Optimization of the Accelerated Solvent Extraction of Caffeoylquinic Acids from Forced Chicory Roots and Antioxidant Activity of the Resulting Extracts
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Analytical Reagents and Chemicals
2.3. Optimization of the Extraction of CQAs
2.3.1. Extraction Protocol
2.3.2. Response Surface Methodology
2.4. FCR Characterization
2.4.1. Moisture Determination
2.4.2. Sugar Determination
2.4.3. Protein Determination
2.4.4. Ash (Dry Basic) Determination
2.5. HPLC Quantification of the CQAs
2.6. Antioxidant Activity
2.6.1. Measurement of Antioxidant Activity
2.6.2. Calculation of Antioxidant Activity
2.7. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Forced Chicory Root
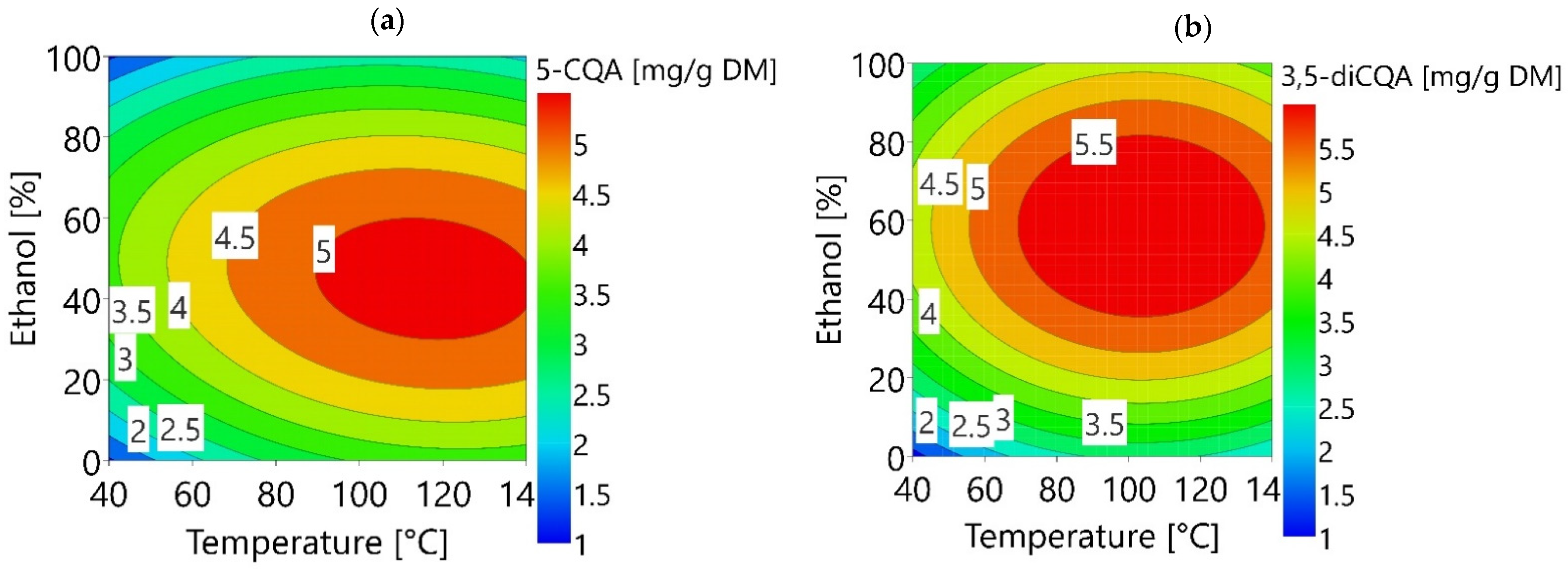
3.2. Optimization of 5-CQA and 3,5-diCQA Extraction Using RSM
3.2.1. Modelling of the Experimental Data
3.2.2. Validation of the Models on the Optimal Extraction Conditions Predicted
3.3. Optimization of the Antioxidant Capacity of Extracts
3.3.1. Modelling of the Experimental Data
3.3.2. Validation of the Model
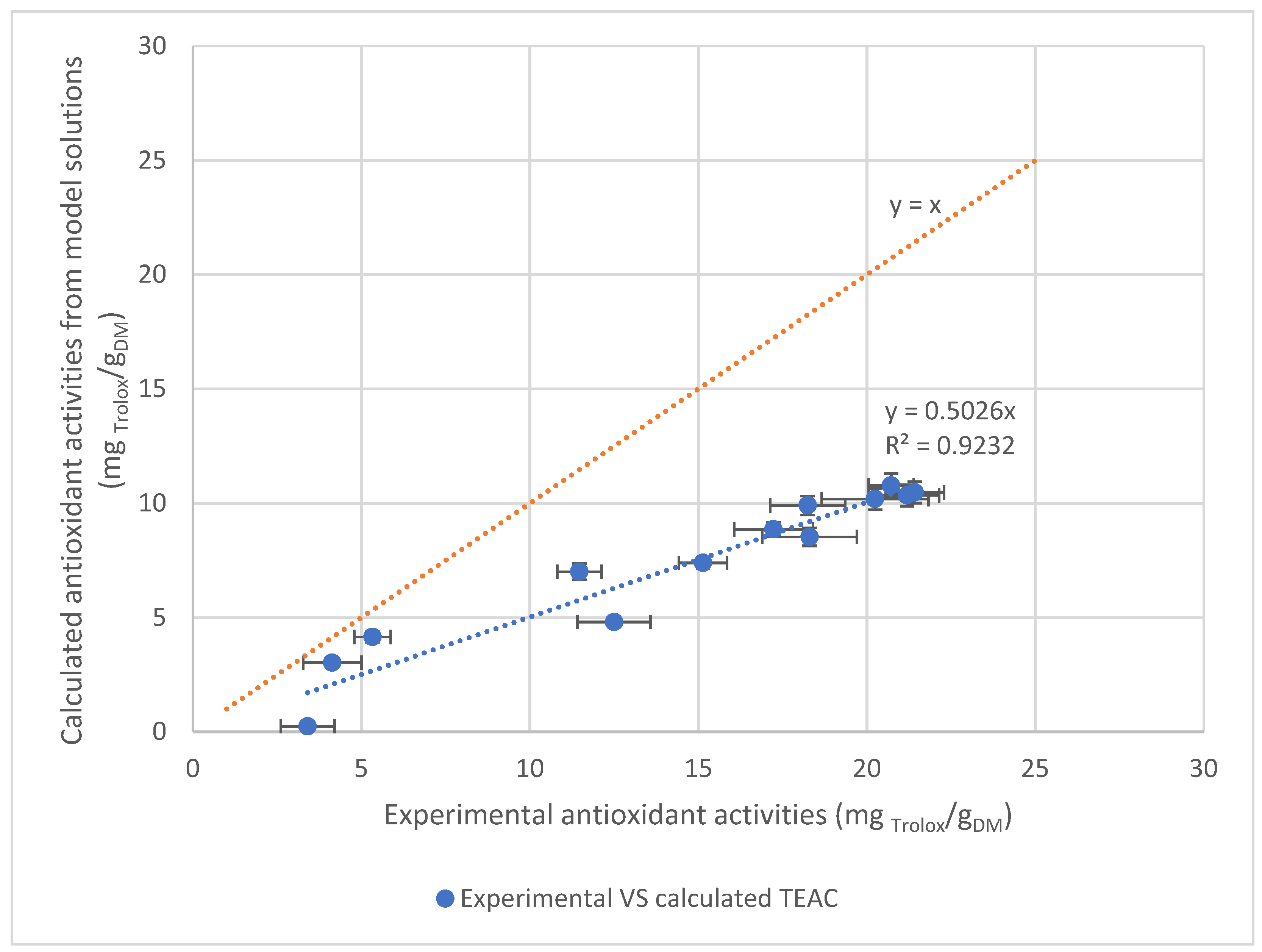
3.3.3. Correlation between Antioxidant Activity of Extracts and Caffeoylquinic Acid Contents
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baert, J.R.A.; Van Bockstaele, E.J. Cultivation and breeding of root chicory for inulin production. Ind. Crops Prod. 1992, 1, 229–234. [Google Scholar] [CrossRef]
- Nwafor, I.C.; Shale, K.; Achilonu, M.C. Chemical Composition and Nutritive Benefits of Chicory (Cichorium intybus) as an Ideal Complementary and/or Alternative Livestock Feed Supplement. Sci. World J. 2017, 2017, e7343928. [Google Scholar] [CrossRef]
- Twarogowska, A.; Van Poucke, C.; Van Droogenbroeck, B. Upcycling of Belgian endive (Cichorium intybus var. foliosum) by-products. Chemical composition and functional properties of dietary fibre root powders. Food Chem. 2020, 332, 127444. [Google Scholar] [CrossRef] [PubMed]
