Single, Subsequent, or Simultaneous Treatments to Mitigate Mycotoxins in Solid Foods and Feeds: A Critical Review
Abstract
1. Introduction
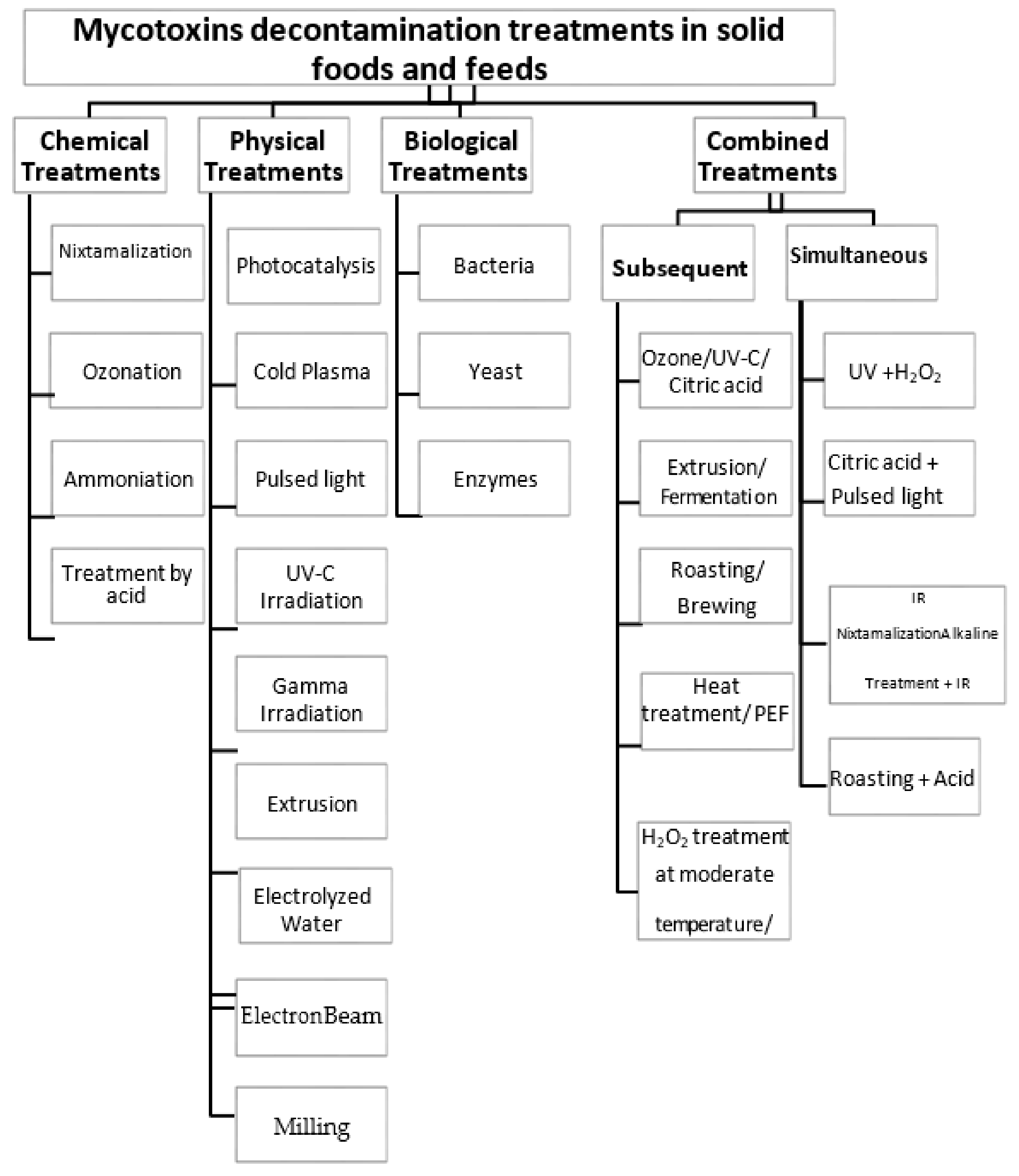
2. Single Detoxification Treatments Used in Solid Foods and Feeds
2.1. Chemical Treatments
2.1.1. Nixtamalization
2.1.2. Ozonation
2.1.3. Ammoniation
2.1.4. Acid
2.2. Physical Treatments
2.2.1. Photocatalytic Treatment
2.2.2. Cold Plasma
2.2.3. Pulsed Light
2.2.4. UV-C Irradiation
2.2.5. Gamma Irradiation
2.2.6. Extrusion
2.2.7. Electrolyzed Oxidizing Water
2.2.8. Electron Beam Irradiation
2.2.9. Milling
2.3. Biological Treatments
| Treatment | Feeds/Foods | Contaminants | Experimental Parameters | Reduction Rates | Advantages | References |
|---|---|---|---|---|---|---|
| Bacteria: ZEN-detoxifying Bacillus (ZDB) strains | Maize | ZEN | The highest level of ZEN degradation | B2 strain-reduction rate = 56% |
| [116] |
| B2 strain detoxifies other mycotoxins | Reduction rates: AFB1: 3.8%; DON: 25%; FB1: 39.5%; T2 toxin: 9.5% |
| ||||
| Bacteria: Bacillus licheniformis spore CotA laccaseapplication of immobilized laccase in contaminated corn meal | Corn meal | ZEN | Treatment with immobilized CotA laccase onto chitosan microspheres for 12-h | Degradation rate: 90% |
| [117] |
| Treatment with free CotA laccase for 12-h | Degradation rate: 70% | |||||
| Reuse of immobilized enzymes for 5 cycles | Decreased degradation rate on each after each cycle: Cycle 1: 90%; Cycle 2: 77%; Cycle 3: 54%; Cycle 4: 30%; Cycle 5: 21% | |||||
| Bacteria—Fermentation: Lactic acid bacteria | Wheat-based products | DON 15 -AcDON AOH D3G, toxins H-2 and HT-2: Enniatin ENNB1 | Pediococcus acidilactici LUHS29 strain | The strongest mycotoxins decontamination effect |
| [118] |
| Prolonged fermentation at 35 °C for 48 h with Pediococcus acidilactici LUHS29 strain | DON: 44–69% 15-AcDON, AOH, D3G, toxins H-2 and HT-2: Removal Enniatin: 5–70% ENNB1: complete removal | |||||
| Combined fermentation (Lactic acid bacteria 7 (JCM 1149) and Pediococcus acidilactici LUHS29 (DSM 20284)) | Complete elimination or effective reduction of DON: 79–100% | |||||
| Enzyme | Maize | FB | FB degradation during dry milling of maize |
| [119] | |
| Fumonisin esterase FumD | Enzyme concentration: 40 U/kg | Reduction rates FBT:
| ||||
| Yeast | Wheat grains and bread | Fusarium Mycotoxins: DON, NIV ZEN | Bread prepared by baking with the addition of an inoculum of the test yeast | Reduction rates: DON: 16.4% to 33.4%; NIV:18.5% to 36.2%; ZEA: 14.3% to 35.4% |
| [120] |
| Yeast | Peanut meal | AFB1 | Peanut samples are heated at 40, 60, 80, 100, or 110 °C for 10 min | [121] | ||
| The residual rates after heat treatment at the following temperature for 10 min: (T:% of residual AFB1 | 80 °C: 61.08%; 100 °C: 63.46%; 110 °C: 49.63% | |||||
| The residual rates after fermentation by Z. rouxii: (Temperature: % of residual AFB1) | (40 °C:32.73%)-(60 °C:20.85%)-(80 °C:16.18%)-(100 °C:5.13%)-(110 °C:5.10%) | |||||
| 100 °C | The optimal temperature achieved the highest reduction rate | |||||
| Peanut samples are heated at 100 °C for 5, 10, 15, or 20 min | ||||||
| The residual rates after heating at 100 °C for different times: (time: % of residual AFB1) | (5 min: 21.06%)-(10 min: 5.13%)-(15 min: 2.48%)-(20 min: 2.44%) | |||||
| 15 min | The optimal time | |||||
| Optimal treatment (100 °C -15 min): | Residual % of AFB1: 2.48% | |||||
3. Subsequent Detoxification Treatments Used in Solid Foods and Feeds
3.1. O3, UV-C, and Citric Acid
3.2. Extrusion and Fermentation
3.3. Roasting and Brewing
3.4. PEF and Thermal Treatment
3.5. H2O2 Treatment at Moderate Temperature after Roasting
4. Simultaneous Detoxification Treatments Used in Solid Foods and Feeds
4.1. UV with H2O2
4.2. Pulsed Light with Citric Acid
4.3. Infrared with Alkaline Treatment
4.4. Roasting with Acid
5. Comparison between the Different Mycotoxin Decontamination Treatments
6. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Medina, A.; Akbar, A.; Baazeem, A.; Rodriguez, A.; Magan, N. Climate Change, Food Security and Mycotoxins: Do We Know Enough? Fungal Biol. Rev. 2017, 31, 143–154. [Google Scholar] [CrossRef]
- Gomez, K.S.; Castañeda Roldán, E.; Ávila Sosa, R.; Munguía-Pérez, R. Mycotoxins and Climate Change. In The Impact of Climate Change on Fungal Diseases; Frías-De-León, M.G., Brunner-Mendoza, C., del Rocío Reyes-Montes, M., Duarte-Escalante, E., Eds.; Fungal Biology Book Series; Springer International Publishing: Cham, Switzerland, 2022; pp. 239–256. ISBN 978-3-030-89664-5. [Google Scholar]
- Matumba, L.; Namaumbo, S.; Ngoma, T.; Meleke, N.; De Boevre, M.; Logrieco, A.F.; De Saeger, S. Five Keys to Prevention and Control of Mycotoxins in Grains: A Proposal. Glob. Food Secur. 2021, 30, 100562. [Google Scholar] [CrossRef]
- Puri, S.; Shingh, S.; Tiwari, P. Mycotoxins: A Threat to Food Security and Health. Int. J. Appl. Sci. Biotechnol. 2019, 7, 298. [Google Scholar] [CrossRef]
- Marc, R.A. Implications of Mycotoxins in Food Safety; IntechOpen: Cluj-Napoca, Romania, 2022; ISBN ISBN 978-1-83962-904-4. [Google Scholar]
- Hassan, H.F.; Koaik, L.; Khoury, A.E.; Atoui, A.; El Obeid, T.; Karam, L. Dietary Exposure and Risk Assessment of Mycotoxins in Thyme and Thyme-Based Products Marketed in Lebanon. Toxins 2022, 14, 331. [Google Scholar] [CrossRef] [PubMed]
- Chilaka, C.A.; Obidiegwu, J.E.; Chilaka, A.C.; Atanda, O.O.; Mally, A. Mycotoxin Regulatory Status in Africa: A Decade of Weak Institutional Efforts. Toxins 2022, 14, 442. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, R.A.; Jebur, A.B.; Kang, W.; El-Demerdash, F.M. An Overview on the Major Mycotoxins in Food Products: Characteristics, Toxicity, and Analysis. J. Future Foods 2022, 2, 91–102. [Google Scholar] [CrossRef]
- Mycotoxins in Lebanese Food Basket—Final.Pdf. 2022. Available online: https://www.usj.edu.lb/intranet/actu/pdf/11610_1952.pdf (accessed on 10 August 2022).
