The Protective Effects of Iron Free Lactoferrin on Lipopolysaccharide-Induced Intestinal Inflammatory Injury via Modulating the NF-κB/PPAR Signaling Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Culture of Primary IECs
2.3. Co-Culture and Treatment of Primary IECs and Immune Cells
2.4. Colon Collection of Young Mouse Models
2.5. Inflammatory Cytokine Determination
2.6. Total RNA Extraction, Transcriptome Sequencing
2.7. RT-qPCR Validation
2.8. Western Blotting (WB)
2.9. Statistical Analyses
3. Results
3.1. Protective Effect of Apo-LF on LPS-Induced Cellular Inflammation
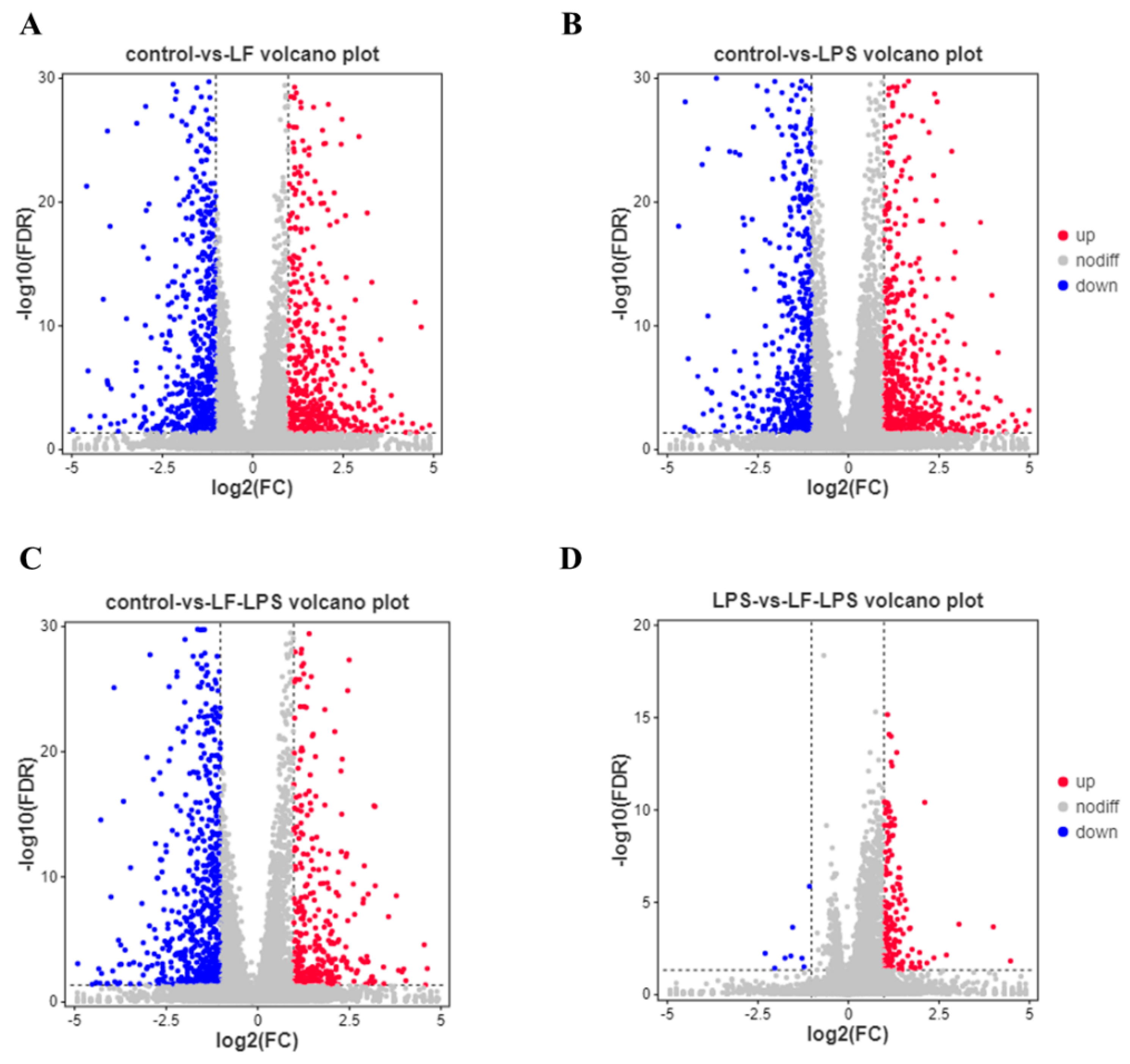
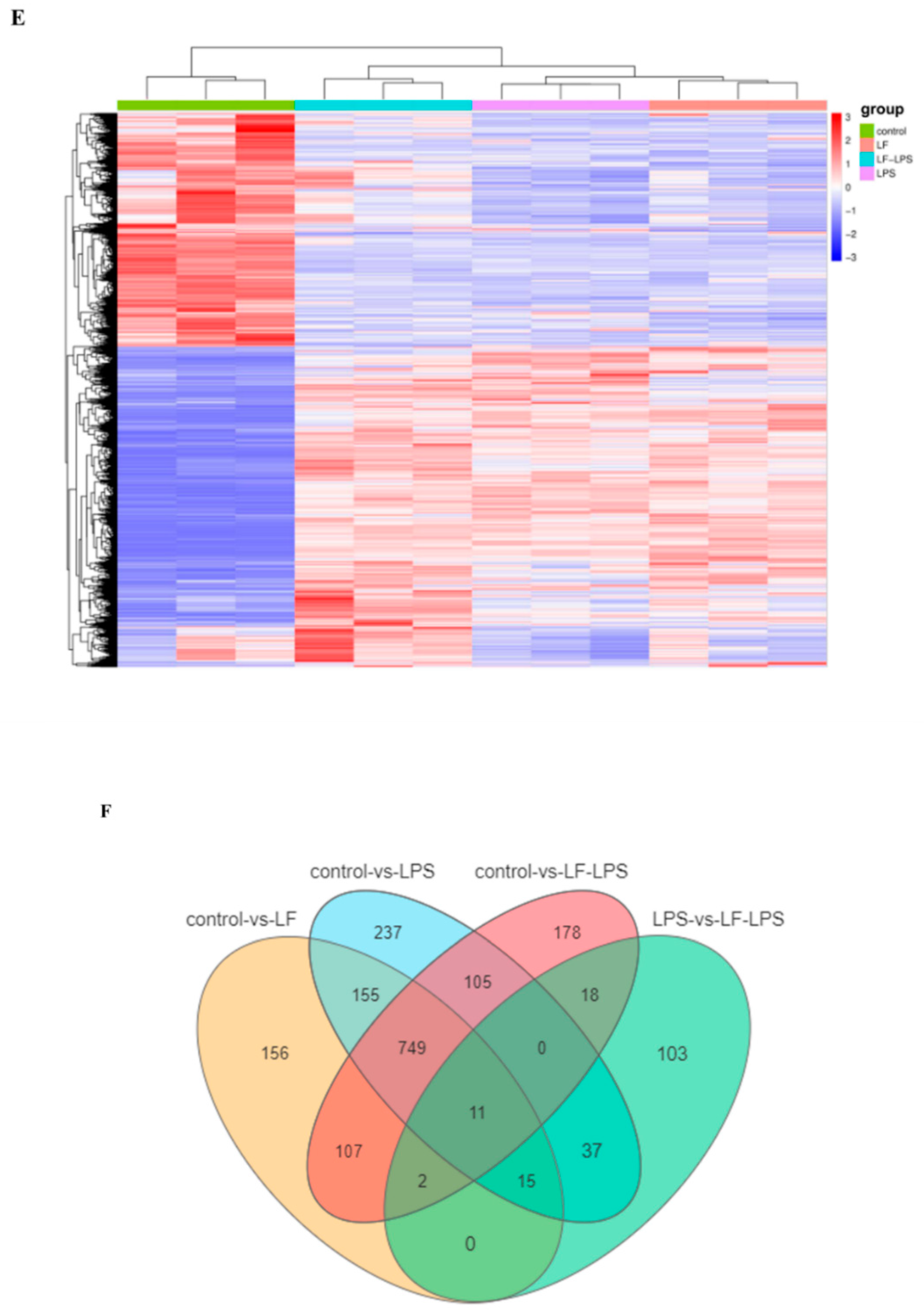
3.2. Apo-LF Effects on the Intestinal Gene Expression Induced by LPS
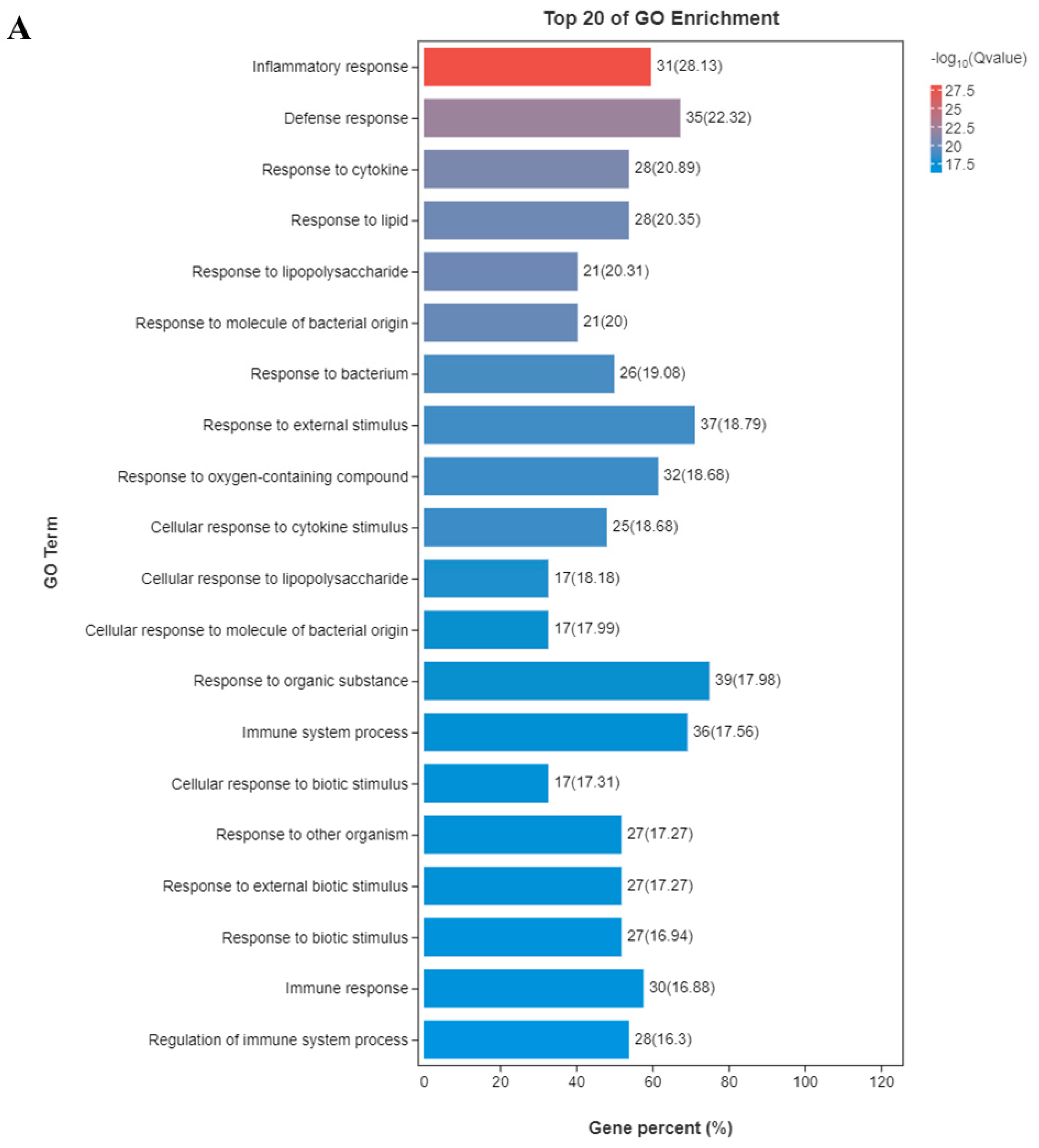
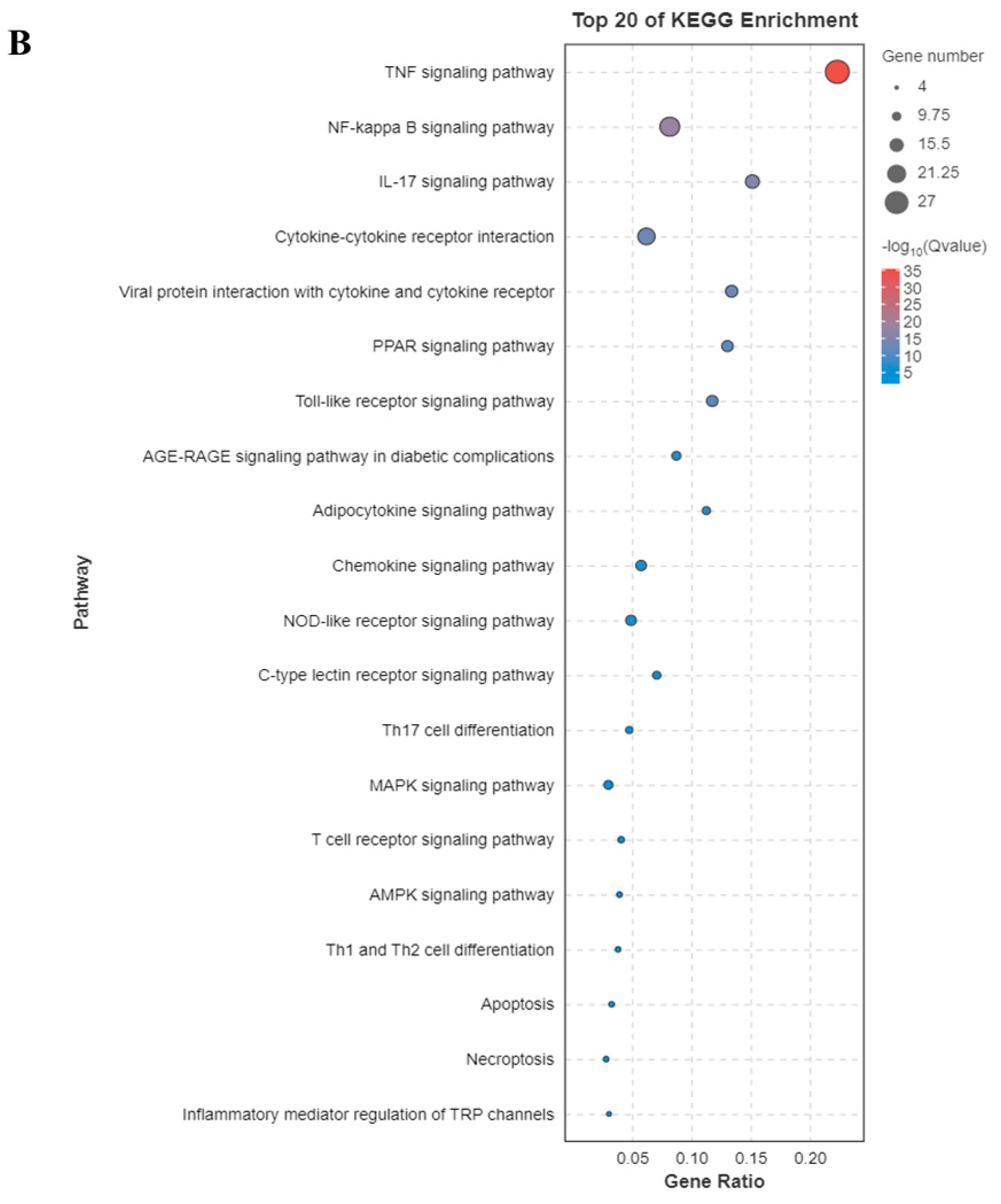
3.3. Functional Enrichment Analysis of GO and KEGG Pathway
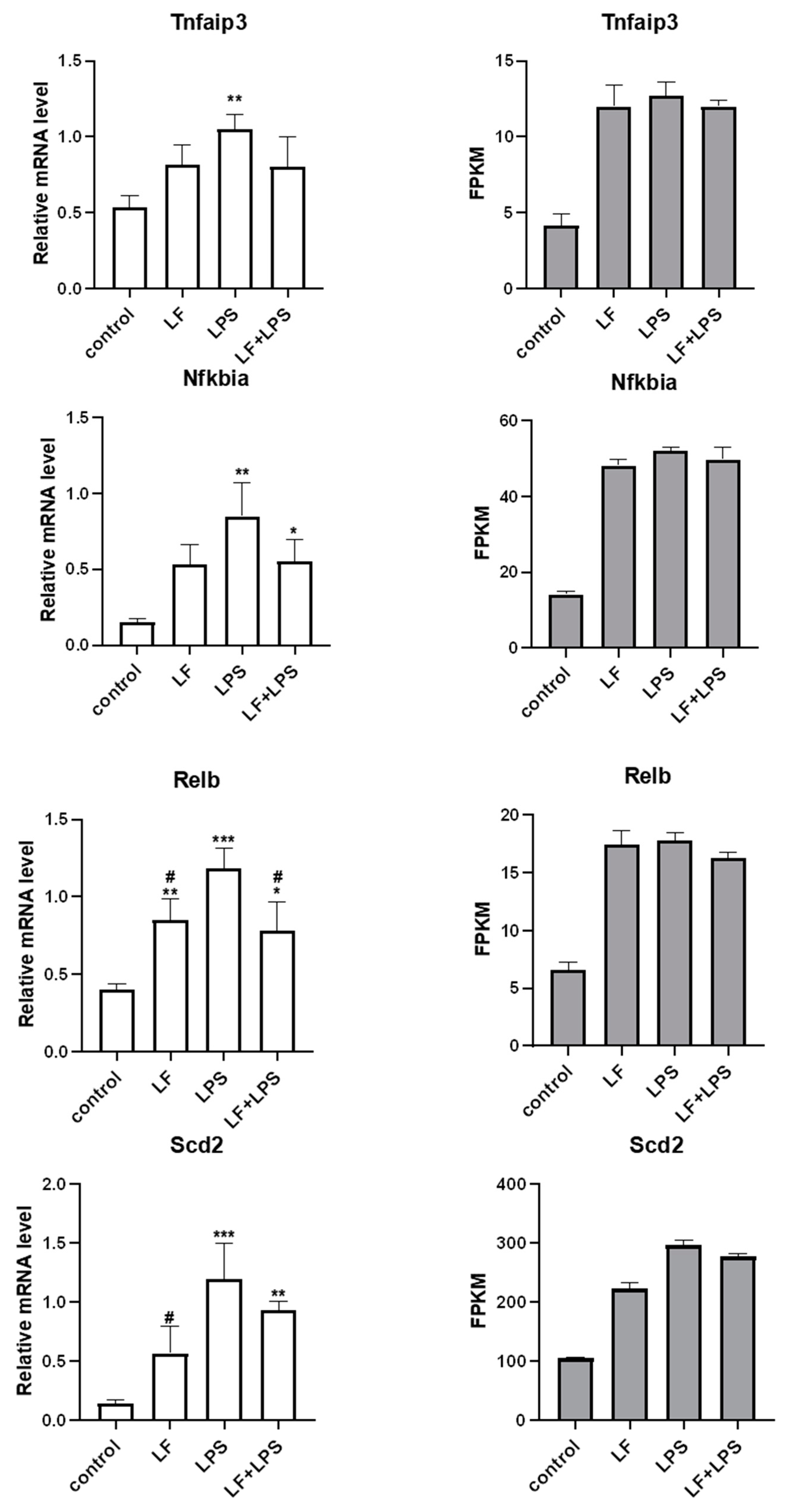
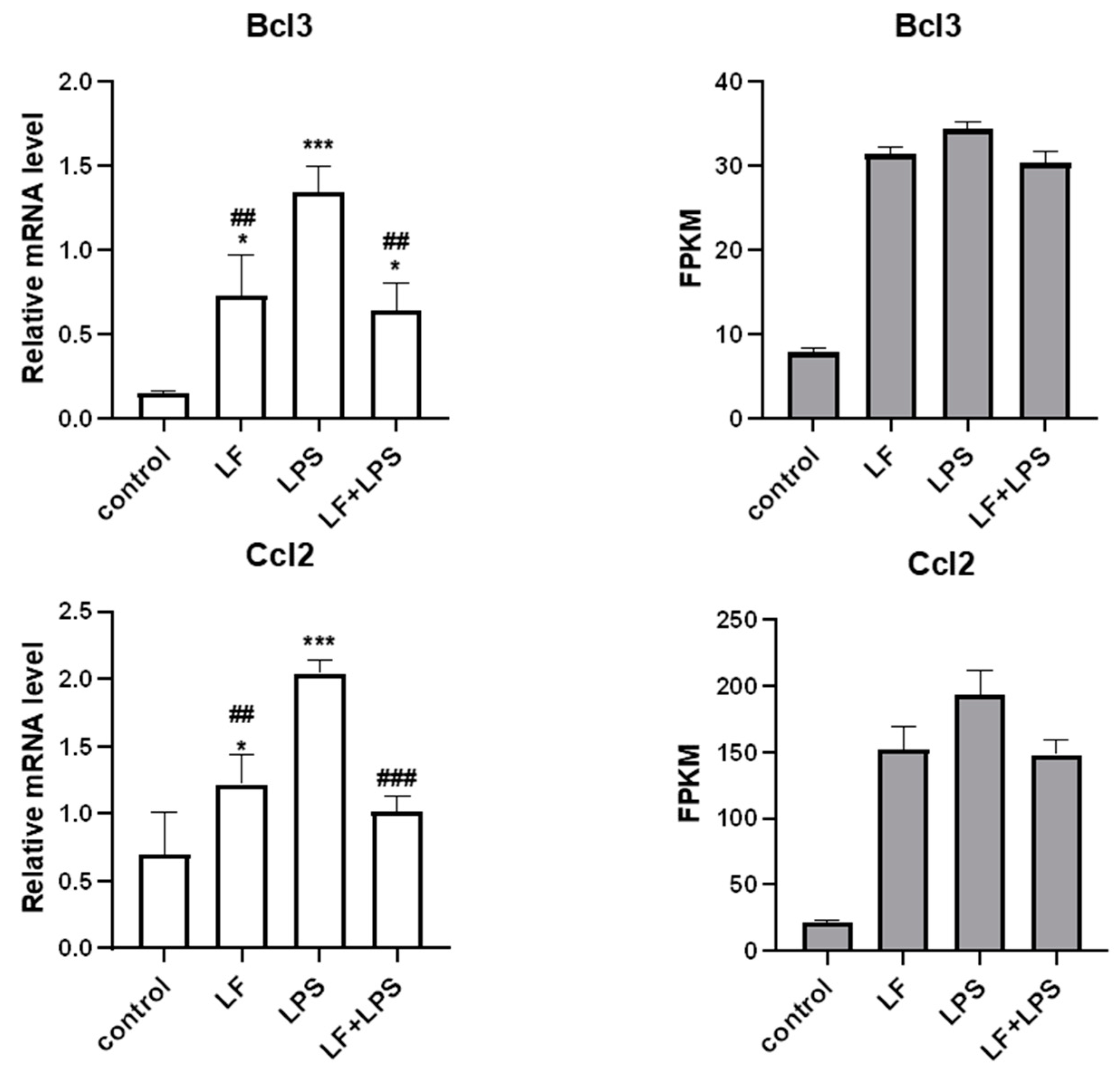
3.4. Effect of Apo-LF on LPS-Induced Cellular Inflammatory Gene Expression
3.5. Apo-LF Inhibited LPS-Induced Colonic Tissue Inflammation in Young Mice
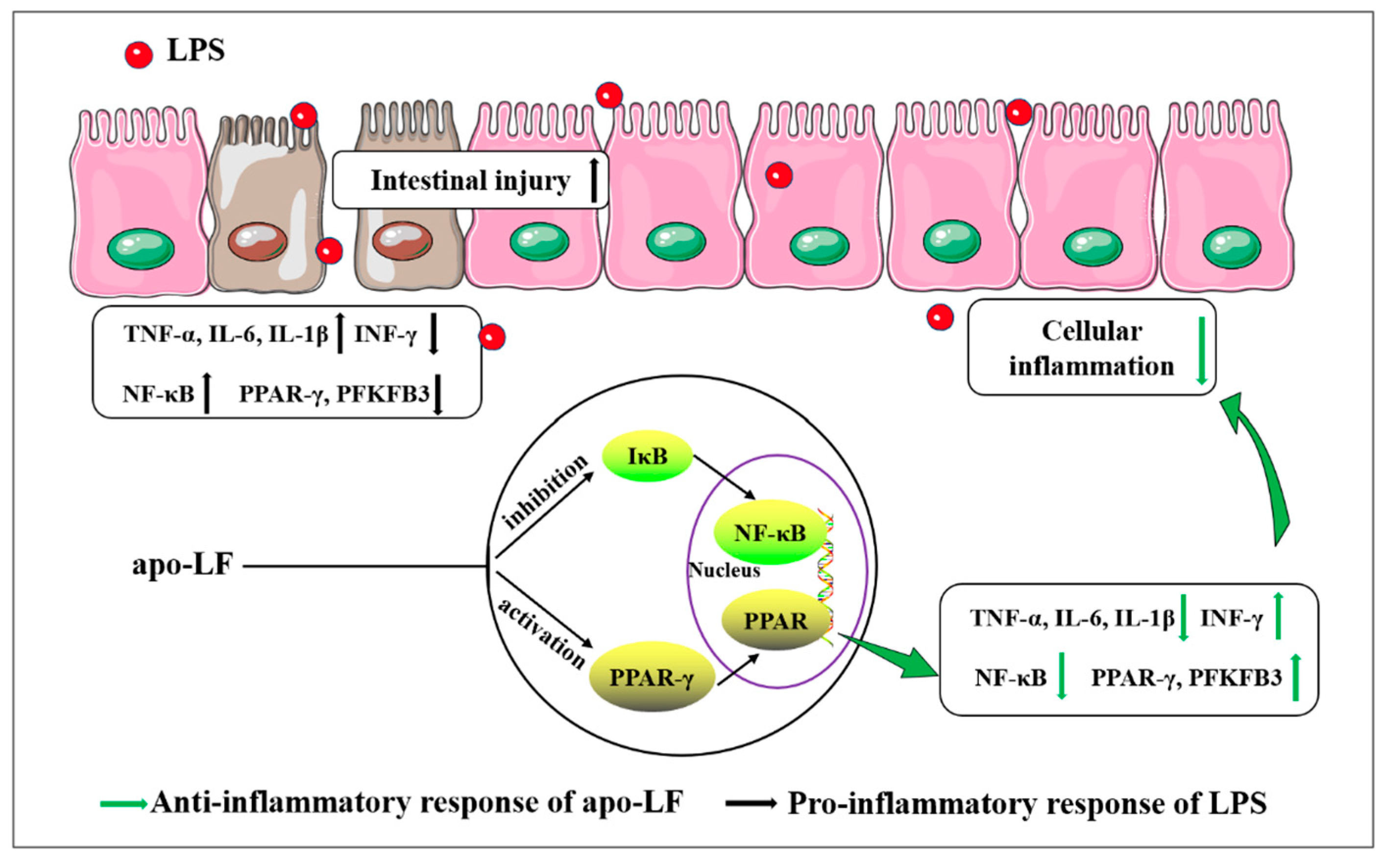
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hou, Q.; Huang, J.; Ayansola, H.; Masatoshi, H.; Zhang, B. Intestinal Stem Cells and Immune Cell Relationships: Potential Therapeutic Targets for Inflammatory Bowel Diseases. Front. Immunol. 2021, 11, 623691. [Google Scholar] [CrossRef] [PubMed]
- Di Tommaso, N.; Gasbarrini, A.; Ponziani, F.R. Intestinal Barrier in Human Health and Disease. Int. J. Env. Res. Public Health 2021, 18, 12836. [Google Scholar] [CrossRef] [PubMed]
- Weström, B.; Arévalo Sureda, E.; Pierzynowska, K.; Pierzynowski, S.G.; Pérez-Cano, F.J. The Immature Gut Barrier and Its Importance in Establishing Immunity in Newborn Mammals. Front. Immunol. 2020, 11, 1153. [Google Scholar] [CrossRef] [PubMed]
