Understanding the Development of Heterocyclic Aromatic Amines in Fried Bacon and in the Remaining Oil after Pan-Frying in Five Different Vegetable Oils
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Bacon Preparation
2.3. Determination of Physicochemical Characteristics of Vegetable Oils
2.3.1. Fatty Acid Composition
2.3.2. Total Free Fatty Acid (FFA) Content
2.3.3. Peroxide Value (POV), Malondialdehyde (MDA) Content, and Carbonyl Group Value (CGV)
2.4. Determination of the Contents of HAA Precursors
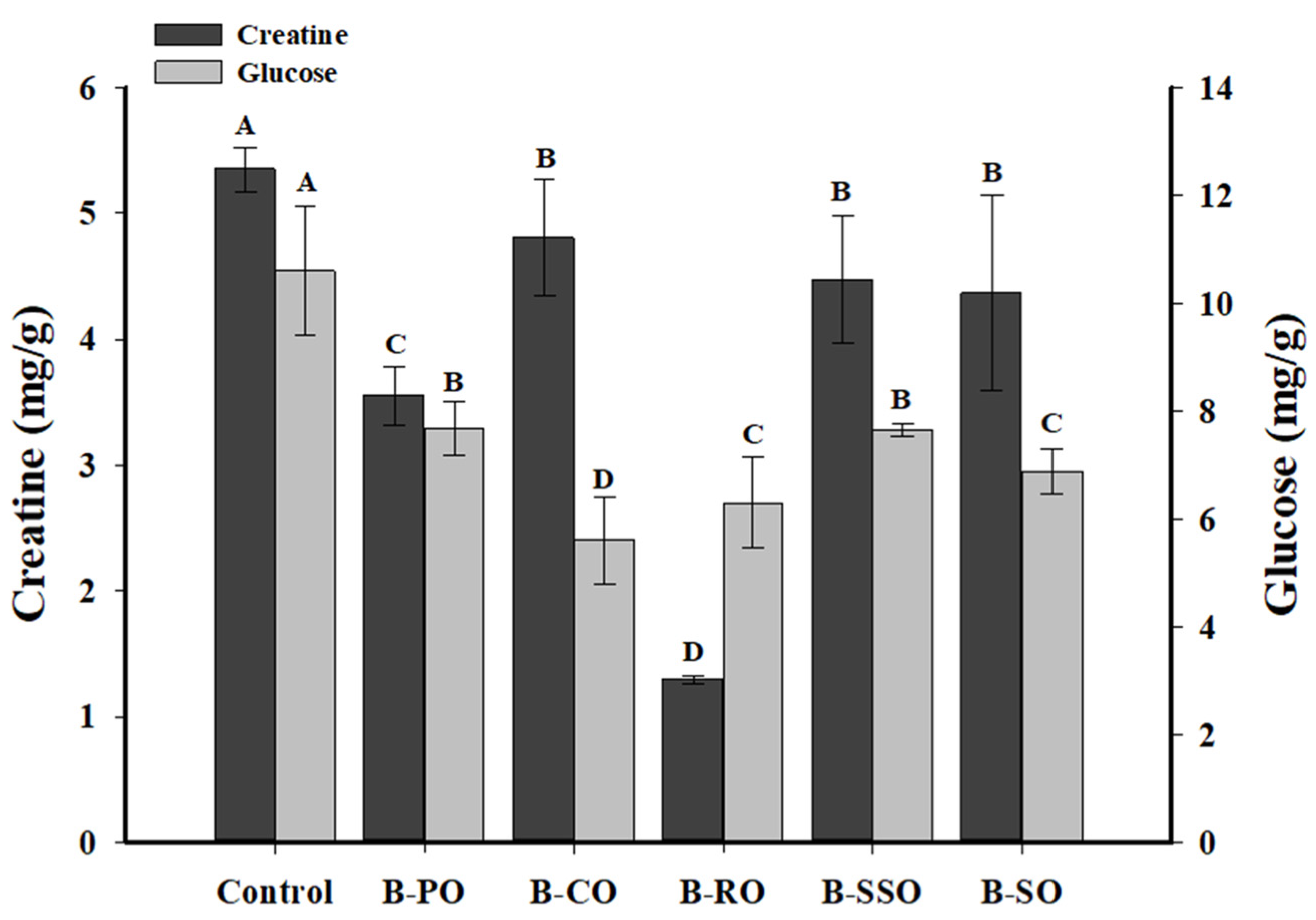
2.4.1. Creatine Content
2.4.2. Glucose Content
2.4.3. FAA Contents
2.5. Extraction and Determination of HAAs in Bacon and Frying Oil
2.6. Sensory Evaluation
2.7. Statistical Analysis
3. Results and Discussion
3.1. Fatty Acid Composition and Quality Characteristics of Vegetable Oils
3.2. The Contents of HAA Precursors in Bacon
3.3. HAA Contents
| Control | B–PO | B–CO | B–RO | B–SSO | B–SO | |
|---|---|---|---|---|---|---|
| Norharman | 5.40 ± 0.33 E | 16.17 ± 1.12 D | 45.66 ± 2.61 A | 21.10 ± 1.28 CD | 25.33 ± 0.66 C | 35.73 ± 1.85 B |
| Harman | 0.19 ± 0.01 E | 6.23 ± 0.43 D | 20.83 ± 1.19 A | 10.32 ± 0.60 C | 8.49 ± 0.22 CD | 14.40 ± 0.75 B |
| AαC | 1.66 ± 0.14 A | 0.38 ± 0.03 B | 0.29 ± 0.03 B | 0.29 ± 0.02 B | 0.27 ± 0.01 B | 0.42 ± 0.02 B |
| MeAαC | n.d. | 7.99 ± 0.55 B | 18.47 ± 1.06 A | 8.15 ± 0.47 B | 5.48 ± 0.14 B | 8.05 ± 0.42 B |
| Non-polar HAAs | 7.25 ± 0.49 D | 30.77 ± 2.13 C | 85.26 ± 4.88 A | 39.86 ± 2.30 C | 39.57 ± 1.03 C | 58.60 ± 3.03 B |
| IQ | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| 7,8-DiMeIQx | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| MeIQx | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| IQx | 0.39 ± 0.02 D | 0.96 ± 0.07 A | 0.89 ± 0.09 A | 0.55 ± 0.03 C | 0.54 ± 0.01 C | 0.68 ± 0.04 B |
| 4,8-DiMeIQx | 0.51 ± 0.03 B | 0.76 ± 0.04 A | 0.71 ± 0.04 A | 0.53 ± 0.03 B | 0.50 ± 0.02 B | 0.59 ± 0.04 B |
| MeIQ | 0.64 ± 0.03 C | 0.70 ± 0.05 C | 1.25 ± 0.13 B | 0.85 ± 0.05 C | 1.10 ± 0.03 B | 1.84 ± 0.09 A |
| PhIP | 1.99 ± 0.14 D | 5.31 ± 0.37 C | 7.05 ± 0.40 B | 9.73 ± 0.51 A | 3.11 ± 0.08 D | 2.66 ± 0.37 D |
| Phe-P-I | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Polar HAAs | 3.52 ± 0.18 E | 7.73 ± 0.51 C | 9.91 ± 0.94 B | 11.66 ± 0.67 A | 5.52 ± 0.12 D | 5.77 ± 0.30 D |