- Chadni, M.; Isidore, E.; Diemer, E.; Ouguir, O.; Brunois, F.; Catteau, R.; Cassan, L.; Ioannou, I. Optimization of Extraction Conditions to Improve Chlorogenic Acid Content and Antioxidant Activity of Extracts from Forced Witloof Chicory Roots. Foods 2022, 11, 1217. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Chen, B.; Yao, S. Application of ultrasonic technique for extracting chlorogenic acid from Eucommia ulmodies Oliv. (E. ulmodies). Ultrason. Sonochem. 2005, 12, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Meinhart, A.D.; da Silveira, T.F.F.; Silva, R.A.; Damin, F.M.; Bruns, R.E.; Godoy, H.T. Multivariate Optimization of Chlorogenic Acid Extraction From Brazilian Coffee. Food Anal. Methods 2017, 10, 2943–2951. [Google Scholar] [CrossRef]
- Mena-García, A.; Rodríguez-Sánchez, S.; Ruiz-Matute, A.I.; Sanz, M.L. Exploitation of artichoke byproducts to obtain bioactive extracts enriched in inositols and caffeoylquinic acids by Microwave Assisted Extraction. J. Chromatogr. A 2020, 1613, 460703. [Google Scholar] [CrossRef] [PubMed]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; FangFang, X.; Modarresi-Ghazani, F.; et al. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef]
- Tajik, N.; Tajik, M.; Mack, I.; Enck, P. The potential effects of chlorogenic acid, the main phenolic components in coffee, on health: A comprehensive review of the literature. Eur. J. Nutr. 2017, 56, 2215–2244. [Google Scholar] [CrossRef]
- Xu, J.G.; Hu, Q.P.; Liu, Y. Antioxidant and DNA-Protective Activities of Chlorogenic Acid Isomers. J. Agric. Food Chem. 2012, 60, 11625–11630. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Q.; Liu, Y.; Chen, G.; Cui, J. Antimicrobial and Antioxidant Activities of Cichorium Intybus Root Extract Using Orthogonal Matrix Design. J. Food Sci. 2013, 78, M258–M263. [Google Scholar] [CrossRef] [PubMed]
- Willeman, H.; Hance, P.; Fertin, A.; Voedts, N.; Duhal, N.; Goossens, J.F.; Hilbert, J.L. A Method for the Simultaneous Determination of Chlorogenic Acid and Sesquiterpene Lactone Content in Industrial Chicory Root Foodstuffs. Sci. World J. 2014, 2014, e583180. [Google Scholar] [CrossRef] [PubMed]
