A Systems Approach, 3rd ed.; Florkowski, W.J., Shewfelt, R., Breuckner, B., Prussia, S.E., Eds.; Academic Press/Elsevier: San Diego, CA, USA, 2014; Volume 9, pp. 217–273. [Google Scholar]
- Gil, M.I.; Garrido, Y. Leafy vegetables: Baby leaves. In Controlled and Modified Atmospheres for Fresh and Fresh-Cut Produce; Academic Press: Cambridge, MA, USA, 2020; pp. 527–536. [Google Scholar]
- Spinardi, A.; Ferrante, A. Effect of storage temperature on quality changes of minimally processed baby lettuce. J. Food Agric. Environ. 2012, 10, 38–42. [Google Scholar]
- Kim, M.J.; Moon, Y.; Tou, J.C.; Mou, B.; Waterland, N.L. Nutritional value, bioactive compounds and health benefits of lettuce (Lactuca sativa L.). J. Food Compos. Anal. 2016, 49, 19–34. [Google Scholar] [CrossRef]
- Damerum, A.; A Chapman, M.; Taylor, G. Innovative breeding technologies in lettuce for improved post-harvest quality. Postharvest Biol. Technol. 2020, 168, 111266. [Google Scholar] [CrossRef]
- Martínez-Ispizua, E.; Calatayud, Á.; Marsal, J.I.; Cannata, C.; Basile, F.; Abdelkhalik, A.; Soler, S.; Valcárcel, J.V.; Martínez-Cuenca, M.-R. The Nutritional Quality Potential of Microgreens, Baby Leaves, and Adult Lettuce: An Underexploited Nutraceutical Source. Foods 2022, 11, 423. [Google Scholar] [CrossRef]
- Koukounaras, A.; Bantis, F.; Karatolos, N.; Melissas, C.; Vezyroglou, A. Influence of Pre-Harvest Factors on Postharvest Quality of Fresh-Cut and Baby Leafy Vegetables. Agronomy 2020, 10, 172. [Google Scholar] [CrossRef]
- Cavaiuolo, M.; Ferrante, A. Nitrates and Glucosinolates as Strong Determinants of the Nutritional Quality in Rocket Leafy Salads. Nutrients 2014, 6, 1519–1538. [Google Scholar] [CrossRef]
- Spadafora, N.D.; Cocetta, G.; Ferrante, A.; Herbert, R.J.; Dimitrova, S.; Davoli, D.; Fernández, M.; Patterson, V.; Vozel, T.; Amarysti, C.; et al. Short-Term Post-Harvest Stress that Affects Profiles of Volatile Organic Compounds and Gene Expression in Rocket Salad during Early Post-Harvest Senescence. Plants 2019, 9, 4. [Google Scholar] [CrossRef] [PubMed]
- Zhan, L.; Fontana, E.; Tibaldi, G.; Nicola, S. Qualitative and physiological response of minimally processed garden cress (Lepidium sativum L.) to harvest handling and storage conditions. J. Food Agric. Environ. 2009, 7, 43–50. [Google Scholar]
- Nicola, S.; Pignata, G.; Casale, M.; Turco, P.E.L.; Gaino, W. Overview of a Lab-scale Pilot Plant for Studying Baby Leaf Vegetables Grown in Soilless Culture. Hortic. J. 2016, 85, 97–104. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Analysis 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Kampfenkel, K.; Van Montagu, M.; Inzé, D. Extraction and determination of ascorbate and dehydroascorbate from plant tissue. Anal. Biochem. 1995, 225, 165–167. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Couture, R.; Cantwell, M.; Ke, D.; Saltveit, M. Physiological Attributes Related to Quality Attributes and Storage Life of Minimally Processed Lettuce. HortScience 1993, 28, 723–725. [Google Scholar] [CrossRef]
- Tardelli, F.; Guidi, L.; Massai, R.; Toivonen, P.M. Effects of 1-methylcyclopropene and post-controlled atmosphere air storage treatments on fresh-cut Ambrosia apple slices. J. Sci. Food Agric. 2012, 93, 262–270. [Google Scholar] [CrossRef]
- Degl’Innocenti, E.; Guidi, L.; Pardossi, A.; Tognoni, F. Biochemical study of leaf browning in minimally processed leaves of lettuce (Lactuca sativa L. Var. acephala). J. Agric, Food Chem. 2005, 53, 9980–9984. [Google Scholar] [CrossRef]
- ISO 4833:2003; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Microorganisms: Colony-Count Technique at 30 °C. ISO: Geneva, Switzerland, 2003.