- Pleadin, J.; Frece, J.; Markov, K. Chapter Eight—Mycotoxins in Food and Feed. In Advances in Food and Nutrition Research; Toldrá, F., Ed.; Academic Press: Cambridge, MA, USA, 2019; Volume 89, pp. 297–345. [Google Scholar]
- Xu, R.; Kiarie, E.G.; Yiannikouris, A.; Sun, L.; Karrow, N.A. Nutritional Impact of Mycotoxins in Food Animal Production and Strategies for Mitigation. J. Anim. Sci. Biotechnol. 2022, 13, 69. [Google Scholar] [CrossRef]
- Smith, M.-C.; Madec, S.; Coton, E.; Hymery, N. Natural Co-Occurrence of Mycotoxins in Foods and Feeds and Their in Vitro Combined Toxicological Effects. Toxins 2016, 8, 94. [Google Scholar] [CrossRef]
- Cinar, A.; Onbaşı, E. Mycotoxins: The Hidden Danger in Foods. In Mycotoxins and Food Safety; IntechOpen: London, UK, 2019; ISBN 978-1-78984-874-8. [Google Scholar]
- Jajić, I.; Dudaš, T.; Krstović, S.; Krska, R.; Sulyok, M.; Bagi, F.; Savić, Z.; Guljaš, D.; Stankov, A. Emerging Fusarium Mycotoxins Fusaproliferin, Beauvericin, Enniatins, and Moniliformin in Serbian Maize. Toxins 2019, 11, 357. [Google Scholar] [CrossRef]
- Fapohunda, S.O.; Anjorin, T.S.; Sulyok, M.; Krska, R. Profile of Major and Emerging Mycotoxins in Sesame and Soybean Grains in the Federal Capital Territory, Abuja, Nigeria. Eur. J. Biol. Res. 2018, 8, 121–130. [Google Scholar]
- Mahato, D.K.; Lee, K.E.; Kamle, M.; Devi, S.; Dewangan, K.N.; Kumar, P.; Kang, S.G. Aflatoxins in Food and Feed: An Overview on Prevalence, Detection and Control Strategies. Front. Microbiol. 2019, 10, 2266. [Google Scholar] [CrossRef]
- Yang, Y.; Li, G.; Wu, D.; Liu, J.; Li, X.; Luo, P.; Hu, N.; Wang, H.; Wu, Y. Recent Advances on Toxicity and Determination Methods of Mycotoxins in Foodstuffs. Trends Food Sci. Technol. 2020, 96, 233–252. [Google Scholar] [CrossRef]
- Eskola, M.; Kos, G.; Elliott, C.T.; Hajšlová, J.; Mayar, S.; Krska, R. Worldwide Contamination of Food-Crops with Mycotoxins: Validity of the Widely Cited ‘FAO Estimate’ of 25%. Crit. Rev. Food Sci. Nutr. 2020, 60, 2773–2789. [Google Scholar] [CrossRef]
- Alshannaq, A.; Yu, J.-H. Occurrence, Toxicity, and Analysis of Major Mycotoxins in Food. Int. J. Environ. Res. Public. Health 2017, 14, 632. [Google Scholar] [CrossRef]
- Altomare, C.; Logrieco, A.F.; Gallo, A. Mycotoxins and Mycotoxigenic Fungi: Risk and Management. A Challenge for Future Global Food Safety and Security. In Encyclopedia of Mycology; Zaragoza, Ó., Casadevall, A., Eds.; Elsevier: Oxford, UK, 2021; pp. 64–93. ISBN 978-0-323-85180-0. [Google Scholar]
- Luo, S.; Du, H.; Kebede, H.; Liu, Y.; Xing, F. Contamination Status of Major Mycotoxins in Agricultural Product and Food Stuff in Europe. Food Control 2021, 127, 108120. [Google Scholar] [CrossRef]
- Stroka, J.; Gonçalves, C. Mycotoxins in Food and Feed: An Overview. In Encyclopedia of Food Chemistry; Elsevier: Amsterdam, The Netherlands, 2019; pp. 401–419. ISBN 978-0-12-814045-1. [Google Scholar]
- Awuchi, C.G.; Ondari, E.N.; Ogbonna, C.U.; Upadhyay, A.K.; Baran, K.; Okpala, C.O.R.; Korzeniowska, M.; Guiné, R.P.F. Mycotoxins Affecting Animals, Foods, Humans, and Plants: Types, Occurrence, Toxicities, Action Mechanisms, Prevention, and Detoxification Strategies—A Revisit. Foods 2021, 10, 1279. [Google Scholar] [CrossRef]
- Cheli, F.; Pinotti, L.; Novacco, M.; Ottoboni, M.; Tretola, M.; Dell’Orto, V. Mycotoxins in Wheat and Mitigation Measures. In Wheat Improvement, Management and Utilization; Wanyera, R., Owuoche, J., Eds.; InTech: Milan, Italy, 2017; ISBN 978-953-51-3151-9. [Google Scholar]
- Wan, J.; Chen, B.; Rao, J. Occurrence and Preventive Strategies to Control Mycotoxins in Cereal-based Food. Compr. Rev. Food Sci. Food Saf. 2020, 19, 928–953. [Google Scholar] [CrossRef]
- Conte, G.; Fontanelli, M.; Galli, F.; Cotrozzi, L.; Pagni, L.; Pellegrini, E. Mycotoxins in Feed and Food and the Role of Ozone in Their Detoxification and Degradation: An Update. Toxins 2020, 12, 486. [Google Scholar] [CrossRef]
- Piotrowska, M. Microbiological Decontamination of Mycotoxins: Opportunities and Limitations. Toxins 2021, 13, 819. [Google Scholar] [CrossRef]
- Liu, M.; Zhao, L.; Gong, G.; Zhang, L.; Shi, L.; Dai, J.; Han, Y.; Wu, Y.; Khalil, M.M.; Sun, L. Invited Review: Remediation Strategies for Mycotoxin Control in Feed. J. Anim. Sci. Biotechnol. 2022, 13, 19. [Google Scholar] [CrossRef]
- Gonçalves, B.L.; Uliana, R.D.; Coppa, C.F.S.C.; Lee, S.H.I.; Kamimura, E.S.; Oliveira, C.A.F.; Corassin, C.H. Aflatoxin M1: Biological Decontamination Methods in Milk and Cheese. Food Sci. Technol. 2022, 42, e22920. [Google Scholar] [CrossRef]
- Assaf, J.C.; Atoui, A.; Khoury, A.E.; Chokr, A.; Louka, N. A Comparative Study of Procedures for Binding of Aflatoxin M1 to Lactobacillus Rhamnosus GG. Braz. J. Microbiol. Publ. Braz. Soc. Microbiol. 2018, 49, 120–127. [Google Scholar] [CrossRef]
- Ragoubi, C.; Quintieri, L.; Greco, D.; Mehrez, A.; Maatouk, I.; D’Ascanio, V.; Landoulsi, A.; Avantaggiato, G. Mycotoxin Removal by Lactobacillus Spp. and Their Application in Animal Liquid Feed. Toxins 2021, 13, 185. [Google Scholar] [CrossRef] [PubMed]
- Assaf, J.C.; Khoury, A.E.; Chokr, A.; Louka, N.; Atoui, A. A Novel Method for Elimination of Aflatoxin M1 in Milk Using Lactobacillus Rhamnosus GG Biofilm. Int. J. Dairy Technol. 2019, 72, 248–256. [Google Scholar] [CrossRef]
- Assaf, J.C.; El Khoury, A.; Atoui, A.; Louka, N.; Chokr, A. A Novel Technique for Aflatoxin M1 Detoxification Using Chitin or Treated Shrimp Shells: In Vitro Effect of Physical and Kinetic Parameters on the Binding Stability. Appl. Microbiol. Biotechnol. 2018, 102, 6687–6697. [Google Scholar] [CrossRef] [PubMed]
- Assaf, J.C.; Nahle, S.; Chokr, A.; Louka, N.; Atoui, A.; El Khoury, A. Assorted Methods for Decontamination of Aflatoxin M1 in Milk Using Microbial Adsorbents. Toxins 2019, 11, 304. [Google Scholar] [CrossRef] [PubMed]
- Pallarés, N.; Berrada, H.; Tolosa, J.; Ferrer, E. Effect of High Hydrostatic Pressure (HPP) and Pulsed Electric Field (PEF) Technologies on Reduction of Aflatoxins in Fruit Juices. LWT 2021, 142, 111000. [Google Scholar] [CrossRef]
- Nan, M.-N.; Bi, Y.; Qiang, Y.; Xue, H.-L.; Yang, L.; Feng, L.-D.; Pu, L.-M.; Long, H.-T.; Prusky, D. Electrostatic Adsorption and Removal Mechanism of Ochratoxin A in Wine via a Positively Charged Nano-MgO Microporous Ceramic Membrane. Food Chem. 2022, 371, 131157. [Google Scholar] [CrossRef]
- Borràs-Vallverdú, B.; Ramos, A.J.; Marín, S.; Sanchis, V.; Rodríguez-Bencomo, J.J. Deoxynivalenol Degradation in Wheat Kernels by Exposition to Ammonia Vapours: A Tentative Strategy for Detoxification. Food Control 2020, 118, 107444. [Google Scholar] [CrossRef]
- Abdel-Aal, E.-S.M.; Miah, K. Kinetics of Deoxynivalenol Flux in Wheat Kernels Steeped in Different Solutions for Improved Food Safety. Food Control 2022, 133, 108606. [Google Scholar] [CrossRef]
- Wu, N.; Ou, W.; Zhang, Z.; Wang, Y.; Xu, Q.; Huang, H. Recent advances in detoxification strategies for zearalenone contamination in food and feed. Chin. J. Chem. Eng. 2021, 29, 168–177. [Google Scholar] [CrossRef]
- Gilbert Sandoval, I.; Wesseling, S.; Rietjens, I.M.C.M. Aflatoxin B1 in Nixtamalized Maize in Mexico; Occurrence and Accompanying Risk Assessment. Toxicol. Rep. 2019, 6, 1135–1142. [Google Scholar] [CrossRef]
- Méndez-Albores, A.; Arámbula-Villa, G.; Loarca-Piña, M.G.F.; Castaño-Tostado, E.; Moreno-Martínez, E. Safety and Efficacy Evaluation of Aqueous Citric Acid to Degrade B-Aflatoxins in Maize. Food Chem. Toxicol. 2005, 43, 233–238. [Google Scholar] [CrossRef]
