Abstract
Given the stochastic complexity of cancer diseases, the development of chemotherapeutic drugs is almost limited by problems of selectivity and side effects. Furthermore, an increasing number of protective approaches have been recently considered as the main way to limit these pathologies. Natural bioactive compounds, and particularly dietary phenolic compounds, showed major protective and therapeutic effects against different types of human cancers. Indeed, phenolic substances have functional groups that allow them to exert several anti-cancer mechanisms, such as the induction of apoptosis, autophagy, cell cycle arrest at different stages, and the inhibition of telomerase. In addition, in vivo studies show that these phenolic compounds also have anti-angiogenic effects via the inhibition of invasion and angiogenesis. Moreover, clinical studies have already highlighted certain phenolic compounds producing clinical effects alone, or in combination with drugs used in chemotherapy. In the present work, we present a major advance in research concerning the mechanisms of action of the different phenolic compounds that are contained in food medicinal plants, as well as evidence from the clinical trials that focus on them.
1. Introduction
Cancer is a complex disease that is linked to several risk factors, such as bacterial and viral infections, oxidative stress, genetic mutation, poor nutrition, and epigenetic disturbance. The transformation mechanisms range from genetic and hormonal disturbances to environmental inducers and metabolic deregulations. This divergence of risk factors gives rise to various forms of cancer, and sometimes, implies therapeutic specificity even for the same type of cancer [1,2,3]. In this regard, the search for anticancer treatments requires the screening of several chemical molecules with functional diversity. Among the candidate molecules studied, we found phenolic compounds. These compounds are recognized for their extensive pharmacological properties, such as anti-inflammatory, antibiotic, antiseptic, antitumor, antiallergic, and cardioprotective properties, among others. In this context, edible medicinal plants are considered to be a very important source of anti-cancer molecules. Indeed, polyphenolic compounds such as flavonoids and acid phenolics are of considerable relevance as anti-cancer drugs [4].
Phenolic compounds are derived from edible plants, particularly medicinal and aromatic plants, which are found in many food products such as vegetables, cereals, legumes, fruits, nuts, and certain beverages. In fact, this chemical family constitutes a group of substances frequently present in the metabolism of medicinal plants, and it contains several subclasses such as phenolic acids, flavonoids, and tannins, which are the most abundant molecules [5,6,7]. These compounds have chemical functional groups that allow them to target the different signaling pathways involved either in cell transformation or in the promotion of cell transformation.
Various investigations have focused on phenolic compounds as anticancer drugs. As a matter of fact, these groups of molecules exert anticancer properties by acting on the multiple checkpoints of cancerous cells, and they can induce apoptosis, autophagy, and cell cycle arrest with high specificity [8,9]. In addition, phenolic compounds exert different mechanisms such as inhibiting telomeres, blocking their expression and inhibiting angiogenesis and metastases [10,11]. These compounds induce remarkable effects against human cancers by acting on molecular targets, notably by reducing the expression of a transcription factor regulating the expression of cytoprotective genes, reducing p53 activation, decreasing Bcl-2 expression and mitochondrial membrane potential, suppressing the expression of HIF-1α, and increasing cellular apoptosis with the downregulation of p-Akt expression [10,11]. Based on previous discussions, this present investigation aims to highlight the potential benefits of dietary phenolic compounds in managing and preventing human cancer; therefore, in this work, we have presented a major recent advance in research concerning the different molecular mechanisms of the substances that contain phenolic compounds, as well as the clinical trials that are carried out on these natural substances which target different human cancers.
2. Research Methodology
A comprehensive systematic review was conducted to highlight all relevant in vivo, in vitro, and clinical research on the potential use of dietary phenolic compounds, and their major secreted secondary metabolites, in order to study their molecular pathways for application as potential natural anticancer drugs. We consulted different credible repositories and scientific databases, such as ScienceDirect, Scopus, Springer Link, Wiley, Web of Science, Google Scholar, and PubMed. Recent comprehensive papers in peer-reviewed publications up to April 2022 were considered. There was no time limit on the year of publication. Only articles written in English were inventoried and used in this article. Exclusion criteria for unscreened articles comprised letters to the editor, non-English language publications, unpublished reports, and conference abstracts. The search strategy was performed using several keywords included within the literature quest, such as ‘Dietary phenolic compounds’, ‘Phenolics compounds and nutraceutical’, ‘Phenolics compounds and drugs’, ‘Phenolics compounds and targeted mechanisms’, ‘Polyphenols in cancer’, ‘Flavonoids in cancer’, ‘Stilbenes in cancer’, ‘Resveratrol in cancer’, ‘Curcumin in cancer’, ‘Phenolic acids in cancer’, ‘Caffeic acid in cancer’, ‘Gallic acid in cancer’, ‘Ellagic acid in cancer, ‘Sinapic acid in cancer’, ‘Rosmarinic acid in cancer’, ‘Flavonoids in cancer’, ‘Flavonols in cancer’, ‘kaempferol in cancer’, ‘Quercetin in cancer’, ‘Flavones in cancer’, ‘Apigenin in cancer’, ‘Genistein in cancer’, ‘Luteolin in cancer’, ‘Epigallocatechin-3-gallate in cancer’, ‘Anthocyanidins in cancer’, ‘apoptosis’, ‘in vitro’, ‘in vivo’, and ‘clinical trials’. A total of 138 peer-reviewed research papers published in the English language were included in this review.
3. Dietary Phenolic Compounds as Sources of Nutraceuticals and Drugs
Currently, an increasing level of attention is being placed on dietary components and their ability to aid the avoidance of certain risk factors that are involved in the genesis of complex pathologies; this is due to their high secondary metabolite content, which includes phenolic compounds. These bioactive agents have powerful antioxidant, anti-inflammatory, and anti-tumor properties, and consequently, they constitute key compounds for promoting healthiness and preventing diseases, especially cancer [12]. Polyphenols can be divided into different categories according to the number of phenolic cycles and organizational structures that bind these cycles to each other. Phenolic acids represent about one-third of the polyphenolic components found in food, and they comprise two principal groups: hydroxycinnamic acid derivatives (Caffeic acid, Coumaric acid, Ferulic acid, Sinapic acid) and hydroxybenzoic acid derivatives (Protocatechuic acid, Gallic acid, p-Hydroxybenzoic acid). Foods with a high phenolic acid content include kiwis, apples, coffee, tea, berries, pears, chicory, and cherries [13]. In addition, flavonoids are among the most common polyphenols consumed in the human diet, and over 4000 varieties of these substances have been recognized [14]. These natural components, which include seven subclasses (anthocyanins, flavonols, flavanols, flavones, flavan-3-ols, chalcones, and isoflavones), are commonly contained in red wine, red cabbage, cherries, black grapes, strawberries, and berries. Flavonols, including Stilbene and Myricetin, have been mainly found in blueberries, onions, leeks, kale, and broccoli. Other important dietary flavonoids are isoflavones, including Genistein; soy products and soybeans are food sources that have the richest levels of these compounds, given their estrogen-like structures [15]. Several phenolic compounds are directly implicated in the sensory characteristics of foods, and are therefore essential to their quality [16].
From the perspective of discovering efficient therapeutic options or combining treatment methods for fighting and preventing heavy and complex illnesses, as well as to guarantee optimum efficacy and outcomes, nutraceuticals are becoming a viable alternative to remedy many physiological and biological deficiencies. Nutraceuticals are described as a group of foods, though they can also be found in some drugs, that are characterized by their similarity to conventional foods, but they offer proven benefits in terms of physiology. Nutraceuticals are products obtained from foods, but they can also be used medicinally in the form of capsules, liquids, or pills, which also possess some physiological advantages. In recent decades, the use of these functional foods has gained considerable interest, and great advances have been made in terms of understanding their nutritional, therapeutic, and safety properties [17]. Recently, it was revealed that various natural bioactive molecules, such as phenolic compounds, exhibit anti-cancer activities, thus enabling them to destroy transformed or cancerous cells in a manner that is not harmful to their healthy homolog; however, this phenomenon appears to be associated with the use of natural ingredients in association with conventional drugs, thereby highlighting the possibility that supplementation with nutraceuticals may enhance the effectiveness of anticancer research and management strategies. Furthermore, nutraceuticals, or functional foods, may be promising candidates for reducing risk factors for cancer development due to their antioxidant and anti-inflammatory properties, and their ability to inhibit cell proliferation [18].
In fact, polyphenols, as phytochemicals consumed as a regular part of the diet (approximately 1 g/day) and available in great abundance in plant-based foods, especially fruits and vegetables, should be regarded as a source of nutrients that assists in the production of nutraceuticals, supplementary treatments, and drugs; therefore, they may be used as part of a new approach that aids the management of several diseases, including cancer.
The purpose of the following section is to highlight, from a mechanistic perspective, the prominent role of dietary phenolic compounds in the different processes that cause tumor transformation, development, and metastasis.
4. Dietary Phenolic Compounds as Anticancer Drugs: Targeted Mechanisms
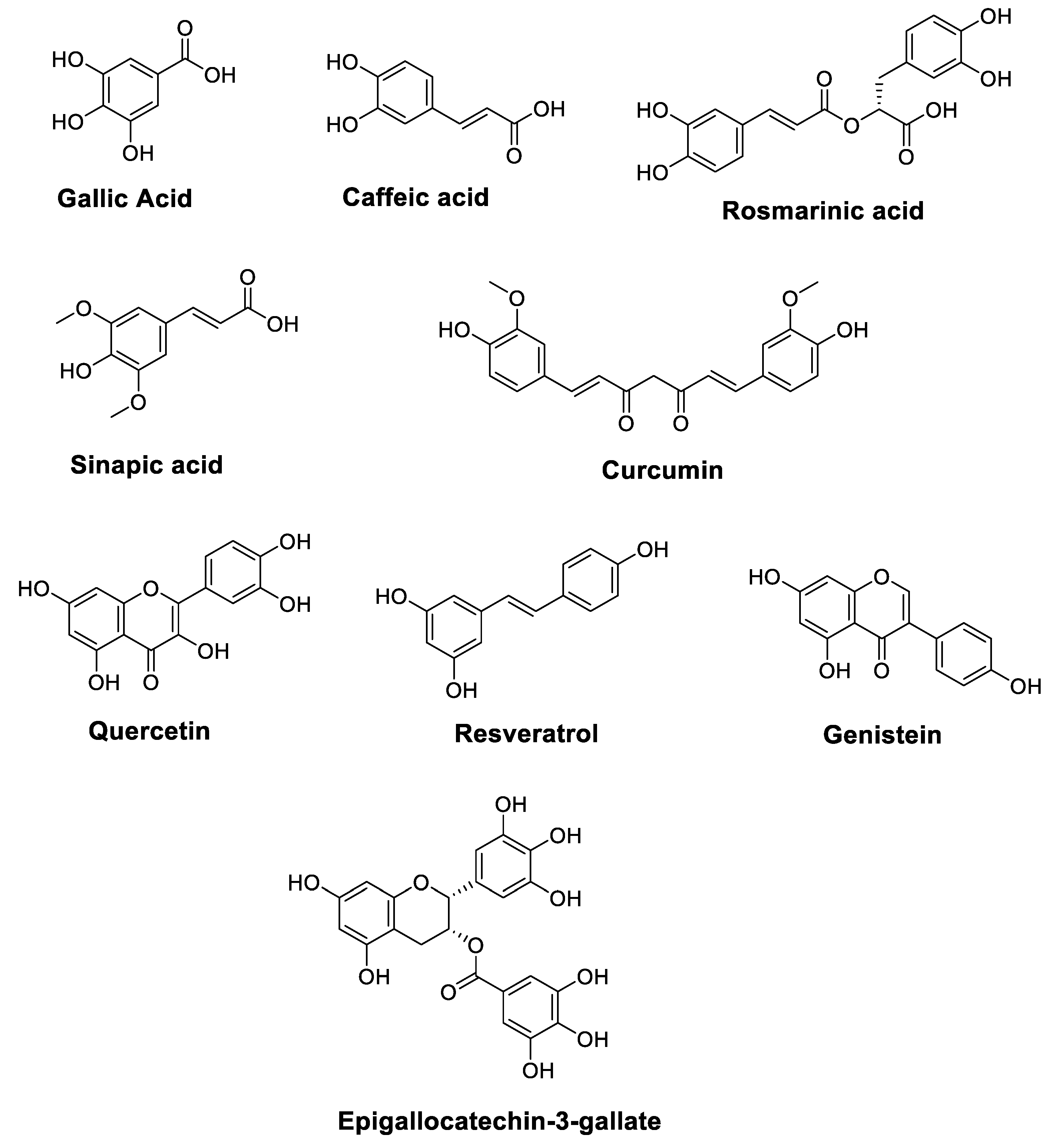
Cancer is one of the leading causes of death worldwide, and it has become a largely preventable disorder with a significant response to modulation via nutritional factors [19,20]. The anticancer characteristics of vegetables and fruits have been partially associated with high levels of polyphenols. Figure 1 illustrates the chemical structures of polyphenols such as Resveratrol, Gallic acid, Caffeic acid, Rosmarinic acid, Sinapic acid, Quercetin, Genistein, Epigallocatechin-3-gallate, and Curcumin, which are essential components of the daily human diet, and they represent the predominant polyphenol content of foods. In addition to vegetables and fruits, bark, seeds, leaves, and flowers also provide abundant sources of polyphenols [21,22].
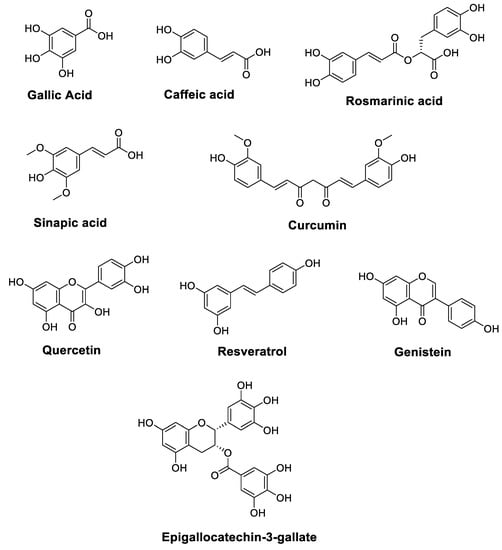
Figure 1.
Chemical structures of polyphenols with anticancer properties.
