Abstract
Enzymatic alcoholysis was performed in an organic medium to synthesize 2-monoacylglycerol (2-MAG) rich in arachidonic acid. The results showed that solvent type and water activity (aw) significantly affected the 2-MAG yield. Under the optimum conditions, 33.58% 2-MAG was produced in the crude product in t-butanol system. Highly pure 2-MAG was obtained after two-stage extraction using 85% ethanol aqueous solution and hexane at first stage and dichloromethane and water at second stage. Isolated 2-MAG was used as substrate to investigate the effect of solvent type and aw on 2-MAG acyl migration in a lipase-inactivated system. The results indicated that non-polar solvents accelerated the acyl migration of 2-MAG, whereas isomerization was inhibited in polar solvent systems. The aw exhibited the strongest inhibition effect on 2-MAG isomerization at 0.97, but also affected the hydrolysis of glycerides and lipase selectivity.
1. Introduction
Structured lipids have modified structures from changing fatty acid composition and/or positional distribution by interesterification, transesterification, acidolysis, or alcoholysis [1,2,3,4]. Structural lipids have wider application and greater added-value than natural lipids. Their enhanced functionality and nutritional value, such as those rich in polyunsaturated fatty acids, can prevent neurodegenerative diseases [5,6,7]. The position of fatty acid on the glycerol backbone is an important factor affecting the physiological function and nutritional value of a structured lipid. 2-Monoacylglycerol (2-MAG) rich in polyunsaturated fatty acids has been demonstrated to have many biological functions [8]. Many studies have shown that the endogenous lipid signaling molecule 2-arachidonoylglycerol (2-AG) plays a vital role in the immune system, cardiovascular system, and central nervous system, with physiological functions including modulation of anxiety, regulation of appetite, and anti-inflammation and anti-tumor effects [9,10,11,12,13]. Thus, synthesis of 2-MAG rich in arachidonic acid (ARA) is of great practical significance.
2-MAG can be prepared by enzymatic or chemical synthesis. Chemical methods for 2-MAG synthesis require higher temperatures or longer reaction times, resulting in promotion of 2-MAG acyl migration and induction of ARA oxidation [14]. In addition, chemical synthesis has the disadvantages of low yield, extremely high raw material costs, and usage of toxic catalyst [15,16]. Thus, 2-MAG is usually prepared by lipase-mediated synthesis [4,17]. Compared to chemical synthesis, enzymatic synthesis has the advantages of milder reaction conditions, easily available raw materials, higher selectivity, limited byproducts, and less waste production, and is more suitable for production of 2-AG [18]. Enzymatic alcoholysis is an efficient way to obtain 2-AG using lipase B from Candida antarctica [17,19,20]. Regardless of the methods used, acyl migration, a spontaneous thermodynamic process, is inevitable in 2-MAG synthesis [20,21,22]. Generally, the fatty acid originally at the sn-2 position is spontaneously transferred to the sn-1 position to form undesirable 1-monoacylglycerol (1-MAG), resulting in decreased yield of 2-MAG and activity loss. Previous studies have shown that acyl migration reaches equilibrium at a 9:1 ratio of 1-MAG to 2-MAG [22]. Thus, to prepare 2-MAG with a high yield, it is necessary to understand the process and inhibition of acyl migration in enzymatic alcoholysis [20,23]. Many factors affect acyl migration of 2-MAG, including aw, temperature, time, type of solvent, and carrier material of the immobilized enzyme [24,25,26]. It is known that temperature and lipase carrier material accelerate acyl migration; the mechanism is relatively clear [22,25]. Few studies have reported the effects of water activity (aw) and solvent type on isomerization of partial acylglycerols [24,25,26,27].
In this study, the effects of the reaction medium and aw on acyl migration in alcoholysis were investigated and the influencing mechanism was discussed. The conditions for preparation of 2-AG by lipase-catalyzed alcoholysis of Mortierella alpina oil were optimized, and the effects of the solvent and aw on alcoholysis were investigated. To further study the effect of medium and aw separately, 2-AG was purified from the crude product prepared by enzymatic alcoholysis and was used as the raw material to investigate the effects of the solvent and aw on acyl migration in the enzyme inactivation system.
