Fabrication of Chitosan/Hydroxyethyl Cellulose/TiO2 Incorporated Mulberry Anthocyanin 3D-Printed Bilayer Films for Quality of Litchis
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
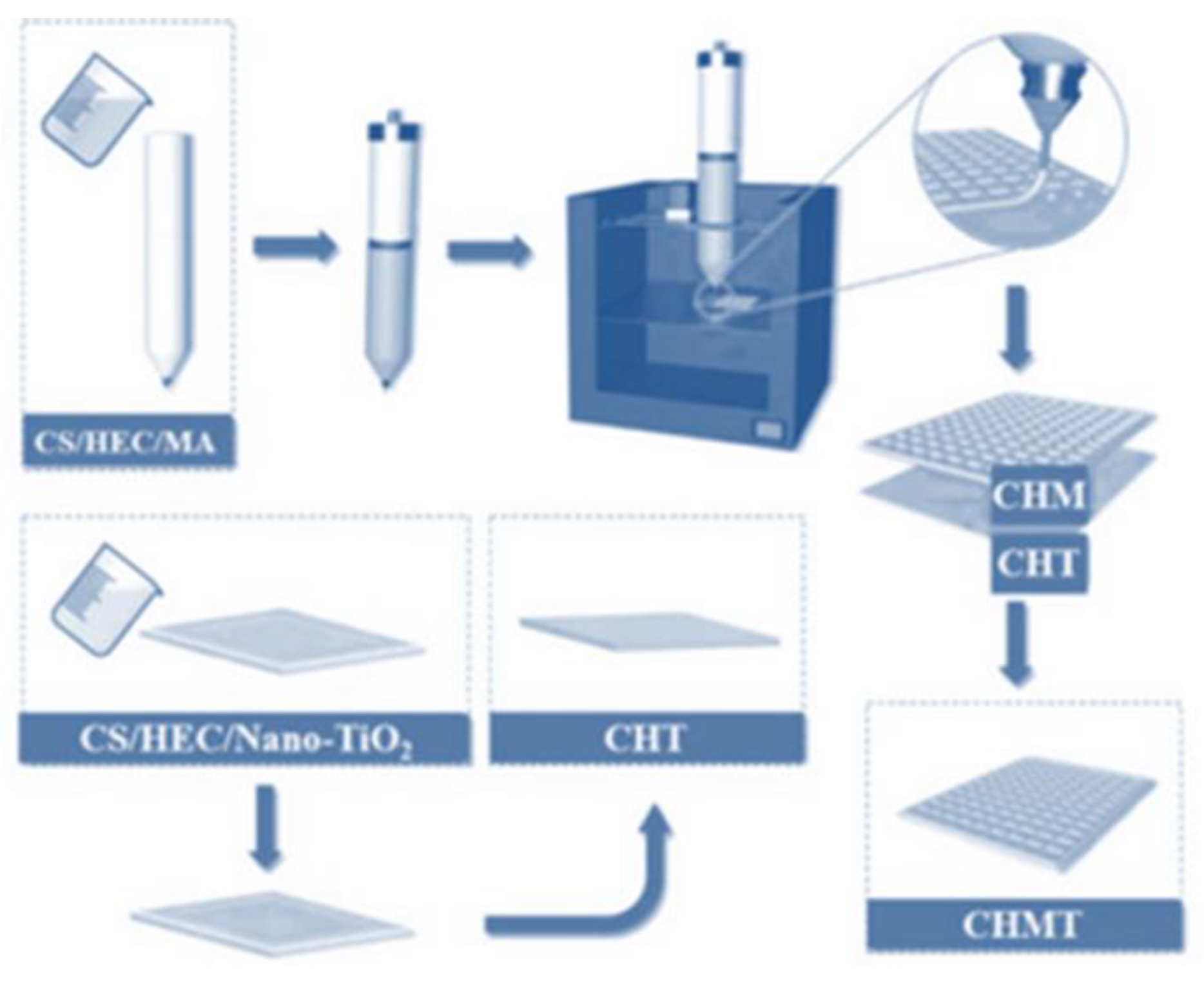
2.2. Preparation of Chitosan/Hydroxyethyl Cellulose Substrate
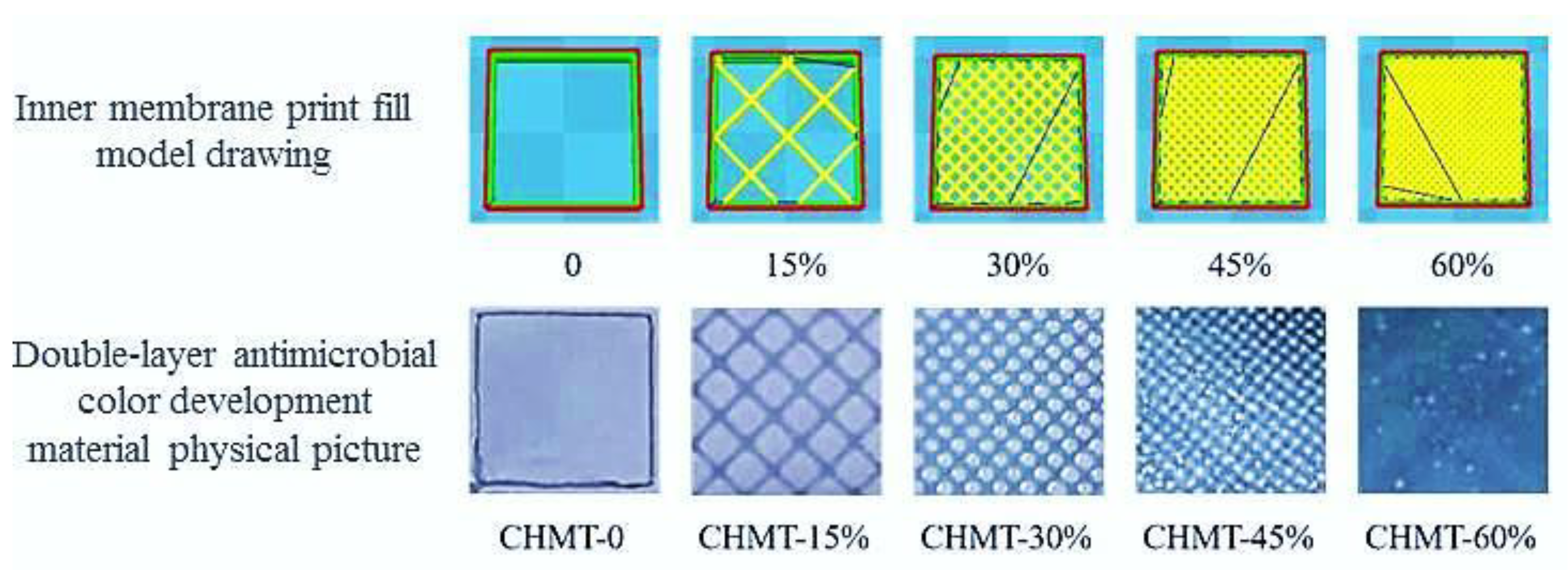
2.3. Preparation of Dual-Layer Antimicrobial Color Development Materials
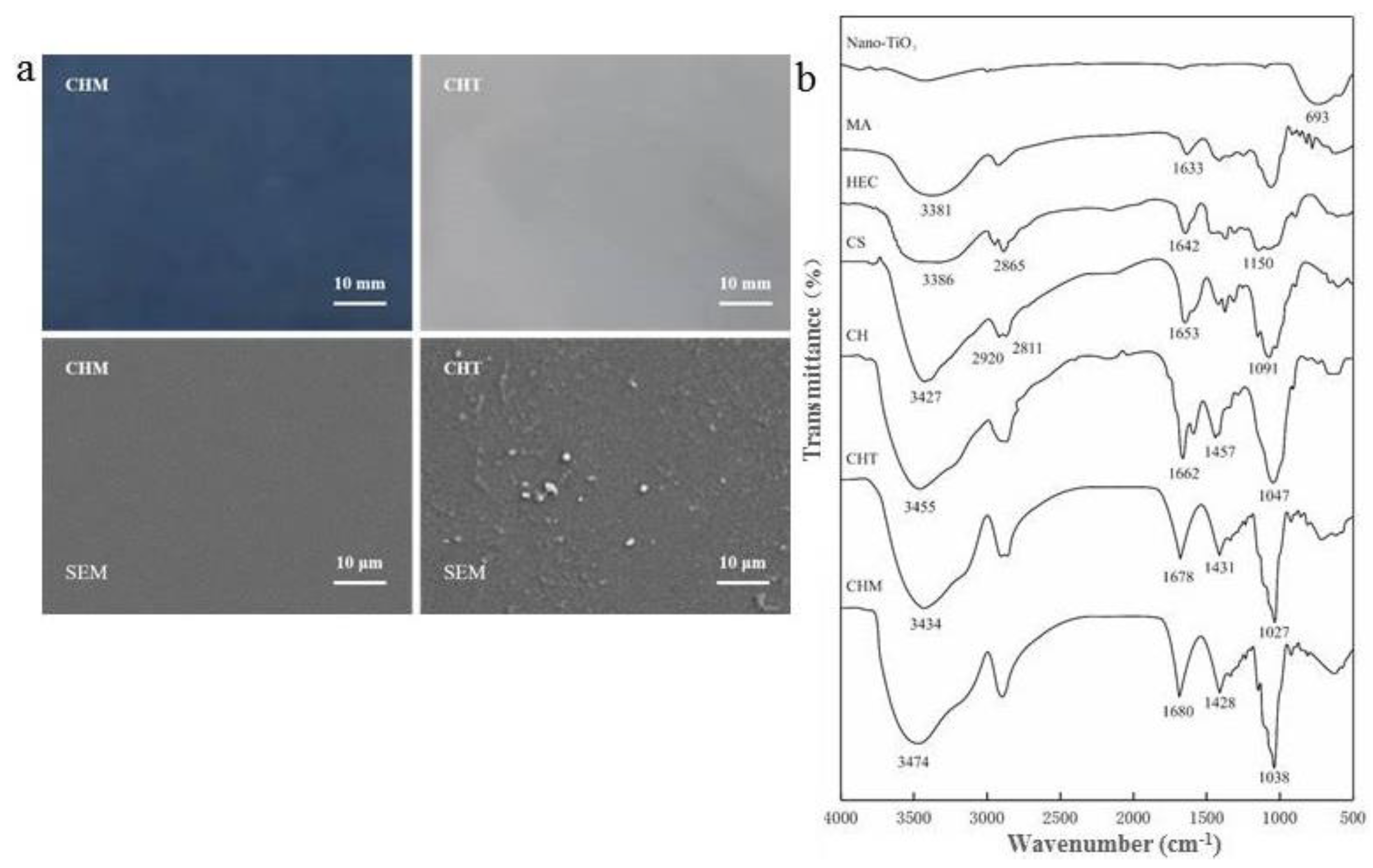
2.4. Scanning Electron Microscopy
2.5. Fourier-Transform Infrared Spectroscopy
2.6. Thickness and Water Vapor Transmission Coefficient
2.7. Mechanical Properties
2.8. Opacity
2.9. Broad-Spectrum Antibacterial
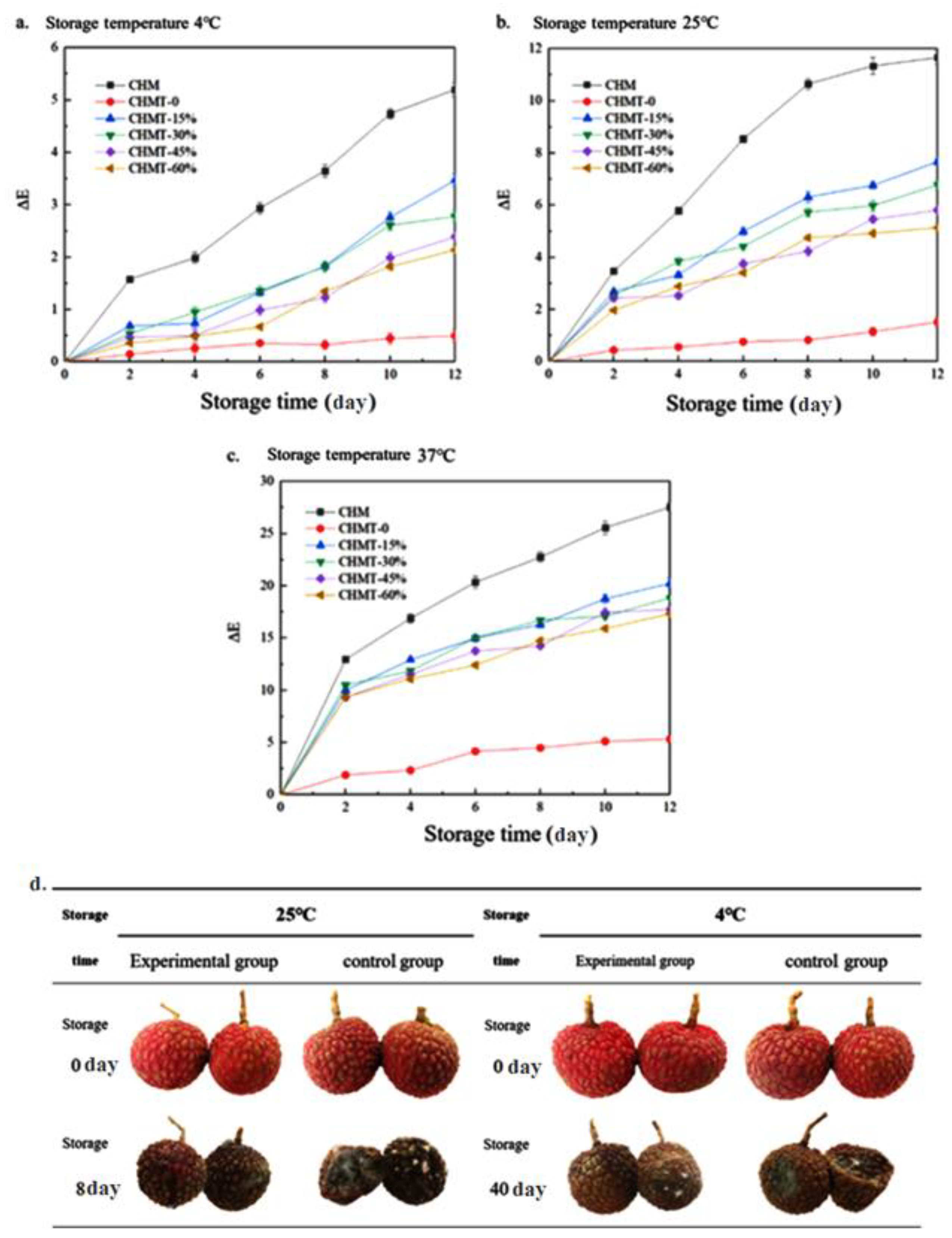
2.10. Color Stability
2.11. Preservation of Litchi Fruit
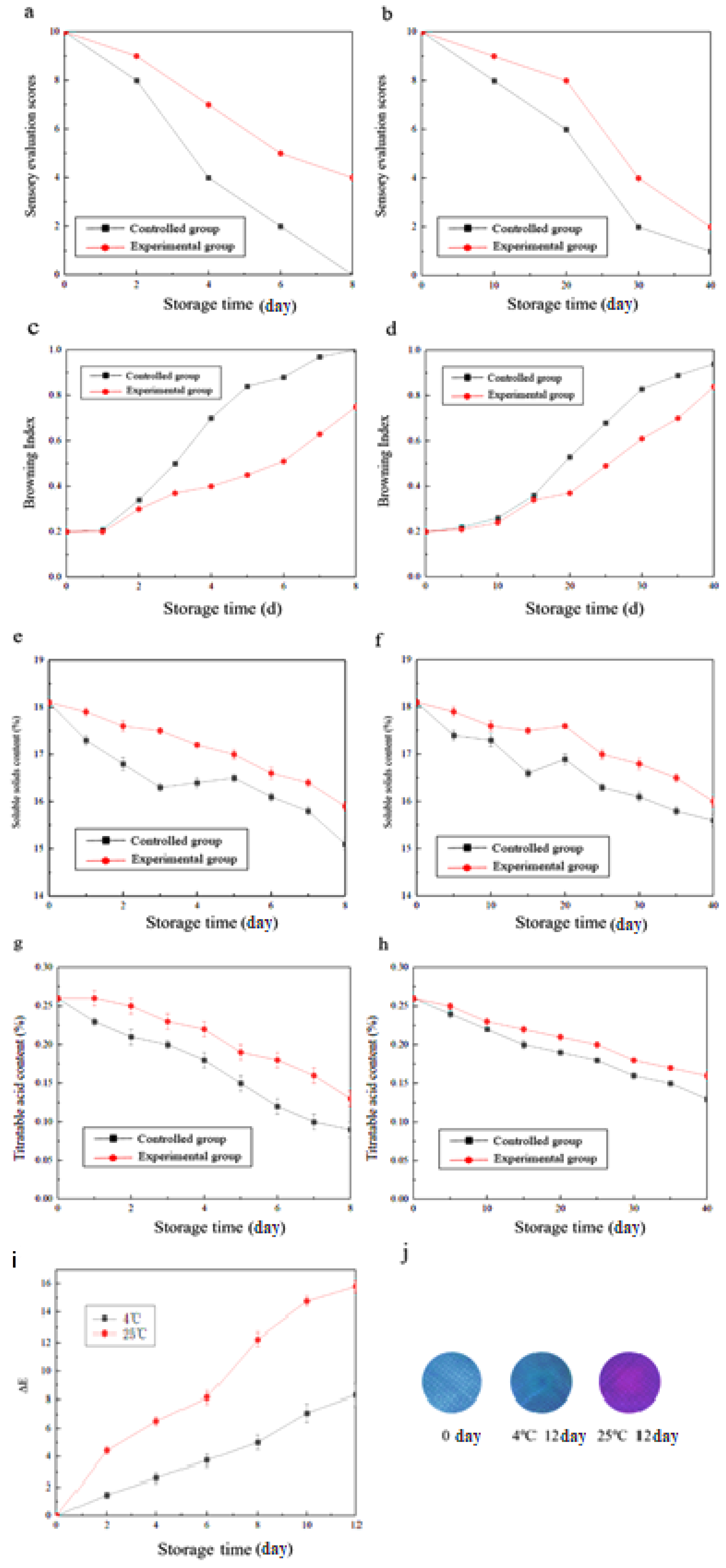
2.12. Sensory Evaluation
2.13. Browning Index
2.14. Determination of Soluble Solids Content
2.15. Determination of Titratable Acid Content
2.16. Evaluation of the Intelligent Color
2.17. Statistical Analysis
3. Results and Discussion
3.1. Performance Testing of the Dual-Layer Antimicrobial Color
3.2. Fourier-Transform Infrared Spectroscopy
3.3. Optical Properties and Appearance
3.4. Water Vapor Transmission Coefficient
3.5. Mechanical Properties
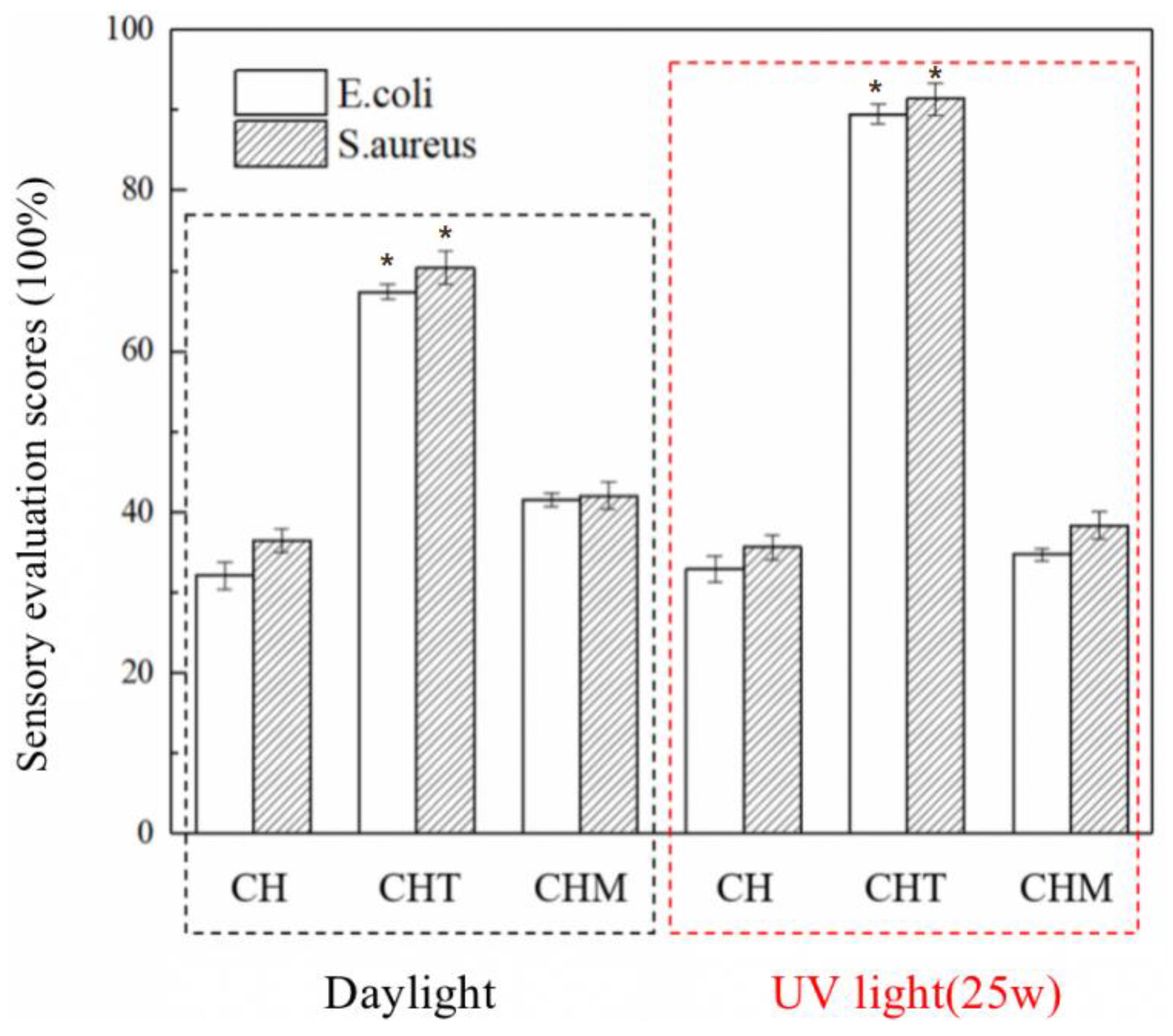
3.6. Broad-Spectrum Antibacterial
3.7. Color Stability of the Double-Layer Antibacterial Color Development Material
3.8. Sensory Evaluation of Litchi Fruit
3.9. Browning Index
3.10. Soluble Solids Content
3.11. Titratable Acid Content
3.12. Litchi Freshness Intelligent Color-Rendering Effect
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, Z.; Huber, D.J.; Qu, H.; Yun, Z.; Wang, H.; Huang, Z.; Huang, H.; Jiang, Y. Enzymatic browning and antioxidant activities in harvested litchi fruit as influenced by apple polyphenols. Food Chem. 2015, 171, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Lin, H.; Lin, M.; Chen, Y.; Wang, H.; Lin, Y.; Shi, J.; Lin, Y. A novel chitosan formulation treatment induces disease resistance of harvested litchi fruit to Peronophythora litchii in association with ROS metabolism. Food Chem. 2018, 266, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Xiao, L.; Yan, H.; Zhang, D.; Wu, F.; Liu, X.; Su, X.; Dong, X.; Wang, J.; Duan, X.; et al. Redox regulation of methionine in calmodulin affects the activity levels of senescence-related transcription factors in litchi. BBA Gen. Subj. 2017, 1861, 1140–1151. