Dissipation, Residue and Dietary Intake Risk Assessment of Penthiopyrad in Eggplants and Its Removal Using Various Household Processing Techniques
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Field Trials
2.3. General Household Processing Treatments
2.4. Instrumentation and Pretreatment
2.5. Method Validation
2.6. Dissipation Kinetics, Processing Factor, Potential Enantioselectivity and Statistical Analysis
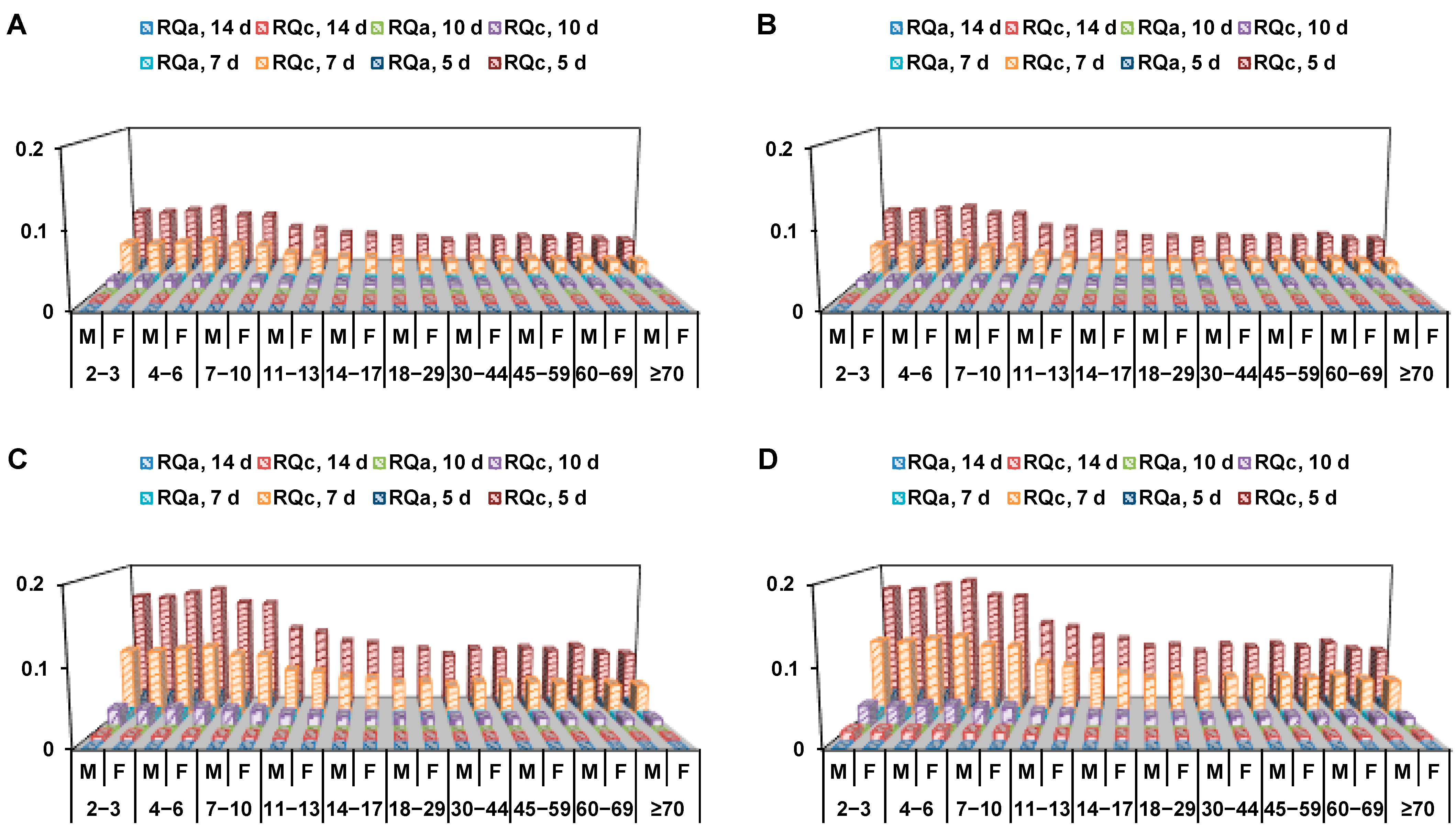
2.7. Dietary Risk Assessment
3. Results
3.1. Method Validation
3.2. Dissipation, Residue Distribution and Dietary Risk Assessment of Penthiopyrad and PAM in Eggplants under Field Conditions and the Potential Stereoselectivity
3.3. The Distribution of Penthiopyrad Residues in Eggplant Samples after Household Processing
3.3.1. Washing Processing
3.3.2. Peeling Processing
3.3.3. Thermal Processing
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reiler, E.; Jørs, E.; Bælum, J.; Huici, O.; Caero, M.M.A.; Cedergreen, N. The influence of tomato processing on residues of organochlorine and organophosphate insecticides and their associated dietary risk. Sci. Total Environ. 2015, 527–528, 262–269. [Google Scholar] [CrossRef]
- Souza, L.P.; Faroni, L.R.D.; Heleno, F.F.; Pinto, F.G.; Queiroz, M.E.L.R.; Prates, L.H.F. Difenoconazole and linuron dissipation kinetics in carrots under open-field conditions. Ecotox. Environ. Safe. 2019, 168, 479–485. [Google Scholar] [CrossRef]
- Zhang, J.; Li, M.M.; Zhang, R.; Jin, N.; Quan, R.; Chen, D.Y.; Francis, F.; Wang, F.Z.; Kong, Z.Q.; Fan, B. Effect of processing on herbicide residues and metabolite formation during traditional Chinese tofu production. LWT Food Sci. Technol. 2020, 131, 109707. [Google Scholar] [CrossRef]
- Oladosu, Y.; Rafii, M.Y.; Arolu, F.; Chukwu, S.C.; Salisu, M.A.; Olaniyan, B.A.; Fagbohun, I.K.; Muftaudeen, T.K. Genetic diversity and utilization of cultivated eggplant germplasm in varietal improvement. Plants 2021, 10, 1714. [Google Scholar] [CrossRef] [PubMed]