- Butzner, J.D.; Gall, D.G. Effects of chronic protein-calorie malnutrition on small intestinal repair after an acute bacterial enteritis: A study in infant rabbits. Pediatr. Res. 1988, 23, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Windster, J.D.; Ouwendijk, W.J.D.; Sloots, C.E.J.; Verjans, G.M.G.M.; Verdijk, R.M. Ileocolic Intussusception as the Presenting Symptom of Primary Enteric Varicella-Zoster Virus Infection in a 7-Month-Old Infant. J. Infect. Dis. 2020, 222, 305–308. [Google Scholar] [CrossRef] [PubMed]
- Chatterton, D.E.; Nguyen, D.N.; Bering, S.B.; Sangild, P.T. Anti-inflammatory mechanisms of bioactive milk proteins in the intestine of newborns. Int. J. Biochem. Cell Biol. 2013, 45, 1730–1747. [Google Scholar] [CrossRef]
- Kong, X.; Yang, M.; Guo, J.; Feng, Z. Effects of Bovine Lactoferrin on Rat Intestinal Epithelial Cells. J. Pediatr. Gastroenterol. Nutr. 2020, 70, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Majka, G.; Więcek, G.; Śróttek, M.; Śpiewak, K.; Brindell, M.; Koziel, J.; Marcinkiewicz, J.; Strus, M. The impact of lactoferrin with different levels of metal saturation on the intestinal epithelial barrier function and mucosal inflammation. Biometals 2016, 29, 1019–1033. [Google Scholar] [CrossRef]
- Mikulic, N.; Uyoga, M.A.; Mwasi, E.; Stoffel, N.U.; Zeder, C.; Karanja, S.; Zimmermann, M.B. Iron Absorption is Greater from Apo-Lactoferrin and is Similar Between Holo-Lactoferrin and Ferrous Sulfate: Stable Iron Isotope Studies in Kenyan Infants. J. Nutr. 2020, 150, 3200–3207. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Lopez, V.; Kelleher, S.L.; Lönnerdal, B. Apo- and holo-lactoferrin are both internalized by lactoferrin receptor via clathrin-mediated endocytosis but differentially affect ERK-signaling and cell proliferation in Caco-2 cells. J. Cell Physiol. 2011, 226, 3022–3031. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, M.; Shinoda, I.; Samejima, Y.; Miyauchi, H.; Fukuwatari, Y.; Hayasawa, H. Lactoferrin as a suppressor of cell migration of gastrointestinal cell lines. J. Cell Physiol. 1997, 170, 101–105. [Google Scholar] [CrossRef]
- Li, L.; Ren, F.; Yun, Z.; An, Y.; Wang, C.; Yan, X. Determination of the effects of lactoferrin in a preclinical mouse model of experimental colitis. Mol. Med. Rep. 2013, 8, 1125–1129. [Google Scholar] [CrossRef] [PubMed]