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Lin, F.; Yang, G.; Yue, X.; Zhang, Q.; Zhang, Z.; Chen, H. Advantages of immersion freezing for quality preservation of litchi fruit during frozen storage. LWT-Food Sci. Technol. 2015, 60, 948–956. [Google Scholar] [CrossRef]
- Sivakumar, D.; Terry, L.A.; Korsten, L. An overview on litchi fruit quality and alternative postharvest treatments to replace sulfur dioxide fumigation. Food Rev. Int. 2010, 26, 162–188. [Google Scholar] [CrossRef]
- He, M.; Wu, Y.; Hong, M.; Yun, Z.; Li, T.; Jiang, Y. α-Lipoic acid treatment alleviates postharvest pericarp browning of litchi fruit by regulating antioxidant ability and energy metabolism. Postharvest Biol. Technol. 2021, 180, 111629. [Google Scholar] [CrossRef]
- Lin, B.; Du, Y.; Liang, X.; Wang, X.; Wang, X.; Yang, J. Effect of chitosan coating on respiratory behavior and quality of stored litchi under ambient temperature. J. Food Eng. 2010, 102, 94–99. [Google Scholar] [CrossRef]
- Wu, Y.; Lin, H.; Lin, Y.; Shi, J.; Xue, S.; Hung, Y.-C.; Chen, Y.; Wang, H. Effects of biocontrol bacteria Bacillus amyloliquefaciens LY-1 culture broth on quality attributes and storability of harvested litchi fruit. Postharvest Biol. Technol. 2017, 132, 81–87. [Google Scholar] [CrossRef]
- Wang, Y.; Yi, S.; Lu, R.; Sameen DEAhmed, S.; Dai, J.; Qin, W.; Li, S.; Liu, Y. Preparation, characterization, and 3D printing verification of chitosan/halloysite nanotubes/tea polyphenol nanocomposite films. Int. J. Biol. Macromol. 2021, 166, 32–44. [Google Scholar] [CrossRef]
- Liu, Y.; Sameen, D.E.; Ahmed, S.; Dai, J.; Qin, W. Antimicrobial peptides and their application in food packaging. Trends Food Sci. Technol. 2021, 112, 471–483. [Google Scholar] [CrossRef]
- Vilela, C.; Pinto, R.J.B.; Coelho, J.; Domingues, M.R.M.; Daina, S.; Sadocco, P.; Santos, S.A.O.; Freire, C.S.R. Bioactive chitosan/ellagic acid films with UV-light protection for active food packaging. Food Hydrocoll. 2017, 73, 120–128. [Google Scholar] [CrossRef]