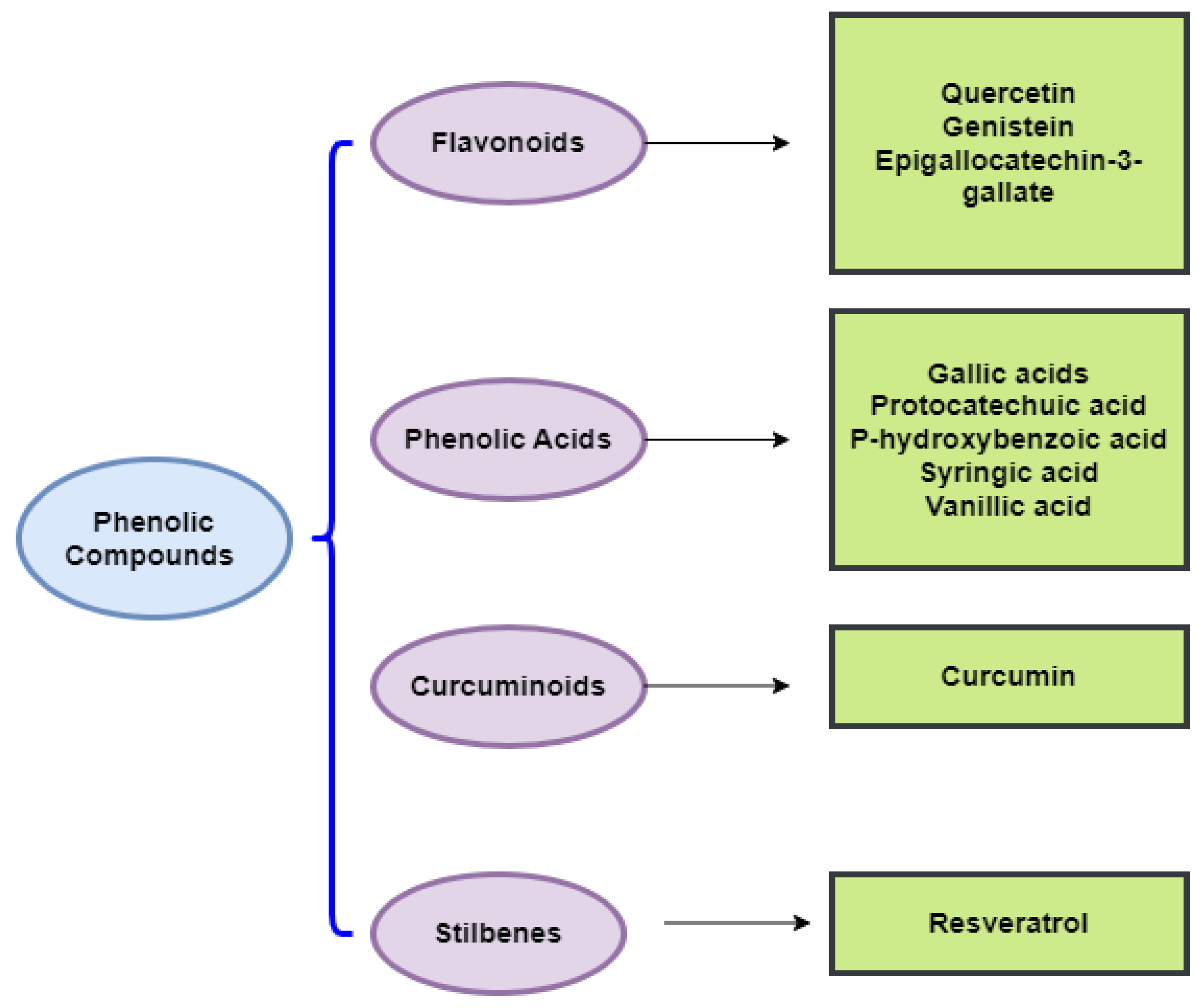
As conventional therapeutic and surgical approaches have failed to adequately manage various types of cancer, the need to identify and develop chemopreventive strategies has become a public health priority [20]. In this respect, the chemopreventive benefits of dietary polyphenols are principally attributed to their anti-angiogenic, cell cycle arrest, anti-metastatic, anti-inflammatory, anti-proliferative, autophagic, and apoptotic activities [20]. Indeed, dietary polyphenols can confer an indigenous defense by promoting cell signaling processes, including activator protein-1 (AP-1) DNA binding, activating the nuclear factor kappa B (NF-κB), phosphoinositide 3-kinase (PI3-kinases)/protein kinase B (PKB)/Akt pathway, biosynthesizing glutathione, and translocating nuclear factor 2 erythroid-related (Nrf2) into the nucleus, as well as mitogen-activated protein kinase (MAPK), extracellular signal-regulated proteinase (ERK), c-jun N-terminal kinase (JNK) (Figure 2), and P38 [23]. To assess the effectiveness of chemoprevention by dietary phytochemicals, the following molecular targets of polyphenols for the mechanistic regulation of chemoprevention pathways are discussed in more detail below. Listed in Table 1 are the dietary polyphenols that act as anticancer agents.
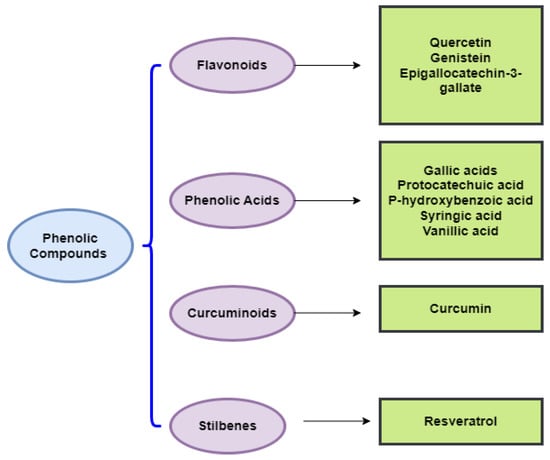
Figure 2.
Chemical classes of dietary phenolic compounds exhibiting anticancer properties.

Table 1.
Dietary polyphenols that act as anticancer agents.
In the following sections, we will discuss the anticancer action of different chemical classes of phenolic compounds, in accordance with Figure 2.
4.1. Phenolic Acids
Phenolic acids are among the most common non-flavonoid plant phenolic components that exist as glycosides or aglycones (free form) [107]. They are widely distributed in plants and are found in oilseeds, grains, legumes, vegetables, fruits, herbs, and beverages. Hydroxybenzoic and hydroxycinnamic acids are two classes of phenolic acids based on C1–C6 and C3–C6 skeletons [108]. Hydroxycinnamic acids include p-coumaric, ferulic, caffeic, cinnamic, and sinapic acids. In contrast, hydroxybenzoic acids are represented by Gallic, Protocatechuic, p-Hydroxybenzoic, Syringic, and Vanillic acids [107]. Hydroxycinnamic and hydroxybenzoic acids, and their derivatives, exhibit high antioxidant and antiproliferative properties in vitro and in vivo by regulating different signaling pathways [109,110].
4.1.1. Gallic Acid
Gallic acid (GA) (3,4,5-Trihydroxybenzoic acid) (Figure 1) exerts potent anticancer properties due to its remarkable antioxidant properties. In a recent study, using an oral squamous cell carcinoma (FaDu and Cal33) cell line, Gallic acid enhanced the anticancer efficacy of Docetaxel, Cisplatin, Doxorubicin, 5-FU, and Paclitaxel in combination with gamma irradiation in vitro, through the superoxide-mediated apoptosis pathway powered by lipophagy inhibition in an NRF2-dependent signaling pathway [31]. In vitro and molecular docking methods were used to investigate the anticancer potential of GA, which was isolated from a Ceriops tagal leaf methanolic extract, against two human cancer cell lines, HeLa (cervical) and MDA-MB-231 (breast) cancer cells. In fact, at 24 h, the IC50 values for GA were 4.18 ± 0.45 and 80.04 ± 0.19 μg/mL for HeLa and MDA-MB-231 cell lines, respectively. Moreover, molecular docking results revealed that GA exhibits an estimated binding free energy (ΔG) of −5.4 kcal/mol to the Bcl-B protein, the drug target. The estimated inhibition constant (Ki) for GA was 110 μM. This molecule may offer a considerable opportunity to block the overexpression of the cancer drug target protein in cancer cells [24].
Ko et al. carried out a recent investigation to examine the anticancer potential of GA against a non-small cell lung carcinoma (NSCLC) cell line A549 in vitro. The 48 h cell viability assay showed that GA treatment inhibited cell proliferation in a dose-dependent manner [25]. Additionally, the findings showed that the inhibition of the PI3K/Akt pathway was mediated by AG upregulation in p53 (tumor suppressor protein), which subsequently regulated intrinsic apoptotic proteins such as Bcl-2, Bax, and it cleaved caspase-3 and cell cycle-related proteins such as p27, p21, Cyclin E1, and D1, as evidenced by the Western blot analysis. In the in vivo model, AG treatment for four weeks reduced tumor mass size in the A549-derived tumor mouse model. Moreover, GA lowered the expression of p-Akt and the proliferating cell nuclear antigen and increased the expression of cleaved caspase-3 expression in tumor tissues.
GA is one of the major bioactive compounds in Dovyalis caffra. In this regard, the methanol extract of this plant was found to exert a potential anticancer action (58.90% toxicity) on HepG2 cells at 1000 µg/mL [26]. Furthermore, a recent study by Sanchez-Martin et al. showed that GA could be a promising agent in cancer prevention via its interaction with G-Quadruplexes. Results also revealed that GA exhibits a selective antitumor effect in colorectal cancer [27]. The IC50 values for CRL1790, SW480, and SW620 were >100, 22.39 ± 2.12, and 11.83 ± 1.54 µM, respectively. Moreover, results showed that GA-induced cell cycle arrest at the S and G2/M phases triggers nucleolar stress and downregulates G4-containing genes. In addition, as shown from immunofluorescence being used on BG4 antibodies, GA stabilized G4 in both in vitro and in vivo cellular environments, thus leading to tumor growth inhibition in colorectal cancer xenografts. These researchers also proved that GA binding to G4s is present in 5’ETS and the CMYC promoter.
Similarly, treatment of human prostate carcinoma DU145 cells with GA showed potent cell growth inhibition, apoptotic death, and cell cycle arrest in a dose- and time-dependent manner. It also showed the downregulation of cyclin- and cyclin-dependent kinases, and a marked induction of Cip1/p21. Other investigations indicated that GA causes Tyr15 phosphorylation early in cell division cycle 2 (cdc2). Furthermore, GA induced cdc25A and cdc25C phosphorylation, through ataxia telangiectasia mutated (ATM) and checkpoint kinase 2 (Chk2) activation, when DNA became damaged. Moreover, GA induced the cleavage of caspase-3, caspase-9, and poly (ADP)ribose polymerase [29]. Similarly, GA isolated from grape seed extract could be a promising phytochemical against prostate cancer. It exhibited the significant inhibition of growth and apoptotic death of DU145 cells in a dose- and time-dependent manner, and it caused caspase-9, caspase-3, and PARP cleavages [28].
Inoue et al. examined the cytotoxic effect of GA in different cell lines. These researchers indicated that the IC50 for mouse lymphoid neoplasm (P388- D1), human epithelial carcinoma (HeLa), human hepatoma (PLC/PRF/5), rat hepatoma (dRLh-84), human promyelocytic leukemia (HL60RG), and human epidermoid carcinoma (KB) are 4.8, 6.1, 6.6, 6.2, 5.4, and 13.2 µg/mL, respectively [32]. In contrast, the IC50 values for endothelial cells and fibroblasts were over 20 µg/mL. These findings indicate the ability of GA to promote cell death in tumor cells with comparatively high selectivity. Similarly, Sachithanandam et al. examined the anticancer potential of GA isolated from the Ceriops tagal leaf methanol extract against two human cancer cell lines, namely, HeLa (cervical) and MDA-MB-231 (breast) cancer cells. Indeed, at 24 h, the IC50 values were 99.91 ± 0.18 and 18.29 ± 0.12 µg/mL for the HeLa and MDA-MB-231 cell lines, respectively [24]. Molecular docking results reported that GA has an estimated binding free energy (ΔG) of −5.4 kcal/mol for the drug target protein Bcl-B.
4.1.2. Caffeic Acid
Natural caffeic acid (E)-3-(3,4-Dihydroxyphenyl)prop-2-enoic acid (Figure 1) is well-known to possess several biological properties, including anticancer effects. In this context, Min et al. reported that CA significantly inhibits the growth of H1299 NSCLC cells by inducing apoptosis [41]. CA combined with Paclitaxel (PTX) provides a synergistic anticancer action against H1299 cells. Moreover, these researchers found that the combined treatment of PTX and CA induces a reduction in the proliferation of H1299 NSCLC cells. As evidenced by flow cytometry, CA treatment increased apoptosis, caspase-3 and caspase-9 activities, and the sub-G1 phase arrest of H1299 cells. In addition, CA increased the phosphorylation of kinase1/2 and the c-Jun NH2-terminal protein kinase1/2, and increased the PTX-induced activation of Bid, Bax, and downstream cleaved PARP. The combined treatment showed no significant side effects and strongly suppressed tumor growth in H1299 xenografts. Similarly, Kanimozhi et al. attempted to assess the molecular mechanism of CA as an anticancer agent against Hela and ME-180 cells [42]; therefore, CA increased markers of lipid peroxidation, such as connective dienes, reactive thiobarbituric acid substances, and lipid hydroperoxide. This molecule also enhanced morphological changes, altered mitochondrial membrane potential, and increased ROS levels in CA-treated cells, suggesting that CA possesses anticancer activity due to its pro-oxidant effect [42].
The results of a study by Bhat et al. suggested that CA has a pro-oxidant action through its involvement in the mobilization of endogenous copper, which is likely to be the copper bound to chromatin [43]. In fact, CA induced DNA disruption in human peripheral lymphocytes and produced oxidative stress in lymphocytes, which is suppressed by ROS scavengers and neocuproine [43]. Additionally, Guo et al. reported an easy synthesis of silver nanoparticles (AgNPs) using CA [44]. These particles exhibited potent anticancer activity. Data showed that AgNPs induce cytotoxicity in human hepatoma HepG2 cells in a dose-dependent manner; they could enter cells and inhibit tumor viability through the induction of apoptosis [44]. An investigation by Rosendahl et al. indicated that CA imitates anti-estrogen activity and alters key IGF-IR/pAkt and ER/cyclin D1 growth regulatory signals, thus leading to cell cycle damage and diminished cell proliferation [50]. CA showed anticancer activities against breast cancer ER+ and ER- that might raise tumor cells’ sensitivity to tamoxifen and diminish breast cancer growth [50]. Chen et al. conducted a study to evaluate the impact of CA on the toll-like receptor 4 (TLR4) signaling pathway. CA reduced NF-κB activation and IL-12 synthesis [51]. Nevertheless, it impaired the TLR4 pathway by altering the TLR4/MD2 complex. Results showed that the downregulation of the expression of TLR4, TRIF, and IRAK4 leads to apoptosis in breast cancers [51].
Research findings revealed that CA prevents cancer cell progression by blocking the histone demethylase (HDM) gene. CA suppressed DNA methylation catalyzed by prokaryotic DNA methyltransferase (DNMT) M.SssI and human DNMT1 (IC50 = 3.0 µM) using an enhanced formation of S-adenosyl-L–homocysteine (C14H20N6O5S) (SAH, a potent inhibitor of DNA methylation) during catecholamine-O-methyltransferase (COMT)-mediated O-methylation. Furthermore, CA could inhibit the methylation of the promoter region of the RAR-β gene using cultured MCF-7 and MAD-MB-231 human breast cancer cells [45]. CA has demonstrated its importance as an effective inhibitor of 5-lipooxygenase (5-LO) and it has proven its capacity to diminish NF-κB, Interleukin-6 (IL-6), and IL-1β levels during inflammatory processes, especially global cerebral ischemia-reperfusion injuries in rats [46]. Furthermore, in the study by Jung et al., CA radically inhibited the action of STAT3, which triggers the action of HIF-1α. It strongly inhibited STAT3 and suppressed tumor angiogenesis by blocking the signal transducer and activator of transcription 3 (STAT3) activity, as well as the vascular endothelial growth factor (VEGF) and hypoxia-inducible factor-1α (HIF-1α) expression [47]. Moreover, CA significantly suppressed CT-26 colon cancer cell-induced lung metastasis through the inhibition of ERK phosphorylation. CA also markedly repressed mitogen-activated MEK1 and TOPK functions and directly bound with MEK1 or TOPK. CA blocked TPA-, EGF-, and H-Ras-induced ERK phosphorylation, AP-1, and NF-κB transactivation, and the neoplastic transformation of JB6 P+ cells [48].
Regarding skin cancer, Yang et al. analyzed the molecular mechanisms by which CA could exert chemopreventive action against the EGF-induced neoplastic transformation of HaCaT cells and SUV-mediated skin carcinogenesis [49]. These researchers found that CA effectively inhibits colony formation and suppresses tumor incidence and volume in both in vivo and in vitro models. The CA-treated mouse skin cancer xenograft model showed a reduction in MAPK phosphorylation. Additionally, CA interacted with ERK2 directly in the Q105, D106, and M108 amino acid residues, directly interfered with ERK1/2, and suppressed ERK1/2 activities in vitro [49]. In lung cancer, CA phenethyl ester, a CA derivative representing a natural phenolic compound that is widely abundant in plants and propolis extract, was found to exhibit high chemopreventive effects against A549 lung adenocarcinoma cells. It was highly influential in suppressing TGF-β-promoted cell motility and transforming the growth factor-β (TGF-β)-induced activation of Akt (protein kinase β), and in blocking the phosphatidyl inositol 3-kinase (PI3K)/Akt pathway.