2. Materials and Methods
2.1. Materials
Refined ARA-enriched fungal oil from M. alpina was provided by CABIO Biotechnology Co., Ltd. (Wuhan, China) and stored at −20 °C until use. The major fatty acids in the ARA oil were ARA (46.5%), tetracosanoic acid (11.7%), palmitic acid (9.8%), stearic acid (6.3%) and oleic acid (6.1%). Lipozyme 435 (immobilized lipase B from Candida antarctica, immobilized on acrylic resin, 10 U/mg), Lipozyme RM IM (1,3-specific lipase from Rhizomucor miehei, immobilized on ion-exchange resins, 275 IUN/g), and Lipozyme TL IM (immobilized 1,3-specific lipase from Thermomyces lanuginosus, immobilized on silica gel, 30 U/g) were generously donated by Novozymes (Beijing, China). Lipase CL “Amano” IM (immobilized lipase B from Candida antarctica, 400 U/g), Lipase DF “Amano” IM (immobilized 1,3-specific lipase from Rhizopus oryzae, 600 U/g), Lipase DF “Amano” 15 (free 1,3-specific lipase from Rhizopus oryzae, 150 U/mg), Lipase AY “Amano” S (free lipase from Candida cylindracea, 30 U/mg), and Lipase G “Amano” 50 (free lipase from Penicillium camemberti, 50 U/mg) were generously donated by Amano Enzyme, Inc. (Wuxi, China). One lipase unit (U) was defined as the amount of lipase producing 1 μmol of fatty acid per min at 30 °C at pH 7.0 using triolein as substrate. Specific activity was defined as units per milligram of protein. 1-monopalmitin and 2-monopalmitin standards were obtained from Sigma-Aldrich Chemical Co., Ltd. (Shanghai, China). HPLC-grade hexane, isopropyl alcohol, and methanoic acid were obtained from Beijing J & K Scientific Company (Beijing, China). Other analytical-grade organic solvents were purchased from Sinopharm Chemical Reagent Company (Shanghai, China).
2.2. Preparation of 2-MAG by Enzymatic Alcoholysis at a Controlled aw
Reactants including 2 g of fungal oil and 4 g of ethanol (molar ratio of 1:40, oil to ethanol) were mixed in a 25-mL glass vessel with magnetic agitation at 400 rpm. Lipase was used as a biocatalyst to start the reaction. Alcoholysis was performed at a controlled aw; additional solvent was added to the reaction system to investigate the effect of solvent type. After completion of alcoholysis, the product was centrifuged at 4000 rpm for 4 min to fully remove the lipase. The solvents were removed by rotary evaporation at 40 °C to obtain the final alcoholysis product for HPLC analysis.
To maximize 2-MAG content in the crude product, eight commercial lipases used in the alcoholysis system were evaluated and screened in the following conditions: 1:40 molar ratio of oil to ethanol, lipase load of 8% or 15% (relative to mass of oil), aw of 0.53, 35 °C, without additional solvent. The effects of solvent type, solvent quantity, aw, alcoholysis temperature, and time on 2-MAG content in the crude product were studied. The solvents included ethanol, acetone, t-butanol, dichloromethane, and hexane. The solvent quantity ranged from 1 mL to 4 mL; aw ranged from 0.11 to 0.97. The alcoholysis temperature and time ranged from 25 °C to 40 °C and from 2 h to 8 h, respectively. After a factor optimization was completed, the optimized value was used for the next factor optimization.
During enzymatic alcoholysis, the aw of the reaction system was accurately controlled using a previous method [28]. A certain aw was achieved by equilibrating the reaction mixture and lipase with an aqueous saturated salt solution at room temperature for 24 h in two separated desiccators. The saturated salt solution had a known aw: 0.11 for saturated LiCl solution, 0.53 for saturated Mg(NO3)2 solution, 0.74 for saturated NaNO3 solution, and 0.97 for saturated K2SO4 solution.
2.3. Purification of Crude Product to Prepare High Purity 2-MAG
After removal of the lipase and solvent, fatty acid ethyl ester (FAEE), diacylglycerol (DAG), and triacylglycerol (TAG) in the crude product were removed by solvent extraction to obtain an 85% ethanol aqueous solution containing 2-MAG according to the method used by Zhang et al. [4]. In addition to 2-MAG, the polar ethanol solution may also contain trace amounts of lipase protein and glycerol. Thus, further purification was required. Purified 2-MAG was obtained after removal of water and ethanol from the polar phase. Purified 2-MAG (3 g) was mixed with 100 mL of dichloromethane. An equal amount of distilled water was added for extraction. After two layers were observed, a dichloromethane layer containing 2-MAG was collected. The solvent was removed by evaporation under reduced pressure at 25 °C to obtain the final purified 2-MAG product, which was stored in a refrigerator at −20 °C for further use. The isolated yield of 2-MAG was calculated according to the ratio of the mass of isolated 2-MAG to the theoretical mass of 2-MAG.
2.4. Acyl Migration of 2-MAG in Lipase-Inactivated System
To eliminate interference of other factors and investigate the effects of solvent type and aw on the acyl migration of 2-MAG, a lipase-inactivated system was used. Purified 2-MAG (0.7 g) and inactivated Lipozyme TL IM (0.3 g) were mixed with 7 mL of solvent (ethanol, acetone, t-butanol, dichloromethane, or hexane). Acyl migration was conducted at 30 °C in a sealed vessel containing a saturated salt solution of LiCl, Mg(NO3)2, NaNO3, or K2SO4 with an aw of 0.11, 0.53, 0.74, or 0.97, respectively, for the reaction system. Periodically, 100-μL samples were collected from the reaction crude products and analyzed by a high-performance liquid chromatography refractive index detector (HPLC–RID) after adding 1 mL of mobile phase solvent.