- Karyn, P.; Suguna, J.; Hanxi, B.; Shanyu, M.; Lin, Z.; Arencibia, G.S.; Savin, D.A.; William, P.; Correll, M.; Zhaohui, T. Glycerol-based dendrimer nanocomposite film as a tunable pH-sensor for food packaging. ACS Appl. Mater. Interfaces 2021, 13, 23268–23281. [Google Scholar]
- Ma, Y.; Li, S.; Ji, T.; Wu, W.; Sameen, D.E.; Ahmed, S.; Qin, W.; Dai, J.; Liu, Y. Development and optimization of dynamic gelatin/chitosan nanoparticles incorporated with blueberry anthocyanins for milk freshness monitoring. Carbohydr. Polym. 2020, 247, 116738. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yi, S.; Sameen, D.E.; Hossen, M.A.; Dai, J.; Li, S.; Qin, W.; Lee, K.J. Designing and utilizing 3D printed chitosan/halloysite nanotube/tea polyphenol composites to maintain the quality of fresh blueberries. Innov. Food Sci. Emerg. Technol. 2021, 74, 102808. [Google Scholar] [CrossRef]
- Pereira, V.A.; de Arruda, I.N.Q.; Stefani, R. Active chitosan/PVA films with anthocyanins from Brassica oleraceae (Red Cabbage) as time–temperature indicators for application in intelligent food packaging. Food Hydrocoll. 2015, 43, 180–188. [Google Scholar] [CrossRef]
- Zhang, R.; Lan, W.; Ji, T.; Sameen, D.E.; Qin, W.; Liu, Y. Development of polylactic acid/ZnO composite membranes prepared by ultrasonication and electrospinning for food packaging. LWT 2021, 135, 110072. [Google Scholar] [CrossRef]
- Sameen, D.E.; Ahmed, S.; Lu, R.; Li, R.; Dai, J.; Qin, W.; Zhang, Q.; Li, S.; Liu, Y. Electrospun nanofibers food packaging: Trends and applications in food systems. Crit. Rev. Food Sci. Nutr. 2022, 62, 6238–6251. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Wang, K.; Lan, H.; Wang, Y.; Hu, Z.; Zhao, L. Effect of hybrid gelator systems of beeswax-carrageenan-xanthan on rheological properties and printability of litchi inks for 3D food printing. Food Hydrocoll. 2021, 113, 106482. [Google Scholar] [CrossRef]
- Tohic, C.L.; O’Sullivan, J.J.; Drapala, K.P.; Chartrin, V.; Chan, T.; Morrison, A.P.; Kerry, J.P.; Kelly, A.L. Effect of 3D printing on the structure and textural properties of processed cheese. J. Food Eng. 2018, 220, 56–64. [Google Scholar] [CrossRef]
- Wang, Y.; Ye, Z.; Li, J.; Zhang, Y.; Guo, Y.; Cheng, J.H. Effects of dielectric barrier discharge cold plasma on the activity, structure and conformation of horseradish peroxidase (HRP) and on the activity of litchi peroxidase (POD). LWT 2021, 141, 111078. [Google Scholar] [CrossRef]