- Pradal, D.; Vauchel, P.; Decossin, S.; Dhulster, P.; Dimitrov, K. Kinetics of ultrasound-assisted extraction of antioxidant polyphenols from food by-products: Extraction and energy consumption optimization. Ultrason. Sonochem. 2016, 32, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Cova, C.M.; Boffa, L.; Pistocchi, M.; Giorgini, S.; Luque, R.; Cravotto, G. Technology and Process Design for Phenols Recovery from Industrial Chicory (Chicorium intybus) Leftovers. Molecules 2019, 24, 2681. [Google Scholar] [CrossRef] [PubMed]
- Giergielewicz-Możajska, H.; Dąbrowski, Ł.; Namieśnik, J. Accelerated Solvent Extraction (ASE) in the Analysis of Environmental Solid Samples—Some Aspects of Theory and Practice. Crit. Rev. Anal. Chem. 2001, 31, 149–165. [Google Scholar] [CrossRef]
- Frosi, I.; Montagna, I.; Colombo, R.; Milanese, C.; Papetti, A. Recovery of Chlorogenic Acids from Agri-Food Wastes: Updates on Green Extraction Techniques. Molecules 2021, 26, 4515. [Google Scholar] [CrossRef] [PubMed]
- Baiano, A.; Bevilacqua, L.; Terracone, C.; Contò, F.; Del Nobile, M.A. Single and interactive effects of process variables on microwave-assisted and conventional extractions of antioxidants from vegetable solid wastes. J. Food Eng. 2014, 120, 135–145. [Google Scholar] [CrossRef]
- Ameer, K.; Shahbaz, H.M.; Kwon, J.H. Green Extraction Methods for Polyphenols from Plant Matrices and Their Byproducts: A Review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 295–315. [Google Scholar] [CrossRef] [PubMed]
- Owusu-Ware, S.K.; Chowdhry, B.Z.; Leharne, S.A.; Antonijević, M.D. Quantitative analysis of overlapping processes in the non-isothermal decomposition of chlorogenic acid by peak fitting. Thermochim. Acta. 2013, 565, 27–33. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.O.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Ash in Biomass. Natl. Renew. Energy Lab. 2008, 9, 1–6. [Google Scholar]
- Stökle, K.; Kruse, A. Extraction of sugars from forced chicory roots. Biomass. Convers. Biorefin. 2019, 9, 699–708. [Google Scholar] [CrossRef]
- Twarogowska, A.; Van Droogenbroeck, B. Influence of cultivar and growing location on composition and functionality of dietary fibre concentrates produced from forced roots of Belgian endive (Cichorium intybus var. foliosum). J. Food Compos. Anal. 2021, 106, 104281. [Google Scholar] [CrossRef]