- Chambers, D.C.; Carew, A.M.; Lukowski, S.W.; Powell, J.E. Transcriptomics and single-cell RNA-sequencing. Respirology 2019, 24, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.; Chang, S.Y.; Bogere, P.; Won, K.; Choi, J.Y.; Choi, Y.J.; Lee, H.K.; Hur, J.; Park, B.Y.; Kim, Y.; et al. Beneficial roles of probiotics on the modulation of gut microbiota and immune response in pigs. PLoS ONE 2019, 14, e0220843. [Google Scholar] [CrossRef] [PubMed]
- Arnold, J.W.; Roach, J.; Fabela, S.; Moorfield, E.; Ding, S.; Blue, E.; Dagher, S.; Magness, S.; Tamayo, R.; Bruno-Barcena, J.M.; et al. The pleiotropic effects of prebiotic galacto-oligosaccharides on the aging gut. Microbiome 2021, 9, 31. [Google Scholar] [CrossRef] [PubMed]
- Mahe, M.M.; Sundaram, N.; Watson, C.L.; Shroyer, N.F.; Helmrath, M.A. Establishment of human epithelial enteroids and colonoids from whole tissue and biopsy. J. Vis. Exp. 2015, 97, 52483. [Google Scholar] [CrossRef]
- Staab, J.F.; Lemme-Dumit, J.M.; Latanich, R.; Pasetti, M.F.; Zachos, N.C. Co-Culture System of Human Enteroids/Colonoids with Innate Immune Cells. Curr. Protoc. Immunol. 2020, 131, e113. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.B.; Wang, C.Y.; Yang, L.L.; Cheng, Y.; Yun, Z.Y.; Yang, J.W. A Method for Preparing Lactoferrin with Required Iron Saturation; Application No. 201810713582; Inner Mongolia Yili Industrial Group Co. Ltd.: Hohhot, China, 2020. [Google Scholar]
- Clevers, H. Primary mouse small intestinal epithelial cell cultures. Methods Mol. Biol. 2013, 945, 319–328. [Google Scholar]
- Fan, L.L.; Yao, Q.Q.; Wu, H.M.; Wen, F.; Wang, J.Q.; Li, H.Y.; Zheng, N. Protective effects of recombinant lactoferrin with different iron saturations on enteritis injury in young mice. J. Dairy Sci. 2022, 105, 4791–4803. [Google Scholar] [CrossRef]
- Gao, Y.; Li, S.; Bao, X.; Luo, C.; Yang, H.; Wang, J.; Zhao, S.; Zheng, N. Transcriptional and Proteomic Analysis Revealed a Synergistic Effect of Aflatoxin M1 and Ochratoxin a Mycotoxins on the Intestinal Epithelial Integrity of Differentiated Human Caco-2 Cells. J. Proteome Res. 2018, 17, 3128–3142. [Google Scholar] [CrossRef] [PubMed]