- Ahmed, S.; Sameen, D.E.; Lu, R.; Li, R.; Dai, J.; Qin, W.; Liu, Y. Research progress on antimicrobial materials for food packaging. Crit. Rev. Food Sci. Nutr. 2022, 62, 3088–3102. [Google Scholar] [CrossRef]
- Elgadir, M.A.; Uddin, M.S.; Ferdosh, S.; Adam, A.; Chowdhury, A.J.K.; Sarker, M.Z.I. Impact of chitosan composites and chitosan nanoparticle composites on various drug delivery systems: A review. J. Food Drug Anal. 2015, 23, 619–629. [Google Scholar] [CrossRef]
- Siripatrawan, U.; Vitchayakitti, W. Improving functional properties of chitosan films as active food packaging by incorporating with propolis. Food Hydrocoll. 2016, 61, 695–702. [Google Scholar] [CrossRef]
- Castillo, L.A.; Farenzena, S.; Pintos, E.; Rodríguez, M.S.; Villar, M.A.; García, M.A.; López, O.V. Active films based on thermoplastic corn starch and chitosan oligomer for food packaging applications. Food Packag. Shelf Life 2017, 14, 128–136. [Google Scholar] [CrossRef]
- Niu, X.; Liu, Y.; Song, Y.; Han, J.; Pan, H. Rosin modified cellulose nanofiber as a reinforcing and co-antimicrobial agents in polylactic acid /chitosan composite film for food packaging. Carbohydr. Polym. 2018, 183, 102–109. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, Y.; Liu, Y.; Zhang, J.; Hossen, M.A.; Sameen, D.E.; Dai, J.; Li, S.; Qin, W. Fabrication and characterization of pH-responsive intelligent films based on carboxymethyl cellulose and gelatin/curcumin/chitosan hybrid microcapsules for pork quality monitoring. Food Hydrocoll. 2022, 124, 107224. [Google Scholar] [CrossRef]
- Sun, J.; Jiang, H.; Wu, H.; Tong, C.; Pang, J.; Wu, C. Multifunctional bionanocomposite films based on konjac glucomannan/chitosan with nano-ZnO and mulberry anthocyanin extract for active food packaging. Food Hydrocoll. 2020, 107, 106942. [Google Scholar] [CrossRef]
- Fabra, M.J.; López-Rubio, A.; Lagaron, J.M. Use of the electrohydrodynamic process to develop active/bioactive bilayer films for food packaging applications. Food Hydrocoll. 2016, 55, 11–18. [Google Scholar] [CrossRef]
- Lan, W.; Zhang, R.; Ji, T.; Sameen, D.E.; Ahmed, S.; Qin, W.; Dai, J.; He, H.; Liu, Y. Improving nisin production by encapsulated Lactococcus lactis with starch/carboxymethyl cellulose edible films. Carbohydr. Polym. 2021, 251, 117062. [Google Scholar] [CrossRef]