- Van Den Ende, W.; Mintiens, A.; Speleers, H.; Onuoha, A.A.; Van Laere, A. The metabolism of fructans in roots of Cichorium intybus during growth, storage and forcing. New Phytol. 1996, 132, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Kaur, N. Fructan Metabolism in Jerusalem Artichoke and Chicory. In Developments in Crop Science [Internet]; Elsevier: Amsterdam, The Netherlands, 2000; pp. 223–248. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0378519X00800124 (accessed on 15 March 2022).
- Dawidowicz, A.L.; Typek, R. Thermal Stability of 5-o-Caffeoylquinic Acid in Aqueous Solutions at Different Heating Conditions. J. Agric. Food Chem. 2010, 58, 12578–12584. [Google Scholar] [CrossRef]
- Wianowska, D.; Typek, R.; Dawidowicz, A.L. Chlorogenic Acid Stability in Pressurized Liquid Extraction Conditions. J. AOAC Int. 2015, 98, 415–421. [Google Scholar] [CrossRef]
- Clifford, M.N. Chlorogenic Acids. In Coffee: Volume 1: Chemistry; [Internet]; Clarke, R.J., Macrae, R., Eds.; Springer: Dordrecht, The Netherlands, 1985; pp. 153–202. (accessed on 26 April 2022). [Google Scholar] [CrossRef]
- Ji, W.; Meng, Q.; Ding, L.; Wang, F.; Dong, J.; Zhou, G.; Wang, B. Measurement and correlation of the solubility of caffeic acid in eight mono and water+ethanol mixed solvents at temperatures from (293.15 to 333.15) K. J. Mol. Liq. 2016, 224, 1275–1281. [Google Scholar] [CrossRef]
- Kayahan, S.; Saloglu, D. Nutrition and Sustainable Diets, a section of the journal Frontiers in Sustainable Food Systems Comparison of Phenolic Compounds and Antioxidant Activities of Raw and Cooked Turkish Artichoke Cultivars. Front. Sustain. Food Syst. 2021, 5, 761145. [Google Scholar] [CrossRef]
- Milala, J.; Grzelak, K.; Krol, B.; Juskiewicz, J.; Zdunczyk, Z. Composition and properties of chicory extracts rich in fructans and polyphenols. [Internet]. Pol. J. Food Nutr. Sci. 2009, 59, 35–43. Available online: http://yadda.icm.edu.pl/yadda/element/bwmeta1.element.agro-d71d38e7-f2e1-4b50-81bb-3ae5607e0d54 (accessed on 2 November 2021).