- Karimi, A.; Kazemi, M.; Samani, S.A.; Simal-Gandara, J. Bioactive compounds from by-products of eggplant: Functional properties, potential applications and advances in valorization methods. Trends. Food Sci. Tech. 2021, 112, 518–531. [Google Scholar] [CrossRef]
- Nisha, P.; Abdul Nazar, P.; Jayamurthy, P. A comparative study on antioxidant activities of different varieties of Solanum melongena. Food. Chem. Toxicol. 2009, 47, 2640–2644. [Google Scholar] [CrossRef] [PubMed]
- Specialty Produce. Chinese Eggplant. Available online: https://specialtyproduce.com/produce/chinese_eggplant_4042.php (accessed on 10 September 2022).
- Zero Power Intelligence. Annual Research and Consultation Report of Panorama Survey and Investment Strategy on China Eggplant Industry. Available online: https://www.chinairn.com/report/20220513/101838964.html (accessed on 10 September 2022).
- EFSA (European Food Safety Authority). Reasoned opinion on the setting of new MRLs for penthiopyrad in various crops. EFSA J. 2012, 10, 2948. [Google Scholar] [CrossRef]
- Ren, B.; Zhao, T.T.; Li, Y.H.; Liang, H.L.; Zhao, X.X.; Chen, H.Y.; Li, L.; Liang, H.W. Enantioselective bioaccumulation and toxicity of the novel chiral antifungal agrochemical penthiopyrad in zebrafish (Danio rerio). Ecotox. Environ. Safe. 2021, 228, 113010. [Google Scholar] [CrossRef] [PubMed]
- EFSA (European Food Safety Authority). Review of the existing maximum residue levels for penthiopyrad according to Article 12 of Regulation (EC) No 396/2005. EFSA J. 2021, 19, 6810. [Google Scholar] [CrossRef]
- National Health and Family Planning Commission; Ministry of Agriculture of the People’s Republic of China. National Food Safety Standard–Maximum Residue Limits for Pesticides in Food (GB 2763–2021). Available online: http://www.nhc.gov.cn/sps/s7891/201702/ed7b47492d7a42359f839daf3f70eb4b.shtml (accessed on 20 March 2022).