- Li, S.; Ma, Y.; Ji, T.; Sameen, D.E.; Ahmed, S.; Qin, W.; Dai, J.; Li, S.; Liu, Y. Cassava starch/carboxymethylcellulose edible films embedded with lactic acid bacteria to extend the shelf life of banana. Carbohydr. Polym. 2020, 248, 116805. [Google Scholar] [CrossRef]
- Wu, C.; Sun, J.; Zheng, P.; Kang, X.; Chen, M.; Li, Y.; Ge, Y.; Hu, Y.; Pang, J. Preparation of an intelligent film based on chitosan/oxidized chitin nanocrystals incorporating black rice bran anthocyanins for seafood spoilage monitoring. Carbohydr. Polym. 2019, 222, 115006. [Google Scholar] [CrossRef] [PubMed]
- Otoni, C.G.; de Moura, M.R.; Aouada, F.A.; Camilloto, G.P.; Cruz, R.S.; Lorevice, M.V.; de Nilda, F.F.S.; Mattoso, L.H.C. Antimicrobial and physical-mechanical properties of pectin/papaya puree/cinnamaldehyde nanoemulsion edible composite films. Food Hydrocoll. 2014, 41, 188–194. [Google Scholar] [CrossRef]
- Ezati, P.; Tajik, H.; Moradi, M. Fabrication and characterization of alizarin colorimetric indicator based on cellulose-chitosan to monitor the freshness of minced beef. Sens. Actuators B. Chem. 2019, 285, 519–528. [Google Scholar] [CrossRef]
- Jiang, Y.; Fu, J.; Zauberman, G.; Fuchs, Y. Purification of polyphenol oxidase and the browning control of litchi fruit by glutathione and citric acid. J. Sci. Food Agric. 1999, 79, 950–954. [Google Scholar] [CrossRef]
- Somboonkaew, N.; Terry, L.A. Physiological and biochemical profiles of imported litchi fruit under modified atmosphere packaging. Postharvest Biol. Technol. 2010, 56, 246–253. [Google Scholar] [CrossRef]
- Silva, N.H.C.S.; Vilela, C.; Almeida, A.; Marrucho, I.M.; Freire, C.S.R. Pullulan-based nanocomposite films for functional food packaging: Exploiting lysozyme nanofibers as antibacterial and antioxidant reinforcing additives. Food Hydrocoll. 2018, 77, 921–930. [Google Scholar] [CrossRef]
- Phothisarattana, D.; Wongphan, P.; Promhuad, K.; Promsorn, J.; Harnkarnsujarit, N. Biodegradable poly(butylene adipate-co-terephthalate) and thermoplastic starch-blended TiO2 nanocomposite blown films as functional active packaging of fresh fruit. Polymers 2021, 13, 4192. [Google Scholar] [CrossRef]