- Blackwell, C.; Moscovis, S.; Hall, S.; Burns, C.; Scott, R.J. Exploring the risk factors for sudden infant deaths and their role in inflammatory responses to infection. Front. Immunol. 2015, 6, 44. [Google Scholar] [CrossRef] [PubMed]
- Støy, A.C.F.; Heegaard, P.M.H.; Skovgaard, K.; Bering, S.B.; Bjerre, M.; Sangild, P.T. Increased Intestinal Inflammation and Digestive Dysfunction in Preterm Pigs with Severe Necrotizing Enterocolitis. Neonatology 2017, 111, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Pammi, M.; Suresh, G. Enteral lactoferrin supplementation for prevention of sepsis and necrotizing enterocolitis in preterm infants. Cochrane Database Syst. Rev. 2017, 6, CD007137. [Google Scholar] [CrossRef] [PubMed]
- Asztalos, E.V.; Barrington, K.; Lodha, A.; Tarnow-Mordi, W.; Martin, A. Lactoferrin infant feeding trial_Canada (LIFT_Canada): Protocol for a randomized trial of adding lactoferrin to feeds of very-low-birth-weight preterm infants. BMC Pediatr. 2020, 20, 40. [Google Scholar] [CrossRef] [PubMed]
- Manzoni, P. Clinical Benefits of Lactoferrin for Infants and Children. J. Pediatr. 2016, 173, S43–S52. [Google Scholar] [CrossRef] [PubMed]
- Muraleedharan, C.K.; Mierzwiak, J.; Feier, D.; Nusrat, A.; Quiros, M. Generation of Murine Primary Colon Epithelial Monolayers from Intestinal Crypts. J. Vis. Exp. 2021, 168, e62156. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Sun, R.; Wang, G.; Chen, Z.; Li, Y.; Zhao, Y.; Liu, D.; Zhao, H.; Zhang, F.; Yao, J.; et al. SIRT3-mediated deacetylation of PRDX3 alleviates mitochondrial oxidative damage and apoptosis induced by intestinal ischemia/reperfusion injury. Redox. Biol. 2020, 28, 101343. [Google Scholar] [CrossRef]
- Frick, A.; Khare, V.; Jimenez, K.; Dammann, K.; Lang, M.; Krnjic, A.; Gmainer, C.; Baumgartner, M.; Mesteri, I.; Gasche, C. A Novel PAK1-Notch1 Axis Regulates Crypt Homeostasis in Intestinal Inflammation. Cell Mol. Gastroenterol. Hepatol. 2021, 11, 892–907. [Google Scholar] [CrossRef]