- Wijngaard, H.H.; Brunton, N. The optimisation of solid–liquid extraction of antioxidants from apple pomace by response surface methodology. J. Food Eng. 2010, 96, 134–140. [Google Scholar] [CrossRef]
- Iwai, K.; Kishimoto, N.; Kakino, Y.; Mochida, K.; Fujita, T. In vitro antioxidative effects and tyrosinase inhibitory activities of seven hydroxycinnamoyl derivatives in green coffee beans. J. Agric. Food Chem. 2004, 52, 4893–4898. [Google Scholar] [CrossRef]
- Lo Scalzo, R. Organic acids influence on DPPH scavenging by ascorbic acid. Food Chem. 2008, 107, 40–43. [Google Scholar] [CrossRef]
- Piang-Siong, W.; De Caro, P.; Marvilliers, A.; Chasseray, X.; Payet, B.; Sing, A.S.C.; Illien, B. Contribution of trans-aconitic acid to DPPH scavenging ability in different media. Food Chem. 2017, 214, 447–452. [Google Scholar] [CrossRef]
- Lalljie, S.P.D.; Vindevogel, J.; Sandra, P. Quantitation of organic acids in sugar refinery juices with capillary zone electrophoresis and indirect UV detection. J. Chromatogr. A 1993, 652, 563–569. [Google Scholar] [CrossRef]
- Papetti, A.; Maietta, M.; Corana, F.; Marrubini, G.; Gazzani, G. Polyphenolic profile of green/red spotted Italian Cichorium intybus salads by RP-HPLC-PDA-ESI-MSn. J. Food Compos. Anal. 2017, 63, 189–197. [Google Scholar] [CrossRef]
- Papetti, A.; Mascherpa, D.; Carazzone, C.; Stauder, M.; Spratt, D.A.; Wilson, M.; Pratten, J.; Ciric, L.; Lingström, P.; Zaura, E.; et al. Identification of organic acids in Cichorium intybus inhibiting virulence-related properties of oral pathogenic bacteria. Food Chem. 2013, 138, 1706–1712. [Google Scholar] [CrossRef]
- Lončarić, A.; Pichler, A.; Trtinjak, I.; Piližota, V.; Kopjar, M. Phenolics and antioxidant activity of freeze-dried sour cherry puree with addition of disaccharides. LWT 2016, 73, 391–396. [Google Scholar] [CrossRef]
- Ludwig, I.A.; Bravo, J.; De Peña, M.P.; Cid, C. Effect of sugar addition (torrefacto) during roasting process on antioxidant capacity and phenolics of coffee. LWT—Food Sci. Technol. 2013, 51, 553–559. [Google Scholar] [CrossRef]



| Experiment | Temperature X1 (°C) | Percentage of Ethanol X2 (%) |
| 1 | 40 | 0 |
| 2 | 65 | 0 |
| 3 | 115 | 0 |
| 4 | 140 | 0 |
| 5 | 40 | 50 |
| 6 | 90 | 50 |
| 7 | 140 | 50 |
| 8 | 40 | 100 |
| 9 | 65 | 100 |
| 10 | 115 | 100 |
| 11 | 90 | 50 |
| 12 | 90 | 50 |
| 13 | 90 | 50 |
| Moisture Content (g/100 g DM) | 9.2 ± 0.8 |
|---|---|
| Ash (g/100 g DM) | 6.60 ± 0.21 |
| Proteins (g/100 g DM) | 0.64 ± 0.13 |
| Fructan (g/100 g DM) | 10.18 ± 0.54 |
| D-Fructose (g/100 g DM) | 10.24 ± 0.08 |
| D-glucose (g/100 g DM) | 2.52 ± 0.05 |
| Sucrose (g/100 g DM) | 15.73 ± 1.07 |
| Experiment | Quantity of 5-CQA Extracted (mg/gDM) | Quantity of 3,5-diCQA Extracted (mg/gDM) | Antioxidant Activity of Extracts (mgTrolox/gDM) |
|---|---|---|---|
| 1 | 0.23 | N.D * | 3.41 ± 0.79 |
| 2 | 2.63 | 2.38 | 12.50 ± 1.08 |
| 3 | 3.19 | 2.17 | 15.14 ± 0.71 |
| 4 | 3.38 | 2.72 | 17.23 ± 1.17 |
| 5 | 4.22 | 5.02 | 18.30 ± 1.40 |
| 6 | 4.76 | 5.75 | 20.24 ± 1.58 |
| 7 | 4.59 | 4.64 | 18.24 ± 1.12 |
| 8 | 0.85 | 2.58 | 4.14 ± 0.86 |
| 9 | 1.26 | 3.24 | 5.33 ± 0.54 |
| 10 | 2.82 | 4.50 | 11.47 ± 0.66 |
| 11 | 5.08 | 5.74 | 21.20 ± 0.95 |
| 12 | 4.79 | 6.44 | 20.72 ± 0.67 |
