Application of Cheese Whey Containing Postbiotics of Lactobacillus acidophilus LA5 and Bifidobacterium animalis BB12 as a Preserving Liquid in High-Moisture Mozzarella
Abstract
1. Introduction
2. Materials and Methods
2.1. Probiotic Strains and Cultures Preparation
2.2. Preparation of Postbiotics Solutions
2.3. Cheese Application of Postbiotics
2.4. Cheese Analysis
2.4.1. Microbiological Analysis
2.4.2. pH Measurement
2.4.3. Sensorial Evaluation
2.4.4. Color Measurements
2.5. Statistical Analysis
3. Results and Discussion
3.1. Microbiological Analysis
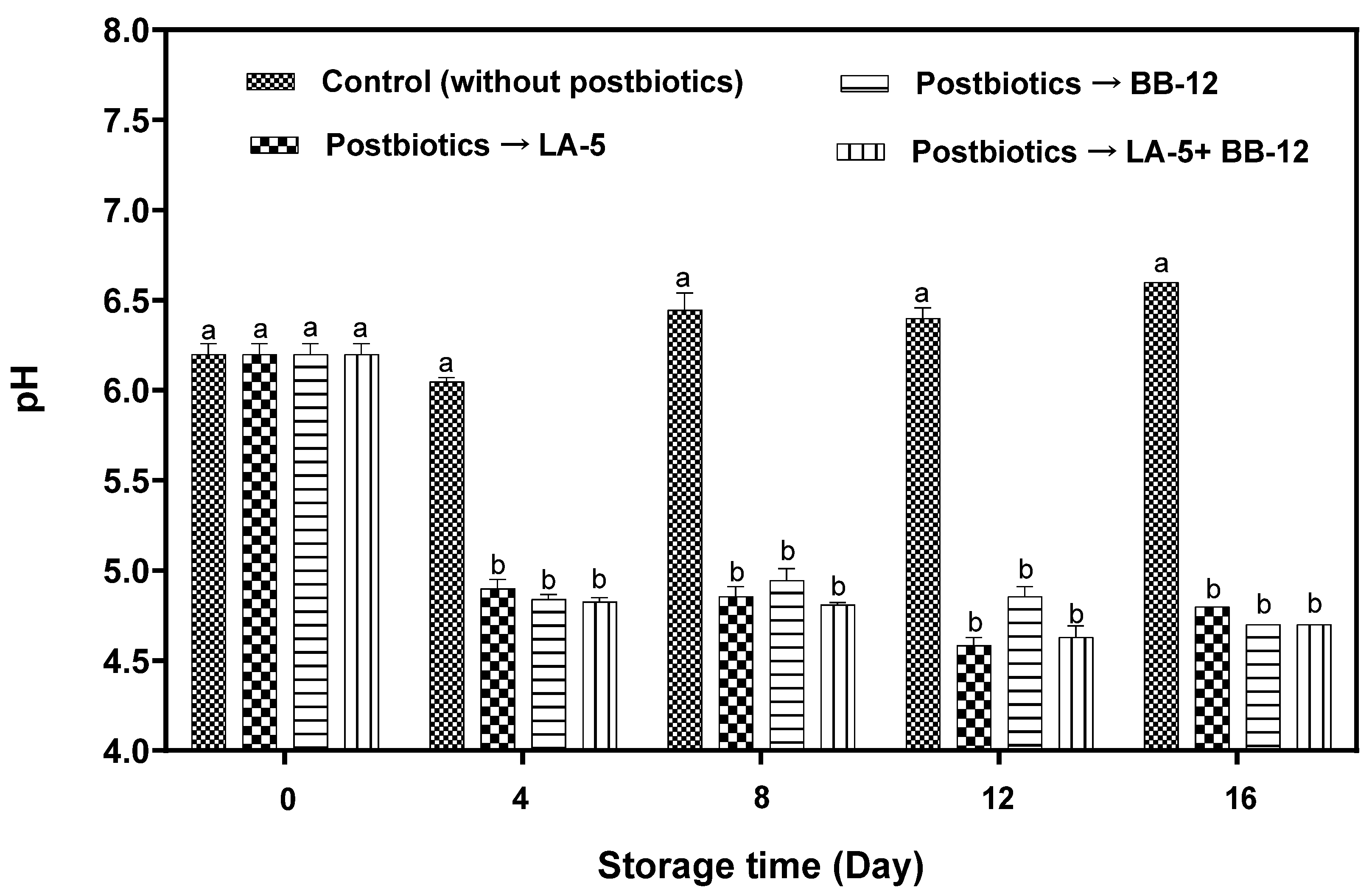
3.2. pH Changes
3.3. Color Measurement
3.4. Sensory Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moradi, M.; Tajik, H.; Mardani, K.; Ezati, P. Efficacy of lyophilized cell-free supernatant of Lactobacillus salivarius (Ls-BU2) on Escherichia coli and shelf life of ground beef. Vet. Res. Forum 2019, 10, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Margalho, L.P.; Kamimura, B.A.; Brexó, R.P.; Alvarenga, V.O.; Cebeci, A.S.; Janssen, P.W.M.; Dijkstra, A.; Starrenburg, M.J.C.; Sheombarsing, R.S.; Cruz, A.G.; et al. High throughput screening of technological and biopreservation traits of a large set of wild lactic acid bacteria from Brazilian artisanal cheeses. Food Microbiol. 2021, 100, 103872. [Google Scholar] [CrossRef] [PubMed]
- Sabahi, S.; Homayouni Rad, A.; Aghebati-Maleki, L.; Sangtarash, N.; Ozma, M.A.; Karimi, A.; Hosseini, H.; Abbasi, A. Postbiotics as the new frontier in food and pharmaceutical research. Crit. Rev. Food Sci. Nutr. 2022, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Thorakkattu, P.; Khanashyam, A.C.; Shah, K.; Babu, K.S.; Mundanat, A.S.; Deliephan, A.; Deokar, G.S.; Santivarangkna, C.; Nirmal, N.P. Postbiotics: Current Trends in Food and Pharmaceutical Industry. Foods 2022, 11, 3094. [Google Scholar] [CrossRef]
- Aguilar-Toalá, J.E.; Arioli, S.; Behare, P.; Belzer, C.; Berni Canani, R.; Chatel, J.-M.; D’Auria, E.; de Freitas, M.Q.; Elinav, E.; Esmerino, E.A.; et al. Postbiotics—When simplification fails to clarify. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 825–826. [Google Scholar] [CrossRef]
- Moradi, M.; Molaei, R.; Guimarães, J.T. A review on preparation and chemical analysis of postbiotics from lactic acid bacteria. Enzym. Microb. Technol. 2021, 143, 109722. [Google Scholar] [CrossRef]
- Wegh, C.A.M.; Geerlings, S.Y.; Knol, J.; Roeselers, G.; Belzer, C. Postbiotics and Their Potential Applications in Early Life Nutrition and Beyond. Int. J. Mol. Sci. 2019, 20, 4673. [Google Scholar] [CrossRef]