- Dutton, J.S.; Hinman, S.S.; Kim, R.; Wang, Y.; Allbritton, N.L. Primary Cell-Derived Intestinal Models: Recapitulating Physiology. Trends Biotechnol. 2019, 37, 744–760. [Google Scholar] [CrossRef]
- Schlottmann, K.; Wachs, F.P.; Grossmann, J.; Vogl, D.; Maendel, M.; Falk, W.; Schölmerich, J.; Andus, T.; Rogler, G. Interferon gamma downregulates IL-8 production in primary human colonic epithelial cells without induction of apoptosis. Int. J. Color. Dis. 2004, 19, 421–429. [Google Scholar] [CrossRef]
- Biton, M.; Haber, A.L.; Rogel, N.; Burgin, G.; Beyaz, S.; Schnell, A.; Ashenberg, O.; Su, C.W.; Smillie, C.; Shekhar, K.; et al. Helper Cell Cytokines Modulate Intestinal Stem Cell Renewal and Differentiation. Cell 2018, 175, 1307–1320. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhu, H.; Li, B.; Robinson, S.C.; Lee, C.; O’Connell, J.S.; Bindi, E.; Zheng, S.; Sherman, P.M.; Pierro, A. Lactoferrin Reduces Necrotizing Enterocolitis Severity by Upregulating Intestinal Epithelial Proliferation. Eur. J. Pediatr. Surg. 2020, 30, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, E.A.; Duffy, L.C.; Schanbacher, F.L.; Qiao, H.; Dryja, D.; Leavens, A.; Rossman, J.; Rich, G.; Dirienzo, D.; Ogra, P.L. In vivo effects of bifidobacteria and lactoferrin on gut endotoxin concentration and mucosal immunity in Balb/c mice. Dig. Dis. Sci. 2004, 49, 579–589. [Google Scholar] [CrossRef]
- Geng, H.; Bu, H.F.; Liu, F.; Wu, L.; Pfeifer, K.; Chou, P.M.; Wang, X.; Sun, J.; Lu, L.; Pandey, A.; et al. In Inflamed Intestinal Tissues and Epithelial Cells, Interleukin 22 Signaling Increases Expression of H19 Long Noncoding RNA, Which Promotes Mucosal Regeneration. Gastroenterology 2018, 155, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Bein, A.; Zilbershtein, A.; Golosovsky, M.; Davidov, D.; Schwartz, B. LPS Induces Hyper-Permeability of Intestinal Epithelial Cells. J. Cell Physiol. 2017, 232, 381–390. [Google Scholar] [CrossRef]
- Angrisano, T.; Pero, R.; Peluso, S.; Keller, S.; Sacchetti, S.; Bruni, C.B.; Chiariotti, L.; Lembo, F. LPS-induced IL-8 activation in human intestinal epithelial cells is accompanied by specific histone H3 acetylation and methylation changes. BMC Microbiol. 2010, 10, 72. [Google Scholar] [CrossRef]