- Mallakian, S.; Rezanezhad, R.; Jalali, M.; Ghobadi, F. The Effect of Ozone Gas on Destruction and Detoxification of Aflatoxin. Bull. Société R. Sci. Liège 2017, 86, 1–6. [Google Scholar] [CrossRef]
- Park, D.; Price, W. Reduction of Aflatoxin Hazards Using Ammoniation. Rev. Environ. Contam. Toxicol. 2001, 171, 139–175. [Google Scholar] [CrossRef]
- Ouf, S.A.; Ali, E.M. Does the Treatment of Dried Herbs with Ozone as a Fungal Decontaminating Agent Affect the Active Constituents? Pollut. 2021, 277, 116715. [Google Scholar] [CrossRef]
- Da Luz, S.R.; Almeida Villanova, F.; Tuchtenhagen Rockembach, C.; Dietrich Ferreira, C.; José Dallagnol, L.; Luis Fernandes Monks, J.; de Oliveira, M. Reduced of Mycotoxin Levels in Parboiled Rice by Using Ozone and Its Effects on Technological and Chemical Properties. Food Chem. 2022, 372, 131174. [Google Scholar] [CrossRef]
- Young, J.C.; Zhu, H.; Zhou, T. Degradation of Trichothecene Mycotoxins by Aqueous Ozone. Food Chem. Toxicol. 2006, 44, 417–424. [Google Scholar] [CrossRef]
- Li, M.M.; Guan, E.Q.; Bian, K. Effect of Ozone Treatment on Deoxynivalenol and Quality Evaluation of Ozonised Wheat. Food Addit. Contam. Part A 2015, 32, 544–553. [Google Scholar] [CrossRef]
- McDonough, M.X.; Campabadal, C.A.; Mason, L.J.; Maier, D.E.; Denvir, A.; Woloshuk, C. Ozone Application in a Modified Screw Conveyor to Treat Grain for Insect Pests, Fungal Contaminants, and Mycotoxins. J. Stored Prod. Res. 2011, 47, 249–254. [Google Scholar] [CrossRef]
- Savi, G.D.; Gomes, T.; Canever, S.B.; Feltrin, A.C.; Piacentini, K.C.; Scussel, R.; Oliveira, D.; Machado-de-Ávila, R.A.; Cargnin, M.; Angioletto, E. Application of Ozone on Rice Storage: A Mathematical Modeling of the Ozone Spread, Effects in the Decontamination of Filamentous Fungi and Quality Attributes. J. Stored Prod. Res. 2020, 87, 101605. [Google Scholar] [CrossRef]
- Maureen, N.; Kaaya, A.N.; Kauffman, J.; Narrod, C.; Atukwase, A. Enhancing Nutritional Benefits and Reducing Mycotoxin Contamination of Maize through Nixtamalization. J. Biol. Sci. 2020, 20, 153–162. [Google Scholar] [CrossRef]
- Rodríguez-Aguilar, M.; Solís-Mercado, J.; Flores-Ramírez, R.; Díaz-Barriga, F.; Zuki-Orozco, A.; Cilia-López, V.G. Aflatoxins and the Traditional Process of Nixtamalisation in Indigenous Communities from the Huasteca Potosina Region. World Mycotoxin J. 2020, 13, 391–399. [Google Scholar] [CrossRef]
- Moreno-Pedraza, A.; Valdés-Santiago, L.; Hernández-Valadez, L.J.; Rodríguez-Sixtos Higuera, A.; Winkler, R.; Guzmán-de Peña, D.L. Reduction of Aflatoxin B1 during Tortilla Production and Identification of Degradation By-Products by Direct-Injection Electrospray Mass Spectrometry (DIESI-MS). Salud Publica Mex. 2015, 57, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Odukoya, J.O.; De Saeger, S.; De Boevre, M.; Adegoke, G.O.; Audenaert, K.; Croubels, S.; Antonissen, G.; Vermeulen, K.; Gbashi, S.; Njobeh, P.B. Effect of Selected Cooking Ingredients for Nixtamalization on the Reduction of Fusarium Mycotoxins in Maize and Sorghum. Toxins 2021, 13, 27. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Contaminants in the Food Chain (CONTAM); Schrenk, D.; Bignami, M.; Bodin, L.; Chipman, J.K.; del Mazo, J.; Grasl-Kraupp, B.; Hoogenboom, L.; Leblanc, J.; Nebbia, C.S.; et al. Assessment of an Application on a Detoxification Process of Groundnut Press Cake for Aflatoxins by Ammoniation. EFSA J. 2021, 19, e07035. [Google Scholar] [CrossRef] [PubMed]
- Sumner, P.; Hammond, C. Treating Aflatoxin-Contaminated Corn with Ammonia; University of Georgia: Athens, GA, USA, 2009. [Google Scholar]
- Nyandieka, H.S.; Maina, J.O.; Nyamwange, C. Detoxification of Aflatoxin in Artificially Contaminated Maize Crop by Ammoniation Procedures. Discov. Innov. 2009, 21, 77. [Google Scholar] [CrossRef]
- Jubeen, F.; Sher, F.; Hazafa, A.; Zafar, F.; Ameen, M.; Rasheed, T. Evaluation and Detoxification of Aflatoxins in Ground and Tree Nuts Using Food Grade Organic Acids. Biocatal. Agric. Biotechnol. 2020, 29, 101749. [Google Scholar] [CrossRef]
- Humer, E.; Lucke, A.; Harder, H.; Metzler-Zebeli, B.; Böhm, J.; Zebeli, Q. Effects of Citric and Lactic Acid on the Reduction of Deoxynivalenol and Its Derivatives in Feeds. Toxins 2016, 8, 285. [Google Scholar] [CrossRef]
- Rushing, B.R.; Selim, M.I. Effect of Dietary Acids on the Formation of Aflatoxin B 2a as a Means to Detoxify Aflatoxin B 1. Food Addit. Contam. Part A 2016, 33, 1456–1467. [Google Scholar] [CrossRef]
- Schaarschmidt, S.; Fauhl-Hassek, C. Mycotoxins during the Processes of Nixtamalization and Tortilla Production. Toxins 2019, 11, 227. [Google Scholar] [CrossRef]
- Anguiano-Ruvalcaba, G.L.; Vargas-Cortina, A.V.y.; Peña, D.G.-D. Inactivation of aflatoxin B1 and aflatoxicol through traditional “nixtamalización” of corn and their regeneration by acidification of corn dough. Salud Pública México 2005, 47, 369–375. [Google Scholar] [CrossRef]
- Guo, Y.; Zhao, L.; Ma, Q.; Ji, C. Novel Strategies for Degradation of Aflatoxins in Food and Feed: A Review. Food Res. Int. 2021, 140, 109878. [Google Scholar] [CrossRef]
- Nunes, V.M.; Moosavi, M.; Mousavi Khaneghah, A.; Oliveira, C.A. Innovative Modifications in Food Processing to Reduce the Levels of Mycotoxins. Curr. Opin. Food Sci. 2021, 38, 155–161. [Google Scholar] [CrossRef]
- Sipos, P.; Peles, F.; Brassó, D.L.; Béri, B.; Pusztahelyi, T.; Pócsi, I.; Győri, Z. Physical and Chemical Methods for Reduction in Aflatoxin Content of Feed and Food. Toxins 2021, 13, 204. [Google Scholar] [CrossRef]
- Cabrera-Meraz, J.; Maldonado, L.; Bianchini, A.; Espinal, R. Incidence of Aflatoxins and Fumonisins in Grain, Masa and Corn Tortillas in Four Municipalities in the Department of Lempira, Honduras. Heliyon 2021, 7, e08506. [Google Scholar] [CrossRef]
- Murugesan, P.; Brunda, D.K.; Moses, J.A.; Anandharamakrishnan, C. Photolytic and Photocatalytic Detoxification of Mycotoxins in Foods. Food Control 2021, 123, 107748. [Google Scholar] [CrossRef]
- Hoffmans, Y.; Schaarschmidt, S.; Fauhl-Hassek, C.; Van der Fels-Klerx, H. (Ine) Factors during Production of Cereal-Derived Feed That Influence Mycotoxin Contents. Toxins 2022, 14, 301. [Google Scholar] [CrossRef]
- Karlovsky, P.; Suman, M.; Berthiller, F.; De Meester, J.; Eisenbrand, G.; Perrin, I.; Oswald, I.P.; Speijers, G.; Chiodini, A.; Recker, T.; et al. Impact of Food Processing and Detoxification Treatments on Mycotoxin Contamination. Mycotoxin Res. 2016, 32, 179–205. [Google Scholar] [CrossRef]
- Wu, S.; Wang, F.; Li, Q.; Wang, J.; Zhou, Y.; Duan, N.; Niazi, S.; Wang, Z. Photocatalysis and Degradation Products Identification of Deoxynivalenol in Wheat Using Upconversion Nanoparticles@TiO2 Composite. Food Chem. 2020, 323, 126823. [Google Scholar] [CrossRef]
- Ott, L.C.; Appleton, H.J.; Shi, H.; Keener, K.; Mellata, M. High Voltage Atmospheric Cold Plasma Treatment Inactivates Aspergillus Flavus Spores and Deoxynivalenol Toxin. Food Microbiol. 2021, 95, 103669. [Google Scholar] [CrossRef]
- Marshall, H.; Meneely, J.P.; Quinn, B.; Zhao, Y.; Bourke, P.; Gilmore, B.F.; Zhang, G.; Elliott, C.T. Novel Decontamination Approaches and Their Potential Application for Post-Harvest Aflatoxin Control. Trends Food Sci. Technol. 2020, 106, 489–496. [Google Scholar] [CrossRef]