- Abadias, M.; Usall, J.; Anguera, M.; Solsona, C.; Viñas, I. Microbiological quality of fresh, minimally-processed fruit and vegetables, and sprouts from retail establishments. Int. J. Food Microbiol. 2008, 123, 121–129. [Google Scholar] [CrossRef] [PubMed]
- ISO 21527-1:2008; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Yeasts and Moulds—Part 1: Colony Count Technique in Products with Water Activity Greater Than 0.95. ISO: Geneva, Switzerland, 2008.
- Tzortzakis, N.; Nicola, S.; Savvas, D.; Voogt, W. Editorial: Soilless Cultivation through an Intensive Crop Production Scheme. Management Strategies, Challenges and Future Directions. Front. Plant Sci. 2020, 11, 363. [Google Scholar] [CrossRef] [PubMed]
- Fontana, E.; Nicola, S. Producing Garden Cress (Lepidium sativum L.) for the Fresh-cut Chain using Soilless Culture System. J. Hort. Sci. Biotechnol. 2008, 83, 23–32. [Google Scholar] [CrossRef]
- Fontana, E.; Nicola, S. Traditional and Soilless Culture Systems to Produce Corn Salad (Valerianella olitoria L.) and Rocket (Eruca sativa Mill.) with Low Nitrate Content. J. Food Agric. Environ. 2009, 7, 405–410. [Google Scholar]
- Nicola, S.; Pignata, G.; Tibaldi, G. The floating growing system can assure a low microbial contamination of baby leaf vegetables at harvest. Acta Hortic. 2018, 1209, 57–64. [Google Scholar] [CrossRef]
- Jia, T.; Ito, H.; Hu, X.; Tanaka, A. Accumulation of the non yellow coloring 1 protein of the chlorophyll cycle requires chlorophyll b in Arabidopsis thaliana. Plant J. 2015, 81, 11. [Google Scholar] [CrossRef]
- Zhan, L.; Li, J.; Huang, W.; Song, C.; Li, J.; Pang, L.; Li, Y. Light irradiation affects the total antioxidant capacity, total phenolic compounds, phenolic acids, and related enzyme activities of minimally processed spinach (Spinacia oleracea L.). J Food Process. Preserv. 2020, 44, e14825. [Google Scholar] [CrossRef]
- Degl’Innocenti, E.; Pardossi, A.; Tognoni, F.; Guidi, L. Physiological basis of sensitivity to enzymatic browning in ‘lettuce’,‘escarole’and ‘rocket salad’when stored as fresh-cut products. Food Chem. 2007, 104, 209–215. [Google Scholar] [CrossRef]
- A Ansah, F.; Amodio, M.L.; Colelli, G. Quality of fresh-cut products as affected by harvest and postharvest operations. J. Sci. Food Agric. 2018, 98, 3614–3626. [Google Scholar] [CrossRef]
- Yousuf, B.; Deshi, V.; Ozturk, B.; Siddiqui, M.W. Fresh-Cut Fruits and Vegetables: Quality Issues and Safety Concerns; Siddiqui, M.W., Ed.; Fresh-Cut Fruits and Vegetables; Academic Press: Cambridge, MA, USA, 2020; pp. 1–15. [Google Scholar]
| O2 (%) | CO2 (%) | Chl. a (mg g−1 FW) | Chl. b (mg g−1 FW) | Car. (mg g−1 FW) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| d1 | d9 | d1 | d9 | d1 | d9 | d1 | d9 | d1 | d9 | |