- Yang, G.Q.; Li, J.M.; Lan, T.T.; Dou, L.; Zhang, K.K. Dissipation, residue, stereoselectivity and dietary risk assessment of penthiopyrad and metabolite PAM on cucumber and tomato in greenhouse and field. Food Chem. 2022, 387, 132875. [Google Scholar] [CrossRef] [PubMed]
- EFSA (European Food Safety Authority). Peer review of the pesticide risk assessment for the active substance penthiopyrad in light of confirmatory data submitted. EFSA J. 2022, 20, 7037. [Google Scholar] [CrossRef]
- Jankowska, M.; Łozowicka, B.; Kaczyński, P. Comprehensive toxicological study over 160 processing factors of pesticides in selected fruit and vegetables after water, mechanical and thermal processing treatments and their application to human health risk assessment. Sci. Total Environ. 2019, 652, 1156–1167. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.S.; Qin, X.X.; Chen, D.; Liu, Z.Y.; Zhang, K.K.; Hu, D.Y. Determination, residue analysis, risk assessment and processing factors of tebufenozide in okra fruits under field conditions. J. Sci. Food Agr. 2020, 100, 1230–1237. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Guidance Document on the Estimation of LOD and LOQ for Measurements in the Field of Contaminants in Feed and Food. Available online: https://ec.europa.eu/food/document/download/09b61576-c430-4888-8c18-4189ae44b5fb_en (accessed on 20 March 2022).
- Lan, T.T.; Yang, G.Q.; Li, J.M.; Chi, D.; Zhang, K.K. Residue, dissipation and dietary intake risk assessment of tolfenpyrad in four leafy green vegetables under greenhouse conditions. Food Chem. X 2022, 13, 100241. [Google Scholar] [CrossRef]
- Tian, F.J.; Liu, X.G.; Wu, Y.B.; Xu, J.; Dong, F.S.; Wu, X.H.; Zheng, Y.Q. Simultaneous determination of penflufen and one metabolite in vegetables and cereals using a modified quick, easy, cheap, effective, rugged, and safe method and liquid chromatography coupled to tandem mass spectrometry. Food Chem. 2016, 213, 410–416. [Google Scholar] [CrossRef]
- Li, J.M.; Lan, T.T.; Yang, G.Q.; Mu, S.Y.; Zhang, K.K. Enantioselective evaluation of the chiral fungicide mandipropamid: Dissipation, distribution and potential dietary intake risk in tomato, cucumber, Chinese cabbage and cowpea. Ecotox. Environ. Safe. 2022, 232, 113260. [Google Scholar] [CrossRef] [PubMed]
- Scholz, R.; Donkersgoed, G.V.; Herrmann, M.; Kittelmann, A.; Schledorn, M.V.; Graven, C.; Mahieu, K.; Velde-Koerts, T.V.D.; Anagnostopoulos, C.; Bempelou, E.; et al. European database of processing factors for pesticides. EFSA Supporting Publ. 2018, 15, EN-1510. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Chen, D.; Han, J.H.; Chen, Y.; Zhang, K.K. Stereoselective degradation behavior of the novel chiral antifungal agrochemical penthiopyrad in soil. Environ. Res. 2021, 194, 110680. [Google Scholar] [CrossRef]
- Song, L.; Zhong, Z.Z.; Han, Y.T.; Zheng, Q.L.; Qin, Y.H.; Wu, Q.; He, X.P.; Pan, C.P. Dissipation of sixteen pesticide residues from various applications of commercial formulations on strawberry and their risk assessment under greenhouse conditions. Ecotox. Environ. Saf. 2020, 188, 109842. [Google Scholar] [CrossRef]
- Jin, S.G. Survey Report on the Nutrition and Health of Chinese Residents: Data Set on the Status of Nutrition and Health in 2002; People’s Hygiene Press: Beijing, China, 2008. [Google Scholar]
- FAO (Food and Agriculture Organization of the United Nations); WHO (World Health Organization). Environmental Health Criteria 240: Principles and Methods for the Risk Assessment of Chemicals in Food; WHO Press: Geneva, Sweden, 2011; pp. 251–252. [Google Scholar]
- European Commission. Guidance Document on Analytical Quality Control and Method Validation Procedures for Pesticide Residues Analysis in Food and Feed (SANTE/11312/2021). Available online: https://ec.europa.eu/food/document/download/d4786faf-c574-4222-a5c6-45086b3920b8_en?filename=pesticides_mrl_guidelines_wrkdoc_2021-11312.pdf (accessed on 20 March 2022).