- Stephens, M.; von der Weid, P.Y. Lipopolysaccharides modulate intestinal epithelial permeability and inflammation in a species-specific manner. Gut Microbes 2020, 11, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Zhao, F.; Wang, J.; Zhu, W. Lactoferrin attenuates lipopolysaccharide-stimulated inflammatory responses and barrier impairment through the modulation of NF-κB/MAPK/Nrf2 pathways in IPEC-J2 cells. Food Funct. 2020, 11, 8516–8526. [Google Scholar] [CrossRef] [PubMed]
- Porcu, E.; Sadler, M.C.; Lepik, K.; Auwerx, C.; Wood, A.R.; Weihs, A.; Sleiman, M.S.B.; Ribeiro, D.M.; Bandinelli, S.; Tanaka, T.; et al. Differentially expressed genes reflect disease-induced rather than disease-causing changes in the transcriptome. Nat. Commun. 2021, 12, 5647. [Google Scholar] [CrossRef] [PubMed]
- Millet, P.; McCall, C.; Yoza, B. RelB: An outlier in leukocyte biology. J. Leukoc. Biol. 2013, 94, 941–951. [Google Scholar] [CrossRef]
- Hsu, K.H.; Wei, C.W.; Su, Y.R.; Chou, T.; Lin, Y.L.; Yang, F.C.; Tsou, A.P.; Hsu, C.L.; Tseng, P.H.; Chen, N.J.; et al. Upregulation of RelB in the miR-122 knockout mice contributes to increased levels of proinflammatory chemokines/cytokines in the liver and macrophages. Immunol. Lett. 2020, 226, 22–30. [Google Scholar] [CrossRef]
- Zhang, S.; Gao, J.; Liu, S.; Yu, L.; Zhang, W.; Liang, Y.; Wang, H. Transcription Coactivator BCL3 Acts as a Potential Regulator of Lipid Metabolism Through the Effects on Inflammation. J. Inflamm. Res. 2021, 14, 4915–4926. [Google Scholar] [CrossRef] [PubMed]
- Wang, V.Y.; Huang, W.; Asagiri, M.; Spann, N.; Hoffmann, A.; Glass, C.; Ghosh, G. The transcriptional specificity of NF-κB dimers is coded within the κB DNA response elements. Cell Rep. 2012, 2, 824–839. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Rojas, M.; Lilly, B.; Tsai, N.T.; Lemtalsi, T.; Liou, G.I.; Caldwell, R.W.; Caldwell, R.B. NAD(P)H oxidase-dependent regulation of CCL2 production during retinal inflammation. Invest. Ophthalmol. Vis. Sci. 2009, 50, 3033–3040. [Google Scholar] [CrossRef]