| Mix | ||||||||||
| 100 LB | 18.75 a z | 16.65 | 1.85 b | 3.10 | 0.18 b | 0.11 b | 0.06 b | 0.04 b | 0.08 b | 0.04 b |
| 75 LB | 16.80 b | 12.93 | 3.43 a | 5.83 | 0.20 ab | 0.26 a | 0.07 b | 0.07 a | 0.09 b | 0.10 a |
| 50 LB | 16.72 b | 16.28 | 3.58 a | 3.47 | 0.27 a | 0.29 a | 0.09 a | 0.08 a | 0.12 a | 0.11a |
| Filling amount | ||||||||||
| F250 | 16.53 b | 14.73 | 3.78 a | 4.79 | 0.24 a | 0.21 | 0.08 a | 0.06 | 0.10 | 0.08 |
| F125 | 18.31 a | 15.84 | 2.13 b | 3.48 | 0.19 b | 0.23 | 0.06 b | 0.07 | 0.08 | 0.09 |
| Mix × Filling amount | ||||||||||
| 100 LB × F250 | 18.07 | 15.83 | 2.60 | 3.80 | 0.17 | 0.11 | 0.05 | 0.03 | 0.08 | 0.04 |
| 100 LB × F125 | 19.43 | 17.47 | 1.10 | 2.40 | 0.18 | 0.11 | 0.06 | 0.05 | 0.08 | 0.04 |
| 75 LB × F250 | 15.23 | 11.50 | 4.83 | 7.10 | 0.22 | 0.22 | 0.07 | 0.06 | 0.09 | 0.08 |
| 75 LB × F125 | 18.37 | 14.37 | 2.03 | 4.57 | 0.19 | 0.30 | 0.06 | 0.08 | 0.09 | 0.12 |
| 50 LB × F250 | 16.30 | 16.87 | 3.90 | 3.47 | 0.34 | 0.31 | 0.11 | 0.09 | 0.14 | 0.11 |
| 50 LB × F125 | 17.13 | 15.70 | 3.27 | 3.47 | 0.20 | 0.27 | 0.07 | 0.07 | 0.09 | 0.10 |
| Mean | 17.42 | 15.29 | 2.96 | 4.13 | 0.21 | 0.22 | 0.07 | 0.06 | 0.09 | 0.08 |
| SE | 0.45 | 2.15 | 0.37 | 1.57 | 0.03 | 0.03 | 0.01 | 0.01 | 0.01 | 0.01 |
| Significance | ||||||||||
| Mix | <0.001 | 0.181 | <0.001 | 0.186 | 0.006 | <0.001 | 0.001 | <0.001 | 0.005 | <0.001 |
| Filling amount | <0.001 | 0.531 | <0.001 | 0.315 | 0.021 | 0.610 | 0.025 | 0.307 | 0.061 | 0.434 |
| Mix × Filling amount | 0.040 | 0.634 | 0.025 | 0.724 | 0.033 | 0.192 | 0.013 | 0.160 | 0.022 | 0.152 |
| Chl. a (mg g−1 FW) | Chl. b (mg g−1 FW) | Car. (mg g−1 FW) | TP (mg Gallic Acid g−1 FW) | AA (mg g−1 FW) | PAL (µmol Cinnamic Acid h−1 g−1 FW) | PO43− (mg g−1 FW) | CaCO3 (mg g−1 FW) | |
|---|---|---|---|---|---|---|---|---|
| Species | ||||||||
| LB | 0.20 b | 0.06 b | 0.09 b | 0.26 b | 0.23 b | 0.63 b | 0.14 a | 0.55 b |
| RO | 0.53 a | 0.15 a | 0.21 a | 0.70 a | 0.38 a | 2.36 a | 0.07 b | 2.16 a |
| Mean | 0.37 | 0.11 | 0.15 | 0.48 | 0.31 | 1.49 | 0.11 | 1.35 |
| Significance | 0.002 | 0.001 | 0.002 | 0.001 | 0.022 | 0.016 | 0.020 | <0.001 |
| AC (μmol Fe2+ g−1 FW) | TP (mg Gallic Acid g−1 FW) | AA (mg g−1 FW) | DHAA (mg g−1 FW) | |||||
|---|---|---|---|---|---|---|---|---|
| d1 | d9 | d1 | d9 | d1 | d9 | d1 | d9 | |
| Mix | ||||||||
| 100 LB | 2.49 b z | 2.07 | 0.22 b | 0.16 c | 0.219 c | 0.115 c | 0.030 b | 0.068 b |
| 75 LB | 2.68 b | 2.10 | 0.25 b | 0.26 b | 0.307 b | 0.164 b | 0.035 ab | 0.064 b |
| 50 LB | 3.44 a | 2.39 | 0.37 a | 0.37 a | 0.330 a | 0.271 a | 0.040 a | 0.083 a |
| Filling amount | ||||||||
| F250 | 2.99 | 2.17 | 0.30 | 0.22 b | 0.296 a | 0.170 b | 0.036 | 0.071 |
| F125 | 2.75 | 2.20 | 0.26 | 0.31 a | 0.275 b | 0.197 a | 0.034 | 0.072 |