| Total HAAs | 10.77 ± 0.66 D | 38.50 ± 2.65 C | 95.17 ± 5.40 A | 51.52 ± 2.98 BC | 44.82 ± 1.14 C | 64.37 ± 3.32 B |
3.4. Sensory Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Merlo, T.C.; Lorenzo, J.M.; Saldana, E.; Patinho, I.; Oliveira, A.C.; Menegali, B.S.; Selani, M.M.; Domínguez, R.; Contreras-Castillo, C.J. Contreras-Castillo. Relationship between volatile organic compounds, free amino acids, and sensory profile of smoked bacon. Meat Sci. 2021, 181, 108596. [Google Scholar] [CrossRef] [PubMed]
- Gibis, M.; Kruwinnus, M.; Weiss, J. Impact of different pan-frying conditions on the formation of heterocyclic aromatic amines and sensory quality in fried bacon. Food Chem. 2015, 168, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.; Fredholm, L.; Bjerne, I.; Jägerstad, M. Influence of frying fat on the formation of heterocyclic amines in fried beefburgers and pan residues. Food Chem. Toxicol. 1995, 33, 993–1004. [Google Scholar] [CrossRef]
- Gibis, M. Heterocyclic aromatic amines in cooked meat products: Causes, formation, occurrence, and risk assessment. Compr. Rev. Food Sci. F. 2016, 15, 269–302. [Google Scholar] [CrossRef]
- Nagao, M.; Honda, M.; Seino, Y.; Yahagi, T.; Sugimura, T. Mutagenicities of smoke condensates and the charred surface of fish and meat. Cancer Lett. 1977, 2, 221–226. [Google Scholar] [CrossRef]
- Rahman, U.; Sahar, A.; Khan, M.I.; Nadeem, M. Production of heterocyclic aromatic amines in meat: Chemistry, health risks and inhibition. A review. LWT-Food Sci. Technol. 2014, 59, 229–233. [Google Scholar] [CrossRef]
- Zamora, R.; Hidalgo, F.J. Formation of heterocyclic aromatic amines with the structure of aminoimidazoazarenes in food products. Food Chem. 2020, 313, 126128. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer (IARC). Some naturally occurring substances: Food items and constituents, heterocyclic aromatic amines and mycotoxins. Monogr. Eval. Carcinog. Risks Hum. 1993, 56, 165–195. [Google Scholar] [CrossRef]
- Knize, M.G.; Sinha, R.; Rothman, N.; Brown, E.D.; Felton, J.S. Heterocyclic amine content in fast-food meat products. Food Chem. Toxicol. 1995, 33, 545–551. [Google Scholar] [CrossRef]