- Phanwipa, W.; Maturin, K.; Thanalee, S.; Nathdanai, H. Novel edible starch films incorporating papain for meat tenderization. Food Packag. Shelf Life 2022, 31, 100787. [Google Scholar]
- Zhang, R.; Ma, Y.; Lan, W.; Sameen, D.E.; Ahmed, S.; Dai, J.; Qin, W.; Li, S.; Liu, Y. Enhanced photocatalytic degradation of organic dyes by ultrasonic-assisted electrospray TiO2/graphene oxide on polyacrylonitrile/β-cyclodextrin nanofibrous membranes. Ultrason. Sonochemistry 2021, 70, 105343. [Google Scholar] [CrossRef]
- Wattinee, K.; Phanwipa, W.; Phatthranit, K.; Nathdanai, H. Thermoplastic starch blown films functionalized by plasticized nitrite blended with PBAT for superior oxygen barrier and active biodegradable meat packaging. Food Chem. 2022, 374, 131709. [Google Scholar]
- Yadav, H.M.; Otari, S.V.; Koli, V.B.; Mali, S.S.; Hong, C.K.; Pawar, S.H.; Delekar, S.D. Preparation and characterization of copper-doped anatase TiO2 nanoparticles with visible light photocatalytic antibacterial activity. J. Photochem. Photobiol. A Chem. 2014, 280, 32–38. [Google Scholar] [CrossRef]
- Ekici, L.; Simsek, Z.; Ozturk, I.; Sagdic, O.; Yetim, H. Effects of temperature, time, and pH on the stability of anthocyanin extracts: Prediction of total anthocyanin content using nonlinear models. Food Anal. Methods 2014, 7, 1328–1336. [Google Scholar] [CrossRef]
- Liu, Y.; Ahmed, S.; Sameen, D.E.; Wang, Y.; Lu, R.; Dai, J.; Li, S.; Qin, W. A review of cellulose and its derivatives in biopolymer-based for food packaging application. Trends Food Sci. Technol. 2021, 112, 532–546. [Google Scholar] [CrossRef]
- Li, S.; Jiang, Y.; Zhou, Y.; Li, R.; Jiang, Y.; Hossen, M.A.; Dai, J.; Qin, W.; Liu, Y. Facile fabrication of sandwich-like anthocyanin/chitosan/lemongrass essential oil films via 3D printing for intelligent evaluation of pork freshness. Food Chem. 2022, 370, 131082. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, Y.; Feng, T.; Luo, J.; Sameen, D.E.; Hossen, M.A.; Dai, J.; Li, S.; Qin, W. Development and characterization of aldehyde-sensitive cellulose/chitosan/beeswax colorimetric papers for monitoring kiwifruit maturity. Int. J. Biol. Macromol. 2021, 187, 566–574. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Li, B.; Xu, Y. Effects of O2 and CO2 concentrations on physiology and quality of litchi fruit in storage. Food Chem. 2005, 91, 659–663. [Google Scholar] [CrossRef]