| Mix × Filling amount | ||||||||
| 100 LB × F250 | 2.84 | 2.09 | 0.25 | 0.15 | 0.227 | 0.111 | 0.035 | 0.072 |
| 100 LB × F125 | 2.13 | 2.05 | 0.18 | 0.17 | 0.211 | 0.120 | 0.025 | 0.065 |
| 75 LB × F250 | 2.62 | 2.27 | 0.28 | 0.25 | 0.324 | 0.146 | 0.035 | 0.062 |
| 75 LB × F125 | 2.73 | 1.93 | 0.23 | 0.27 | 0.290 | 0.183 | 0.035 | 0.066 |
| 50 LB × F250 | 3.50 | 2.15 | 0.36 | 0.26 | 0.337 | 0.254 | 0.037 | 0.079 |
| 50 LB × F125 | 3.39 | 2.63 | 0.37 | 0.48 | 0.323 | 0.288 | 0.043 | 0.087 |
| Mean | 2.87 | 2.19 | 0.28 | 0.26 | 0.285 | 0.183 | 0.035 | 0.072 |
| SE | 0.19 | 0.13 | 0.02 | 0.03 | 0.006 | 0.011 | 0.003 | 0.005 |
| Significance | ||||||||
| Mix | <0.001 | 0.058 | <0.001 | <0.001 | <0.001 | <0.001 | 0.021 | 0.002 |
| Filling amount | 0.130 | 0.764 | 0.085 | <0.001 | <0.001 | 0.005 | 0.563 | 0.734 |
| Mix × Filling amount | 0.092 | 0.017 | 0.232 | 0.001 | 0.258 | 0.372 | 0.082 | 0.291 |
| BP (Abs340) | So-Q (Abs437) | PAL (µmol Cinnamic Acid h−1 g−1 FW) | PPO (Units g−1 FW) | |||||
|---|---|---|---|---|---|---|---|---|
| d1 | d9 | d1 | d9 | d1 | d9 | d1 | d9 | |
| Mix | ||||||||
| 100 LB | 0.15 c | 0.17 c | 0.10 c | 0.10 b | 0.83 b | 0.97 c | 18.41 ab z | 25.30 b |
| 75 LB | 0.35 b | 0.32 b | 0.12 b | 0.14 ab | 1.12 b | 1.45 b | 17.80 b | 25.23 b |
| 50 LB | 0.68 a | 0.69 a | 0.17 a | 0.21 a | 2.19 a | 2.28 a | 20.38 a | 27.80 a |
| Filling amount | ||||||||
| F250 | 0.41 | 0.33 b | 0.12 | 0.13 | 1.46 | 1.31 b | 19.14 | 28.54 a |
| F125 | 0.37 | 0.45 a | 0.13 | 0.18 | 1.30 | 1.83 a | 18.59 | 23.69 b |
| Mix × Filling amount | ||||||||
| 100 LB × F250 | 0.15 | 0.14 | 0.09 | 0.08 | 0.79 | 0.97 | 19.39 | 29.55 |
| 100 LB × F125 | 0.14 | 0.19 | 0.10 | 0.13 | 0.88 | 0.97 | 17.42 | 21.06 |
| 75 LB × F250 | 0.35 | 0.24 | 0.12 | 0.09 | 1.20 | 1.11 | 17.12 | 26.82 |
| 75 LB × F125 | 0.34 | 0.40 | 0.13 | 0.20 | 1.04 | 1.79 | 18.48 | 23.64 |
| 50 LB × F250 | 0.72 | 0.61 | 0.16 | 0.21 | 2.40 | 1.85 | 20.91 | 29.24 |
| 50 LB × F125 | 0.64 | 0.77 | 0.17 | 0.20 | 1.99 | 2.71 | 19.85 | 26.36 |
| Mean | 0.39 | 0.39 | 0.13 | 0.15 | 1.38 | 1.57 | 18.86 | 26.11 |
| SE | 0.03 | 0.04 | 0.01 | 0.03 | 0.12 | 0.17 | 1.02 | 0.79 |
| Significance | ||||||||
| Mix | <0.001 | <0.001 | <0.001 | 0.005 | <0.001 | <0.001 | 0.045 | 0.004 |
| Filling amount | 0.142 | <0.001 | 0.141 | 0.053 | 0.133 | 0.001 | 0.512 | <0.001 |
| Mix × Filling amount | 0.294 | 0.252 | 0.930 | 0.176 | 0.158 | 0.041 | 0.260 | 0.002 |
| NO3− (mg g−1 FW) | PO43− (mg g−1 FW) | CaCO3 (mg g−1 FW) | TB (cfu g−1 FW) | Y + M (cfu g−1 FW) | ||||
|---|---|---|---|---|---|---|---|---|
| d1 | d9 | d1 | d9 | d1 | d9 | d9 | d9 | |
| Mix | ||||||||
| 100 LB | 0.49 z | 0.28 b | 0.14 a | 0.15 a | 0.47 c | 0.35 c | 5.91 × 103 b | 4.63 × 101 b |