In prostate cancer, evidence showed that the CA phenethyl ester can block NF-κB activation in prostate cancer-3 (PC-3) cells by inhibiting the ability of the Tumor necrosis factor-α (TNF-α) and Paclitaxel to activate NF-κB; however, this action is also linked to lower cellular levels of apoptosis-inhibiting proteins (cIAP1, cIAP-2, and XIAP), and the downregulation of elevated levels of spontaneous apoptosis and cIAP-1 expression [33].
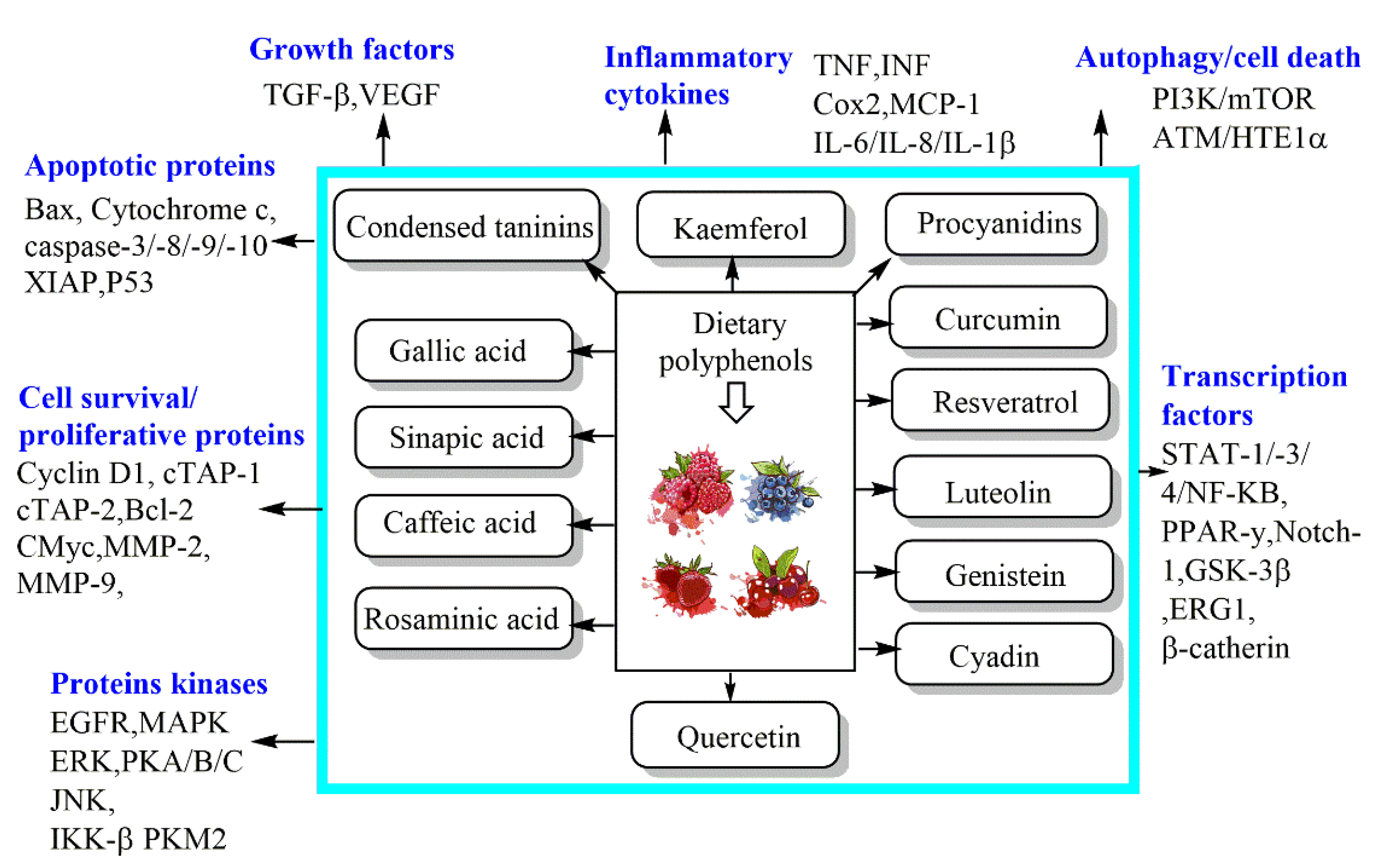
Similarly, CA phenethyl ester exerted a strong anticancer effect against androgen-independent prostate cancer cells. It induced a protein abundance of p21Cip1, p27Kip1, ATF4, cyclin E, p53, TRIB3, phospho-p53 (Ser6, Ser33, Ser46, Ser392), phospho-p38 MAPK Thr180/Tyr182, Chk1, Chk2, phospho-ATM S1981, phospho-ATR S428, and phospho-p90RSK Ser380; however, it markedly decreased the protein abundance of Skp2, Cdk2, Cdk4, Cdk7, Rb, phospho-Rb S807/811, cyclin A, cyclin D1, cyclin H, E2F1, c-Myc, SGK, phospho-p70S6kinase T421/S424, phospho-mTOR Ser2481, and phospho-GSK3α Ser21. This investigation established that CA phenethyl ester induces cell cycle arrest and growth inhibition in castration-resistant prostate cancer cells through the regulation of Skp2, p53, p21Cip1, and p27Kip1 (Figure 3) [34].
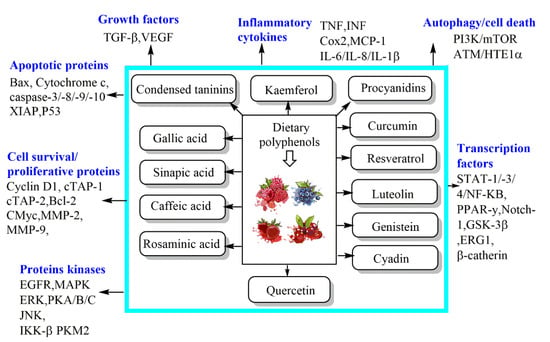
Figure 3.
Mechanisms of dietary phenolic bioactive compounds against cancer cells.
Due to the high resistance of melanoma to conventional chemotherapy, several investigations have been undertaken that are aimed at new therapeutic strategies and the development of co-adjuvants. Using A2058 human melanoma cells, CA phenethyl ester was proposed to inhibit a ROS-induced DNA strand break [35]. Results revealed that the administration of CA phenethyl ester (10 mg/kg/day) causes intracellular glutathione (GSH) depletion, triggers a five to seven fold increase in B16-F0 cell apoptosis, and inhibits tumor size growth [36]. Moreover, CA exerted cell toxicity at concentrations above 0.35 mM and suppressed (in vitro) melanin production and casein kinase 2 (CK2)-induced tyrosinase phosphorylation in a dose-dependent manner [37]. In their study, Wadhwa et al. found that CA phenethyl ester exerts strong antitumor and antimetastatic potential, particularly when complexed with gamma-cyclodextrin (γCD). As shown via molecular docking analysis, this compound can disrupt mortalin-p53 complexes [38]. Furthermore, the study’s findings revealed that the disruption of the CA phenethyl ester-induced mortal-p53 complexes leads to the nuclear translocation and activation of p53, thus causing cancer cell growth arrest. Researchers also found that CA phenethyl ester is cytotoxic to several cancer cells (IC50 varies from 5 to 80 µM) when complexed with γCD [39].
To find new treatments for osteosarcoma, Pagnan et al. explored the anticancer effect of CA phenethyl ester, and its precursor CA, on murine osteosarcoma UMR-106. CA phenethyl ester (IC25 = 1.3 μM/IC50 = 2.7 μM) was found to diminish mitochondrial ROS production, cell migration, and cell viability when compared with CA alone (IC25 = 91.0 μM/IC50 = 120.0 μM) [40]. CA phenethyl ester showed greater selectivity towards tumor cells, thus protecting normal cells. These results illustrate the potential anticancer properties of CA phenethyl ester, and they highlight ways in which incorporating a phenethyl ester could lead to improved drug performance over its CA predecessor [40].
4.1.3. Rosmarinic Acid
Rosmarinic acid (RA), (2R)-3-(3,4-Dihydroxyphenyl)-2-{[(E)-3-(3,4-dihydroxyphenyl)prop-2-enoyl]oxy}propanoic acid, one of the esters of caffeic acid, is a secondary metabolite of plants and is mainly found in plants belonging to the Lamiaceae family, such as Rosmarinus officinalis (rosemary) [111]. To explore the anticancer effects of RA, Luo and collaborators conducted a study using human oral cancer cells. They recorded the inhibition of cell proliferation, induction of apoptosis, cell cycle arrest at the G2/M phase, and the negative effect of cancer cell migration potential, in a concentration-dependent manner, while oxidative stress was being promoted in the endoplasmic reticulum [52]. Moreover, RA reduced levels of dextran sulphate sodium in (DSS)-induced colitis-associated colon cancer by triggering TLR4/NF-κB/STAT3 activation in the mouse model. RA dramatically improved the colitis severity, tumor incidence, colorectal adenoma progression, anti-apoptotic factors, and inflammatory markers [53].
To study the anticancer effects of RA against prostate tumors, Jang et al. conducted a study using prostate cancer cell lines [54]. The data obtained revealed that AR inhibits colony and spheroid formation and decreases cell proliferation in OC3 and DU145 cell lines. Additionally, RA treatment effectively suppressed a histone deacetylase enzyme that regulated apoptotic pathway-related mitochondrial intrinsic gene expression, including Bcl-2, Bax, caspase-3, and poly (ADP-ribose) polymerase-1 (PARP-1), through the enhancement of p53 levels. Furthermore, RA induced early and late apoptosis by downregulating the proliferating cell nuclear antigen, cyclin D1, and cyclin E1, and increasing p21 expression. Nevertheless, further studies are needed to assess AR-mediated cytotoxicity in normal cells [54]. Another study by Chao et al. investigated the RA’s anticancer effects on hepatocellular carcinoma [55]. Rosmarinic acid is among the significant polyphenolic components of the ethyl acetate fractionated extract (EAFE) of Glechoma hederacea L., which was found to be effective in inhibiting HepG2 cell proliferation, inducing apoptosis, and it also triggered cell death and arrest at the S phase. The apoptogenic activity of EAFE involves Ca2+ accumulation, ROS induction, MMP disruption, caspase 3, nine activations, an increased Bax/Bcl-2 ratio, and glutathione depletion.
In the same context, RA markedly suppressed SMMC-7721 cell proliferation and promoted G1 arrest and apoptosis in a dose-dependent manner. Additionally, RA appears to prevent cell invasion by controlling the epithelial–mesenchymal transition. RA treatment significantly inhibited the PI3K/AKT/mTOR signaling pathway. The findings above provide a basis for considering RA as an anti-cancer drug [56]. Concerning malignant bone tumors, the results obtained by Ma et al. indicated an anticancer property of RA in U2OS and MG63 osteosarcoma cells by suppressing DJ-1 expression via regulation of the PTEN/PI3K/Akt signaling pathway [57]. RA induced apoptosis by upregulating caspase-8, caspase-9, and caspase-3 cleavage rates, thus enhancing the Bax/Bcl-2 ratio, triggering ROS generation, and reducing the matrix metalloproteinase (MMP); therefore, a biological target of RA in osteosarcoma cells could be DJ-1 [57]. The anticancer activity of RA to prevent breast cancer has also been tested. In the study carried out by Messeha et al., the results revealed significant dose- and time-dependent antiproliferative and cytotoxic effects in the cell lines that were tested [58]. Using MDA-MB-231 cells, arrested AR cells in the G0/G1 phase, induced apoptosis that was related to cell cycle arrest, and differential alterations, many genes were involved in apoptosis expression [58].
In the MDA-MB-468 cell line, RA caused the significant transcriptional activation of TNF, growth arrest, DNA-damage-inducible 45 alpha (GADD45A), and BNIP3. In contrast, RA markedly upregulated the mRNA expression of harakiri (HRK), tumor necrosis factor receptor superfamily 25 (TNFRSF25), and BCL-2 interacting protein 3 (BNIP3) in MDA-MB-231 cells. In addition, RA suppressed TNF superfamily 11B receptor (TNFRSF11B) expression in MDA-MB-231 cells. Evidence indicates that RA may be a promising candidate for triple-negative breast cancer strategies, especially in MDA-MB-468 cells [58]. Similarly, using mouse models of breast cancer, RA exhibited chemopreventive benefits and antitumor effects alone and in combination with Paclitaxel. AR markedly repressed VEGF, NF-κB, and TNF-α expression and restored Bcl-2, p53, caspase-3, and Bax levels, thus causing apoptosis. Furthermore, RA and/or Paclitaxel markedly repressed tumor growth with increasing p53 and caspase-3 apoptotic marker levels and repressed the Bcl2/Bax ratio in mice with Ehrlich solid tumors [59].
To evaluate the anti-cancer effects in melanoma cells, Huang et al. reported that RA inhibits the invasion, proliferation, and migration of human melanoma A375 cells, promotes apoptosis, and increases theCisplatin sensitivity of melanoma cells by inhibiting the ADAM17/EGFR/AKT/GSK3β pathway [60]. Recently, Zhou et al. reported that RA restricts Gli1 nuclear translocation and promotes proteasome-mediated Gli1 degradation. RA resulted in G1/S cell cycle arrest and apoptosis in pancreatic ductal adenocarcinoma cells and inhibited the invasion and migration of these cells via MMP-9 and E-cadherin. These results strongly support RA as a beneficial therapeutic agent for pancreatic ductal adenocarcinoma cells [61]. In addition, RA treatment downregulated the oncogenic transcription factor forkhead box M1 (FOXM1) and targeted genes involved in tumor growth and abnormal cell proliferation. Furthermore, a combination of Cisplatin and RA methyl ester reversed Cisplatin resistance by potentially inhibiting FOXM1 [62] in ovarian cancer cells.
Moreover, RA dose-dependently suppressed non-small cell lung cancer (NSCLC) growth and cell colony formation, induced G1 phase cell cycle arrest and apoptosis, and increased the sensitivity of the Cisplatin-resistant cell line. The molecular mechanism of RA involved the inhibition of NSCLC cell growth, cell cycle arrest, and the induction of apoptosis through the activation of MAPK and suppression of P-gp and MDR1 expression, subsequently resulting in higher expression levels of p21 and p53 [63]. Interestingly, RA combined with anti-MUC1 showed stronger anticancer effects than monotherapy. Combined treatment significantly suppressed fucose, Tn, sialyl T, and cancer-related sugar antigen expression, as well as the mRNA expression of enzymes that are involved in their formation: ppGalNAcT2, C1GalT1, ST6GalNAcT2, ST3GalT1, and FUT4. Furthermore, C1GalT1 was suppressed at the protein level. Gal-3, which is a factor that is likely to be involved in metastasis, was effectively repressed at the mRNA level by administering RA and anti-MUC1. Bax protein and pro-apoptotic Bad mRNA were induced to a significant extent, and the expression of anti-apoptotic Bcl-2 mRNA was suppressed by this treatment [64].