- Gonçalves Lemos, J.; Stefanello, A.; Olivier Bernardi, A.; Valle Garcia, M.; Nicoloso Magrini, L.; Cichoski, A.J.; Wagner, R.; Venturini Copetti, M. Antifungal Efficacy of Sanitizers and Electrolyzed Waters against Toxigenic Aspergillus. Food Res. Int. 2020, 137, 109451. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, C.D.; Lang, G.H.; da SIlva Lindemann, I.; da Silva, N.T.; Hoffmann, J.F.; Ziegler, V.; de Oliveira, M. Postharvest UV-C Irradiation for Fungal Control and Reduction of Mycotoxins in Brown, Black, and Red Rice during Long-Term Storage. Food Chem. 2021, 339, 127810. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Santaescolástica, C.; Fraeye, I.; Barba, F.J.; Gómez, B.; Tomasevic, I.; Romero, A.; Moreno, A.; Toldrá, F.; Lorenzo, J.M. Application of Non-Invasive Technologies in Dry-Cured Ham: An Overview. Trends Food Sci. Technol. 2019, 86, 360–374. [Google Scholar] [CrossRef]
- Liu, Y.; Joseph Hubert, G.; Gong, Y.Y.; Orfila, C. A Review of Post-Harvest Approaches to Reduce Fungal and Mycotoxin Contamination of Foods. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1521–1560. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Wang, F.; Li, Q.; Zhou, Y.; He, C.; Duan, N. Detoxification of DON by Photocatalytic Degradation and Quality Evaluation of Wheat. RSC Adv. 2019, 9, 34351–34358. [Google Scholar] [CrossRef]
- Hojnik, N.; Modic, M.; Walsh, J.L.; Žigon, D.; Javornik, U.; Plavec, J.; Žegura, B.; Filipič, M.; Cvelbar, U. Unravelling the Pathways of Air Plasma Induced Aflatoxin B1 Degradation and Detoxification. J. Hazard. Mater. 2021, 403, 123593. [Google Scholar] [CrossRef]
- Kiš, M.; Milošević, S.; Vulić, A.; Herceg, Z.; Vukušić, T.; Pleadin, J. Efficacy of Low Pressure DBD Plasma in the Reduction of T-2 and HT-2 Toxin in Oat Flour. Food Chem. 2020, 316, 126372. [Google Scholar] [CrossRef]
- Wielogorska, E.; Ahmed, Y.; Meneely, J.; Graham, W.G.; Elliott, C.T.; Gilmore, B.F. A Holistic Study to Understand the Detoxification of Mycotoxins in Maize and Impact on Its Molecular Integrity Using Cold Atmospheric Plasma Treatment. Food Chem. 2019, 301, 125281. [Google Scholar] [CrossRef]
- Casas-Junco, P.P.; Solís-Pacheco, J.R.; Ragazzo-Sánchez, J.A.; Aguilar-Uscanga, B.R.; Bautista-Rosales, P.U.; Calderón-Santoyo, M. Cold Plasma Treatment as an Alternative for Ochratoxin A Detoxification and Inhibition of Mycotoxigenic Fungi in Roasted Coffee. Toxins 2019, 11, 337. [Google Scholar] [CrossRef]
- Woldemariam, H.W.; Harmeling, H.; Emire, S.; Teshome, P.G.; Toepfl, S.; Aganovic, K. Pulsed Light Treatment Reduces Microorganisms and Mycotoxins Naturally Present in Red Pepper (Capsicum annuum L.) Powder. J. Food Process Eng. 2021, 45, e13948. [Google Scholar] [CrossRef]
- Wang, B.; Mahoney, N.E.; Khir, R.; Wu, B.; Zhou, C.; Pan, Z.; Ma, H. Degradation Kinetics of Aflatoxin B 1 and B 2 in Solid Medium by Using Pulsed Light Irradiation: Degradation Kinetics of Aflatoxins in Solid Medium Using Pulsed Light Irradiation. J. Sci. Food Agric. 2018, 98, 5220–5224. [Google Scholar] [CrossRef]
- Wang, B.; Mahoney, N.E.; Pan, Z.; Khir, R.; Wu, B.; Ma, H.; Zhao, L. Effectiveness of Pulsed Light Treatment for Degradation and Detoxification of Aflatoxin B1 and B2 in Rough Rice and Rice Bran. Food Control 2016, 59, 461–467. [Google Scholar] [CrossRef]
- Udovicki, B.; Stankovic, S.; Tomic, N.; Djekic, I.; Smigic, N.; Trifunovic, B.S.; Milicevic, D.; Rajkovic, A. Evaluation of Ultraviolet Irradiation Effects on Aspergillus Flavus and Aflatoxin B1 in Maize and Peanut Using Innovative Vibrating Decontamination Equipment. Food Control 2022, 134, 108691. [Google Scholar] [CrossRef]
- Shen, M.-H.; Singh, R.K. Effect of Rotating Peanuts on Aflatoxin Detoxification by Ultraviolet C Light and Irradiation Uniformity Evaluated by AgCl-Based Dosimeter. Food Control 2021, 120, 107533. [Google Scholar] [CrossRef]
- Khalil, O.A.A.; Hammad, A.A.; Sebaei, A.S. Aspergillus Flavus and Aspergillus Ochraceus Inhibition and Reduction of Aflatoxins and Ochratoxin A in Maize by Irradiation. Toxicon 2021, 198, 111–120. [Google Scholar] [CrossRef]
- Calado, T.; Fernández-Cruz, M.L.; Cabo Verde, S.; Venâncio, A.; Abrunhosa, L. Gamma Irradiation Effects on Ochratoxin A: Degradation, Cytotoxicity and Application in Food. Food Chem. 2018, 240, 463–471. [Google Scholar] [CrossRef]
- Ben Amara, A.; Mehrez, A.; Ragoubi, C.; Romero-González, R.; Garrido Frenich, A.; Landoulsi, A.; Maatouk, I. Fungal Mycotoxins Reduction by Gamma Irradiation in Naturally Contaminated Sorghum. J. Food Process. Preserv. 2022, 46, e16345. [Google Scholar] [CrossRef]
- Janić Hajnal, E.; Babic, J.; Pezo, L.; Banjac, V.; Colovic, R.; Kos, J.; Krulj, J.; Vrtač, K.; Jakovac-Strajn, B. Effects of Extrusion Process on Fusarium and Alternaria Mycotoxins in Whole Grain Triticale Flour. LWT 2021, 155, 112926. [Google Scholar] [CrossRef]
- Massarolo, K.C.; Mendoza, J.R.; Verma, T.; Kupski, L.; Badiale-Furlong, E.; Bianchini, A. Fate of Aflatoxins in Cornmeal during Single-Screw Extrusion: A Bioaccessibility Approach. LWT 2021, 138, 110734. [Google Scholar] [CrossRef]
- Lyu, F.; Gao, F.; Zhou, X.; Zhang, J.; Ding, Y. Using Acid and Alkaline Electrolyzed Water to Reduce Deoxynivalenol and Mycological Contaminations in Wheat Grains. Food Control 2018, 88, 98–104. [Google Scholar] [CrossRef]
- Woldemariam, H.W.; Kießling, M.; Emire, S.A.; Teshome, P.G.; Töpfl, S.; Aganovic, K. Influence of Electron Beam Treatment on Naturally Contaminated Red Pepper (Capsicum annuum L.) Powder: Kinetics of Microbial Inactivation and Physicochemical Quality Changes. Innov. Food Sci. Emerg. Technol. 2021, 67, 102588. [Google Scholar] [CrossRef]
- Scarpino, V.; Vanara, F.; Sulyok, M.; Krska, R.; Blandino, M. Fate of Regulated, Masked, Emerging Mycotoxins and Secondary Fungal Metabolites during Different Large-Scale Maize Dry-Milling Processes. Food Res. Int. 2021, 140, 109861. [Google Scholar] [CrossRef]
- Vanara, F.; Scarpino, V.; Blandino, M. Fumonisin Distribution in Maize Dry-Milling Products and By-Products: Impact of Two Industrial Degermination Systems. Toxins 2018, 10, 357. [Google Scholar] [CrossRef]
- Sun, S.; Zhao, R.; Xie, Y.; Liu, Y. Photocatalytic Degradation of Aflatoxin B1 by Activated Carbon Supported TiO2 Catalyst. Food Control 2019, 100, 183–188. [Google Scholar] [CrossRef]
- Wu, Y.; Cheng, J.-H.; Sun, D.-W. Blocking and Degradation of Aflatoxins by Cold Plasma Treatments: Applications and Mechanisms. Trends Food Sci. Technol. 2021, 109, 647–661. [Google Scholar] [CrossRef]
- Gómez-López, V.; Noguera-Artiaga, L.; Figueroa, F.; Girón, F.; Carbonell-Barrachina, A.; Gabaldon, J.; Perez-Lopez, A. Effect of Pulsed Light on Quality of Shelled Walnuts. Foods 2022, 11, 1186. [Google Scholar] [CrossRef]
- Bhavya, M.L.; Umesh Hebbar, H. Pulsed Light Processing of Foods for Microbial Safety. Food Qual. Saf. 2017, 1, 187–202. [Google Scholar] [CrossRef]
- Deng, L.-Z.; Tao, Y.; Mujumdar, A.S.; Pan, Z.; Chen, C.; Yang, X.-H.; Liu, Z.-L.; Wang, H.; Xiao, H.-W. Recent Advances in Non-Thermal Decontamination Technologies for Microorganisms and Mycotoxins in Low-Moisture Foods. Trends Food Sci. Technol. 2020, 106, 104–112. [Google Scholar] [CrossRef]
- Shen, M.; Singh, R. Detoxification of Aflatoxins in Foods by Ultraviolet Irradiation, Hydrogen Peroxide, and Their Combination—A Review. LWT 2021, 142, 110986. [Google Scholar] [CrossRef]