- Aguilar-Toalá, J.E.; Garcia-Varela, R.; Garcia, H.S.; Mata-Haro, V.; González-Córdova, A.F.; Vallejo-Cordoba, B.; Hernández-Mendoza, A. Postbiotics: An evolving term within the functional foods field. Trends Food Sci. Technol. 2018, 75, 105–114. [Google Scholar] [CrossRef]
- Yilmaz, N.; Özogul, F.; Moradi, M.; Fadiloglu, E.E.; Šimat, V.; Rocha, J.M. Reduction of biogenic amines formation by foodborne pathogens using postbiotics in lysine-decarboxylase broth. J. Biotechnol. 2022, 358, 118–127. [Google Scholar] [CrossRef]
- Nataraj, B.H.; Ali, S.A.; Behare, P.V.; Yadav, H. Postbiotics-parabiotics: The new horizons in microbial biotherapy and functional foods. Microb. Cell Fact. 2020, 19, 168. [Google Scholar] [CrossRef]
- Żółkiewicz, J.; Marzec, A.; Ruszczyński, M.; Feleszko, W. Postbiotics—A step beyond Pre- and Probiotics. Nutrients 2020, 12, 2189. [Google Scholar] [CrossRef] [PubMed]
- Moradi, M.; Kousheh, S.A.; Almasi, H.; Alizadeh, A.; Guimarães, J.T.; Yılmaz, N.; Lotfi, A. Postbiotics produced by lactic acid bacteria: The next frontier in food safety. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3390–3415. [Google Scholar] [CrossRef] [PubMed]
- Shafipour Yordshahi, A.; Moradi, M.; Tajik, H.; Molaei, R. Design and preparation of antimicrobial meat wrapping nanopaper with bacterial cellulose and postbiotics of lactic acid bacteria. Int. J. Food Microbiol. 2020, 321, 108561. [Google Scholar] [CrossRef] [PubMed]
- Rasouli, Y.; Moradi, M.; Tajik, H.; Molaei, R. Fabrication of anti-Listeria film based on bacterial cellulose and Lactobacillus sakei-derived bioactive metabolites; application in meat packaging. Food Biosci. 2021, 42, 101218. [Google Scholar] [CrossRef]
- İncili, G.K.; Karatepe, P.; Akgöl, M.; Tekin, A.; Kanmaz, H.; Kaya, B.; Çalıcıoğlu, M.; Hayaloğlu, A.A. Impact of chitosan embedded with postbiotics from Pediococcus acidilactici against emerging foodborne pathogens in vacuum-packaged frankfurters during refrigerated storage. Meat Sci. 2022, 188, 108786. [Google Scholar] [CrossRef]
- İncili, G.K.; Karatepe, P.; Akgöl, M.; Kaya, B.; Kanmaz, H.; Hayaloğlu, A.A. Characterization of Pediococcus acidilactici postbiotic and impact of postbiotic-fortified chitosan coating on the microbial and chemical quality of chicken breast fillets. Int. J. Biol. Macromol. 2021, 184, 429–437. [Google Scholar] [CrossRef]
- Jutinico-Shubach, A.; Gutiérrez-Cortés, C.; Suarez, H. Antilisterial activity of chitosan-based edible coating incorporating cell-free supernatant from Pediococcus pentosaceus 147 on the preservation of fresh cheese. J. Food Process. Preserv. 2020, 44, e14715. [Google Scholar] [CrossRef]