4.1.4. Sinapic Acid
Sinapic acid (SA), (2E)-3-(4-Hydroxy-3,5-dimethoxyphenyl)prop-2-enoic acid, can be found in a wide variety of fruits and vegetables, and is derived from hydroxycinnamic acid. To assess the role of SA as an anticancer agent for the prevention of prostate cancer, Eroğlu et al. have shown that SA has the potential to become a promising molecule that works against prostate cancer cells [65]. In LNCaP cells, BAX, caspase-3, caspase-7, and CYCS expressions markedly increased; however, reduced expressions of CDH2, MMP-2, and MMP-9 were observed in AS therapy. In PC-3 cells, treatment with AS enhanced BAX, caspase-3, caspase-8, CYCS, FAS, TIMP-1, and CDH1 expression, but significantly reduced the expression of MMP-9. The IC50 value of SA in both PC-3 and LNCaP cells was determined at 1000 μM for 72 h [65]. SA provided substantial protection against lipopolysaccharide (LPS)/D-galactosamine (D-GalN)-induced acute liver failure (ALF) in rats via the downregulation of NF-κB and upregulation of Nrf2/heme oxygenase (HO)-1 pathways [66]. SA is a histone deacetylase (HDAC) inhibitor that exerts its antitumor activity through apoptosis activation and autophagy induction, cell cycle arrest, enhanced ROS release leading to oxidative stress, the inhibition of angiogenesis, and induction of mitotic cell death in cancer cells [67]. Moreover, SA suppressed pancreatic cancer proliferation, migration, and invasion through the downregulation of the AKT/Gsk-3β signaling pathway [68].
Recently, Hu et al. examined the possible role of SA in combating lung cancer in vitro and in vivo [69]. The in vitro model showed that SA exhibits significant cytotoxic potential against human lung cancer, and it exhibited potential cytotoxic and apoptotic activity by increasing ROS levels, caspase-3, and caspase-9 activity. In the in vivo model, SA improved B[a]P-mediated lung cancer exposure in Swiss albino mice by reducing IgG and IgM levels, the number of leukocytes, assays of neutrophil function, soluble immune complexes, tumor markers (AHH, LDH, GGT, 5’NT, and CEA), pro-inflammatory cytokines, and by improving the activity index, phagocytic index, and the enzymes involved with antioxidant defense [69]. In addition, the effect of SA on the activation of apoptosis in the human laryngeal carcinoma cell line (HEp-2) was investigated in vitro by Janakiraman et al. [70]. As evidenced by the dichlorofluorescein diacetate (DCFH-DA) fluorometric method, SA-treated HEp-2 cells showed increased ROS levels (108.32 ± 7.32) compared with untreated cells (76.41 ± 7.09) for 48 h. The results also revealed a decrease in mitochondrial membrane potential in cancer cells with enhanced fluorescence intensity (134.78 ± 9.76) versus treated cells (123.76 ± 7.81), as shown in the fluorescence assay. The IC50 value of SA was 125.23 μM/mL after 24 h and 117.81 µM/mL after 48 h. SA promoted cell apoptosis with associated cell viability loss, modulated ROS, and arrested cell cycles by triggering G0/G1 phase arrest for HEp-2 cells.
In another study, Balaji mice injected with SA highlighted the chemopreventive properties of SA against DMH-induced colon carcinogenesis in vivo. The findings showed that SA showed a stronger action at a dose of 40 mg/kg body weight, which was measured by the increase in antioxidant defense (superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx)), a reduction in tumor incidence, and the modulation of LPO markers, including phase I (CYP450 and CYP2E1) and phase II Glutathione-S-transferase (GST), DT-Diaphorase (DTD), and UDP-Glucuronyl transferase (UDP-GT) detoxification enzymes [71]. Furthermore, the combination of SA and Cisplatin suppressed hepatocellular carcinoma cell migration and proliferation, as evidenced by the MTT assay, and it promoted autophagy and apoptosis, thus providing anti-hepatocellular carcinoma action [72].
4.2. Curcuminoids
Curcumin
The polyphenolic phytochemical Curcumin ((1E,6E)1,7-Bis(4-hydroxy-3-methoxyphenyl)hepta-1,6-diene-3,5-dione), extracted from Curcuma longa, is a powerful anti-proliferative, anti-angiogenic, anti-metastatic, pro-apoptotic, and antioxidant promoter of anticancer activities in several types of cancer cell lines [112]. Masuelli et al. explored the possible anticancer effects of Curcumin against human and mouse MM cell lines. Curcumin was found to induce DNA damage, suppress MM cell survival in a dose- and time-dependent manner, and increase ROS production [73]. It also activated apoptosis inductions via increased p53 expression, PARP-1 cleavage, caspase-9 activation, an increased Bax/Bcl-2 ratio, and cell cycle arrest. Curcumin treatment promoted ERK1/2 and p38 MAPK phosphorylation, blocked p54 JNK and AKT phosphorylation, enhanced c-Jun expression and phosphorylation, and prevented NF-κB nuclear translocation [73].
Chronic inflammation is one of the leading underlying causes of several types of cancer. In this sense, it was found that the activity of IκB kinase is reduced by curcumin, thereby inhibiting the degradation of IκBα and resulting in the blockage of NF-κB subunit nuclear translocation. Additionally, Curcumin can block the shift of subunit NF-κB to the nucleus and attenuate the expression of other pro-inflammatory genes, which are usually activated in cancer [113]. Moreover, it regulated the NF-κβ-dependent signaling pathways to induce apoptosis. Curcumin has also been documented to suppress p38 and JNK activation that is induced by TNF-α or IL-1β, thus leading to the potential suppression of IκBα degradation in HT29 cells [74].
Using head and neck squamous cell carcinoma cancer, Curcumin repressed inflammatory cytokines such as TNF-α, IKKβ kinase, IL-6, and IL-8. Additionally, Curcumin can suppress protein kinase activity, including PKA, PhK, mTOR, and MAPK, which are critical for regulating cell proliferation, growth, survival, and death [90]. In human glioblastoma, Curcumin has the potential to modulate critical pathways, including apoptosis, cell proliferation, cell cycle arrest, autophagy, tumor cell motility, and oxidative stress. The mechanisms of action involved in the anticancer effect of Curcumin in human glioblastoma include modulation of the Rb, p53, MAPK, P13K/Akt, JAK/STAT, Shh, and NF-κB pathways [75]. Similarly, curcumin reinforced nimustine hydrochloride’s (UNA) anticancer effect by deleting IκB, p65, and p50 phosphorylation and reducing the expression of cyclooxygenase-2 (COX-2) [76]. In addition, Curcumin’s antiproliferative activity could be mediated by the downregulation of cyclin D1, since NF-κB regulates the cyclin D1 promoter [77].
In the survey undertaken by Tzuu-Yuan et al., using GBM 8401 cells, Curcumin upregulated the inhibition of transcription factor NF-κB in a concentration-dependent manner [78]. It induced apoptosis through a caspase and mitochondrial-dependent pathway. Similarly, Curcumin promoted gefitinib’s antitumor activity in the xenografted NSCLC cell lines and mouse model via suppression of NSCLC proliferation, EGFR phosphorylation, EGFR ubiquitination, and apoptosis induction. In addition, Curcumin mitigated gefitinib-induced gastrointestinal side effects by altering p38 activation [79].
In lung adenocarcinoma cells, by suppressing the expression of ERK1/2 activities, as well as EGFR, and COX-2, Curcumin exhibited pro-apoptotic activity, which is consistent with high apoptosis and the reduced survival of pulmonary adenocarcinoma cells [91]. Similarly, in A549 human lung cancer cell lines, Curcumin downregulated NF-κB activity and acted on the JAK2/STAT3 signaling pathway by suppressing JAK2 activity; thus, Curcumin is a successful therapeutic drug for treating lung cancer [92]. Interestingly, a new catanionic lipid nanosystem (CLN) incorporating Curcumin was found to possess enhanced cytotoxicity in Lewis lung cancer cells, inducing cell cycle arrest, pro-apoptotic, antiproliferative, and anti-invasive effects. This novel nanosystem triggered Lewis lung cancer cell apoptosis with Curcumin via the cellular target PI3K/Akt/FoxO1/Bim [93].
In ML-1a human myeloid cells, researchers discovered that Curcumin represses TNF-α-induced nuclear translocation and NF-κB DNA binding by suppressing IκBα phosphorylation and degradation [94]. There is no cure for chronic lymphocytic leukemia (CLL) with current chemotherapy treatments; however, Curcumin was found to induce apoptosis in B-cell chronic lymphocytic leukemia (B-CLL) via the upregulation of the proapoptotic protein BIM, and the downregulation of the AKT, STAT3, and XIAP (X-linked inhibitor of apoptosis) proteins and NF-κB [95]. Using colony formation and MTT methods, Li et al. demonstrated that Curcumin exhibits anticancer activity against leukemic cell lines [96]. This compound suppressed clonogenicity and cell proliferation and arrested the cell cycle at the G2/M phase in a time- and dose-dependent manner through the inhibition of Wilms tumor protein 1 (WT1) [96].
The second most common hematological malignancy, multiple myeloma, may be incurable; however, in a recent paper, Curcumin was found to potently block MM cell proliferation by promoting apoptosis in a time- and concentration-dependent manner by blocking EZH2 expression in RPMI8226 and U266 cell lines. Curcumin enhanced miR-101, and after that, lower EZH2 expression was observed. Conversely, the expression of EZH2 resulted in a softer expression of miR-101. These results indicated Curcumin’s impact and mode of action on multiple myeloma via the EZH2-miR-101 regulating feedback circuit [96]. In breast cancer, it is essential to note that recent research has reported Curcumin’s potential to effectively target many breast cancer-related signaling pathways, including Wnt/β-Catenin, Hedgehog, Notch, and PI3K/mTOR, as well as JAK-STAT [81]. In addition, this phytochemical can suppress cell growth in breast cancer cells by epigenetic modifications and DNA methylation mechanisms [80]. On the other hand, a mixture of Curcumin and arabinogalactan markedly reduced the growth of breast cancer cells with no appreciable effect on normal cells. The combined treatment enhanced cell apoptosis by affecting the membrane potential, elevating the ROS level, and reducing glutathione. Furthermore, the combination of Curcumin and arabinogalactan inhibited breast tumor progression through the overexpression of p53 levels in mice [82].
Additionally, Curcumin reduced cell proliferation in MCF-7 breast cancer cells by arresting cells in the G1 phase. This cell cycle arrest is mediated by enhancing cyclin E proteaosomal degradation and increasing the upregulation of CDK inhibitors p21, p53, and p27 [83]. In human mammary epithelial carcinoma cells, Curcumin-induced apoptosis via a selective increase in G-phase p53 expression (2) generated cytochrome c from mitochondria, downregulated cyclin D1 expression, and blocked the Cdk4/Cdk6 association [84]. Furthermore, Curcumin showed anticancer effects by inhibiting invasion and inducing apoptosis via ROS production, thus decreasing MMP-2 and Bcl-2 activity, and increasing Bax and caspase-3 activity in H-ras-transformed MCF10A human mammary epithelial cells [85]. Garcea et al. showed in their investigation that Curcumin administration causes a downregulation of M(1)G protein levels in colorectal cancer, whereas COX-2 protein levels remain unaltered in malignant colorectal tissue [86]. It also effectively suppressed the growth of the HT-29 cell line and DMH (1,2-dimethylhydrazine)-induced colorectal carcinogenesis in rats by inhibiting the signal transduction pathway PPARγ [87]. Furthermore, Curcumin repressed (COX-2) the pre-RNA processing factor 4B (Prp4B) and p53 expression [88].
In liver cancer, findings showed that Curcumin improves stability and the cytoprotective effect against tert-butyl hydroperoxide (t-BHP)-induced HepG2 cell death, and it promotes the nuclear transport of transcription factor Nrf-2, which controls the antioxidant signaling pathway [98]. Curcumin also targets the Notch-1 signaling pathway. Indeed, notch-1 signaling was damaged by Curcumin in the intracellular Notch domain in HEP3B, SK-Hep-1, and SNU449 cell lines. It also showed a protective effect against diethylnitrosamine (DENA)-induced hyperplasia and HCC in rodents by reducing p21-Ras, p53, and NF-κB expression [97].
4.3. Flavonoids
Quercetin
Quercetin (3,3′,4′,5,7-Pentahydroxyflavone) is the main component of the flavonoid subclass, flavonols. It is found in numerous fruits and vegetables, and it constitutes one of the most common flavonols in the Western diet. This phytochemical plays a pivotal role in cancer prevention [114]. Within this context, Lei et al. explored the therapeutic effect of Quercetin combined with irinotecan/SN-38 on the human gastric cancer cell line AGS [99]. Consequently, Quercetin decreased cell viability in a concentration-dependent manner. At a dose of 50 µM, it inhibited 50% of tumor growth. Combined with SN-38, it improved apoptosis, enhanced the anti-proliferation action, and synergistically modulated GSK-3β/β-catenin signaling with SN-38. Quercetin, in combination with irinotecan (10 mg/kg once a week), or alone (three times a week), mediated a substantial decrease in tumor size by day 28, it downregulated tumors VEGF-R and VEGF-A, and decreased COX-2 gene expression [99].
Jia et al. indicated that Quercetin could block breast cancer progression by preventing the cell motility and glycolysis mediated by the Akt-mTOR pathway; thus, it may be a promising candidate for the treatment of breast cancer [100]. Quercetin induces autophagy through Akt-mTOR pathway inactivation. Moreover, by applying IGF-1, an Akt-mTOR pathway inducer, and 3-MA, an autophagy inhibitor, these authors showed that Quercetin exhibits an effective suppressive effect on glycolysis and cell motility through the activation of autophagy, which is mediated by the Akt-mTOR pathway. In addition, Quercetin downregulated MMP-2 and MMP-9, VEGF suppressed glucose uptake and lactate production, as well as suppressing PKM2, LDHA, and GLUT1 expression. Furthermore, Quercetin repressed the progression and metastasis of breast cancer in mice that were injected with MCF-7 cells. It also downregulated PKM2, p-AKT, and VEGF levels in tumor tissue [100].
Furthermore, Quercetin, at a physiologically relevant concentration (0–10 μM, 4-day incubation), dose-dependently suppressed the growth of breast cancer cell lines SK-Br3 and MDA-MB-453. Although a low dose of Quercetin exhibited a modest cytotoxic effect, the primary mechanism of Quercetin’s antiproliferative effect was cell cycle arrest in the G1 phase. This event occurred via the downregulation of cyclin-dependent kinase 1 (CDK1) and cyclin B1, which are critical ingredients for G2/M cell cycle progression, and via the activation of phosphorylation for the retinoblastoma tumor suppressor protein, pRb [102]. Moreover, Quercetin reduced cell proliferation in a concentration-dependent manner (0.6 to 300 μΜ) and inhibited five glycolysis pathway molecules that are related to ovarian and breast cancer cell lines, specifically GLUT1, HKII, PFKFB3, PDHK1, and LDH [101].
To show the beneficial effects of Quercetin against prostate cancer, Erdogan and colleagues showed that this substance, at a dose of 40 Μm, upregulates Bax, Bcl-2, p21Cip1, p27Kip1, and Cyt c, caspase 3, caspase 8, and p53. It also blocked cell survival in a dose- and time-dependent manner. This polyphenol was also found to enhance midkine’s apoptotic effect, increase caspase 3, and decrease survivin gene expression. It stimulated cell cycle arrest in the G1 phase and reduced S phase cells. Similarly, Quercetin downregulated PI3K, Akt, ERK1/2, p38, NF-κB, and survivin protein phosphorylation, and upregulated PTEN expression. In combination with midkine, Quercetin exhibited a stronger action on ERK1/2, p38, NFκB, and survivin [106]. In contrast, at a dose of 100 and 150 μM (added every 24 h for 72 h), Quercetin suppressed proliferation in human prostate adenocarcinoma cell lines (PC-3) by 83% and 64.17%. It regulated the gene expression associated with DNA repair, tumor invasion and matrix degradation, apoptosis, cell cycle arrest, glycolysis, and metabolism, without cytotoxicity [105].