- Skog, K.; Eneroth, A.; Svanberg, M. Effect of different cooking methods on the formation of food mutagens in meat. Int. J. Food Sci. Tech. 2003, 38, 313–323. [Google Scholar] [CrossRef]
- Costa, M.; Viegas, O.; Melo, A.; Petisca, C.; Pinho, O.; Ferreira, I.M.P.L.V.O. Heterocyclic aromatic amine formation in barbecued sardines (Sardina pilchardus) and atlantic salmon (Salmo salar). J. Agr. Food Chem. 2009, 57, 3173–3179. [Google Scholar] [CrossRef] [PubMed]
- Alaejos, M.S.; Afonso, A.M. Factors that affect the content of heterocyclic aromatic amines in foods. Compr. Rev. Food Sci. Food Saf. 2011, 10, 52–108. [Google Scholar] [CrossRef]
- Xue, C.; Chen, Q.; He, Z.; Wang, Z.; Qin, F.; Yang, T.; Chen, J.; Zeng, M. Non-precursors amino acids can inhibit β-carbolines through free radical scavenging pathways and competitive inhibition in roast beef patties and model food systems. Meat Sci. 2020, 169, 108203. [Google Scholar] [CrossRef] [PubMed]
- Oz, F.; Kaya, M. Heterocyclic aromatic amines in meat. J. Food Process. Pres. 2011, 35, 739–753. [Google Scholar] [CrossRef]
- Khan, M.R.; Busquets, R.; Azam, M. Blueberry, raspberry, and strawberry extracts reduce the formation of carcinogenic heterocyclic amines in fried camel, beef and chicken meats. Food Control 2021, 123, 107852. [Google Scholar] [CrossRef]
- Ekiz, E.; Oz, F. The effects of different frying oils on the formation of heterocyclic aromatic amines in meatballs and the changes in fatty acid compositions of meatballs and frying oils. J. Sci. Food Agr. 2019, 99, 1509–1518. [Google Scholar] [CrossRef]
- Li, Y.; Quan, W.; Wang, J.; He, Z.; Qin, F.; Wang, Z.; Zeng, M.; Chen, J. Effects of ten vegetable oils on heterocyclic amine profiles in roasted beef patties using UPLC-MS/MS combined with principal component analysis. Food Chem. 2021, 347, 128996. [Google Scholar] [CrossRef]
- Du, H.; Chen, Q.; Liu, Q.; Wang, Y.; Kong, B. Evaluation of flavor characteristics of bacon smoked with different woodchips by HS-SPME-GC-MS combined with an electronic tongue and electronic nose. Meat Sci. 2021, 182, 108626. [Google Scholar] [CrossRef]
- ISO 12966-2; Animal and Vegetable Fats and Oils Gas Chromatography of Fatty Acid 341 Methylesters, Part 2: Preparation of Methylesters of Fatty Acids. International Organization for Standardization: Geneva, Switzerland, 2011.