- Udomkun, P.; Wiredu, A.N.; Nagle, M.; Müller, J.; Vanlauwe, B.; Bandyopadhyay, R. Innovative Technologies to Manage Aflatoxins in Foods and Feeds and the Profitability of Application—A Review. Food Control 2017, 76, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, N.; Yusop, S.M.; Rahman, I.A.; Dauqan, E.; Abdullah, A. Efficacy of Gamma Irradiation in Improving the Microbial and Physical Quality Properties of Dried Chillies (Capsicum Annuum L.): A Review. Foods 2022, 11, 97. [Google Scholar] [CrossRef]
- Awuchi, C.; Nyakundi Ondari, E.; Ofoedu, C.; Chacha, J.; Rasaq, W.; Morya, S.; Okpala, C. Grain Processing Methods’ Effectiveness to Eliminate Mycotoxins: An Overview. Asian J. Chem. 2021, 33, 2267–2275. [Google Scholar] [CrossRef]
- Villarreal-Barajas, T.; Vázquez-Durán, A.; Méndez-Albores, A. Effectiveness of Electrolyzed Oxidizing Water on Fungi and Mycotoxins in Food. Food Control 2022, 131, 108454. [Google Scholar] [CrossRef]
- Rebezov, M.; Saeed, K.; Khaliq, A.; Rahman, S.J.U.; Sameed, N.; Semenova, A.; Khayrullin, M.; Dydykin, A.; Abramov, Y.; Thiruvengadam, M.; et al. Application of Electrolyzed Water in the Food Industry: A Review. Appl. Sci. 2022, 12, 6639. [Google Scholar] [CrossRef]
- Mousavi Khaneghah, A.; Hashemi Moosavi, M.; Oliveira, C.A.F.; Vanin, F.; Sant’Ana, A.S. Electron Beam Irradiation to Reduce the Mycotoxin and Microbial Contaminations of Cereal-Based Products: An Overview. Food Chem. Toxicol. 2020, 143, 111557. [Google Scholar] [CrossRef]
- Mohammadi, X.; Matinfar, G.; Khaneghah, A.M.; Singh, A.; Pratap-Singh, A. Emergence of Cold Plasma and Electron Beam Irradiation as Novel Technologies to Counter Mycotoxins in Food Products. World Mycotoxin J. 2021, 14, 75–83. [Google Scholar] [CrossRef]
- Kim, G.-R.; Ramakrishnan, S.R.; Ameer, K.; Chung, N.; Kim, Y.-R.; Kwon, J.-H. Irradiation Effects on Chemical and Functional Qualities of Ready-to-Eat Saengshik, a Cereal Health Food. Radiat. Phys. Chem. 2020, 171, 108692. [Google Scholar] [CrossRef]
- Milani, J.; Maleki, G. Effects of Processing on Mycotoxin Stability in Cereals. J. Sci. Food Agric. 2014, 94, 2372–2375. [Google Scholar] [CrossRef]
- Reis, T.A.; Oliveira, T.D.; Zorzete, P.; Faria, P.; Corrêa, B. A Non-Toxigenic Aspergillus Flavus Strain Prevents the Spreading of Fusarium Verticillioides and Fumonisins in Maize. Toxicon 2020, 181, 6–8. [Google Scholar] [CrossRef]
- Molo, M.S.; Heiniger, R.W.; Boerema, L.; Carbone, I. Trial Summary on the Comparison of Various Non-Aflatoxigenic Strains of Aspergillus Flavus on Mycotoxin Levels and Yield in Maize. Agron. J. 2019, 111, 942–946. [Google Scholar] [CrossRef]
- Du, G.; Liu, L.; Guo, Q.; Cui, Y.; Chen, H.; Yuan, Y.; Wang, Z.; Gao, Z.; Sheng, Q.; Yue, T. Microbial Community Diversity Associated with Tibetan Kefir Grains and Its Detoxification of Ochratoxin A during Fermentation. Food Microbiol. 2021, 99, 103803. [Google Scholar] [CrossRef]
- Ul Hassan, Z.; Al Thani, R.; Atia, F.A.; Alsafran, M.; Migheli, Q.; Jaoua, S. Application of Yeasts and Yeast Derivatives for the Biological Control of Toxigenic Fungi and Their Toxic Metabolites. Environ. Technol. Innov. 2021, 22, 101447. [Google Scholar] [CrossRef]
- Li, X.; Tang, H.; Yang, C.; Meng, X.; Liu, B. Detoxification of Mycotoxin Patulin by the Yeast Rhodotorula Mucilaginosa. Food Control 2019, 96, 47–52. [Google Scholar] [CrossRef]
- Nahle, S.; El Khoury, A.; Savvaidis, I.; Chokr, A.; Louka, N.; Atoui, A. Detoxification Approaches of Mycotoxins: By Microorganisms, Biofilms and Enzymes. Int. J. Food Contam. 2022, 9, 3. [Google Scholar] [CrossRef]
- Chen, S.-W.; Wang, H.-T.; Shih, W.-Y.; Ciou, Y.-A.; Chang, Y.-Y.; Ananda, L.; Wang, S.-Y.; Hsu, J.-T. Application of Zearalenone (ZEN)-Detoxifying Bacillus in Animal Feed Decontamination through Fermentation. Toxins 2019, 11, 330. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, Y.; Liu, Y.; Ma, Q.; Ji, C.; Zhao, L. Detoxification of the Mycoestrogen Zearalenone by Bacillus Licheniformis Spore CotA Laccase and Application of Immobilized Laccase in Contaminated Corn Meal. LWT 2022, 163, 113548. [Google Scholar] [CrossRef]
- Zadeike, D.; Vaitkeviciene, R.; Bartkevics, V.; Bogdanova, E.; Bartkiene, E.; Lele, V.; Juodeikiene, G.; Cernauskas, D.; Valatkeviciene, Z. The Expedient Application of Microbial Fermentation after Whole-Wheat Milling and Fractionation to Mitigate Mycotoxins in Wheat-Based Products. LWT 2021, 137, 110440. [Google Scholar] [CrossRef]
- Alberts, J.F.; Davids, I.; Moll, W.-D.; Schatzmayr, G.; Burger, H.-M.; Shephard, G.S.; Gelderblom, W.C.A. Enzymatic Detoxification of the Fumonisin Mycotoxins during Dry Milling of Maize. Food Control 2021, 123, 107726. [Google Scholar] [CrossRef]
- Podgórska-Kryszczuk, I.; Solarska, E.; Kordowska-Wiater, M. Reduction of the Fusarium Mycotoxins: Deoxynivalenol, Nivalenol and Zearalenone by Selected Non-Conventional Yeast Strains in Wheat Grains and Bread. Molecules 2022, 27, 1578. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Chen, Y.; Kong, Q.; Ma, Y.; Liu, Y. Detoxification of Aflatoxin B1 by Zygosaccharomyces Rouxii with Solid State Fermentation in Peanut Meal. Toxins 2017, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Gavahian, M.; Mathad, G.N.; Oliveira, C.A.F.; Mousavi Khaneghah, A. Combinations of Emerging Technologies with Fermentation: Interaction Effects for Detoxification of Mycotoxins? Food Res. Int. 2021, 141, 110104. [Google Scholar] [CrossRef] [PubMed]
- Babaee, R.; Karami-Osboo, R.; Mirabolfathy, M. Evaluation of the Use of Ozone, UV-C and Citric Acid in Reducing Aflatoxins in Pistachio Nut. J. Food Compos. Anal. 2022, 106, 104276. [Google Scholar] [CrossRef]
- Zokaityte, E.; Lele, V.; Starkute, V.; Zavistanaviciute, P.; Klupsaite, D.; Bartkevics, V.; Pugajeva, I.; Bērziņa, Z.; Gruzauskas, R.; Sidlauskiene, S.; et al. The Influence of Combined Extrusion and Fermentation Processes on the Chemical and Biosafety Parameters of Wheat Bran. LWT 2021, 146, 111498. [Google Scholar] [CrossRef]
- Al Attiya, W.; Hassan, Z.U.; Al-Thani, R.; Jaoua, S. Prevalence of Toxigenic Fungi and Mycotoxins in Arabic Coffee (Coffea Arabica): Protective Role of Traditional Coffee Roasting, Brewing and Bacterial Volatiles. PLoS ONE 2021, 16, e0259302. [Google Scholar] [CrossRef]
- Subramanian, V.; Shanmugam, N.; Ranganathan, K.; Kumar, S.; Reddy, R. Effect of Combination Processing on Aflatoxin Reduction: Process Optimization by Response Surface Methodology. J. Food Process. Preserv. 2017, 41, e13230. [Google Scholar] [CrossRef]
- Shen, M.-H.; Singh, R.K. Detoxifying Aflatoxin Contaminated Peanuts by High Concentration of H2O2 at Moderate Temperature and Catalase Inactivation. Food Control 2022, 142, 109218. [Google Scholar] [CrossRef]
- Shen, M.-H.; Singh, R.K. Decomposing Aflatoxins in Peanuts Using Advanced Oxidation Processes by UV and H2O2. Food Bioprocess Technol. 2022, 15, 1647–1657. [Google Scholar] [CrossRef]
- Abuagela, M.; Marshall, M.; Yagiz, Y.; Mostafa, H.; Iqdiam, B. Combined Effects of Citric Acid and Pulsed Light Treatments to Degrade B-Aflatoxins in Peanut. Food Bioprod. Process. 2019, 117, 396–403. [Google Scholar] [CrossRef]
- Zavala-Franco, A.; Arámbula-Villa, G.; Ramírez-Noguera, P.; Salazar, A.M.; Sordo, M.; Marroquín-Cardona, A.; de Dios Figueroa-Cárdenas, J.; Méndez-Albores, A. Aflatoxin Detoxification in Tortillas Using an Infrared Radiation Thermo-Alkaline Process: Cytotoxic and Genotoxic Evaluation. Food Control 2020, 112, 107084. [Google Scholar] [CrossRef]