To find new alternatives to treat primary effusion lymphoma (PEL), Granato et al. demonstrated that Quercetin suppresses the cytokine release of both IL-6 and IL-10, thus leading to PEL cell death. At the same time, Quercetin inhibited the PI3K/AKT/mTOR and STAT3 pathways in PEL cells, thereby decreasing the level of cell survival proteins, including c-FLIP, cyclin D1, and cMyc [104]. Similarly, Sturza et al. evaluated the beneficial activities of Quercetin on the bioenergetic profile of the B164A5 murine melanoma cell line [103]. Results showed that Quercetin might represent an alternative strategy to treat cancer cells by modulating mitochondrial and glycolytic pathways to produce ATP. Moreover, Quercetin could reduce the extracellular acidification rate (ECAR) and oxygen consumption rate (OCR) by 50, 100, and 150 µM after a 48 h treatment [103].
5. Dietary Phenolic Compounds as Anticancer Drugs: Clinical Trials
5.1. Curcuminoids
Curcumin
Curcumin was reported in the literature as a chemotherapeutic, chemopreventive, and chemosensitizer agent [115] due to its various anticancer properties, as shown in the study by Chapman et al. [116]. With this in mind, Dhillon et al. assessed the oral administration of 8 g/day of curcumin to patients with pancreatic cancer [117]. The antitumor effects of curcumin were observed in two patients; one showed progress where curcumin treatment reduced the tumor by 73% without toxicity at 8 g/day for up to 18 months, however, the limited bioavailability of curcumin likely attenuated the activity. Additionally, Sung et al. tested curcumin on multiple myeloma (MM) patients in phase I/II clinical trials [118]. The study revealed that all doses (2, 4, 8, and 12 g/day) showed no side effects and inhibited the activation of NF-κB, COX-2, and STAT3 in the peripheral blood mononuclear cells (PBMC) that were isolated from MM patients. Interestingly, Cruz et al. reported that the combined treatment of Curcumin and Quercetin (480 mg and 20 mg, respectively, thrice a day, for six months) exerted adenoma regression in five patients with familial adenomatous polyposis (FAP) [119]. This treatment significantly reduced the number and size of ileal and rectal adenomas, compared with the baseline (60.4% and 50.9%, respectively), without toxicity. Other studies demonstrated that a daily dose of curcumin (3.6 g in capsule form) improves the general health of colorectal cancer patients by increasing p53 molecule expression in tumor cells, and consequently, inducing tumor cell apoptosis [86,120].
5.2. Stilbenes
Resveratrol
Resveratrol, also called trans-3,5,4′-Trihydroxystilbene and 5-[(E)-2-(4-Hydroxyphenyl)ethen-1-yl]benzene-1,3-diol, is a remarkable bioactive compound. In recent decades, several clinical studies have been conducted to provide clear data on the pharmacokinetics, safety, and effectiveness of Resveratrol to prove its therapeutic efficacy against different cancers; however, most of these investigations focus primarily on assessing Resveratrol’s bioavailability as a chemopreventive agent rather than its potential efficacy as a therapeutic agent for cancer patients. In this respect, a closed phase I clinical trial has shown the role of this molecule in the prevention of colon cancer [121]. Results demonstrated the potential effect of Resveratrol on the Wnt pathway, which is a pivotal pathway that is activated in more than 80% of colon cancers. Furthermore, in a phase I clinical trial, Resveratrol significantly reduced the levels of circulating cancer biomarkers, including Insulin-like growth factor 1 and Insulin-like growth factor-binding protein 3 [122]. In another phase I, double-blind, randomized clinical trial, Resveratrol increased the levels of cleaved caspase-3 in malignant hepatic tissue [123].
Generally, most clinical trials that are concerned with using this Stilbene against cancers in colorectal cancer patients note that Resveratrol could easily reach tumors located along the gastrointestinal tract at higher doses after oral administration. In their clinical study, Patel et al. showed that a concentration of 0.5 g Resveratrol effectively decreased the proliferation of colorectal cells [124]. On the other hand, ongoing clinical trials in phases I and II that either use Resveratrol alone or in combination with SRT501, demonstrated promising results in patients with colon and colorectal cancer [125]. After oral administration, the safety of micronized Resveratrol SRT501 has also been reported, and it showed a positive effect in colorectal cancer patients with hepatic metastases [125].
5.3. Flavonoids
5.3.1. Isoflavones
Genistein
Genistein (4′,5,7-Trihydroxyisoflavone), 5,7-Dihydroxy-3-(4-hydroxyphenyl))-4H-1-benzopyran-4-one is a natural isoflavone that has been isolated from soybeans and is known as an antimetastatic agent. It inhibited tumor growth and metastasis in vivo and exhibited significant antiproliferative activity on several cancer cells in vitro [126,127,128]. Case-control studies showed that dietary soybean intake may reduce one’s cancer risk [129,130]. The clinical outcome proves the efficacy of Genistein as an antimetastatic agent for patients with prostate cancer. In a randomized, placebo-controlled, double-blind assay, Genistein significantly decreased serum prostate-specific antigen (PSA) levels in prostate cancer patients without affecting hormones [131]. Phase I and Phase II clinical trials have indicated that this soy isoflavone may reduce the risk of both hormone-dependent and hormone-independent cancers, including prostate, breast, gastric, non-small cell lung, and colon cancers [132]. The use of this molecule as a dietary supplement in the treatment of patients with stage II, III, or IV prostate cancer is under evaluation [129]. Additionally, the combinatory treatment of the soy isoflavone Genistein with Gemcitabine, a known cancer drug, is also being studied in women with stage IV breast cancer. A phase II, randomized, placebo-controlled trial, recruited 59 patients with urothelial bladder cancer, and showed that daily oral intake of Genistein (300 or 600 mg/d as the purified soy extract G-2535) for 14–21 days prior to surgery exhibits a bimodal effect on bladder cancer tissue, which is noticeable at the lowest concentration [133].
5.3.2. Flavonols
Epigallocatechin-3-Gallate (EGCG)
Epigallocatechin-3-gallate (EGCG) is one of the phenolic compounds found in green tea, and it is considered a potent chemopreventive agent. It has been identified as an important constituent of polyphenol E, a standardized decaffeinated green tea catechin mixture that is currently used in numerous clinical trials [134,135]. To this end, EGCG is the subject of several investigations that aim to illustrate its chemosensitizing potential. It enhanced the therapeutic efficacy of the available chemotherapy treatments and reduced its associated adverse effects. In this respect, several clinical human trial-based studies in phases I and II have proven the critical role of EGCG in cancer prevention; however, further clinical trials are still needed to better understand the effectiveness of EGCG in cancer management.
The results of a study by McLarty et al., conducted on 26 men with positive prostate biopsies, showed that treatment with polyphenol E (800 mg EGCG for a total of 1.3 g of green tea) led to a significant reduction in serum levels of PSA, HGF, and VEGF, thus suggesting the efficacy of polyphenol E in the treatment or prevention of prostate cancer [136]. Similarly, Zhao et al. investigated the tolerability, safety, and efficacy of topical EGCG for radiation dermatitis in women with breast cancer undergoing radiotherapy [137]. These researchers indicated the good tolerance of EGCG following topical administration, although the maximum tolerated concentration was not reported. A phase I clinical trial with EGCG, in combination with standard chemoradiotherapy in unresectable stage III lung cancer, showed that oral administration of EGCG is feasible, safe, and effective [138]. Clinical phase II suggested a dose of 440 µmol/L [138].
6. Conclusions and Perspectives
At present, the use of foodstuffs in treating and preventing diseases, including cancer, is attracting attention. This is due to the presence of bioactive compounds such as polyphenols, among others, in our diet. These natural compounds are gaining popularity in cancer treatment due to their lower side effects, cost, and accessibility compared with conventional drugs. In this review, we have shown through published research that dietary phenolic compounds are an excellent source of natural anticancer substances, and they provide a range of preventive and therapeutic options against several types of cancer. These compounds could be used alone or in combination with other anticancer drugs. Certain phenolic compounds, such as Quercetin and Gallic acid, have well-known mechanisms of action. These molecules act specifically on the various checkpoints of cancerous cells; therefore, exploring these mechanisms of action could further improve therapeutic efficacy. However, further investigations that could involve human subjects and different pharmacokinetic parameters are required to ensure the safety of these compounds before they can be used as prescription drugs. In addition, the development of a standardized extract or dosage could also be studied in clinical trials. In summary, the polyphenols present in our food can be useful in complementary medicine for the prevention and treatment of different types of cancers due to their natural origin, safety, and low cost compared with cancer drugs. In our opinion, these molecules, that are contained in plants and used in our alimentary system, can be considered as functional foods or nutraceuticals. As a result of the different mechanisms demonstrated, phenolic compounds can prevent the appearance of certain cancers and reduce their progression towards later stages; however, further investigations need to be carried out on other cancers, as well as the risk factors that are associated with these cancers.
Author Contributions
Conceptualization, A.B; methodology, A.B., S.B., N.E.O. and N.E.H.; software, A.B.; validation, A.B., Y.B. and L.-H.L.; formal analysis, A.B; investigation, S.B. and A.B.; resources, S.B.; data curation, S.B.; writing—original draft preparation, S.B., A.B. and N.E.H.; writing—review and editing, A.B., S.B., Y.B., N.E.O. and L.-H.L.; visualization, A.B., Y.B., N.E.O. and L.-H.L.; supervision, A.B. and Y.B.; project administration, A.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| 5-LO | 5-LipoOxygenase |
| 5’ ETS | 5’ External transcribed spacer |
| 5-FU | 5-Fluorouracil |
| 5’-NT | 5’-Nucleotidase |
| Akt | Protein Kinase B |
| ADAM17 | Disintegrin and metalloprotease 17 |
| ATF4 | Activating transcription factor 4 |
| AHH | Aryl hydrocarbon hydroxylase |
| ATM | Ataxia telangiectasia mutated |
| ALF | Acute Liver Failure |
| AP-1 | Activator Protein-1 |
| BNIP3 | BCL-2-Interacting Protein 3 |
| BAX | Bcl-2-associated X protein |
| BCL-2 | B-cell leukemia/lymphoma-2 |
| CA | Caffeic acid |
| CEA | Carcinoembryonic antigen |
| Chk2 | Checkpoint kinase 2 |
| CAT | Catalase |
| CDK1 | Cyclin-Dependent Kinase 1 |
| CK2 | Casein Kinase 2 |
| CLL | Chronic Lymphocytic Leukemia |
| CLN | Catanionic Lipid Nanosystem |
| CDH-2 | Cadherin-2 |
| CYCS | Cytochrome C, Somatic |
| CMYC | C-myc proto-oncogene protein |
| COMT | Catecholamine-O-Methyltransferase |
| COX-2 | Cyclooxygenase-2 |
| CRC | Colorectal Carcinoma |
| DNMT | DNA Methyltransferase |
| DCFH-DA | Dichlorofluorescein diacetate |
| DSS | Dextran Sulphate Sodium |
| DTD | DT-Diaphorase |
| ECAR | Extracellular Acidification Rate |
| EGCG | Epigallocatechin-3-gallate |
| EGFR | Epidermal growth factor receptor |
| EMT | Epithelial-Mesenchymal Transition |
| ERK | Extracellular Signal-Regulated Kinase |
| FAP | Familial Adenomatous Polyposis |
| FoxM1 | Forkhead box M1 |
| FAS | Fatty acid synthase |
| FUT4 | Fucosyltransferase 4 |
| GA | Gallic acid |
| GGT | r-glutamyltranspeptidase |
| GADD45A | DNA-Damage-Inducible 45 Alpha |
| GPx | Glutathione Peroxidase |
| GST | Glutathione-S-Transferase |
| GSK3β | Glycogen synthase kinase-3 beta |
| GLUT1 | Glucose transporter 1 |
| HDAC | Histone deacetylase |
| HRK | Harakiri |
| HCC | Hepatocellular Carcinoma |
| HDM | Histone Demethylase |
| HIF-1α | Hypoxia-Inducible Factor-1α |
| HO | Heme Oxygenase |
| IL | Interleukin |
| IAP | Inhibitors of apoptosis proteins |
| INF | Interferon |
| IKKβ | Factor-kappa-B kinase subunit beta |
| IgM | Immunoglobulin M |
| IgG | Immunoglobulin G |
| JNK | C-Jun N-Terminal Kinase |
| JAK2 | Janus kinase 2 |