- Chen, Y.; Jia, X.; Sun, F.; Jiang, S.; Liu, H.; Liu, Q.; Kong, B. Using a stable pre-emulsified canola oil system that includes porcine plasma protein hydrolysates and oxidized tannic acid to partially replace pork fat in frankfurters. Meat Sci. 2019, 160, 107968. [Google Scholar] [CrossRef]
- AOCS (American Oil Chemists’ Society). Method Ca5a-40, Free Fatty Acid. Official Methods and Recommended Practices by the American Oil Chemists’ Society; AOCS: Champaign, IL, USA, 2005. [Google Scholar]
- AOCS Method Ja 8-87. Peroxide value. In Official Methods and Recommended Practices of the American Oil Chemists’ Society, 4th ed.; Firestone, D.E., Ed.; AOCS Press: Champaign, IL, USA, 1994. [Google Scholar]
- GB/T 5009.181-2016; Chinese National food Safety Standard, Determination of Malondialdehyde in Food. National and Family Planning Commission of the People’s Republic of China: Beijing, China, 2016.
- GB/T 5009.230-2016; Chinese National Food Safety Standard, Determination of Carbonyl Group Value in Food. National and Family Planning Commission of the People’s Republic of China: Beijing, China, 2016.
- Du, H.; Li, X.; Wang, Q.; Liu, Q.; Chen, Q.; Kong, B. Influence of partial replacements of NaCl by KCl on quality characteristics and the heterocyclic aromatic amine contents of bacon. Foods 2022, 11, 143. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Q.; Wang, Z.; Chen, Q.; Sun, F.; Xu, M.; Kong, B. Influence of different ratios of sucrose and green tea leaves on heterocyclic aromatic amine formation and quality characteristics of smoked chicken drumsticks. Food Control 2021, 133, 108613. [Google Scholar] [CrossRef]
- Du, H.; Liu, Q.; Chen, Q.; Xia, X.; Xu, M.; Kong, B. Effect of woodchip types on heterocyclic aromatic amine formation and quality characteristics of smoked bacon. Food Biosci. 2022, 47, 101709. [Google Scholar] [CrossRef]
- Hu, F.B.; Manson, J.A.E.; Willett, W.C. Types of dietary fat and risk of coronary heart disease: A critical review. J. Am. Coll. of Nutr. 2001, 20, 5–19. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Fan, Y.; Li, J.; Tang, L.; Hu, J.; Deng, Z. Evaluating and predicting the oxidative stability of vegetable oils with different fatty acid compositions. J. Food Sci. 2013, 78, 633–641. [Google Scholar] [CrossRef] [PubMed]
- GB 2716-2018; Chinese National Food Safety Standard, Vegetable oil. National Health Commission of the People’s Republic of China: Beijing, China, 2018.