- Rastegar, H.; Shoeibi, S.; Yazdanpanah, H.; Amirahmadi, M.; Khaneghah, A.M.; Campagnollo, F.B.; Anderson, S.S. Removal of Aflatoxin B1 by Roasting with Lemon Juice and/or Citric Acid in Contaminated Pistachio Nuts. Food Control 2017, 71, 279–284. [Google Scholar] [CrossRef]
| Technique | Feeds/Foods | Contaminants | Experimental Parameters | Reduction Rate | Advantages/Disadvantages | References |
|---|---|---|---|---|---|---|
| Ozonation | Powdered sun-dried herbs and spices | AFs | Ozone concentration = 3 ppm/time 210 min | Highest level of aflatoxin reduction: 93.75% for licorice 90% for peppermint | Advantages: Fumigation with Ozone: 3 ppm/time: 280 min— Sanitation and reduction of microbial load; Active against a wide range of microorganisms, viruses, Gram-negative and Gram-positive bacteria, spores, and fungi; Instability of Ozone—transformation into O2-O3 has a Gras status; The major biologically active constituent attributed to the medical properties of the chamomile flower was increased. Disadvantages: Reduction of chamomile essential oil by 57.14% and peppermint by 26.67%. | [44] |
| Ozonation | Parboiled Rice | Mycotoxins | Parboiled rice grains treated with ozone | Significant reduction of mycotoxins contamination, regardless of the time and period of application and the mycotoxin evaluated | Advantages: After soaking samples in ozone for 3 and 5 h: Higher head rice yield, luminosity and hardness, decreased cooking time, percentage of defective grains, and soluble protein. | [45] |
| Ozonation | Aqueous medium | TrichotheceneMycotoxins (TC) | Saturated aqueous ozone (≈25 ppm) | Degradation of TC mycotoxins to materials that were not detected by UV or MS | Disadvantages: Ozone is a toxic gas, so all preparations were conducted in a fume hood. | [46] |
| At lower levels (≈0.25 ppm) of aqueous ozone | Intermediate products were observed | |||||
| Ozonation was sensitive to pH. | ||||||
| pH 4 to 6 | Maximum reduction rates | |||||
| pH 9 | No reaction | |||||
| Ozonation | Wheat | DON | ↓ initial concentrations of DON solution treated with ↑ concentrations of ozone, and ↑ times | ↑ DON degradation rates | Advantages: No significant changes in the protein content, sedimentation value, pasting properties, and water absorption; Improvement in the flour quality. Slight ↑ in dough development time and stability time; No decrease in the quality of wheat for end-users; Products produced from ozone-treated wheat flour (noodles) have a longer shelf life, lower darkening rate, and microbial growth; No harmful residues, easy to use, and no waste. Disadvantages: Ozone treatment in solution is faster than gaseous treatment of scabbed wheat. | [47] |
| In Solution: Processing time = 30 s; Ozone concentration = 1 mg L−1 | Degradation rate of DON = 54.2% | |||||
| In scabbed wheat: Processing time = 12h; Moisture content = 17%; Ozone gas concentration = 60 mg L−1 | Degradation rate of DON = 57.3% | |||||
| Gaseous ozone | Effective against DON in scabbed wheat | |||||
| ↑ Ozone concentration and ↑ processing time | ↑ Degradation rate of DON | |||||
| Ozonation | Grains | AFs | Ozone concentration = 47,800 ppm The average retention time = 1.8 min. Screw Conveyor System | Decreased Aspergillus flavus counts in a single pass through the screw conveyor: ↓ 96%; Reduction rate of aflatoxin: 20–30% | Advantages: Treatments with humidified and dry ozone: similar effects on fungi and insects; ↑ residence time: ↑ insect mortality and mold reduction. Disadvantages: The total electricity cost for running the equipment at maximum load was USD 3.98/h based on an electricity rate of USD 0.11/kWh; The reduction was not sufficient enough to be of commercial value; Electricity and equipment are needed. | [48] |
| Ozonation | Rice | Filamentous fungi | An application of 0.393 kg O3 m−3 rice | Different concentrations of ozone along the silo: 10−1, 10−2, and 10−3 (mol m−3) for the portions IP, CP, and SP, respectively; | Advantages: No damage to grain quality; No significant alteration of the quality of rice, starch modifications, lipid peroxidation, protein profile, and microstructure alterations. | [49] |
| highest concentration of ozone in the inferior part of the silo at the ozone inlet = Strong fungi reduction | ||||||
| Nixtamalization | Maize | AF and Fumonisins | Soaking in a solution of:
| AF: up to 90% | Advantages:
| |
| Fumonisins: up to 80% | [50] | |||||
| | ||||||
| Nixtamalization | Maize | AF | Traditional Nixtamalization Process-TNP | Not efficient enough to eliminate aflatoxins present in contaminated maize | Disadvantages:
| [51] |
| Nixtamalization | Tortilla | AFB1 | Alkaline pH of the maize-dough = 10.2, Resting time = 30–40 min of resting at room temperature | AFB1: 100% | [52] | |
| Nixtamalization | Maize and Sorghum | FBs, DON, NIV, and ZEN | The use of 5 cooking ingredients—1 g of cooking ingredient/400 mL of water at 92 °C for 40 min | Advantages: Sodium hydroxide and potassium hydroxide are good alternatives to calcium hydroxide; Sodium hydroxide could be used in the industrial nixtamalization process. Disadvantages: Environmental concerns about using calcium hydroxide; The high pH of the byproducts and wastewater when using calcium hydroxide; Calcium chloride is not effective in reducing mycotoxins. | [53] | |
| Calcium chloride as a cooking ingredient | The least effect on mycotoxin reduction | |||||
| Ammoniation | Groundnut press cake | AFs | Ammoniation at (0.5–2.0%) to feed materials/moisture content: 12–16%, at 45–55 psi, and at 80–100 °C for 20–60 min | Reductions in the levels of aflatoxin of between 96% and 99% | Disadvantages: Insufficient information was available to conclude on the safety and efficacy of the proposed decontamination process; No evidence that the proposed process is sufficient to ensure irreversibility in acid medium (GIT). | [54] |
| Ammoniation | Wheat kernels | DON | Treatment with Ammonia vapor at 90 °C for 2 h | Degradation of DON >75% | Advantages: In silico evaluation estimated a decrease in toxicity and biological effects. | [37] |
| With an initial level of DON up to 2000 μg/kg | Treatment efficacy is not affected | |||||
| Ammoniation | Corn | AFs | The use of aqua-ammonia | Effective and inexpensive | Advantages: Effective and inexpensive, and it can be applied on the farm at low cost by sealing the grain in plastic. Disadvantages: Corn treated with ammonia turns dark because the sugar (altrose) is caramelized and the grain temperature increases by about 10 °F at the time of treatment; Not an FDA-approved process and treated corn cannot be legally shipped out of state;
| [55] |
| | ||||||
| Ammoniation | Maize | AFs | The effect of ammonia | More destructive to aflatoxins G1 and G2 compared with aflatoxin B1 and B2 | [56] | |
| Highest detoxification rate | Aflatoxins G1 (95%) Aflatoxin G2 (93%) | |||||
| Lowest degradation rate | Aflatoxin B1 (85%) Aflatoxin B2 (83%) | |||||
| Acid | Selected Nuts | AFs | Moisture Levels: walnut (10 ± 3 and 16 ± 3%); pistachio (10 ± 3%); peanuts (10 ± 3%) Citric, Lactic and propionic acid at 9% Time: 15 min | Reduction rate of aflatoxins: citric acid (99%); lactic acid (99.9%); propionic acid (96.07%) | Advantages: Food-grade organic acids do not affect the nuts’ quality. | [57] |