- Fu, L.; Majeed, Y.; Zhang, X.; Karkee, M.; Zhang, Q. Faster R–CNN–based apple detection in dense-foliage fruiting-wall trees using RGB and depth features for robotic harvesting. Biosyst. Eng. 2020, 197, 245–256. [Google Scholar] [CrossRef]
| Abbreviation | Materials |
|---|---|
| CS | Chitosan |
| HEC | Hydroxyethyl cellulose |
| MA | Mulberry anthocyanins |
| CH | Chitosan and hydroxyethyl cellulose composite |
| CHT | Chitosan, hydroxyethyl cellulose and nano-TiO2 composite |
| CHM | Chitosan, hydroxyethyl cellulose and mulberry anthocyanins composite |
| CHMT | Chitosan, hydroxyethyl cellulose, mulberry anthocyanins and nano-TiO2 composite |
| Level | State of the Skin | State of the Pulp | Smell | Taste | Score |
|---|---|---|---|---|---|
| I | Bright color, normal appearance | Full and springy | Fragrant and sweet | Taste excellent | 100 |
| II | Dark color, slight loss of water | Neither too hard, nor too soft | Natural fragrance | Taste good | 80–90 |
| III | Large area browning and water loss | Water softening | Light herbal scent | Poor taste | 60–70 |
| IV | Complete browning and large loss of water | The flesh is yellow and shriveled | Slight abnormal taste | Taste sour | 40–50 |
| V | Mildew spots and severe water loss appear | Atrophy and slight decay | Abnormal taste | Almost inedible | 20–30 |
| VI | Severe mildew and water loss | Putrid | Obvious abnormal taste | Cannot eat | 0–10 |
| Sample | L* | a* | b* | ΔE | Opacity |
|---|---|---|---|---|---|
| CHMT-0 | 88.78 ± 0.43 a | 0.77 ± 0.01 a | 0.41 ± 0.07 a | 4.82 ± 0.19 a | 21.53 ± 0.04 a |
| CHMT-15% | 80.39 ± 0.79 b | 11.02 ± 0.77 b | −4.66 ± 0.34 b | 12.86 ± 0.20 b | 22.03 ± 0.09 a |
| CHMT-30% | 72.64 ± 0.53 c | 13.84 ± 0.67 c | −6.33 ± 0.42 c | 20.34 ± 0.02 c | 33.55 ± 0.56 b |
| CHMT-45% | 69.69 ± 1.01 d | 15.53 ± 0.32 d | −7.35 ± 0.42 d | 23.78 ± 0.89 d | 39.40 ± 0.41 c |
| CHMT-60% | 63.56 ± 0.89 e | 16.33 ± 0.24 d | −8.69 ± 0.31 e | 29.36 ± 0.93 e | 46.34 ± 0.47 d |
| Sample | Thickness (μm) | WVP·10−12 (g·cm/(cm2·s·Pa)) | Tensile Strength (Mpa) | Elongation at Break (%) |
|---|---|---|---|---|
| CHMT-0 | 40.01 ± 0.28 a | 7.81 ± 0.25 a | 21.55 ± 0.58 a | 17.01 ± 0.11 a |
| CHMT-15% | 63.24 ± 1.22 b | 7.23 ± 0.53 a | 22.32 ± 0.65 a | 18.01 ± 1.18 a |