| 13 | 4.93 | 5.56 | 21.42 ± 0.86 |
| Factors | Coefficients Scaled and Centred | ||
|---|---|---|---|
| Constant | α | 4.9974 | 5.6817 |
| Temp. (X1) | Β1 | 0.8004 | 0.472837 * |
| Eth. (X2) | Β2 | −0.3283 * | 0.767482 |
| Temp*Temp (X12) | Β11 | −0.8071 * | −1.10511 |
| Eth*Eth (X22) | Β22 | −2.4638 | −2.65103 |
| Temp*Eth (X1X2) | Β12 | 0.3253 * | 0.17629 * |
| Validation parameters | |||
| R2 | 0.928 | 0.930 | |
| R2adj | 0.877 | 0.881 | |
| Regression (p value) | 0.001 | 0.001 | |
| Condition number | 3.6463 | 3.6463 | |
| 5-CQA Yield (mg/gDM) | 3,5-diCQA Yield (mg/gDM) | |
|---|---|---|
| Optimal conditions for 5-CQA (107 °C, 46% ethanol) | ||
| Predicted values | 5.20 ± 0.14 | 5.64 ± 0.69 |
| Observed values | 4.95 ± 0.48 | 4.94 ± 0.70 |
| p-value (Student test) | 0.23 | 0.14 |
| Optimal conditions for 3,5-diCQA (95 °C, 57% ethanol) | ||
| Predicted values | 4.73 ± 0.14 | 5.77 ± 0.70 |
| Observed values | 4.90 ± 0.64 | 5.41 ± 0.79 |
| p-value (Student test) | 0.33 | 0.30 |
| Factors | Coefficients Scaled and Centred | DPPH Coefficients Values |
|---|---|---|
| Constant | α | 21.1548 |
| Temp. (X1) | Β1 | 2.95503 |
| Eth. (X2) | Β2 | −2.5831 |
| Temp*Temp (X12) | Β11 | −3.40446 * |
| Eth*Eth (X22) | Β22 | −9.54013 |
| Temp*Eth (X1X2) | Β12 | −1.90696 * |
| Validation parameters | ||
| R2 | 0.916 | |
| R2adj | 0.856 | |
| Regression (p value) | 0.006 | |
| Condition number | 3.64 | |
| TEAC (mgTrolox/gDM) | TEAC (mgTrolox/gDM) | |
|---|---|---|
| Optimal conditions | 107 °C, 46% ethanol | 95 °C, 57% ethanol |
| Predicted values | 21.96 ± 2.68 | 20.84 ± 2.72 |
| Observed values | 24.34 ± 2.46 | 23.36 ± 3.14 |
| p-value (Student test) | 0.16 | 0.18 |
| Molecules | TEAC (mgTrolox/mmolmolecule) |
| 5-CQA | 271.7 ± 9.4 |
| 3,5-diCQA | 508.7 ± 7.1 |
| 4,5-diCQA | 543.2 ± 4.7 |
| 3,4-diCQA | 607.8 ± 1.3 |
| CA | 283.8 ± 2.8 |
| Compounds | Concentration of Real Extract (mg/gDM) | Concentration of Model Solution (mg/gDM) |
|---|---|---|
| 5-CQA | 5.28 | 5.60 |
| 3,5-diCQA | 5.91 | 6.41 |
| 4,5-diCQA | 0.92 | 1.02 |
| 3,4-diCQA | 0.34 | 0.24 |
| CA | 0.05 | 0.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diemer, E.; Chadni, M.; Grimi, N.; Ioannou, I. Optimization of the Accelerated Solvent Extraction of Caffeoylquinic Acids from Forced Chicory Roots and Antioxidant Activity of the Resulting Extracts. Foods 2022, 11, 3214. https://doi.org/10.3390/foods11203214
Diemer E, Chadni M, Grimi N, Ioannou I. Optimization of the Accelerated Solvent Extraction of Caffeoylquinic Acids from Forced Chicory Roots and Antioxidant Activity of the Resulting Extracts. Foods. 2022; 11(20):3214. https://doi.org/10.3390/foods11203214
Chicago/Turabian StyleDiemer, Etienne, Morad Chadni, Nabil Grimi, and Irina Ioannou. 2022. "Optimization of the Accelerated Solvent Extraction of Caffeoylquinic Acids from Forced Chicory Roots and Antioxidant Activity of the Resulting Extracts" Foods 11, no. 20: 3214. https://doi.org/10.3390/foods11203214
APA StyleDiemer, E., Chadni, M., Grimi, N., & Ioannou, I. (2022). Optimization of the Accelerated Solvent Extraction of Caffeoylquinic Acids from Forced Chicory Roots and Antioxidant Activity of the Resulting Extracts. Foods, 11(20), 3214. https://doi.org/10.3390/foods11203214