| LDH | Lactate dehydrogenase |
| MAPK | Mitogen-Activated Protein Kinase |
| MM | Multiple Myeloma |
| MMP | Matrix Metalloproteinase |
| MCP-1 | Monocyte chemoattractant protein-1 |
| MDR1 | Multidrug Resistance 1 |
| MUC1 | Mucin 1 |
| NF-κB | Nuclear Factor Kappa B |
| Nrf2 | Nuclear factor erythroid-related factor 2 |
| Notch-1 | Neurogenic locus notch homolog protein 1 |
| mTOR | mammalian target of rapamycin |
| NSCLC | Non-Small Cell Lung Cancer |
| OC | Ovarian Cancer |
| OCR | Oxygen Consumption Rate |
| PARP-1 | Poly (ADP-ribose) Polymerase-1 |
| PBMC | Peripheral Blood Mononuclear Cell |
| PEL | Primary Effusion Lymphoma |
| P-gp | P-glycoprotein |
| PI3-kinases | Phosphoinositide 3-Kinase |
| PSA | Prostate-Specific Antigen |
| PTEN | Phosphatase and Tensin Homolog |
| PTX | Paclitaxel |
| PKB | Protein kinase B |
| PKM-2 | Pyruvate kinase muscle-2 |
| p53 | Tumor protein 53 |
| PFKFB3 | Phosphofructo-2-kinase/fructose-2,6-biphosphatase 3 |
| PDHK1 | Pyruvate dehydrogenase kinase1 |
| RA | Rosmarinic acid |
| ROS | Reactive Oxygen Species |
| SA | Sinapic acid |
| SOD | Superoxide Dismutase |
| STAT3 | Signal Transducer and Activator of Transcription 3 |
| t-BHP | tert-Butyl HydroPeroxide |
| TLR4 | Toll-Like Receptor 4 |
| TNFRSF11B | TNF Superfamily 11B Receptor |
| TNFRSF25 | Tumor Necrosis Factor Receptor Superfamily 25 |
| TNF-α | Tumor Necrosis Factor-α |
| TIMP-1 | Tissue inhibitor of metalloproteinase-1 |
| TGF-β | Transforming growth factor beta |
| TRIB3 | Tribbles homolog 3 |
| UDP-GT | UDP-Glucuronyl Transferase |
| VEGF | Vascular Endothelial Growth Factor |
| WT1 | Wilms Tumor Protein 1 |
| γCD | Gamma-Cyclodextrin |
| XIAP | X-linked inhibitor of apoptosis |
References
- Hurson, A.N.; Ahearn, T.U.; Keeman, R.; Abubakar, M.; Jung, A.Y.; Kapoor, P.M.; Koka, H.; Yang, X.R.; Chang-Claude, J.; Martínez, E. Systematic Literature Review of Risk Factor Associations with Breast Cancer Subtypes in Women of African, Asian, Hispanic, and European Descents. Cancer Res. 2022, 82, 3670. [Google Scholar] [CrossRef]
- Stopsack, K.H.; Nandakumar, S.; Arora, K.; Nguyen, B.; Vasselman, S.E.; Nweji, B.; McBride, S.M.; Morris, M.J.; Rathkopf, D.E.; Slovin, S.F. Differences in Prostate Cancer Genomes by Self-Reported Race: Contributions of Genetic Ancestry, Modifiable Cancer Risk Factors, and Clinical FactorsRacial Differences in Prostate Cancer Genomes. Clin. Cancer Res. 2022, 28, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.A.; Dayu, A.R.B. Menopausal Hormone Therapy: Why We Should No Longer Be Afraid of the Breast Cancer Risk. Climacteric 2022, 25, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Maruca, A.; Catalano, R.; Bagetta, D.; Mesiti, F.; Ambrosio, F.A.; Romeo, I.; Moraca, F.; Rocca, R.; Ortuso, F.; Artese, A. The Mediterranean Diet as Source of Bioactive Compounds with Multi-Targeting Anti-Cancer Profile. Eur. J. Med. Chem. 2019, 181, 111579. [Google Scholar] [CrossRef] [PubMed]
- Dobroslavić, E.; Repajić, M.; Dragović-Uzelac, V.; Elez Garofulić, I. Isolation of Laurus Nobilis Leaf Polyphenols: A Review on Current Techniques and Future Perspectives. Foods 2022, 11, 235. [Google Scholar] [CrossRef]
- Mitra, S.; Tareq, A.M.; Das, R.; Emran, T.B.; Nainu, F.; Chakraborty, A.J.; Ahmad, I.; Tallei, T.E.; Idris, A.M.; Simal-Gandara, J. Polyphenols: A First Evidence in the Synergism and Bioactivities. Food Rev. Int. 2022, 1–23. [Google Scholar] [CrossRef]
- Rosero, S.; Del Pozo, F.; Simbaña, W.; Álvarez, M.; Quinteros, M.F.; Carrillo, W.; Morales, D. Polyphenols and Flavonoids Composition, Anti-Inflammatory and Antioxidant Properties of Andean Baccharis Macrantha Extracts. Plants 2022, 11, 1555. [Google Scholar] [CrossRef]
- Islam, B.U.; Suhail, M.; Khan, M.K.; Zughaibi, T.A.; Alserihi, R.F.; Zaidi, S.K.; Tabrez, S. Polyphenols as Anticancer Agents: Toxicological Concern to Healthy Cells. Phytother. Res. 2021, 35, 6063–6079. [Google Scholar] [CrossRef]
- Rauf, A.; Shariati, M.A.; Imran, M.; Bashir, K.; Khan, S.A.; Mitra, S.; Emran, T.B.; Badalova, K.; Uddin, M.; Mubarak, M.S. Comprehensive Review on Naringenin and Naringin Polyphenols as a Potent Anticancer Agent. Environ. Sci. Pollut. Res. 2022, 29, 1–17. [Google Scholar] [CrossRef]
- Mottaghi, S.; Abbaszadeh, H. Natural Lignans Honokiol and Magnolol as Potential Anticarcinogenic and Anticancer Agents. A Comprehensive Mechanistic Review. Nutr. Cancer 2022, 74, 761–778. [Google Scholar] [CrossRef]
- Yoganathan, S.; Alagaratnam, A.; Acharekar, N.; Kong, J. Ellagic Acid and Schisandrins: Natural Biaryl Polyphenols with Therapeutic Potential to Overcome Multidrug Resistance in Cancer. Cells 2021, 10, 458. [Google Scholar] [CrossRef]
- Ksouri, R.; Ksouri, W.M.; Jallali, I.; Debez, A.; Magné, C.; Hiroko, I.; Abdelly, C. Medicinal Halophytes: Potent Source of Health Promoting Biomolecules with Medical, Nutraceutical and Food Applications. Crit. Rev. Biotechnol. 2012, 32, 289–326. [Google Scholar] [CrossRef]
- Bahadoran, Z.; Mirmiran, P.; Azizi, F. Dietary Polyphenols as Potential Nutraceuticals in Management of Diabetes: A Review. J. Diabetes Metab. Disord. 2013, 12, 1–9. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food Sources and Bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef]
- Adlercreutz, H. Lignans and Human Health. Crit. Rev. Clin. Lab. Sci. 2007, 44, 483–525. [Google Scholar] [CrossRef]
- Yordi, E.G.; Pérez, E.M.; Matos, M.J.; Villares, E.U. Antioxidant and Pro-Oxidant Effects of Polyphenolic Compounds and Structure-Activity Relationship Evidence. Nutr. Well-Health 2012, 2, 23–48. [Google Scholar]
- Karimi, Z.; Firouzi, M.; Dadmehr, M.; Javad-Mousavi, S.A.; Bagheriani, N.; Sadeghpour, O. Almond as a Nutraceutical and Therapeutic Agent in Persian Medicine and Modern Phytotherapy: A Narrative Review. Phytother. Res. 2021, 35, 2997–3012. [Google Scholar] [CrossRef]
- Maiuolo, J.; Gliozzi, M.; Carresi, C.; Musolino, V.; Oppedisano, F.; Scarano, F.; Nucera, S.; Scicchitano, M.; Bosco, F.; Macri, R. Nutraceuticals and Cancer: Potential for Natural Polyphenols. Nutrients 2021, 13, 3834. [Google Scholar] [CrossRef]
- Arrigoni, R.; Ballini, A.; Santacroce, L.; Cantore, S.; Inchingolo, A.; Inchingolo, F.; Di Domenico, M.; Quagliuolo, L.; Boccellino, M. Another Look at Dietary Polyphenols: Challenges in Cancer Prevention and Treatment. Curr. Med. Chem. 2022, 29, 1061–1082. [Google Scholar] [CrossRef]
- Patra, S.; Pradhan, B.; Nayak, R.; Behera, C.; Das, S.; Patra, S.K.; Efferth, T.; Jena, M.; Bhutia, S.K. Dietary Polyphenols in Chemoprevention and Synergistic Effect in Cancer: Clinical Evidences and Molecular Mechanisms of Action. Phytomedicine 2021, 90, 153554. [Google Scholar] [CrossRef]
- Nichenametla, S.N.; Taruscio, T.G.; Barney, D.L.; Exon, J.H. A Review of the Effects and Mechanisms of Polyphenolics in Cancer. Crit. Rev. Food Sci. Nutr. 2006, 46, 161–183. [Google Scholar] [CrossRef]
- Ramos, S. Cancer Chemoprevention and Chemotherapy: Dietary Polyphenols and Signalling Pathways. Mol. Nutr. Food Res. 2008, 52, 507–526. [Google Scholar] [CrossRef]
- Han, X.; Shen, T.; Lou, H. Dietary Polyphenols and Their Biological Significance. Int. J. Mol. Sci. 2007, 8, 950–988. [Google Scholar] [CrossRef]
- Sachithanandam, V.; Parthiban, A.; Lalitha, P.; Muthukumaran, J.; Jain, M.; Elumalai, D.; Jayabal, K.; Sridhar, R.; Ramachandran, P.; Ramachandran, R. Biological Evaluation of Gallic Acid and Quercetin Derived from Ceriops Tagal: Insights from Extensive in Vitro and in Silico Studies. J. Biomol. Struct. Dyn. 2022, 40, 1490–1502. [Google Scholar] [CrossRef]
- Ko, E.-B.; Jang, Y.-G.; Kim, C.-W.; Go, R.-E.; Lee, H.K.; Choi, K.-C. Gallic Acid Hindered Lung Cancer Progression by Inducing Cell Cycle Arrest and Apoptosis in A549 Lung Cancer Cells via PI3K/Akt Pathway. Biomol. Ther. 2022, 30, 151. [Google Scholar] [CrossRef]
- Qanash, H.; Yahya, R.; Bakri, M.M.; Bazaid, A.S.; Qanash, S.; Shater, A.F.; Abdelghany, T.M. Anticancer, Antioxidant, Antiviral and Antimicrobial Activities of Kei Apple (Dovyalis Caffra) Fruit. Sci. Rep. 2022, 12, 1–15. [Google Scholar]
- Sanchez-Martin, V.; Plaza-Calonge, M.d.C.; Soriano-Lerma, A.; Ortiz-Gonzalez, M.; Linde-Rodriguez, A.; Perez-Carrasco, V.; Ramirez-Macias, I.; Cuadros, M.; Gutierrez-Fernandez, J.; Murciano-Calles, J. Gallic Acid: A Natural Phenolic Compound Exerting Antitumoral Activities in Colorectal Cancer via Interaction with G-Quadruplexes. Cancers 2022, 14, 2648. [Google Scholar] [CrossRef]
- Veluri, R.; Singh, R.P.; Liu, Z.; Thompson, J.A.; Agarwal, R.; Agarwal, C. Fractionation of Grape Seed Extract and Identification of Gallic Acid as One of the Major Active Constituents Causing Growth Inhibition and Apoptotic Death of DU145 Human Prostate Carcinoma Cells. Carcinogenesis 2006, 27, 1445–1453. [Google Scholar] [CrossRef]
- Agarwal, C.; Tyagi, A.; Agarwal, R. Gallic Acid Causes Inactivating Phosphorylation of Cdc25A/Cdc25C-Cdc2 via ATM-Chk2 Activation, Leading to Cell Cycle Arrest, and Induces Apoptosis in Human Prostate Carcinoma DU145 Cells. Mol. Cancer Ther. 2006, 5, 3294–3302. [Google Scholar] [CrossRef]
- Locatelli, C.; Filippin-Monteiro, F.B.; Centa, A.; Creczinsky-Pasa, T.B. Antioxidant, Antitumoral and Anti-Inflammatory Activities of Gallic Acid. In Handbook on Gallic Acid: Natural Occurrences, Antioxidant Properties and Health Implications; Nova Publishers: Hauppauge, NY, USA, 2013; pp. 1–23. [Google Scholar]
- Patra, S.; Bhol, C.S.; Panigrahi, D.P.; Praharaj, P.P.; Pradhan, B.; Jena, M.; Bhutia, S.K. Gamma Irradiation Promotes Chemo-Sensitization Potential of Gallic Acid through Attenuation of Autophagic Flux to Trigger Apoptosis in an NRF2 Inactivation Signalling Pathway. Free Radic. Biol. Med. 2020, 160, 111–124. [Google Scholar] [CrossRef]
- Inoue, M.; Suzuki, R.; Sakaguchi, N.; Li, Z.; Takeda, T.; Ogihara, Y.; Jiang, B.Y.; Chen, Y. Selective Induction of Cell Death in Cancer Cells by Gallic Acid. Biol. Pharm. Bull. 1995, 18, 1526–1530. [Google Scholar] [CrossRef]
- Ozturk, G.; Ginis, Z.; Akyol, S.; Erden, G.; Gurel, A.; Akyol, O. The Anticancer Mechanism of Caffeic Acid Phenethyl Ester (CAPE): Review of Melanomas, Lung and Prostate Cancers. Eur. Rev. Med. Pharmacol. Sci. 2012, 16, 2064–2068. [Google Scholar]
- Lin, H.-P.; Lin, C.-Y.; Huo, C.; Hsiao, P.-H.; Su, L.-C.; Jiang, S.S.; Chan, T.-M.; Chang, C.-H.; Chen, L.-T.; Kung, H.-J. Caffeic Acid Phenethyl Ester Induced Cell Cycle Arrest and Growth Inhibition in Androgen-Independent Prostate Cancer Cells via Regulation of Skp2, P53, P21Cip1 and P27Kip1. Oncotarget 2015, 6, 6684. [Google Scholar] [CrossRef]
- Chen, C.-N.; Wu, C.-L.; Lin, J.-K. Apoptosis of Human Melanoma Cells Induced by the Novel Compounds Propolin A and Propolin B from Taiwenese Propolis. Cancer Lett. 2007, 245, 218–231. [Google Scholar] [CrossRef]
- Kudugunti, S.K.; Vad, N.M.; Ekogbo, E.; Moridani, M.Y. Efficacy of Caffeic Acid Phenethyl Ester (CAPE) in Skin B16-F0 Melanoma Tumor Bearing C57BL/6 Mice. Investig. New Drugs 2011, 29, 52–62. [Google Scholar] [CrossRef]
- Maruyama, H.; Kawakami, F.; Lwin, T.-T.; Imai, M.; Shamsa, F. Biochemical Characterization of Ferulic Acid and Caffeic Acid Which Effectively Inhibit Melanin Synthesis via Different Mechanisms in B16 Melanoma Cells. Biol. Pharm. Bull. 2018, 41, 806–810. [Google Scholar] [CrossRef]
- Wadhwa, R.; Nigam, N.; Bhargava, P.; Dhanjal, J.K.; Goyal, S.; Grover, A.; Sundar, D.; Ishida, Y.; Terao, K.; Kaul, S.C. Molecular Characterization and Enhancement of Anticancer Activity of Caffeic Acid Phenethyl Ester by γ Cyclodextrin. J. Cancer 2016, 7, 1755. [Google Scholar] [CrossRef]
- Ishida, Y.; Gao, R.; Shah, N.; Bhargava, P.; Furune, T.; Kaul, S.C.; Terao, K.; Wadhwa, R. Anticancer Activity in Honeybee Propolis: Functional Insights to the Role of Caffeic Acid Phenethyl Ester and Its Complex with γ-Cyclodextrin. Integr. Cancer Ther. 2018, 17, 867–873. [Google Scholar] [CrossRef]
- Pagnan, A.L.; Pessoa, A.S.; Tokuhara, C.K.; Fakhoury, V.S.; Oliveira, G.S.N.; Sanches, M.L.R.; Inacio, K.K.; Ximenes, V.F.; Oliveira, R.C. Anti-Tumour Potential and Selectivity of Caffeic Acid Phenethyl Ester in Osteosarcoma Cells. Tissue Cell 2022, 74, 101705. [Google Scholar] [CrossRef]
- Min, J.; Shen, H.; Xi, W.; Wang, Q.; Yin, L.; Zhang, Y.; Yu, Y.; Yang, Q.; Wang, Z. Synergistic Anticancer Activity of Combined Use of Caffeic Acid with Paclitaxel Enhances Apoptosis of Non-Small-Cell Lung Cancer H1299 Cells in Vivo and in Vitro. Cell. Physiol. Biochem. 2018, 48, 1433–1442. [Google Scholar] [CrossRef]
- Kanimozhi, G.; Prasad, N.R. Anticancer Effect of Caffeic Acid on Human Cervical Cancer Cells. In Coffee in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2015; pp. 655–661. [Google Scholar]