| CHMT-30% | 70.12 ± 1.34 c | 6.54 ± 0.33 b | 25.61 ± 0.39 b | 20.01 ± 0.25 b |
| CHMT-45% | 79.81 ± 1.76 d | 4.73 ± 0.45 c | 29.52 ± 0.77 c | 21.01 ± 0.32 b |
| CHMT-60% | 87.21 ± 1.53 e | 2.16 ± 0.15 d | 34.43 ± 1.12 d | 24.01 ± 0.73 c |
| Sample | Inhibitory Circle Size in Daylight (mm) | Post-UV Inhibition Circle Size (mm) | ||
|---|---|---|---|---|
| E. coli | S. aureus | E. coli | S. aureus | |
| CHMT-0 | 14.58 ± 0.11 a | 16.44 ± 0.13 a | 16.12 ± 0.19 a | 18.96 ± 0.24 a |
| CHMT-15% | 13.43 ± 0.15 b | 15.38 ± 0.16 b | 15.07 ± 0.10 b | 17.52 ± 0.17 b |
| CHMT-30% | 11.26 ± 0.20 c | 14.11 ± 0.09 c | 12.15 ± 0.23 c | 15.67 ± 0.19 c |
| CHMT-45% | 9.33 ± 0.08 d | 11.19 ± 0.17 d | 10.14 ± 0.12 d | 11.98 ± 0.25 d |
| CHMT-60% | 8.24 ± 0.09 e | 9.27 ± 0.05 e | 8.43 ± 0.04 e | 9.66 ± 0.12 e |
| Freshness of Litchi | |||||
|---|---|---|---|---|---|
| Fresh | Relatively Fresh | Stale | Almost Rot | Spoilage | |
| Sensory evaluation score | 100 | 80–90 | 60–70 | 40–50 | 0–30 |
| Red (R) | 145–155 | 124–132 | 140–142 | 150–159 | 155–163 |
| Green (G) | 159–162 | 144–156 | 139–146 | 130–141 | 122–131 |
| Blue (B) | 171–179 | 162–170 | 163–169 | 167–175 | 168–173 |
| Freshness colorimetric card |  |  |  |  |  |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, J.; Xia, G.; Liu, L.; Ji, A.; Luo, Q. Fabrication of Chitosan/Hydroxyethyl Cellulose/TiO2 Incorporated Mulberry Anthocyanin 3D-Printed Bilayer Films for Quality of Litchis. Foods 2022, 11, 3286. https://doi.org/10.3390/foods11203286
Luo J, Xia G, Liu L, Ji A, Luo Q. Fabrication of Chitosan/Hydroxyethyl Cellulose/TiO2 Incorporated Mulberry Anthocyanin 3D-Printed Bilayer Films for Quality of Litchis. Foods. 2022; 11(20):3286. https://doi.org/10.3390/foods11203286
Chicago/Turabian StyleLuo, Jinjie, Guofeng Xia, Lizi Liu, Anping Ji, and Qiang Luo. 2022. "Fabrication of Chitosan/Hydroxyethyl Cellulose/TiO2 Incorporated Mulberry Anthocyanin 3D-Printed Bilayer Films for Quality of Litchis" Foods 11, no. 20: 3286. https://doi.org/10.3390/foods11203286
APA StyleLuo, J., Xia, G., Liu, L., Ji, A., & Luo, Q. (2022). Fabrication of Chitosan/Hydroxyethyl Cellulose/TiO2 Incorporated Mulberry Anthocyanin 3D-Printed Bilayer Films for Quality of Litchis. Foods, 11(20), 3286. https://doi.org/10.3390/foods11203286