- Comstock, S.S.; Reznikov, E.A.; Contractor, N.; Donovan, S.M. Dietary Bovine Lactoferrin Alters Mucosal and Systemic Immune Cell Responses in Neonatal Piglets. J. Nutr. 2014, 144, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Drago-Serrano, M.E.; Campos-Rodríguez, R.; Carrero, J.C.; de la Garza, M. Lactoferrin: Balancing Ups and Downs of Inflammation Due to Microbial Infections. Int. J. Mol. Sci. 2017, 18, 501. [Google Scholar] [CrossRef]
- Lawrence, T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.L.; Liu, Y.H.; Liu, C.; Qi, M.P.; Liu, R.N.; Zhu, X.F.; Zhou, Q.G.; Chen, Y.Y.; Guo, A.Z.; Hu, C.M. Indirubin Inhibits LPS-Induced Inflammation via TLR4 Abrogation Mediated by the NF-kB and MAPK Signaling Pathways. Inflammation 2017, 40, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Vallabhapurapu, S.; Karin, M. Regulation and function of NF-kappaB transcription factors in the immune system. Annu. Rev. Immunol. 2009, 27, 693–733. [Google Scholar] [CrossRef]
- Spagnuolo, P.A.; Hoffman-Goetz, L. Dietary lactoferrin does not prevent dextran sulfate sodium induced murine intestinal lymphocyte death. Exp. Biol. Med. 2008, 233, 1099–1108. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, Y.; Zhang, J.; Wang, R.; Cheng, B.; Kalambhe, D.; Wang, Y.; Gu, Z.; Chen, D.; Wang, B.; et al. Lactoferrin-mediated macrophage targeting delivery and patchouli alcohol-based therapeutic strategy for inflammatory bowel diseases. Acta Pharm. Sin. B 2020, 10, 1966–1976. [Google Scholar] [CrossRef] [PubMed]
- Christofides, A.; Konstantinidou, E.; Jani, C.; Boussiotis, V.A. The role of peroxisome proliferator-activated receptors (PPAR) in immune responses. Metabolism 2021, 114, 154338. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Zhang, W.; Feng, S.J.; Yu, H.P. Emodin suppresses LPS-induced inflammation in RAW264.7 cells through a PPARγ-dependent pathway. Int. Immunopharmacol. 2016, 34, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Dammann, K.; Khare, V.; Lang, M.; Claudel, T.; Harpain, F.; Granofszky, N.; Evstatiev, R.; Williams, J.M.; Pritchard, D.M.; Watson, A.; et al. PAK1 modulates a PPARγ/NF-κB cascade in intestinal inflammation. Biochim. Biophys. Acta Mol. Cell Res. 2015, 1853, 2349–2360. [Google Scholar] [CrossRef] [PubMed]