- de Lima Marques, J.; Funck, G.D.; da Silva Dannenberg, G.; dos Santos Cruxen, C.E.; El Halal, S.L.M.; Dias, A.R.G.; Fiorentini, Â.M.; da Silva, W.P. Bacteriocin-like substances of Lactobacillus curvatus P99: Characterization and application in biodegradable films for control of Listeria monocytogenes in cheese. Food Microbiol. 2017, 63, 159–163. [Google Scholar] [CrossRef]
- Mohammadi, R.; Moradi, M.; Tajik, H.; Molaei, R. Potential application of postbiotics metabolites from bioprotective culture to fabricate bacterial nanocellulose based antimicrobial packaging material. Int. J. Biol. Macromol. 2022, 220, 528–536. [Google Scholar] [CrossRef]
- Zappia, A.; Branca, M.L.; Piscopo, A.; Poiana, M. Evaluation of different salted governing liquids on shelf life extension of lacto-fermented mozzarella cheese. J. Food Sci. Technol. 2020, 57, 4293–4298. [Google Scholar] [CrossRef]
- Faccia, M.; Gambacorta, G.; Natrella, G.; Caponio, F. Shelf life extension of Italian mozzarella by use of calcium lactate buffered brine. Food Control 2019, 100, 287–291. [Google Scholar] [CrossRef]
- Lucera, A.; Mastromatteo, M.; Conte, A.; Zambrini, A.V.; Faccia, M.; Del Nobile, M.A. Effect of active coating on microbiological and sensory properties of fresh mozzarella cheese. Food Packag. Shelf Life 2014, 1, 25–29. [Google Scholar] [CrossRef]
- Chang, S.; Mohammadi Nafchi, A.; Baghaie, H. Development of an active packaging based on polyethylene containing linalool or thymol for mozzarella cheese. Food Sci. Nutr. 2021, 9, 3732–3739. [Google Scholar] [CrossRef] [PubMed]
- Segat, A.; Biasutti, M.; Iacumin, L.; Comi, G.; Baruzzi, F.; Carboni, C.; Innocente, N. Use of ozone in production chain of high moisture mozzarella cheese. LWT—Food Sci. Technol. 2014, 55, 513–520. [Google Scholar] [CrossRef]
- Azhdari, S.; Moradi, M. Application of antimicrobial coating based on carboxymethyl cellulose and natamycin in active packaging of cheese. Int. J. Biol. Macromol. 2022, 209, 2042–2049. [Google Scholar] [CrossRef]
- Zappia, A.; Branca, M.L.; Piscopo, A.; Poiana, M. Shelf life extension of mozzarella cheese packed in preserving liquid with calcium lactate and bergamot juice concentrate. J. Dairy Res. 2020, 87, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Amiri, S.; Rezaei Mokarram, R.; Sowti Khiabani, M.; Rezazadeh Bari, M.; Alizadeh Khaledabad, M. Characterization of antimicrobial peptides produced by Lactobacillus acidophilus LA-5 and Bifidobacterium lactis BB-12 and their inhibitory effect against foodborne pathogens. LWT 2022, 153, 112449. [Google Scholar] [CrossRef]