- FAO (Food and Agriculture Organization of the United Nations); WHO (World Health Organization). Guidelines on Performance Criteria for Methods of Analysis for the Determination of Pesticide Residues in Food and Feed (CXG 90-2017). Available online: https://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXG%2B90-2017%252FCXG_090e.pdf (accessed on 20 March 2022).
- Ye, J.; Zhao, M.R.; Liu, J.; Liu, W.P. Enantioselectivity in environmental risk assessment of modern chiral pesticides. Environ. Pollut. 2010, 158, 2371–2383. [Google Scholar] [CrossRef]
- Chang, W.X.; Nie, J.Y.; Yan, Z.; Wang, Y.J.; Farooq, S. Systemic stereoselectivity study of etoxazole: Stereoselective bioactivity, acute toxicity, and environmental behavior in fruits and soils. J. Agric. Food Chem. 2019, 67, 6708–6715. [Google Scholar] [CrossRef] [PubMed]
- FAO (Food and Agriculture Organization of the United Nations). Evaluation of Pesticide Residues for Estimation of Maximum Residue Levels and Calculation of Dietary Intake: Training Manual. Available online: https://www.fao.org/3/i5545e/i5545e.pdf (accessed on 20 March 2022).
- Quan, R.; Li, M.M.; Liu, Y.G.; Jin, N.; Zhang, J.; Li, R.X.; Wang, F.Z.; Wang, Z.D.; Francis, F.; Kong, Z.Q.; et al. Residues and enantioselective behavior of cyflumetofen from apple production. Food Chem. 2020, 321, 126687. [Google Scholar] [CrossRef]
- Bonnechère, A.; Hanot, V.; Jolie, R.; Hendrickx, M.; Bragard, C.; Bedoret, T.; Loco, J.V. Processing factors of several pesticides and degradation products in carrots by household and industrial processing. J. Food Res. 2012, 1, 68–83. [Google Scholar] [CrossRef][Green Version]
- Radwan, M.A.; Abu-Elamayem, M.M.; Shiboob, M.H.; Abdel-Aal, A. Residual behaviour of profenofos on some field-grown vegetables and its removal using various washing solutions and household processing. Food. Chem Toxicol. 2005, 43, 553–557. [Google Scholar] [CrossRef] [PubMed]
- Miyahara, M.; Saito, Y. Effects of the processing steps in tofu production on pesticide residues. J. Agric. Food Chem. 1994, 42, 369–373. [Google Scholar] [CrossRef]