| 75 LB | 0.44 | 0.28 b | 0.13 a | 0.13 b | 0.93 b | 0.71 b | 7.74 × 104 a | 8.00 × 101 a |
| 50 LB | 0.43 | 0.36 a | 0.10 b | 0.10 c | 1.22 a | 1.22 a | 4.62 × 104 ab | 1.01 × 102 a |
| Filling amount | ||||||||
| F250 | 0.45 | 0.30 | 0.12 | 0.13 | 0.86 | 0.69 b | 2.65 × 104 | 8.43 × 101 |
| F125 | 0.46 | 0.32 | 0.13 | 0.12 | 0.89 | 0.83 a | 5.98 × 104 | 6.69 × 101 |
| Mix × Filling amount | ||||||||
| 100 LB × F250 | 0.47 | 0.30 | 0.13 | 0.15 | 0.43 | 0.30 | 6.82 × 103 | 5.53 × 101 |
| 100 LB × F125 | 0.51 | 0.26 | 0.15 | 0.15 | 0.51 | 0.40 | 5.00 × 103 | 3.73 × 101 |
| 75 LB × F250 | 0.46 | 0.27 | 0.13 | 0.14 | 0.89 | 0.57 | 3.63 × 104 | 7.93 × 101 |
| 75 LB × F125 | 0.41 | 0.29 | 0.14 | 0.11 | 0.96 | 0.84 | 1.18 × 105 | 8.07 × 101 |
| 50 LB × F250 | 0.41 | 0.33 | 0.10 | 0.09 | 1.25 | 1.20 | 3.64 × 104 | 1.18 × 102 |
| 50 LB × F125 | 0.45 | 0.39 | 0.11 | 0.10 | 1.19 | 1.23 | 5.59 × 104 | 8.27 × 101 |
| Mean | 0.45 | 0.31 | 0.13 | 0.12 | 0.87 | 0.76 | 4.31 × 104 | 7.56 × 101 |
| SE | 0.04 | 0.03 | 0.01 | 0.01 | 0.07 | 0.06 | 2.03 × 104 | 1.20 × 101 |
| Significance | ||||||||
| Mix | 0.252 | 0.005 | 0.001 | <0.001 | <0.001 | <0.001 | 0.006 | <0.001 |
| Filling amount | 0.727 | 0.457 | 0.068 | 0.066 | 0.649 | 0.011 | 0.055 | 0.085 |
| Mix × Filling amount | 0.437 | 0.125 | 0.479 | 0.049 | 0.578 | 0.132 | 0.119 | 0.318 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhan, L.; Bulgari, R.; Pignata, G.; Casale, M.; Nicola, S. The Mixing Ratio and Filling-Amount Affect the Tissue Browning and Antioxidant Properties of Fresh-Cut Baby Leaf Lettuce (Lactuca sativa L.) and Rocket (Eruca sativa Mill.) Grown in Floating Growing Systems. Foods 2022, 11, 3515. https://doi.org/10.3390/foods11213515
Zhan L, Bulgari R, Pignata G, Casale M, Nicola S. The Mixing Ratio and Filling-Amount Affect the Tissue Browning and Antioxidant Properties of Fresh-Cut Baby Leaf Lettuce (Lactuca sativa L.) and Rocket (Eruca sativa Mill.) Grown in Floating Growing Systems. Foods. 2022; 11(21):3515. https://doi.org/10.3390/foods11213515
Chicago/Turabian StyleZhan, Lijuan, Roberta Bulgari, Giuseppe Pignata, Manuela Casale, and Silvana Nicola. 2022. "The Mixing Ratio and Filling-Amount Affect the Tissue Browning and Antioxidant Properties of Fresh-Cut Baby Leaf Lettuce (Lactuca sativa L.) and Rocket (Eruca sativa Mill.) Grown in Floating Growing Systems" Foods 11, no. 21: 3515. https://doi.org/10.3390/foods11213515
APA StyleZhan, L., Bulgari, R., Pignata, G., Casale, M., & Nicola, S. (2022). The Mixing Ratio and Filling-Amount Affect the Tissue Browning and Antioxidant Properties of Fresh-Cut Baby Leaf Lettuce (Lactuca sativa L.) and Rocket (Eruca sativa Mill.) Grown in Floating Growing Systems. Foods, 11(21), 3515. https://doi.org/10.3390/foods11213515