- Guillén, M.D.; Uriarte, P.S. Monitoring by 1H nuclear magnetic resonance of the changes in the composition of virgin linseed oil heated at frying temperature. Comparison with the evolution of other edible oils. Food Control 2012, 28, 59–68. [Google Scholar] [CrossRef]
- Chen, X.; Jia, W.; Zhu, L.; Mao, L.; Zhang, Y. Recent advances in heterocyclic aromatic amines: An update on food safety and hazardous control from food processing to dietary intake. Compr. Rev. Food Sci. F. 2019, 19, 1–24. [Google Scholar] [CrossRef]
- Gibis, M.; Weiss, J. Impact of precursors creatine, creatinine, and glucose on the formation of heterocyclic aromatic amines in grilled patties of various animal species. J. Food Sci. 2015, 80, C2430–C2439. [Google Scholar] [CrossRef]
- Jägerstad, M.; Skog, K. Formation of meat mutagens. Adv. Exp. Med. Biol. 1991, 289, 83–105. [Google Scholar] [CrossRef]
- Skog, K.; Solyakov, A.; Jägerstad, M. Effects of heating conditions and additives on the formation of heterocyclic amines with reference to amino-carbolines in a meat juice model system. Food Chem. 2000, 68, 299–308. [Google Scholar] [CrossRef]
- Johansson, M.; Skog, K.; Jägerstad, M. Effects of edible oils and fatty-acids on the formation of mutagenic heterocyclic amines in a model system. Carcinogenesis 1993, 14, 89–94. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, M.; Wang, H.; Mujumdar, A.S. Monitoring of free fatty acid content in mixed frying oils by means of LF-NMR and NIR combined with BP-ANN. Food Control 2022, 133, 108599. [Google Scholar] [CrossRef]
- Persson, E.; Graziani, G.; Ferracane, R.; Fogliano, V.; Skog, K. Influence of antioxidants in virgin olive oil on the formation of heterocyclic amines in fried beefburgers. Food Chem. Toxicol. 2003, 41, 1587–1597. [Google Scholar] [CrossRef]
- Liu, W.; Yang, Z.; Shi, L.; Cui, Z.; Li, Y. Degradation of β-carbolines harman and norharman in edible oils during heating. Molecules 2021, 26, 7018. [Google Scholar] [CrossRef] [PubMed]
- Randel, G.; Balzer, M.; Grupe, S.; Drusch, S.; Kaina, B.; Platt, K.L.; Schwarz, K. Degradation of heterocyclic aromatic amines in oil under storage and frying conditions and reduction of their mutagenic potential. Food Chem. Toxicol. 2007, 45, 2245–2253. [Google Scholar] [CrossRef] [PubMed]
| PO | CO | RO | SSO | SO | |
|---|---|---|---|---|---|
| Tetradecanoic acid | 0.02 ± 0.00 C | 0.02 ± 0.00 C | 0.04 ± 0.00 B | 0.06 ± 0.00 A | 0.07 ± 0.00 A |