- Amiri, S.; Rezaei Mokarram, R.; Sowti Khiabani, M.; Rezazade Bari, M.; Alizadeh Khaledabad, M. Production of lactic acid by Lactobacillus acidophilus LA5 and Bifidobacterium lactis BB12 in batch fermentation of cheese whey and milk permeate. J. Food Res. 2020, 30, 33–49. [Google Scholar]
- Amiri, S.; Rezaei Mokarram, R.; Sowti Khiabani, M.; Rezazadeh Bari, M.; Alizadeh Khaledabad, M. In situ production of conjugated linoleic acid by Bifidobacterium lactis BB12 and Lactobacillus acidophilus LA5 in milk model medium. LWT 2020, 132, 109933. [Google Scholar] [CrossRef]
- Amiri, S.; Rezaei Mokarram, R.; Sowti Khiabani, M.; Rezazadeh Bari, M.; Alizadeh Khaledabad, M. Exopolysaccharides production by Lactobacillus acidophilus LA5 and Bifidobacterium animalis subsp. lactis BB12: Optimization of fermentation variables and characterization of structure and bioactivities. Int. J. Biol. Macromol. 2019, 123, 752–765. [Google Scholar] [CrossRef]
- Conte, A.; Scrocco, C.; Sinigaglia, M.; Del Nobile, M.A. Innovative active packaging systems to prolong the shelf life of mozzarella cheese. J. Dairy Sci. 2007, 90, 2126–2131. [Google Scholar] [CrossRef] [PubMed]
- Jafarzadeh, S.; Rhim, J.; Alias, A.K.; Ariffin, F.; Mahmud, S. Application of antimicrobial active packaging film made of semolina flour, nano zinc oxide and nano-kaolin to maintain the quality of low-moisture mozzarella cheese during low-temperature storage. J. Sci. Food Agric. 2019, 99, 2716–2725. [Google Scholar] [CrossRef] [PubMed]
- Torres-Frenzel, P.; DeMarsh, T.A.; Alcaine, S.D. Investigation of the surface-application of lactose oxidase to fresh mozzarella cheese as a potential means of inhibiting blue discoloration. Food Control 2021, 130, 108289. [Google Scholar] [CrossRef]
- Zhong, Y.; Cavender, G.; Zhao, Y. Investigation of different coating application methods on the performance of edible coatings on mozzarella cheese. LWT—Food Sci. Technol. 2014, 56, 1–8. [Google Scholar] [CrossRef]
- Amiri, S.; Rezazadeh Bari, M.; Alizadeh Khaledabad, M.; Rezaei Mokarram, R.; Sowti Khiabani, M. Co-production of parabiotic metabolites by Lactobacillus acidophilus LA5 and Bifidobacterium animalis subsp. lactis BB12 in dairy effluents. Chem. Rev. Lett. 2021, 4, 66–76. [Google Scholar]
- Moradi, M.; Mardani, K.; Tajik, H. Characterization and application of postbiotics of Lactobacillus spp. on Listeria monocytogenes in vitro and in food models. LWT 2019, 111, 457–464. [Google Scholar] [CrossRef]