- Sarangdhar, M.; Yacyshyn, M.B.; Gruenzel, A.R.; Engevik, M.A.; Harris, N.L.; Aronow, B.J.; Yacyshyn, B.R. Therapeutic Opportunities for Intestinal Angioectasia-Targeting PPARγ and Oxidative Stress. Clin. Transl. Sci. 2021, 14, 518–528. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, W.; Xing, W.; Li, H.; Si, T.; Mu, H. MicroRNA-498 disturbs the occurrence and aggression of colon cancer through targeting MDM2 to mediate PPARγ ubiquitination. Life Sci. 2021, 277, 119225. [Google Scholar] [CrossRef]
- Guo, X.; Li, H.; Xu, H.; Halim, V.; Thomas, L.N.; Woo, S.L.; Huo, Y.; Chen, Y.E.; Sturino, J.M.; Wu, C. Disruption of inducible 6-phosphofructo-2-kinase impairs the suppressive effect of PPARγ activation on diet-induced intestine inflammatory response. J. Nutr. Biochem. 2013, 24, 770–775. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, H.; Fan, L.; Gao, Y.; Wang, J.; Zheng, N. The Protective Effects of Iron Free Lactoferrin on Lipopolysaccharide-Induced Intestinal Inflammatory Injury via Modulating the NF-κB/PPAR Signaling Pathway. Foods 2022, 11, 3378. https://doi.org/10.3390/foods11213378
Wu H, Fan L, Gao Y, Wang J, Zheng N. The Protective Effects of Iron Free Lactoferrin on Lipopolysaccharide-Induced Intestinal Inflammatory Injury via Modulating the NF-κB/PPAR Signaling Pathway. Foods. 2022; 11(21):3378. https://doi.org/10.3390/foods11213378
Chicago/Turabian StyleWu, Hongya, Linlin Fan, Yanan Gao, Jiaqi Wang, and Nan Zheng. 2022. "The Protective Effects of Iron Free Lactoferrin on Lipopolysaccharide-Induced Intestinal Inflammatory Injury via Modulating the NF-κB/PPAR Signaling Pathway" Foods 11, no. 21: 3378. https://doi.org/10.3390/foods11213378
APA StyleWu, H., Fan, L., Gao, Y., Wang, J., & Zheng, N. (2022). The Protective Effects of Iron Free Lactoferrin on Lipopolysaccharide-Induced Intestinal Inflammatory Injury via Modulating the NF-κB/PPAR Signaling Pathway. Foods, 11(21), 3378. https://doi.org/10.3390/foods11213378