| Citric acid | Considerable reduction of the 4 aflatoxins; No formation of hazardous residues | |||||
| Lactic acid | Significant reduction of AFB1 and Total Afs; Increase in AFB2 and AFG2; Lactic acid converts AFB1 into AFB2 (less toxic) | |||||
| Propionic acid | More efficient to reduce AFB1 | |||||
| Acid | Feeds/Foods | DON | 5% solutions of lactic acid and citric acid | Reduction of the concentration of common trichothecene mycotoxins, especially DON and its derivate 15Ac-DON | [58] | |
| 5% solutions of lactic acid and citric acid | No or only small effects on zearalenone, fumonisins, and culmorin | |||||
| Lactic acid treatment | Decreased concentration of nivalenol | |||||
| Acid | - | AFB1 | 1 M citric acid—at Room temperature—Time: 96 h | conversion of AFB1 to AFB2a >97% | Advantages: Organic acids have few detrimental effects; Under these conditions, > 71% of AFB1 was hydrated to AFB2a and did not show any reversion to the parent compound after being transferred to a neutral solution; Conversion of AFB1 to AFB2a in a gastric environment can be enhanced by the addition of citric acid. Disadvantages: Discoloration of various types of meats including beef, pork, and fish along with minor alterations in odor and taste. | [59] |
| 0.1 and 1 M citric acid—at boiling temperature—Time: 20 min | Conversion of AFB1 to AFB2a > 98% | |||||
| Technique | Feeds/Foods | Contaminants | Experimental Parameters | Reduction Rate | Advantages/Disadvantages | References |
|---|---|---|---|---|---|---|
| Photocatalysis | Wheat | DON | In solution: DON concentration = 10 μg/mL, time = 60 min, simulated sunlight: using NaYF4:Yb,Tm@TiO2 (6 mg/mL), pH = 8.0 | Rate of DON degradation ≈ 100% | Disadvantages: Decreased efficiency caused by shielding effect. | [69] |
| 3 photocatalytic degradation products were identified | C15H20O8, C15H20O7, and C15H20O5 | |||||
| In wheat:1 mL of 50 μg/mL DON standard solution + 5 g wheat-soaked and naturally dried. | Degradation rate at 120 min = 69.8% | |||||
| Toxic grains + UCNPs aqueous solution/ratio 1:1 | ||||||
| After 1 h of adsorption equilibrium, the wheat samples were illuminated by Xe lamp (200–2500 nm) for 5, 15, 30, 60, 90, and 120 min, respectively | ||||||
| Photocatalysis | Wheat | DON | In wheat: The dosage of photocatalyst UCNP@TiO2 was 8 mg mL−1 Time: 90 minRatio of wheat to liquid: 1:2 | Degradation rate at 90 min = 72.8% | Advantages:
| [76] |
| Plasma | Corn | AFB1 | CAP is generated by a Surface Barrier Discharge (SBD) system operating in ambient air, yielding RONS by a generation of non-equilibrium atmospheric pressure plasma in ambient air | Reduction rate of AFB1 after 60 s: 96% | Advantages:
| [77] |
| Initial concentration of AFB1 = 35 μg/ml | 100% AFB1 decontamination in less than 120 s of treatment | |||||
| Plasma | Oat Flour | T-2 and HT-2 | Low-pressure dielectric barrier discharge (DBD) plasma/different gases/time: 10–30 min | Disadvantages:
| [78] | |
| Exposure to nitrogen for 30 min | The maximal reduction of T-2 toxin degradation (43.25%) | |||||
| Exposure to nitrogen for 30 min | The maximal reduction of HT-2 toxin degradation (29.23%) | |||||
| Mean degradation rate of T-2 toxins in all experiments | 25.01% | |||||
| Mean degradation rate of HT-2 toxins in all experiments | 20.98% | |||||
| Oxygen and air as working gas | No significant reduction of T-2 and HT-2 | |||||
| Plasma | Maize | AFB1 and FB1 | Pulsed dielectric barrier discharge (DBD) jet: | Advantages:
| [79] | |
| Spiked maize grains are placed at 12 mm beneath plasma jet—Time = 10 min | ||||||
| Concentration of AFB1 = 1.25 ng/g | Degradation rate after 10 min of plasma exposure = 65% | |||||
| Concentration of FB1= 259 ng/g | Degradation rate after 10 min of plasma exposure = 64% | |||||
| Plasma | Roasted coffee | OTA | Treatment with cold plasma: Imput power = 30 W/output voltage = 850 V/Helium flow = 1.5 L/min for 30 min | OTA reduction rate = 50% | [80] | |
| Using the brine shrimp (Artemia salina) lethality assay | Untreated roasted coffee = Toxic Treated roasted coffee = Slightly Toxic | |||||
| Pulsed Light | Red pepper powder | AFB1, Total AF, OTA | The highest fluence applied (9.1 J/cm2, 61 pulses, 20 s) | 2.7, 3.1, and 4.1 log CFU/g reduction of yeasts, molds, and total plate counts (TPC), where initial microbial loads were 4.6, 5.5, and 6.5 log CFU/g, respectively |
| [81] |
| The highest fluence applied (9.1 J/cm2, 61 pulses, 20 s) | A maximum reduction of 67.2, 50.9, and 36.9% of (AFB1), (AF), and (OTA) was detected, respectively | |||||
| Pulsed Light | Solid medium | AFB1 and AFB2 | PL at different initial concentrations of AFB1 (229.9, 30.7 and 17.8 μg/kg) and AFB2 (248.2, 32.2 and 19.5 μg/kg) and irradiation intensities (2.86, 1.60 and 0.93 W/cm2) of PL | The degradation of AFB1 and AFB2 followed the second-order reaction kinetic model well (R2 > 0.97); The degradation rate was proportional to the intensities of PL irradiation and the initial concentrations of aflatoxins | [82] | |
| Pulsed Light | Rice | AFB1 and AFB2 | PL treatment of 0.52 J/cm2/pulse for 80 s to rough rice | AFB1 reduction rate = 75% AFB2 reduction rate = 39.2% | Advantages:
| [83] |
| PL treatment of 0.52 J/cm2/pulse for 15 s to rice bran | AFB1 reduction rate = 90.3%AFB2 reduction rate = 86.7% | |||||
| UV-C Irradiation | Brown, black, and red rice (Moisture content = 13%) | Aflatoxin (B1,B2, G1, and G2), DON, OTA, and ZEN | In black and red rice–the UV-C irradiation treatment (dosage of 2.06 kJ/cm2) for 1 h | Effective in fungal decontamination, photo-degradation of mycotoxins | Advantages: (dosage of 2.06 kJ/cm2) for 1 h:
(dosage of 6.18 kJ/cm2) for 3 h:
| [73] |
| In black and red rice—the UV-C irradiation treatment (dosage of 6.18 kJ/cm2) for 3 h | Increased the efficiency of fungal decontamination and reduced mycotoxins | |||||
| In brown rice, the treatment conditions need to be optimized since only the dosage of 6.18 kJ/cm2 | Reduction of fungal contamination | |||||
| UV-C Irradiation | Maize and peanut | AFB1 | After ten days of incubation and irradiation treatment delivering a dose of 8370 mJ/cm2 | The highest reduction of A. flavus count was 4.4 log CFU/g in maize and 3.1 log CFU/g in peanut | Advantages:
| [84] |
| Depending on the treatment | AFB1 reduction level:In maize ranged from 17 to 43% In peanut ranged from 14 to 51% | |||||
| UV-C Irradiation | Peanut | AFB1 | The darkening of the UV indicator (AgCl) | Linearly proportional to the UV dosage from 0 to 120 mJ/cm2 delivered on peanuts | Advantages:
| [85] |
| Rotation at 11 rpm in the cylindrical chamber | Significant improvement in UV uniformity | |||||
| UV irradiation: 2.3 mW/cm2 UV-C for 2 h with rotation at 11 rpm | Reduction percentage by 23.4% (from 14.3 ± 3.4% to 17.7 ± 4.5%) | |||||
| UV irradiation: 2.3 mW/cm2 UV-C for 2 h with rotation at 11 rpm | Increased AFB1 degradation rate from 60.8 ± 15.3 pmol g−1h−1 to 75.0 ± 10.9 pmol g−1h−1 | |||||
| Gamma Irradiation | Maize | AF and OTA | Gamma irradiation dose of 6.0 kGy | Completely inhibited the growth of the two molds | [86] | |
| Gamma irradiation dose of 4.5 kGy | Reduced the production of their mycotoxins | |||||
| Gamma irradiation dose of 20 kGy | Maximum reduction rate is as follows:
| |||||
| Gamma Irradiation | Wheat flourgrape juiceandwine | OTA | In wheat flour, a radiation dose of 30.5 kGy | OTA reduction rate = 24% | Advantages:
| [87] |
| In grape juice, a radiation dose of 30.5 kGy | OTA reduction rate = 12% | |||||
| In wine, a radiation dose of 30.5 kGy | OTA reduction rate = 23% | |||||
| Gamma Irradiation | Sorghum | OTA and AFB1 | Gamma irradiation dose of 3 kGy | Sufficient to eliminate 90% of the natural fungal load of sorghum | [88] | |
| At a radiation dose of 10 kGy | The maximum reduction rate of AFB1 = 59% | |||||
| At a radiation dose of 10 kGy | The maximum reduction rate of OTA = 32% | |||||
| Extrusion | Whole grain triticale flour | DON, 3- and 15-AcDON, HT-2, TEN, AME | Optimal parameters of co-rotating twin-screw extruder for lowering the concentration of each investigated mycotoxins in naturally contaminated flour were: SS = 650 rpm, FR = 30 kg/h, MC = 20 g/100 g | Reduction rate of mycotoxins: DON: 9.5%; 3-AcDON: 27.8%; 15-AcDON: 28.4%; HT-2: 60.5%; TEN: 12.3%; AME: 85.7% | [89] | |
| Extrusion | Cornmeal | AF: B1, B2, G1, G2 | Extrusion in the absence of high-amylose cornstarch | A reduction in aflatoxins level: (B1: 83.7%, B2: 80.5%, G1: 74.7%, and G2: 87.1%) | Disadvantages:
| [90] |
Extrusion in the presence of high-amylose cornstarch | Higher aflatoxins reductions were observed: (B1-89.9%, B2-88.6%, G1-75.0%, and G2-89.9%) | |||||
| Electrolyzed Water | Wheat grains | DON | For AcidEW | Advantages:
| [91] | |
| pH 5.5 | Optimal pH for DON elimination | |||||
| pH 2.5 | Optimal pH for fungal reduction | |||||
| For AlkEW | ||||||
| pH 9.5 | Optimal pH for DON elimination | |||||
pH from 8.5 to 12.5 | Strong elimination activity on fungi | |||||
| Electron Beam | Red pepper powder | OTA | Treatment at 6 kGy |
| Advantages:
| [92] |
| Treatment at 10 kGy for 23 s |
| |||||
| Treatment at 30 kGy |
| |||||
| Milling | Maize | Mycotoxins | Grain cleaning |
| Advantages:
| [93] |
| Milling | Maize | B-series fumonisins (FBs) | Grain cleaning |
| Advantages:
| [94] |
| Dry-degermination process of uncleaned kernels | Reduction rates:
| |||||
| Tempering degermination Process of uncleaned kernels | Reduction rates:
| |||||
| Combination | Feeds /Foods | Contaminants | Experimental Parameters | Reduction Rate | Advantages/Disadvantages | References | ||
|---|---|---|---|---|---|---|---|---|
| Ozone/UV-C/Citric acid | Pistachio nuts | Aflatoxins | Combination of the immersion of the samples in 3 N CA, 30 min exposure to O3, and 36 h exposed to UV-C radiation | AFB1 and AFB2 > 90% AFG1 and AFG2 > 99% | Advantages:
| [123] | ||
| The UV-C | More effect on AFB2 and AFG2 | |||||||
| The O3 treatment | Degradation of AFB1 and AFG1, more than AFB2 and AFG2 | |||||||
| Acid treatment | More effect on AFB1 and AFG1, against AFB2 and AFG2 | |||||||
| Extrusion/Fermentation | Wheat bran | Mycotoxins | Extrusion at 130 °C—Screw speed: 20 rpm + fermentation with L. casei and L paracasei strains at 30 ± 2 °C for 24 h. | The lowest overall concentration of the tested mycotoxins. | Advantages:
| [124] | ||
| Extrusion at 130 °C—Screw speed: 25 rpm + fermentation with L. casei and L paracasei strains at 30 ± 2 °C for 24 h. | ||||||||
| Roasting/Brewing | Coffee | AF and OTA | Treatment | OTA reduction | Advantages:
| [125] | ||
| Low roasting: | Medium roasting: | High roasting: | ||||||
| After roasting | 15.17% | 46.78% | 57.43% | |||||
| After reduction after brewing | 43.57% | 4.11% | 7.28% | |||||
| After roasting and brewing | 58.74% | 60.88% | 64.7% | |||||
| Treatment | AF reduction | |||||||
| Low roasting: | Medium roasting: | High roasting: | ||||||
| After roasting | 31.98% | 46.36% | 61.52% | |||||
| After brewing | 10.19% | 1.50% | 0.86% | |||||
| After roasting and brewing | 40.18% | 47.86% | 62.38% | |||||
| Heat treatment/PEF | Artificially spiked potato dextrose agar (PDA) | AFT—AFB1 | Thermal process: | Disadvantages:
| ||||
| At pH 10 | The effect of process time was observed to affect both AFB1 and AFT content more significantly than the temperature. | [126] | ||||||
| At pH 4 and 7 | The effect of temperature on toxin reduction was more evident. | |||||||
| PEF: Fixed parameters: pulse frequency (50 Hz), burst (10), energy (1 KJ) Time: 10 s | ||||||||
| Variables: pulse width (ms) and output voltage (%), and pH of the PDA/different combination. (20 μs 10%; 51 μs 26% and 65 μs 26% for pH 4, 7, and 10 respectively) | Reduction rates of AFB1: 79–96% | |||||||
| Combined effect of Thermal process + PEF: | ||||||||
| Thermal process: T = 110 °C +t = 15 min + PEF (65 μs 26%, pH 10) | The maximum degradation: AFB1 = 96.881%; AFT = 95.721% | |||||||
| High Conc. H2O2 at Moderate temperature/roasting | Peanuts | AF | 30 g/hg H2O2 at 20 °C | AF reduction rate = 30% |
| [127] | ||
| 30 g/hg H2O2 at 50 °C | AF reduction rate = 73% | |||||||
| 30 g/hg H2O2 at 50 °C for 8 h —unroasted peanuts | AF reduction rate = 86% | |||||||
| Combined effect: 30 g/hg H2O2 at 50 °C for 8 h + roasted peanuts at 140 °C for 10 min | AF reduction rate = 90% | |||||||
| Combination | Feeds/Foods | Mycotoxins | Experimental Parameters | Reduction Rate | Advantages/Disadvantages | References |
|---|---|---|---|---|---|---|
| UV + H2O2 | Peanuts | AFs | Advanced Oxidation Processes by UV and H2O2 | Advantages:
| [128] | |
| 1 h AOP (2.76 mW/cm2 UV-C, 1 g/hg H2O2) of whole peanut kernels | Degradation rate of AF = 33% | |||||
| 1 h AOP (2.76 mW/cm2 UV-C, 1 g/hg H2O2) of milled kernels | Degradation rate of AF = 60% | |||||
| Citric acid + Pulsed light | Peanuts | AFB | PL + CA treatment | AFT ≈ 98.2% AFB1 ≈ 98.9% AFB2 ≈ 98.1% | Advantages:
| [129] |
| IR Nixtamalization (alkaline treatment + IR) | Maize tortillas | AFs | The infrared nixtamalization process (IRNP)—Cooking in a cooker that generates infrared radiation (14.2 A, 1704 W) | The degradation rate of AF: 93.82% | Advantages:
| [130] |
| Traditional nixtamalization process (TNP) | The degradation rate of AF: 98.35% | |||||
| Roasting + acid | Pistachio nuts | AFB1 | Treatment 1: 50 g Pistachio—addition of 30 mL water + 30 mL lemon juice + 6 g of citric acid—roasting at 120 °C for 1 h | AFB1 = 93.1 ± 8.2% | Advantages:
| [131] |
| Treatment 2: 50 g Pistachio—addition of 30 mL water + 15 mL lemon juice + 2.25 g of citric acid—Roasting at 120 °C for 1 h | AFB1 = 49.2 ± 3.5% | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abou Dib, A.; Assaf, J.C.; El Khoury, A.; El Khatib, S.; Koubaa, M.; Louka, N. Single, Subsequent, or Simultaneous Treatments to Mitigate Mycotoxins in Solid Foods and Feeds: A Critical Review. Foods 2022, 11, 3304. https://doi.org/10.3390/foods11203304
Abou Dib A, Assaf JC, El Khoury A, El Khatib S, Koubaa M, Louka N. Single, Subsequent, or Simultaneous Treatments to Mitigate Mycotoxins in Solid Foods and Feeds: A Critical Review. Foods. 2022; 11(20):3304. https://doi.org/10.3390/foods11203304
Chicago/Turabian StyleAbou Dib, Alaa, Jean Claude Assaf, André El Khoury, Sami El Khatib, Mohamed Koubaa, and Nicolas Louka. 2022. "Single, Subsequent, or Simultaneous Treatments to Mitigate Mycotoxins in Solid Foods and Feeds: A Critical Review" Foods 11, no. 20: 3304. https://doi.org/10.3390/foods11203304
APA StyleAbou Dib, A., Assaf, J. C., El Khoury, A., El Khatib, S., Koubaa, M., & Louka, N. (2022). Single, Subsequent, or Simultaneous Treatments to Mitigate Mycotoxins in Solid Foods and Feeds: A Critical Review. Foods, 11(20), 3304. https://doi.org/10.3390/foods11203304