- Jalili, H.; Razavi, S.H.; Safari, M.; Malcata, F.X. Enhancement of growth rate and β-galactosidase activity, and variation in organic acid profile of Bifidobacterium animalis subsp. lactis Bb 12. Enzym. Microb. Technol. 2009, 45, 469–476. [Google Scholar] [CrossRef]
- International Commission on Microbiological Specifications for Foods—ICMSF. (1986). Sampling for microbiological analysis: Principles and specific applications Toronto: International Commission on Microbiological Specifications for Foods Publisher. [CrossRef]
- Koohestani, M.; Moradi, M.; Tajik, H.; Badali, A. Effects of cell-free supernatant of Lactobacillus acidophilus LA5 and Lactobacillus casei 431 against planktonic form and biofilm of Staphylococcus aureus. Vet. Res. Forum Int. Q. J. 2018, 9, 301–306. [Google Scholar] [CrossRef]
- İncili, G.K.; Karatepe, P.; Akgöl, M.; Güngören, A.; Koluman, A.; İlhak, O.İ.; Kanmaz, H.; Kaya, B.; Hayaloğlu, A.A. Characterization of lactic acid bacteria postbiotics, evaluation in-vitro antibacterial effect, microbial and chemical quality on chicken drumsticks. Food Microbiol. 2022, 104, 104001. [Google Scholar] [CrossRef]
- Kure, C.F.; Skaar, I. The fungal problem in cheese industry. Curr. Opin. Food Sci. 2019, 29, 14–19. [Google Scholar] [CrossRef]
- Marino, M.; Dubsky de Wittenau, G.; Saccà, E.; Cattonaro, F.; Spadotto, A.; Innocente, N.; Radovic, S.; Piasentier, E.; Marroni, F. Metagenomic profiles of different types of Italian high-moisture Mozzarella cheese. Food Microbiol. 2019, 79, 123–131. [Google Scholar] [CrossRef]
- Faccia, M.; Natrella, G.; Gambacorta, G. Analysis of the water-soluble compounds as a tool for discriminating traditional and industrial high moisture mozzarella made with citric acid. Int. J. Food Sci. Technol. 2021, 56, 5352–5361. [Google Scholar] [CrossRef]
- Gómez-Narváez, F.; Medina-Pineda, Y.; Contreras-Calderón, J. Evaluation of the heat damage of whey and whey proteins using multivariate analysis. Food Res. Int. 2017, 102, 768–775. [Google Scholar] [CrossRef] [PubMed]
- Arrioja-Bretón, D.; Mani-López, E.; Palou, E.; López-Malo, A. Antimicrobial activity and storage stability of cell-free supernatants from lactic acid bacteria and their applications with fresh beef. Food Control 2020, 115, 107286. [Google Scholar] [CrossRef]
- Faccia, M.; Gambacorta, G.; Pasqualone, A.; Summo, C.; Caponio, F. Quality Characteristics and Consumer Acceptance of High-Moisture Mozzarella Obtained from Heat-Treated Goat Milk. Foods 2021, 10, 833. [Google Scholar] [CrossRef]