- Wang, Z.W.; Huang, J.X.; Chen, J.Y.; Li, F.L. Effectiveness of dishwashing liquids in removing chlorothalonil and chlorpyrifos residues from cherry tomatoes. Chemosphere 2013, 92, 1022–1028. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.W.C. How effective are common household preparations on removing pesticide residues from fruit and vegetables? A review. J. Sci. Food Agric. 2018, 98, 2857–2870. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.H.; Hu, J.; Qian, M.R.; Wang, Q.; Zhang, H. Degradation of triadimefon and residue levels of metabolite triadimenol: Tracing rapeseed from harvesting and storage to household oil processing. J. Sci. Food Agric. 2018, 99, 1484–1491. [Google Scholar] [CrossRef]
- Todaro, A.; Cimino, F.; Rapisarda, P.; Catalano, A.E.; Barbagallo, R.N.; Spagna, G. Recovery of anthocyanins from eggplant peel. Food Chem. 2009, 114, 434–439. [Google Scholar] [CrossRef]
- Cavalcanti, R.N.; Santos, D.T.; Meireles, M.A.A. Non-thermal stabilization mechanisms of anthocyanins in model and food systems—An overview. Food Res. Int. 2011, 44, 499–509. [Google Scholar] [CrossRef]
- Martini, S.; Conte, A.; Cattivelli, A.; Tagliazucchi, D. Domestic cooking methods affect the stability and bioaccessibility of dark purple eggplant (Solanum melongena) phenolic compounds. Food Chem. 2021, 341, 128298. [Google Scholar] [CrossRef] [PubMed]

| Matrix | Analyte | Regression Equation | R2 | ME | LOD (µg/kg) | LOQ (µg/kg) |
|---|---|---|---|---|---|---|
| Acetonitrile | R-(−)-stereoisomer | y = 417,350x + 18,241 | 0.9985 | / | / | / |
| S-(+)-stereoisomer | y = 420,162x + 17,019 | 0.9989 | / | / | / | |
| PAM | y = 2,000,000x + 136,295 | 0.9959 | / | / | / | |
| Eggplant | R-(−)-stereoisomer | y = 434,925x + 19,769 | 0.9980 | 1.04 | 3.3 | 10 |
| S-(+)-stereoisomer | y = 430,125x + 21,207 | 0.9977 | 1.02 | 3.3 | 10 | |
| PAM | y = 2,000,000x + 127,874 | 0.9960 | 1.00 | 3.3 | 10 |
| Analyte | Matrix | Spiked Level (µg/kg) | Intra-Day Recovery, RSD (%, n = 5) | Inter-Day Recovery, RSD (%, n = 15) | ||
|---|---|---|---|---|---|---|
| Day 1 | Day 2 | Day 3 | ||||
| R | Eggplant/raw | 10 | 81.8, 7.6 | 105.7, 8.4 | 113.8, 4.9 | 100.4, 7.0 |
| 100 | 86.6, 1.5 | 89.4, 6.5 | 86.3, 3.8 | 87.5, 3.9 | ||
| 1000 | 98.7, 1.2 | 90.5, 5.1 | 97.7, 1.9 | 95.6, 2.7 | ||
| 2000 | 85.3, 0.6 | 87.1, 3.1 | 82.0, 1.7 | 84.8, 1.8 | ||
| S | Eggplant/raw | 10 | 81.9, 8.3 | 97.2, 7.7 | 101.1, 3.5 | 93.4, 6.5 |
| 100 | 85.6, 0.6 | 84.5, 4.2 | 88.6, 3.2 | 86.2, 2.7 | ||
| 1000 | 96.4, 2.3 | 87.7, 4.8 | 93.7, 1.2 | 92.6, 2.8 | ||
| 2000 | 86.7, 1.9 | 84.1, 2.5 | 82.6, 2.2 | 84.5, 2.2 | ||
| PAM | Eggplant/raw | 10 | 85.4, 4.5 | 82.2, 3.0 | 79.1, 3.9 | 82.2, 3.8 |
| 100 | 87.5, 2.3 | 86.6, 2.2 | 85.4, 2.1 | 86.5, 2.2 | ||