| Pentadecanoic acid | 0.01 ± 0.00 C | n.d. | 0.02 ± 0.00 A | 0.01 ± 0.00 B | 0.01 ± 0.00 B |
| Hexadecanoic acid | 10.38 ± 0.42 A | 11.34 ± 0.33 A | 4.49 ± 0.16 C | 6.70 ± 0.31 B | 10.77 ± 0.31 A |
| Heptadecanoic acide | 0.06 ± 0.00 B | 0.06 ± 0.00 B | 0.05 ± 0.00 C | 0.03 ± 0.00 D | 0.09 ± 0.01 A |
| Stearic acid | 3.23 ± 0.13 C | 1.78 ± 0.05 D | 1.81 ± 0.06 D | 3.88 ± 0.18 B | 4.54 ± 0.13 A |
| Arachidic acid | 1.50 ± 0.06 A | 0.42 ± 0.01 C | 0.67 ± 0.02 B | 0.27 ± 0.01 D | 0.44 ± 0.01 C |
| Heneicosanoic acid | 0.01 ± 0.00 C | n.d. | 0.02 ± 0.00 B | n.d. | 0.03 ± 0.00 A |
| Behenic acid | 3.40 ± 0.13 A | 0.12 ± 0.01 D | 0.34 ± 0.01 C | 0.82 ± 0.04 B | 0.45 ± 0.01 C |
| Tricosanoic acid | 0.03 ± 0.00 B | 0.01 ± 0.00 D | 0.02 ± 0.00 C | 0.03 ± 0.00 B | 0.04 ± 0.00 A |
| Lignoceric acid | 1.55 ± 0.06 A | 0.15 ± 0.01 B | 0.14 ± 0.01 B | 0.27 ± 0.01 C | 0.15 ± 0.01 B |
| ∑SFA | 20.20 ± 0.82 A | 13.90 ± 0.40 C | 7.60 ± 0.26 E | 12.06 ± 0.56 D | 16.56 ± 0.48 B |
| Cis-9-hexadecenoic acid | 0.05 ± 0.00 D | 0.07 ± 0.00 C | 0.20 ± 0.01 A | 0.10 ± 0.01 B | 0.07 ± 0.00 C |
| Cis-9-Octadecenic acid | 39.78 ± 1.61 B | 24.22 ± 0.70 C | 56.88 ± 1.97 A | 26.98 ± 1.25 C | 23.03 ± 0.66 C |
| Cis-11-eicosenoic acid | 1.24 ± 0.05 A | 0.25 ± 0.01 B | 1.27 ± 0.04 A | 0.13 ± 0.01 C | 0.27 ± 0.01 B |
| Cis-13-docosaenoic acid | 0.09 ± 0.00 B | n.d. | 0.16 ± 0.01 A | n.d. | n.d. |
| Cis-15-catecosenoic acid | n.d. | n.d. | 0.14 ± 0.01 A | n.d. | n.d. |
| ∑MUFA | 41.16 ± 1.66 B | 24.54 ± 0.71 C | 58.64 ± 2.03 A | 27.20 ± 1.26 C | 23.37 ± 0.67 C |
| Cis-9,12-octadecadienoic acid | 36.46 ± 1.47 B | 53.86 ± 1.55 A | 18.97 ± 0.66 C | 56.32 ± 2.60 A | 49.59 ± 1.43 A |
| Cis-9,12,15-octadecatrienoic acid | 0.08 ± 0.01 C | 0.80 ± 0.04 B | 5.74 ± 0.34 A | 0.09 ± 0.01 C | 6.07 ± 0.30 A |
| Cis-11,14-eicosadienoic acid | 0.02 ± 0.00 B | 0.02 ± 0.00 B | 0.05 ± 0.00 A | n.d. | 0.03 ± 0.00 B |
| ∑PUFA | 36.56 ± 1.48 B | 54.69 ± 1.58 A | 24.76 ± 0.86 C | 56.41 ± 2.61 A | 55.69 ± 1.61 A |
| ∑MUFA/∑SFA | 2.04 ± 0.08 BC | 1.77 ± 0.05 CD | 7.71 ± 0.27 A | 2.26 ± 0.10 B | 1.41 ± 0.07 D |
| ∑PUFA/∑SFA | 1.81 ± 0.07 D | 3.93 ± 0.11 B | 3.26 ± 0.11 C | 4.68 ± 0.22 A | 3.36 ± 0.10 C |
| Free fatty acid | 0.29 ± 0.01 A | 0.10 ± 0.01 BC | 0.06 ± 0.01 D | 0.09 ± 0.02 C | 0.11 ± 0.01 B |
| Peroxide value | 0.19 ± 0.02 B | 0.12 ± 0.01 C | 0.07 ± 0.01 D | 0.23 ± 0.02 A | 0.16 ± 0.01 B |
| Malondialdehyde | 0.41 ± 0.05 A | 0.28 ± 0.02 BC | 0.34 ± 0.03 B | 0.25 ± 0.02 C | 0.31 ± 0.01 BC |