- Bhat, S.H.; Azmi, A.S.; Hadi, S.M. Prooxidant DNA Breakage Induced by Caffeic Acid in Human Peripheral Lymphocytes: Involvement of Endogenous Copper and a Putative Mechanism for Anticancer Properties. Toxicol. Appl. Pharmacol. 2007, 218, 249–255. [Google Scholar] [CrossRef]
- Guo, D.; Dou, D.; Ge, L.; Huang, Z.; Wang, L.; Gu, N. A Caffeic Acid Mediated Facile Synthesis of Silver Nanoparticles with Powerful Anti-Cancer Activity. Colloids Surf. B Biointerfaces 2015, 134, 229–234. [Google Scholar] [CrossRef]
- Lee, W.J.; Zhu, B.T. Inhibition of DNA Methylation by Caffeic Acid and Chlorogenic Acid, Two Common Catechol-Containing Coffee Polyphenols. Carcinogenesis 2006, 27, 269–277. [Google Scholar] [CrossRef]
- Liang, G.; Shi, B.; Luo, W.; Yang, J. The Protective Effect of Caffeic Acid on Global Cerebral Ischemia-Reperfusion Injury in Rats. Behav. Brain Funct. 2015, 11, 1–10. [Google Scholar] [CrossRef]
- Jung, J.E.; Kim, H.S.; Lee, C.S.; Park, D.-H.; Kim, Y.-N.; Lee, M.-J.; Lee, J.W.; Park, J.-W.; Kim, M.-S.; Ye, S.K. Caffeic Acid and Its Synthetic Derivative CADPE Suppress Tumor Angiogenesis by Blocking STAT3-Mediated VEGF Expression in Human Renal Carcinoma Cells. Carcinogenesis 2007, 28, 1780–1787. [Google Scholar] [CrossRef]
- Kang, N.J.; Lee, K.W.; Kim, B.H.; Bode, A.M.; Lee, H.-J.; Heo, Y.-S.; Boardman, L.; Limburg, P.; Lee, H.J.; Dong, Z. Coffee Phenolic Phytochemicals Suppress Colon Cancer Metastasis by Targeting MEK and TOPK. Carcinogenesis 2011, 32, 921–928. [Google Scholar] [CrossRef]
- Yang, G.; Fu, Y.; Malakhova, M.; Kurinov, I.; Zhu, F.; Yao, K.; Li, H.; Chen, H.; Li, W.; Lim, D.Y. Caffeic Acid Directly Targets ERK1/2 to Attenuate Solar UV-Induced Skin Carcinogenesis. Cancer Prev. Res. 2014, 7, 1056–1066. [Google Scholar] [CrossRef]
- Rosendahl, A.H.; Perks, C.M.; Zeng, L.; Markkula, A.; Simonsson, M.; Rose, C.; Ingvar, C.; Holly, J.M.; Jernström, H. Caffeine and Caffeic Acid Inhibit Growth and Modify Estrogen Receptor and Insulin-like Growth Factor I Receptor Levels in Human Breast Cancer. Clin. Cancer Res. 2015, 21, 1877–1887. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Kao, C.-L.; Liu, C.-M. The Cancer Prevention, Anti-Inflammatory and Anti-Oxidation of Bioactive Phytochemicals Targeting the TLR4 Signaling Pathway. Int. J. Mol. Sci. 2018, 19, 2729. [Google Scholar] [CrossRef]
- Luo, Y.; Ma, Z.; Xu, X.; Qi, H.; Cheng, Z.; Chen, L. Anticancer Effects of Rosmarinic Acid in Human Oral Cancer Cells Is Mediated via Endoplasmic Reticulum Stress, Apoptosis, G2/M Cell Cycle Arrest and Inhibition of Cell Migration. J BUON 2020, 25, 1245–1250. [Google Scholar]
- Jin, B.-R.; Chung, K.-S.; Hwang, S.; Hwang, S.N.; Rhee, K.-J.; Lee, M.; An, H.-J. Rosmarinic Acid Represses Colitis-Associated Colon Cancer: A Pivotal Involvement of the TLR4-Mediated NF-ΚB-STAT3 Axis. Neoplasia 2021, 23, 561–573. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.-G.; Hwang, K.-A.; Choi, K.-C. Rosmarinic Acid, a Component of Rosemary Tea, Induced the Cell Cycle Arrest and Apoptosis through Modulation of HDAC2 Expression in Prostate Cancer Cell Lines. Nutrients 2018, 10, 1784. [Google Scholar] [CrossRef] [PubMed]
- Chao, W.-W.; Liou, Y.-J.; Ma, H.-T.; Chen, Y.-H.; Chou, S.-T. The Antitumor Mechanism of the Polyphenol-Enriched Ethyl Acetate Fraction Extract of Glechoma Hederacea (Lamiaceae) against HepG2 Cells Involves Apoptosis Pathways; ResearchGate: Berlin, Germany, 2020. [Google Scholar]
- Wang, L.; Yang, H.; Wang, C.; Shi, X.; Li, K. Rosmarinic Acid Inhibits Proliferation and Invasion of Hepatocellular Carcinoma Cells SMMC 7721 via PI3K/AKT/MTOR Signal Pathway. Biomed. Pharmacother. 2019, 120, 109443. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Yang, J.; Yang, Y.; Wang, X.; Chen, G.; Shi, A.; Lu, Y.; Jia, S.; Kang, X.; Lu, L. Rosmarinic Acid Exerts an Anticancer Effect on Osteosarcoma Cells by Inhibiting DJ-1 via Regulation of the PTEN-PI3K-Akt Signaling Pathway. Phytomedicine 2020, 68, 153186. [Google Scholar] [CrossRef]
- Messeha, S.S.; Zarmouh, N.O.; Asiri, A.; Soliman, K.F. Rosmarinic Acid-Induced Apoptosis and Cell Cycle Arrest in Triple-Negative Breast Cancer Cells. Eur. J. Pharmacol. 2020, 885, 173419. [Google Scholar] [CrossRef]
- Mahmoud, M.A.; Okda, T.M.; Omran, G.A.; Abd-Alhaseeb, M.M. Rosmarinic Acid Suppresses Inflammation, Angiogenesis, and Improves Paclitaxel Induced Apoptosis in a Breast Cancer Model via NF3 ΚB-P53-Caspase-3 Pathways Modulation. J. Appl. Biomed. 2021, 19, 202–209. [Google Scholar] [CrossRef]
- Huang, L.; Chen, J.; Quan, J.; Xiang, D. Rosmarinic Acid Inhibits Proliferation and Migration, Promotes Apoptosis and Enhances Cisplatin Sensitivity of Melanoma Cells through Inhibiting ADAM17/EGFR/AKT/GSK3β Axis. Bioengineered 2021, 12, 3065–3076. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, W.; Li, Z.; Chen, L.; Wen, C.; Ruan, Q.; Xu, Z.; Liu, R.; Xu, J.; Bai, Y. Rosmarinic Acid Decreases the Malignancy of Pancreatic Cancer through Inhibiting Gli1 Signaling. Phytomedicine 2022, 95, 153861. [Google Scholar] [CrossRef]
- Lim, S.H.; Nam, K.H.; Kim, K.; Yi, S.A.; Lee, J.; Han, J.-W. Rosmarinic Acid Methyl Ester Regulates Ovarian Cancer Cell Migration and Reverses Cisplatin Resistance by Inhibiting the Expression of Forkhead Box M1. Pharmaceuticals 2020, 13, 302. [Google Scholar] [CrossRef]
- Liao, X.-Z.; Gao, Y.; Sun, L.-L.; Liu, J.-H.; Chen, H.-R.; Yu, L.; Chen, Z.-Z.; Chen, W.-H.; Lin, L.-Z. Rosmarinic Acid Reverses Non-Small Cell Lung Cancer Cisplatin Resistance by Activating the MAPK Signaling Pathway. Phytother. Res. 2020, 34, 1142–1153. [Google Scholar] [CrossRef]
- Radziejewska, I.; Supruniuk, K.; Bielawska, A. Anti-Cancer Effect of Combined Action of Anti-MUC1 and Rosmarinic Acid in AGS Gastric Cancer Cells. Eur. J. Pharmacol. 2021, 902, 174119. [Google Scholar] [CrossRef] [PubMed]
- Eroğlu, C.; Avcı, E.; Vural, H.; Kurar, E. Anticancer Mechanism of Sinapic Acid in PC-3 and LNCaP Human Prostate Cancer Cell Lines. Gene 2018, 671, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.A.; Raish, M.; Jardan, Y.A.B.; Ahmad, A.; Shahid, M.; Ahmad, S.F.; Haq, N.; Khan, M.R.; Bakheet, S.A. Sinapic Acid Ameliorates D-Galactosamine/Lipopolysaccharide-Induced Fulminant Hepatitis in Rats: Role of Nuclear Factor Erythroid-Related Factor 2/Heme Oxygenase-1 Pathways. World J. Gastroenterol. 2021, 27, 592. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Bishayee, A.; Pandey, A.K. Targeting Histone Deacetylases with Natural and Synthetic Agents: An Emerging Anticancer Strategy. Nutrients 2018, 10, 731. [Google Scholar] [CrossRef]
- Huang, Z.; Chen, H.; Tan, P.; Huang, M.; Shi, H.; Sun, B.; Cheng, Y.; Li, T.; Mou, Z.; Li, Q. Sinapic Acid Inhibits Pancreatic Cancer Proliferation, Migration, and Invasion via Downregulation of the AKT/Gsk-3β Signal Pathway. Drug Dev. Res. 2022, 83, 721–734. [Google Scholar] [CrossRef]
- Hu, X.; Geetha, R.V.; Surapaneni, K.M.; Veeraraghavan, V.P.; Chinnathambi, A.; Alahmadi, T.A.; Manikandan, V.; Manokaran, K. Lung Cancer Induced by Benzo (A) Pyrene: ChemoProtective Effect of Sinapic Acid in Swiss Albino Mice. Saudi J. Biol. Sci. 2021, 28, 7125–7133. [Google Scholar] [CrossRef]
- Janakiraman, K.; Kathiresan, S.; Mariadoss, A.V. Influence of Sinapic Acid on Induction of Apoptosis in Human Laryngeal Carcinoma Cell Line. Int. J. Mod. Res. Rev. 2014, 2, 165–170. [Google Scholar]
- Balaji, C.; Muthukumaran, J.; Nalini, N. Chemopreventive Effect of Sinapic Acid on 1, 2-Dimethylhydrazine-Induced Experimental Rat Colon Carcinogenesis. Hum. Exp. Toxicol. 2014, 33, 1253–1268. [Google Scholar] [CrossRef]
- Zhao, J.; Li, H.; Li, W.; Wang, Z.; Dong, Z.; Lan, H.; Wang, C.; Song, J.-L. Effects of Sinapic Acid Combined with Cisplatin on the Apoptosis and Autophagy of the Hepatoma Cells HepG2 and SMMC-7721. Evid. Based Complement. Alternat. Med. 2021, 2021, 6095963. [Google Scholar] [CrossRef]
- Masuelli, L.; Benvenuto, M.; Di Stefano, E.; Mattera, R.; Fantini, M.; De Feudis, G.; De Smaele, E.; Tresoldi, I.; Giganti, M.G.; Modesti, A. Curcumin Blocks Autophagy and Activates Apoptosis of Malignant Mesothelioma Cell Lines and Increases the Survival of Mice Intraperitoneally Transplanted with a Malignant Mesothelioma Cell Line. Oncotarget 2017, 8, 34405. [Google Scholar] [CrossRef]
- Moon, D.-O.; Jin, C.-Y.; Lee, J.-D.; Choi, Y.H.; Ahn, S.-C.; Lee, C.-M.; Jeong, S.-C.; Park, Y.-M.; Kim, G.-Y. Curcumin Decreases Binding of Shiga-like Toxin-1B on Human Intestinal Epithelial Cell Line HT29 Stimulated with TNF-α and IL-1β: Suppression of P38, JNK and NF-ΚB P65 as Potential Targets. Biol. Pharm. Bull. 2006, 29, 1470–1475. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.C.; Kamarudin, M.N.A.; Naidu, R. Anticancer Mechanism of Curcumin on Human Glioblastoma. Nutrients 2021, 13, 950. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhu, J.; Lv, X.; Xing, J.; Liu, S.; Chen, C.; Xu, Y. Curcumin Potentiates the Potent Antitumor Activity of ACNU against Glioblastoma by Suppressing the PI3K/AKT and NF-ΚB/COX-2 Signaling Pathways. OncoTargets Ther. 2017, 10, 5471. [Google Scholar] [CrossRef] [PubMed]
- Nagai, S.; Kurimoto, M.; Washiyama, K.; Hirashima, Y.; Kumanishi, T.; Endo, S. Inhibition of Cellular Proliferation and Induction of Apoptosis by Curcumin in Human Malignant Astrocytoma Cell Lines. J. Neurooncol. 2005, 74, 105–111. [Google Scholar] [CrossRef]
- Huang, T.-Y.; Tsai, T.-H.; Hsu, C.-W.; Hsu, Y.-C. Curcuminoids Suppress the Growth and Induce Apoptosis through Caspase-3-Dependent Pathways in Glioblastoma Multiforme (GBM) 8401 Cells. J. Agric. Food Chem. 2010, 58, 10639–10645. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Lee, Y.-M.; Chang, G.-C.; Yu, S.-L.; Hsieh, W.-Y.; Chen, J.J.; Chen, H.-W.; Yang, P.-C. Curcumin Induces EGFR Degradation in Lung Adenocarcinoma and Modulates P38 Activation in Intestine: The Versatile Adjuvant for Gefitinib Therapy. PLoS ONE 2011, 6, e23756. [Google Scholar] [CrossRef]
- Fabianowska-Majewska, K.; Kaufman-Szymczyk, A.; Szymanska-Kolba, A.; Jakubik, J.; Majewski, G.; Lubecka, K. Curcumin from Turmeric Rhizome: A Potential Modulator of DNA Methylation Machinery in Breast Cancer Inhibition. Nutrients 2021, 13, 332. [Google Scholar] [CrossRef]
- Dandawate, P.R.; Subramaniam, D.; Jensen, R.A.; Anant, S. Targeting Cancer Stem Cells and Signaling Pathways by Phytochemicals: Novel Approach for Breast Cancer Therapy. In Proceedings of the Seminars in Cancer Biology; Elsevier: Amsterdam, The Netherlands, 2016; Volume 40, pp. 192–208. [Google Scholar]
- Moghtaderi, H.; Sepehri, H.; Attari, F. Combination of Arabinogalactan and Curcumin Induces Apoptosis in Breast Cancer Cells in Vitro and Inhibits Tumor Growth via Overexpression of P53 Level in Vivo. Biomed. Pharmacother. 2017, 88, 582–594. [Google Scholar] [CrossRef]
- Song, X.; Zhang, M.; Dai, E.; Luo, Y. Molecular Targets of Curcumin in Breast Cancer. Mol. Med. Rep. 2019, 19, 23–29. [Google Scholar] [CrossRef]
- Choudhuri, T.; Pal, S.; Das, T.; Sa, G. Curcumin Selectively Induces Apoptosis in Deregulated Cyclin D1-Expressed Cells at G2 Phase of Cell Cycle in a P53-Dependent Manner. J. Biol. Chem. 2005, 280, 20059–20068. [Google Scholar] [CrossRef]
- Kim, M.-S.; Kang, H.-J.; Moon, A. Inhibition of Invasion and Induction of Apoptosis by Curcumin in H-Ras-Transformed MCF10A Human Breast Epithelial Cells. Arch. Pharm. Res. 2001, 24, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Garcea, G.; Berry, D.P.; Jones, D.J.; Singh, R.; Dennison, A.R.; Farmer, P.B.; Sharma, R.A.; Steward, W.P.; Gescher, A.J. Consumption of the Putative Chemopreventive Agent Curcumin by Cancer Patients: Assessment of Curcumin Levels in the Colorectum and Their Pharmacodynamic Consequences. Cancer Epidemiol. Biomark. Prev. 2005, 14, 120–125. [Google Scholar] [CrossRef]
- Liu, L.B.; Duan, C.N.; Ma, Z.Y.; Xu, G. Curcumin Inhibited Rat Colorectal Carcinogenesis by Activating PPAR-γ: An Experimental Study. Zhongguo Zhong Xi Yi Jie He Za Zhi Zhongguo Zhongxiyi Jiehe Zazhi Chin. J. Integr. Tradit. West. Med. 2015, 35, 471–475. [Google Scholar]
- Goel, A.; Boland, C.R.; Chauhan, D.P. Specific Inhibition of Cyclooxygenase-2 (COX-2) Expression by Dietary Curcumin in HT-29 Human Colon Cancer Cells. Cancer Lett. 2001, 172, 111–118. [Google Scholar] [CrossRef]
- Shehzad, A.; Lee, J.; Huh, T.-L.; Lee, Y.S. Curcumin Induces Apoptosis in Human Colorectal Carcinoma (HCT-15) Cells by Regulating Expression of Prp4 and P53. Mol. Cells 2013, 35, 526–532. [Google Scholar] [CrossRef]
- Ghosh, S.; Banerjee, S.; Sil, P.C. The Beneficial Role of Curcumin on Inflammation, Diabetes and Neurodegenerative Disease: A Recent Update. Food Chem. Toxicol. 2015, 83, 111–124. [Google Scholar] [CrossRef]