| 1000 | 88.4, 2.7 | 83.4, 1.8 | 81.7, 1.4 | 84.5, 1.9 | ||
| 2000 | 91.4, 3.0 | 87.6, 0.9 | 86.6, 1.6 | 88.5, 1.8 | ||
| R | Eggplant/steamed | 10 | 89.0, 1.7 | 106.8, 3.7 | 107.8, 2.5 | 101.2, 2.6 |
| 100 | 93.0, 5.1 | 91.6, 3.2 | 90.4, 2.5 | 91.7, 3.6 | ||
| 1000 | 73.8, 2.0 | 85.0, 2.5 | 84.2, 1.9 | 81.0, 2.1 | ||
| 2000 | 79.2, 1.6 | 89.8, 2.3 | 87.2, 2.1 | 85.4, 2.0 | ||
| S | Eggplant/steamed | 10 | 85.3, 1.2 | 94.8, 3.4 | 93.6, 6.9 | 91.2, 3.9 |
| 100 | 76.7, 1.7 | 92.6, 1.7 | 92.4, 2.6 | 87.2, 2.0 | ||
| 1000 | 72.5, 1.9 | 84.7, 4.6 | 82.1, 2.3 | 79.8, 2.9 | ||
| 2000 | 79.8, 1.7 | 89.8, 2.8 | 87.0, 1.0 | 85.6, 1.8 | ||
| PAM | Eggplant/steamed | 10 | 91.1, 8.8 | 87.4, 7.6 | 84.0, 7.2 | 87.5, 7.8 |
| 100 | 86.1, 1.8 | 86.8, 6.7 | 87.0, 6.8 | 86.7, 5.1 | ||
| 1000 | 92.6, 7.1 | 88.7, 7.1 | 87.1, 8.4 | 89.5, 7.6 | ||
| 2000 | 91.9, 3.9 | 87.8, 3.9 | 89.0, 3.8 | 89.6, 3.9 | ||
| R | Eggplant/boiled | 10 | 94.2, 4.1 | 95.6, 8.1 | 96.1, 7.2 | 95.3, 6.4 |
| 100 | 93.3, 4.5 | 91.4, 3.7 | 94.5, 3.7 | 93.0, 4.0 | ||
| 1000 | 77.6, 8.2 | 84.1, 2.9 | 81.3, 1.8 | 81.0, 4.3 | ||
| 2000 | 78.7, 0.4 | 91.7, 10.4 | 82.1, 10.3 | 84.2, 7.0 | ||
| S | Eggplant/boiled | 10 | 90.4, 3.6 | 88.3, 4.2 | 90.1, 6.2 | 89.6, 4.7 |
| 100 | 77.1, 0.9 | 95.5, 2.0 | 90.2, 1.6 | 87.6, 1.5 | ||
| 1000 | 72.1, 1.9 | 84.1, 3.3 | 80.5, 2.3 | 78.9, 2.5 | ||
| 2000 | 79.5, 0.7 | 90.2, 4.4 | 86.9, 0.7 | 85.5, 1.9 | ||
| PAM | Eggplant/boiled | 10 | 85.8, 4.7 | 85.8, 5.2 | 90.6, 6.9 | 87.4, 5.6 |
| 100 | 86.8, 5.9 | 89.9, 4.2 | 93.2, 4.5 | 90.0, 4.9 | ||
| 1000 | 97.7, 8.6 | 93.6, 9.0 | 92.0, 7.8 | 94.4, 8.5 | ||
| 2000 | 96.4, 4.1 | 91.4, 4.6 | 91.5, 4.7 | 93.1, 4.5 | ||
| Dose (g a.i./ha) | Spraying Time | Stereoisomer | r2 | P1 | k (d−1) | P2 | t1/2 (d) | P3 |
|---|---|---|---|---|---|---|---|---|
| 75 | 2 | R | 0.9551 ± 0.0038 c | <0.001 | 0.2806 ± 0.0040 b | <0.001 | 2.47 ± 0.04 a | <0.001 |
| S | 0.9476 ± 0.0042 c | 0.2712 ± 0.0117 b | 2.56 ± 0.11 a | |||||
| 3 | R | 0.9654 ± 0.0107 b | 0.3772 ± 0.0325 a | 1.85 ± 0.16 b | ||||
| S | 0.9648 ± 0.0067 b | 0.3686 ± 0.0178 a | 1.88 ± 0.09 b | |||||
| 99 | 2 | R | 0.9871 ± 0.0025 a | 0.3665 ± 0.0198 a | 1.89 ± 0.10 b | |||
| S | 0.9866 ± 0.0021 a | 0.3581 ± 0.0204 a | 1.94 ± 0.11 b | |||||
| 3 | R | 0.9842 ± 0.0038 a | 0.2789 ± 0.0069 b | 2.49 ± 0.06 a | ||||
| S | 0.9832 ± 0.0037 a | 0.2751 ± 0.0050 b | 2.52 ± 0.05 a |