| Carbonyl group value | 0.42 ± 0.06 E | 0.86 ± 0.04 D | 1.23 ± 0.06 C | 1.97 ± 0.07 B | 2.26 ± 0.18 A |
| Control | B–PO | B–CO | B–RO | B–SSO | B–SO | |
|---|---|---|---|---|---|---|
| Asp | 0.29 ± 0.02 A | 0.17 ± 0.02 C | 0.10 ± 0.01 D | 0.20 ± 0.02 B | 0.20 ± 0.01 B | 0.16 ± 0.01 C |
| Glu | 1.42 ± 0.08 A | 1.44 ± 0.05 A | 1.42 ± 0.04 A | 1.45 ± 0.03 A | 1.47 ± 0.03 A | 1.42 ± 0.04 A |
| Ser | 0.19 ± 0.01 D | 0.30 ± 0.01 B | 0.22 ± 0.01 C | 0.32 ± 0.01 B | 0.47 ± 0.02 A | 0.30 ± 0.01 B |
| Gly | 1.19 ± 0.07 A | 0.21 ± 0.01 B | 0.12 ± 0.01 C | 0.22 ± 0.02 B | 0.25 ± 0.01 B | 0.21 ± 0.01 B |
| His | 1.32 ± 0.08 A | 1.03 ± 0.04 B | 0.78 ± 0.02 C | 1.11 ± 0.03 B | 0.84 ± 0.02 C | 0.99 ± 0.03 B |
| Thr | 1.34 ± 0.08 A | 0.74 ± 0.03 BC | 0.53 ± 0.02 D | 0.80 ± 0.02 B | 0.72 ± 0.02 BC | 0.63 ± 0.02 CD |
| Ala | 2.03 ± 0.06 A | 1.36 ± 0.05 BC | 0.66 ± 0.02 D | 1.48 ± 0.03 B | 1.34 ± 0.03 BC | 1.21 ± 0.03 C |
| Arg | 3.24 ± 0.19 A | 2.39 ± 0.08 B | 2.68 ± 0.08 B | 2.65 ± 0.06 B | 2.08 ± 0.05 C | 1.74 ± 0.05 D |
| Pro | 0.16 ± 0.01 A | 0.13 ± 0.01 C | 0.12 ± 0.01 D | 0.16 ± 0.01 A | 0.14 ± 0.01 B | n.d. |
| Tyr | 0.11 ± 0.01 AB | 0.12 ± 0.01 A | 0.12 ± 0.01 A | 0.12 ± 0.01 A | 0.10 ± 0.01 B | 0.11 ± 0.01 AB |
| Val | 0.11 ± 0.01 C | 0.21 ± 0.02 B | 0.23 ± 0.02 B | 0.25 ± 0.01 A | 0.22 ± 0.01 B | 0.21 ± 0.01 B |
| Met | 0.02 ± 0.01 E | 0.09 ± 0.01 C | 0.07 ± 0.01 D | 0.27 ± 0.01 B | 0.28 ± 0.01 B | 0.30 ± 0.01 A |
| Cys | 0.68 ± 0.04 BC | 0.72 ± 0.03 AB | 0.46 ± 0.01 D | 0.65 ± 0.02 BC | 0.61 ± 0.01 C | 0.76 ± 0.02 A |
| Ile | 0.39 ± 0.02 B | 0.46 ± 0.02 A | 0.42 ± 0.01 B | 0.41 ± 0.01 B | 0.39 ± 0.01 B | 0.40 ± 0.01 B |
| Leu | 0.09 ± 0.01 E | 0.21 ± 0.02 A | 0.16 ± 0.01 CD | 0.19 ± 0.01 B | 0.17 ± 0.01 C | 0.15 ± 0.01 D |
| Phe | 0.27 ± 0.02 A | 0.16 ± 0.01 B | 0.16 ± 0.02 B | 0.16 ± 0.01 B | 0.14 ± 0.01 B | 0.13 ± 0.01 B |
| Lys | 1.66 ± 0.10 A | 1.68 ± 0.06 A | 1.60 ± 0.08 A | 1.65 ± 0.07 A | 1.65 ± 0.07 A | 1.69 ± 0.04 A |
| Total FAA | 14.50 ± 0.83 A | 11.44 ± 0.40 BC | 9.84 ± 0.28 C | 12.10 ± 0.28 B | 11.09 ± 0.26 BC | 10.44 ± 0.27 BC |
| B–PO | B–CO | B–RO | B–SSO | B–SO | |
|---|---|---|---|---|---|
| Norharman | 77.15 ± 4.21 A | 23.74 ± 1.64 C | 12.82 ± 0.74 D | 31.86 ± 0.88 B | 12.57 ± 0.72 D |
| Harman | 28.00 ± 1.53 A | 8.52 ± 0.59 B | 5.88 ± 0.34 C | 9.98 ± 0.28 B | 4.55 ± 0.26 C |
| AαC | 0.52 ± 0.03 B | 0.48 ± 0.03 B | 0.14 ± 0.01 D | 0.87 ± 0.02 A | 0.33 ± 0.02 C |