- Lev-Ari, S.; Starr, A.; Vexler, A.; Karaush, V.; Loew, V.; Greif, J.; Fenig, E.; Aderka, D.; Ben-Yosef, R. Inhibition of Pancreatic and Lung Adenocarcinoma Cell Survival by Curcumin Is Associated with Increased Apoptosis, down-Regulation of COX-2 and EGFR and Inhibition of Erk1/2 Activity. Anticancer Res. 2006, 26, 4423–4430. [Google Scholar]
- Zhang, B.-Y.; Shi, Y.-Q.; Chen, X.; Dai, J.; Jiang, Z.-F.; Li, N.; Zhang, Z.-B. Protective Effect of Curcumin against Formaldehyde-Induced Genotoxicity in A549 Cell Lines. J. Appl. Toxicol. 2013, 33, 1468–1473. [Google Scholar] [CrossRef]
- Li, S.; Fang, C.; Zhang, J.; Liu, B.; Wei, Z.; Fan, X.; Sui, Z.; Tan, Q. Catanionic Lipid Nanosystems Improve Pharmacokinetics and Anti-Lung Cancer Activity of Curcumin. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 1567–1579. [Google Scholar] [CrossRef]
- Anand, P.; Sundaram, C.; Jhurani, S.; Kunnumakkara, A.B.; Aggarwal, B.B. Curcumin and Cancer: An “Old-Age” Disease with an “Age-Old” Solution. Cancer Lett. 2008, 267, 133–164. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Kay, N.E.; Secreto, C.R.; Shanafelt, T.D. Curcumin Inhibits Prosurvival Pathways in Chronic Lymphocytic Leukemia B Cells and May Overcome Their Stromal Protection in Combination with EGCG. Clin. Cancer Res. 2009, 15, 1250–1258. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, J.; Li, X.; Jia, Y.; Huai, L.; He, K.; Yu, P.; Wang, M.; Xing, H.; Rao, Q. Role of the Wilms’ Tumor 1 Gene in the Aberrant Biological Behavior of Leukemic Cells and the Related Mechanisms. Oncol. Rep. 2014, 32, 2680–2686. [Google Scholar] [CrossRef] [PubMed]
- Darvesh, A.S.; Aggarwal, B.B.; Bishayee, A. Curcumin and Liver Cancer: A Review. Curr. Pharm. Biotechnol. 2012, 13, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Tu, Z.-S.; Wang, Q.; Sun, D.-D.; Dai, F.; Zhou, B. Design, Synthesis, and Evaluation of Curcumin Derivatives as Nrf2 Activators and Cytoprotectors against Oxidative Death. Eur. J. Med. Chem. 2017, 134, 72–85. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.-S.; Hou, Y.-C.; Pai, M.-H.; Lin, M.-T.; Yeh, S.-L. Effects of Quercetin Combined with Anticancer Drugs on Metastasis-Associated Factors of Gastric Cancer Cells: In Vitro and in Vivo Studies. J. Nutr. Biochem. 2018, 51, 105–113. [Google Scholar] [CrossRef]
- Jia, L.; Huang, S.; Yin, X.; Zan, Y.; Guo, Y.; Han, L. Quercetin Suppresses the Mobility of Breast Cancer by Suppressing Glycolysis through Akt-MTOR Pathway Mediated Autophagy Induction. Life Sci. 2018, 208, 123–130. [Google Scholar] [CrossRef]
- Xintaropoulou, C.; Ward, C.; Wise, A.; Marston, H.; Turnbull, A.; Langdon, S.P. A Comparative Analysis of Inhibitors of the Glycolysis Pathway in Breast and Ovarian Cancer Cell Line Models. Oncotarget 2015, 6, 25677. [Google Scholar] [CrossRef]
- Jeong, J.-H.; An, J.Y.; Kwon, Y.T.; Rhee, J.G.; Lee, Y.J. Effects of Low Dose Quercetin: Cancer Cell-Specific Inhibition of Cell Cycle Progression. J. Cell. Biochem. 2009, 106, 73–82. [Google Scholar] [CrossRef]
- Sturza, A.; Pavel, I.; Ancușa, S.; Danciu, C.; Dehelean, C.; Duicu, O.; Muntean, D. Quercetin Exerts an Inhibitory Effect on Cellular Bioenergetics of the B164A5 Murine Melanoma Cell Line. Mol. Cell. Biochem. 2018, 447, 103–109. [Google Scholar] [CrossRef]
- Granato, M.; Rizzello, C.; Montani, M.S.G.; Cuomo, L.; Vitillo, M.; Santarelli, R.; Gonnella, R.; D’Orazi, G.; Faggioni, A.; Cirone, M. Quercetin Induces Apoptosis and Autophagy in Primary Effusion Lymphoma Cells by Inhibiting PI3K/AKT/MTOR and STAT3 Signaling Pathways. J. Nutr. Biochem. 2017, 41, 124–136. [Google Scholar] [CrossRef]
- Noori-Daloii, M.R.; Momeny, M.; Yousefi, M.; Shirazi, F.G.; Yaseri, M.; Motamed, N.; Kazemialiakbar, N.; Hashemi, S. Multifaceted Preventive Effects of Single Agent Quercetin on a Human Prostate Adenocarcinoma Cell Line (PC-3): Implications for Nutritional Transcriptomics and Multi-Target Therapy. Med. Oncol. 2011, 28, 1395–1404. [Google Scholar] [CrossRef] [PubMed]
- Erdogan, S.; Turkekul, K.; Dibirdik, I.; Doganlar, O.; Doganlar, Z.B.; Bilir, A.; Oktem, G. Midkine Downregulation Increases the Efficacy of Quercetin on Prostate Cancer Stem Cell Survival and Migration through PI3K/AKT and MAPK/ERK Pathway. Biomed. Pharmacother. 2018, 107, 793–805. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekara, A.; Shahidi, F. Herbal Beverages: Bioactive Compounds and Their Role in Disease Risk Reduction-A Review. J. Tradit. Complement. Med. 2018, 8, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Goel, N. Phenolic Acids: Natural Versatile Molecules with Promising Therapeutic Applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar]
- Süntar, I.; Yakıncı, Ö.F. Potential Risks of Phytonutrients Associated with High-Dose or Long-Term Use. In Phytonutrients in Food; Elsevier: Amsterdam, The Netherlands, 2020; pp. 137–155. [Google Scholar]
- Thakur, M.; Singh, K.; Khedkar, R. Phytochemicals: Extraction Process, Safety Assessment, Toxicological Evaluations, and Regulatory Issues. In Functional and Preservative Properties of Phytochemicals; Elsevier: Amsterdam, The Netherlands, 2020; pp. 341–361. [Google Scholar]
- Tsimogiannis, D.; Oreopoulou, V. Classification of Phenolic Compounds in Plants. In Polyphenols in Plants; Elsevier: Amsterdam, The Netherlands, 2019; pp. 263–284. [Google Scholar]
- Haghi, A.; Azimi, H.; Rahimi, R. A Comprehensive Review on Pharmacotherapeutics of Three Phytochemicals, Curcumin, Quercetin, and Allicin, in the Treatment of Gastric Cancer. J. Gastrointest. Cancer 2017, 48, 314–320. [Google Scholar] [CrossRef]
- Weng, W.; Goel, A. Curcumin and Colorectal Cancer: An Update and Current Perspective on This Natural Medicine. In Proceedings of the Seminars in Cancer Biology; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Reyes-Farias, M.; Carrasco-Pozo, C. The Anti-Cancer Effect of Quercetin: Molecular Implications in Cancer Metabolism. Int. J. Mol. Sci. 2019, 20, 3177. [Google Scholar] [CrossRef]
- Vinod, B.S.; Maliekal, T.T.; Anto, R.J. Phytochemicals as Chemosensitizers: From Molecular Mechanism to Clinical Significance. Antioxid. Redox Signal. 2013, 18, 1307–1348. [Google Scholar] [CrossRef]
- Chapman, P.B.; Einhorn, L.H.; Meyers, M.L.; Saxman, S.; Destro, A.N.; Panageas, K.S.; Begg, C.B.; Agarwala, S.S.; Schuchter, L.M.; Ernstoff, M.S. Phase III Multicenter Randomized Trial of the Dartmouth Regimen versus Dacarbazine in Patients with Metastatic Melanoma. J. Clin. Oncol. 1999, 17, 2745. [Google Scholar] [CrossRef]
- Dhillon, N.; Aggarwal, B.B.; Newman, R.A.; Wolff, R.A.; Kunnumakkara, A.B.; Abbruzzese, J.L.; Ng, C.S.; Badmaev, V.; Kurzrock, R. Phase II Trial of Curcumin in Patients with Advanced Pancreatic Cancer. Clin. Cancer Res. 2008, 14, 4491–4499. [Google Scholar] [CrossRef]
- Sung, B.; Kunnumakkara, A.B.; Sethi, G.; Anand, P.; Guha, S.; Aggarwal, B.B. Curcumin Circumvents Chemoresistance in Vitro and Potentiates the Effect of Thalidomide and Bortezomib against Human Multiple Myeloma in Nude Mice Model. Mol. Cancer Ther. 2009, 8, 959–970. [Google Scholar] [CrossRef]
- Cruz–Correa, M.; Shoskes, D.A.; Sanchez, P.; Zhao, R.; Hylind, L.M.; Wexner, S.D.; Giardiello, F.M. Combination Treatment with Curcumin and Quercetin of Adenomas in Familial Adenomatous Polyposis. Clin. Gastroenterol. Hepatol. 2006, 4, 1035–1038. [Google Scholar] [CrossRef]
- He, Z.-Y.; Shi, C.-B.; Wen, H.; Li, F.-L.; Wang, B.-L.; Wang, J. Upregulation of P53 Expression in Patients with Colorectal Cancer by Administration of Curcumin. Cancer Investig. 2011, 29, 208–213. [Google Scholar] [CrossRef]
- Bishayee, A. Cancer Prevention and Treatment with Resveratrol: From Rodent Studies to Clinical TrialsResveratrol and Cancer: In Vivo and Clinical Studies. Cancer Prev. Res. 2009, 2, 409–418. [Google Scholar] [CrossRef]
- Brown, V.A.; Patel, K.R.; Viskaduraki, M.; Crowell, J.A.; Perloff, M.; Booth, T.D.; Vasilinin, G.; Sen, A.; Schinas, A.M.; Piccirilli, G. Repeat Dose Study of the Cancer Chemopreventive Agent Resveratrol in Healthy Volunteers: Safety, Pharmacokinetics, and Effect on the Insulin-like Growth Factor Axis. Cancer Res. 2010, 70, 9003–9011. [Google Scholar] [CrossRef]
- Howells, L.M.; Berry, D.P.; Elliott, P.J.; Jacobson, E.W.; Hoffmann, E.; Hegarty, B.; Brown, K.; Steward, W.P.; Gescher, A.J. Phase I Randomized, Double-Blind Pilot Study of Micronized Resveratrol (SRT501) in Patients with Hepatic Metastases—Safety, Pharmacokinetics, and Pharmacodynamics. Cancer Prev. Res. 2011, 4, 1419–1425. [Google Scholar] [CrossRef]
- Patel, K.R.; Brown, V.A.; Jones, D.J.; Britton, R.G.; Hemingway, D.; Miller, A.S.; West, K.P.; Booth, T.D.; Perloff, M.; Crowell, J.A. Clinical Pharmacology of Resveratrol and Its Metabolites in Colorectal Cancer PatientsResveratrol in Colorectal Cancer Patients. Cancer Res. 2010, 70, 7392–7399. [Google Scholar] [CrossRef]
- Sirerol, J.A.; Rodríguez, M.L.; Mena, S.; Asensi, M.A.; Estrela, J.M.; Ortega, A.L. Role of Natural Stilbenes in the Prevention of Cancer. Oxid. Med. Cell. Longev. 2016, 2016, 3128951. [Google Scholar] [CrossRef]
- Khongsti, K.; Das, K.B.; Das, B. MAPK Pathway and SIRT1 Are Involved in the Down-Regulation of Secreted Osteopontin Expression by Genistein in Metastatic Cancer Cells. Life Sci. 2021, 265, 118787. [Google Scholar] [CrossRef]
- Ono, M.; Takeshima, M.; Nishi, A.; Higuchi, T.; Nakano, S. Genistein Suppresses V-Src-Driven Proliferative Activity by Arresting the Cell-Cycle at G2/M through Increasing P21 Level in Src-Activated Human Gallbladder Carcinoma Cells. Nutr. Cancer 2021, 73, 1471–1479. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, D.; Yang, S.; Wang, Y.; Tang, Z.; Fu, X. Co-Administration of Genistein with Doxorubicin-Loaded Polypeptide Nanoparticles Weakens the Metastasis of Malignant Prostate Cancer by Amplifying Oxidative Damage. Biomater. Sci. 2018, 6, 827–835. [Google Scholar] [CrossRef]
- Abbaszadeh, H.; Keikhaei, B.; Mottaghi, S. A Review of Molecular Mechanisms Involved in Anticancer and Antiangiogenic Effects of Natural Polyphenolic Compounds. Phytother. Res. 2019, 33, 2002–2014. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.-Y.; Qin, L.-Q. Soy Isoflavones Consumption and Risk of Breast Cancer Incidence or Recurrence: A Meta-Analysis of Prospective Studies. Breast Cancer Res. Treat. 2011, 125, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Lazarevic, B.; Boezelijn, G.; Diep, L.M.; Kvernrod, K.; Ogren, O.; Ramberg, H.; Moen, A.; Wessel, N.; Berg, R.E.; Egge-Jacobsen, W. Efficacy and Safety of Short-Term Genistein Intervention in Patients with Localized Prostate Cancer Prior to Radical Prostatectomy: A Randomized, Placebo-Controlled, Double-Blind Phase 2 Clinical Trial. Nutr. Cancer 2011, 63, 889–898. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-Y.; Kim, H.S.; Song, Y.-S. Genistein as a Potential Anticancer Agent against Ovarian Cancer. J. Tradit. Complement. Med. 2012, 2, 96–104. [Google Scholar] [CrossRef]
- Messing, E.; Gee, J.R.; Saltzstein, D.R.; Kim, K.; diSant’Agnese, A.; Kolesar, J.; Harris, L.; Faerber, A.; Havighurst, T.; Young, J.M. A Phase 2 Cancer Chemoprevention Biomarker Trial of Isoflavone G-2535 (Genistein) in Presurgical Bladder Cancer PatientsChemoprevention Trial of Genistein in Bladder Cancer. Cancer Prev. Res. 2012, 5, 621–630. [Google Scholar] [CrossRef]
- Almatroodi, S.A.; Almatroudi, A.; Khan, A.A.; Alhumaydhi, F.A.; Alsahli, M.A.; Rahmani, A.H. Potential Therapeutic Targets of Epigallocatechin Gallate (EGCG), the Most Abundant Catechin in Green Tea, and Its Role in the Therapy of Various Types of Cancer. Molecules 2020, 25, 3146. [Google Scholar] [CrossRef]
- Russo, M.; Spagnuolo, C.; Tedesco, I.; Russo, G.L. Phytochemicals in Cancer Prevention and Therapy: Truth or Dare? Toxins 2010, 2, 517–551. [Google Scholar] [CrossRef]
- McLarty, J.; Bigelow, R.L.; Smith, M.; Elmajian, D.; Ankem, M.; Cardelli, J.A. Tea Polyphenols Decrease Serum Levels of Prostate-Specific Antigen, Hepatocyte Growth Factor, and Vascular Endothelial Growth Factor in Prostate Cancer Patients and Inhibit Production of Hepatocyte Growth Factor and Vascular Endothelial Growth Factor in Vitro. Cancer Prev. Res. 2009, 2, 673–682. [Google Scholar]
- Zhao, H.; Zhu, W.; Jia, L.; Sun, X.; Chen, G.; Zhao, X.; Li, X.; Meng, X.; Kong, L.; Xing, L. Phase I Study of Topical Epigallocatechin-3-Gallate (EGCG) in Patients with Breast Cancer Receiving Adjuvant Radiotherapy. Br. J. Radiol. 2016, 89, 20150665. [Google Scholar] [CrossRef]
- Zhao, H.; Zhu, W.; Xie, P.; Li, H.; Zhang, X.; Sun, X.; Yu, J.; Xing, L. A Phase I Study of Concurrent Chemotherapy and Thoracic Radiotherapy with Oral Epigallocatechin-3-Gallate Protection in Patients with Locally Advanced Stage III Non-Small-Cell Lung Cancer. Radiother. Oncol. 2014, 110, 132–136. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).