| Processing Variety | Number | Concentration | Time/min | PF (R) | PF (S) | PF (Rac) | |
|---|---|---|---|---|---|---|---|
| Washing | Tap water rinsing | W1 | 30 | 0.07 | 0.06 | 0.06 | |
| Stir and soaking | W2 | 30 | 0.25 | 0.25 | 0.25 | ||
| Static and soaking | W3 | 30 | 0.38 | 0.35 | 0.36 | ||
| Sodium bicarbonate | W4 | 1% | 30 | 0.01 | 0.01 | 0.01 | |
| 0.5% | 0.02 | 0.02 | 0.02 | ||||
| 0.2% | 0.15 | 0.15 | 0.15 | ||||
| Acetic acid | W5 | 1% | 30 | 0.03 | 0.03 | 0.03 | |
| 0.5% | 0.09 | 0.09 | 0.09 | ||||
| 0.2% | 0.45 | 0.42 | 0.43 | ||||
| Sodium chloride | W6 | 1% | 30 | 0.02 | 0.02 | 0.02 | |
| 0.5% | 0.07 | 0.07 | 0.07 | ||||
| 0.2% | 0.11 | 0.10 | 0.10 | ||||
| Ethanol | W7 | 1% | 30 | 0.01 | 0.01 | 0.01 | |
| 0.5% | 0.04 | 0.04 | 0.04 | ||||
| 0.2% | 0.13 | 0.12 | 0.12 | ||||
| Sodium dodecyl benzene sulfonate | W8 | 1% | 30 | 0 | 0 | 0 | |
| 0.5% | 0.06 | 0.06 | 0.06 | ||||
| 0.2% | 0.09 | 0.09 | 0.09 | ||||
| Peeling | Raw | P1 | 0 | 0 | 0 | ||
| Steaming | P2 | 10 | 0 | 0 | 0 | ||
| Boiling | P3 | 10 | 0 | 0 | 0 | ||
| Steaming | S1 | 5 | 0.04 | 0.04 | 0.04 | ||
| S2 | 10 | 0.01 | 0.01 | 0.01 | |||
| S3 | 20 | 0.01 | 0.01 | 0.01 | |||
| Boiling | B1 | 5 | 0.60 | 0.58 | 0.59 | ||
| B2 | 10 | 0.51 | 0.50 | 0.51 | |||
| B3 | 20 | 0.30 | 0.29 | 0.30 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dou, L.; Mu, S.; Yang, G.; Chang, J.; Zhang, K. Dissipation, Residue and Dietary Intake Risk Assessment of Penthiopyrad in Eggplants and Its Removal Using Various Household Processing Techniques. Foods 2022, 11, 3327. https://doi.org/10.3390/foods11213327
Dou L, Mu S, Yang G, Chang J, Zhang K. Dissipation, Residue and Dietary Intake Risk Assessment of Penthiopyrad in Eggplants and Its Removal Using Various Household Processing Techniques. Foods. 2022; 11(21):3327. https://doi.org/10.3390/foods11213327
Chicago/Turabian StyleDou, Li, Shiyin Mu, Guangqian Yang, Jinming Chang, and Kankan Zhang. 2022. "Dissipation, Residue and Dietary Intake Risk Assessment of Penthiopyrad in Eggplants and Its Removal Using Various Household Processing Techniques" Foods 11, no. 21: 3327. https://doi.org/10.3390/foods11213327
APA StyleDou, L., Mu, S., Yang, G., Chang, J., & Zhang, K. (2022). Dissipation, Residue and Dietary Intake Risk Assessment of Penthiopyrad in Eggplants and Its Removal Using Various Household Processing Techniques. Foods, 11(21), 3327. https://doi.org/10.3390/foods11213327