| MeAαC | 7.17 ± 0.39 A | 6.17 ± 0.43 B | 5.57 ± 0.32 B | 5.58 ± 0.16 B | 1.79 ± 0.10 C |
| Non-polar HAAs | 112.83 ± 6.16 A | 38.92 ± 2.70 B | 24.41 ± 1.41 C | 48.29 ± 1.34 B | 19.24 ± 1.10 C |
| IQ | n.d. | n.d. | n.d. | n.d. | n.d. |
| 7,8-DiMeIQx | n.d. | n.d. | n.d. | n.d. | n.d. |
| MeIQx | n.d. | n.d. | n.d. | n.d. | n.d. |
| IQx | 0.79 ± 0.04 A | 0.52 ± 0.03 B | 0.77 ± 0.05 A | 0.48 ± 0.01 B | 0.26 ± 0.02 C |
| 4,8-DiMeIQx | 0.66 ± 0.05 A | 0.56 ± 0.04 A | 0.43 ± 0.03 B | 0.30 ± 0.01 C | 0.44 ± 0.04 B |
| MeIQ | 0.22 ± 0.01 D | 0.39 ± 0.03 B | 0.31 ± 0.02 C | 1.31 ± 0.03 A | 0.26 ± 0.02 CD |
| PhIP | 2.60 ± 0.14 B | 3.08 ± 0.21 A | 1.16 ± 0.07 C | 1.34 ± 0.03 C | 0.74 ± 0.04 D |
| Phe-P-I | n.d. | n.d. | n.d. | n.d. | n.d. |
| Polar HAAs | 4.28 ± 0.16 A | 4.55 ± 0.31 A | 2.66 ± 0.16 C | 3.43 ± 0.08 B | 1.71 ± 0.06 D |
| Total HAAs | 117.11 ± 6.32 A | 43.47 ± 3.01 B | 27.07 ± 1.57 C | 51.72 ± 1.42 B | 20.95 ± 1.15 C |
| B–PO | B–CO | B–RO | B–SSO | B–SO | |
|---|---|---|---|---|---|
| Color | 5.41 ± 0.23 A | 5.60 ± 0.19 A | 5.01 ± 0.16 AB | 5.00 ± 0.28 AB | 4.46 ± 0.24 B |
| Flavor | 4.91 ± 0.18 A | 5.28 ± 0.18 A | 4.97 ± 0.17 A | 5.43 ± 0.20 A | 5.00 ± 0.20 A |
| Taste | 5.38 ± 0.14 A | 5.36 ± 0.17 A | 5.20 ± 0.17 A | 5.38 ± 0.16 A | 5.21 ± 0.20 A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, H.; Wang, Z.; Li, Y.; Liu, Q.; Chen, Q.; Kong, B. Understanding the Development of Heterocyclic Aromatic Amines in Fried Bacon and in the Remaining Oil after Pan-Frying in Five Different Vegetable Oils. Foods 2022, 11, 3491. https://doi.org/10.3390/foods11213491
Du H, Wang Z, Li Y, Liu Q, Chen Q, Kong B. Understanding the Development of Heterocyclic Aromatic Amines in Fried Bacon and in the Remaining Oil after Pan-Frying in Five Different Vegetable Oils. Foods. 2022; 11(21):3491. https://doi.org/10.3390/foods11213491
Chicago/Turabian StyleDu, Hongzhen, Ziyi Wang, Yuexin Li, Qian Liu, Qian Chen, and Baohua Kong. 2022. "Understanding the Development of Heterocyclic Aromatic Amines in Fried Bacon and in the Remaining Oil after Pan-Frying in Five Different Vegetable Oils" Foods 11, no. 21: 3491. https://doi.org/10.3390/foods11213491
APA StyleDu, H., Wang, Z., Li, Y., Liu, Q., Chen, Q., & Kong, B. (2022). Understanding the Development of Heterocyclic Aromatic Amines in Fried Bacon and in the Remaining Oil after Pan-Frying in Five Different Vegetable Oils. Foods, 11(21), 3491. https://doi.org/10.3390/foods11213491