| Treatment | Day | L* | a* | b* |
|---|---|---|---|---|
| Control | 0 | 94.50 ± 0.80 a | −2.51 ± 0.34 a | 10.06 ± 1.40 a |
| 4 | 95.26 ± 0.30 a | −3.16 ± 0.37 a | 10.03 ± 0.97 a | |
| 8 | 95.06 ± 0.95 a | −3.41 ± 0.51 a | 10.60 ± 1.47 a | |
| 12 | 94.86 ± 4.78 a | −3.16 ± 2.21 a | 11.46 ± 1.22 a | |
| 16 | 94.76 ± 0.90 a | −2.91 ± 0.50 a | 12.16 ± 0.95 a | |
| P-LA-5 | 0 | 94.50 ± 0.80 a | −2.51 ± 0.34 a | 10.06 ± 1.40 a |
| 4 | 91.36 ± 2.82 b | −2.31 ± 0.26 b | 18.76 ± 0.50 b | |
| 8 | 92.06 ± 2.61 b | −1.21 ± 1.44 b | 18.50 ± 0.78 b | |
| 12 | 92.16 ± 3.15 b | −1.62 ± 0.81 b | 18.66 ± 0.66 b | |
| 16 | 90.53 ± 1.73 b | −0.93 ± 0.57 b | 17.46 ± 0.61 b | |
| P-BB-12 | 0 | 94.50 ± 0.80 a | −2.51 ± 0.34 a | 10.06 ± 1.40 a |
| 4 | 92.21 ± 2.56 b | −2.41 ± 0.78 b | 20.03 ± 0.75 b | |
| 8 | 93.23 ± 1.05 b | −1.73 ± 0.66 b | 19.16 ± 1.05 b | |
| 12 | 92.73 ± 0.43 b | −1.81 ± 0.40 b | 18.53 ± 1.06 b | |
| 16 | 91.14 ± 2.17 b | −1.43 ± 0.76 b | 18.33 ± 1.20 b | |
| P-LA-5 + BB-12 | 0 | 94.50 ± 0.80 a | −2.51 ± 0.34 a | 10.06 ± 1.40 a |
| 4 | 91.52 ± 2.38 b | −2.16 ± 0.56 b | 19.40 ± 0.75 b | |
| 8 | 90.15 ± 0.78 b | −0.96 ± 0.20 c | 18.90 ± 1.15 b | |
| 12 | 90.23 ± 1.20 c | −1.36 ± 1.67 b | 18.93 ± 0.72 b | |
| 16 | 90.16 ± 2.45 b | −0.91 ± 1.13 b | 18.70 ± 0.55 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharafi, H.; Moradi, M.; Amiri, S. Application of Cheese Whey Containing Postbiotics of Lactobacillus acidophilus LA5 and Bifidobacterium animalis BB12 as a Preserving Liquid in High-Moisture Mozzarella. Foods 2022, 11, 3387. https://doi.org/10.3390/foods11213387
Sharafi H, Moradi M, Amiri S. Application of Cheese Whey Containing Postbiotics of Lactobacillus acidophilus LA5 and Bifidobacterium animalis BB12 as a Preserving Liquid in High-Moisture Mozzarella. Foods. 2022; 11(21):3387. https://doi.org/10.3390/foods11213387
Chicago/Turabian StyleSharafi, Houshmand, Mehran Moradi, and Saber Amiri. 2022. "Application of Cheese Whey Containing Postbiotics of Lactobacillus acidophilus LA5 and Bifidobacterium animalis BB12 as a Preserving Liquid in High-Moisture Mozzarella" Foods 11, no. 21: 3387. https://doi.org/10.3390/foods11213387
APA StyleSharafi, H., Moradi, M., & Amiri, S. (2022). Application of Cheese Whey Containing Postbiotics of Lactobacillus acidophilus LA5 and Bifidobacterium animalis BB12 as a Preserving Liquid in High-Moisture Mozzarella. Foods, 11(21), 3387. https://doi.org/10.3390/foods11213387