To ensure that the lipase was totally inactivated, alcoholysis was conducted in the following conditions: 1:40 molar ratio of oil to ethanol, 15% inactivated Lipozyme TL IM, 3 mL t-butanol as solvent, aw of 0.53, and 30 °C. After 24 h of reaction, samples were collected from the crude product and analyzed by HPLC–RID. We concluded that the lipase was totally inactivated when no FAEE or partial acylglycerol was observed.
2.5. Analysis of Alcoholysis Product by HPLC–RID
Identification and quantification of the reaction crude product and purified 2-MAG were determined by HPLC–RID (Waters Corp., Milford, MA, USA) using a Sepax HP-Silica column (particle size of 5 μm, 4.6 mm × 250 mm, Sigma-Aldrich Corp., K.K., Tokyo, Japan). The samples were eluted with solvent at 1.0 mL/min and the column temperature was maintained at 30 °C. The solvent was a mixture containing hexane, isopropanol, and methanoic acid with a ratio of 15:1:0.003 (v/v/v). The glyceride standards were used to identify the HPLC peaks of the samples. The standard curve obtained from the peak areas and the standard concentrations were used to quantify the glyceride product. All reactions and their corresponding analyses were carried out in triplicate.
2.6. Statistical Analysis
All data were analyzed by one-way analysis of variance (one-way ANOVA) using Origin 2018 (Origin Lab, Northampton, MA, USA). Tukey’s test was conducted to determine significant differences at the p < 0.05 level. Data presented in the figures represent average values with standard deviations.
3. Results and Discussion
3.1. Effect of Lipase Type and Load on Alcoholysis
Eight lipases from two enzyme preparation companies exhibited different catalytic activities in alcoholysis for synthesis of 2-MAG. When the added amount of lipase (relative to the mass of oil) was 8%, as shown in Figure 1a, three immobilized lipases, Lipozyme 435, Lipozyme TL IM, and Lipase CL IM exhibited considerably higher catalytic activities than the other lipases. Lipozyme 435 and Lipase CL IM were derived from Candida antarctica lipase B; the 2-MAG contents in the crude product reached 23.02% and 19.34%, respectively, after 8 h with these lipases as biocatalysts. In the Lipozyme TL IM-catalyzed reaction system, 11.73% 2-MAG was produced in the crude product. For other lipases as catalysts, no 2-MAG was produced in the system with ethanol as substrate. In this study, 1 mol TAG can theoretically be converted to 2 mol FAEE and 1 mol 2-MAG. Thus, the maximum 33.3% molar content of 2-MAG in the crude product is equal to a 100% 2-MAG yield.
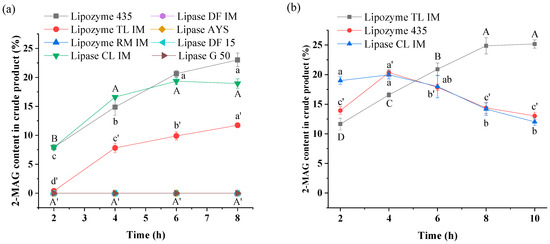
Figure 1.
Effects of lipase type on the content of 2-MAG in the crude product with 8% (a) and 15% lipase load (b). Reaction conditions: (a) molar ratio of 1:40 (fungal oil/ethanol), lipase load of 8% (relative to the mass of oil), aw of 0.53, 35 °C; (b) molar ratio of 1:40 (fungal oil /ethanol), lipase load of 15% (relative to the mass of oil), aw of 0.53, 35 °C. Different letters in the figure indicated significant differences at the p < 0.05 level.
Previous studies have demonstrated that lipases from Lipozyme 435 and Lipozyme TL IM show strong 1,3-specificity in alcoholysis [29,30]. In this study, we also found that another commercial lipase from Candida antarctica lipase B (Lipase CL IM) also had 1,3-specificity in alcoholysis. Thus, these are suitable biocatalysts to prepare 2-MAG. Lipozyme RM IM, Lipase DF IM, and Lipase DF 15 were also considered as 1,3-specific lipases in acidolysis [31] but showed no catalytic activities in alcoholysis, indicating that they may have low tolerance to polar ethanol [32,33]. This result is in agreement with results from our previous study [34].
According to the results in Figure 1a, Lipozyme 435 seemed to be the best choice for preparation of 2-MAG by alcoholysis, followed by Lipozyme TL IM. A low 2-MAG content in the Lipozyme TL IM-mediated system may be attributed to a low lipase load. Thus, these three lipases were selected for further experiments with the lipase load increased to 15%. Figure 1b shows that the highest 2-MAG content (24.87%) obtained in the crude product after 8 h was observed in the Lipozyme TL IM-catalyzed system, considerably higher than the contents in Lipozyme 435- and Lipase CL IM-mediated systems. The 2-MAG content in alcoholysis product obtained with Lipozyme 435 or Lipase CL IM as biocatalysts reached the maximum at 4 h before decreasing, possibly due to a high load of immobilized material and a long reaction time, increasing the probability of 2-MAG isomerization to 1-MAG, which was further converted to FAEE and glycerol by a 1,3-specific lipase, ultimately leading to a decrease in 2-MAG yield [20]. Compared to Lipozyme 435 and Lipase CL IM (approximately 2300 USD/kg), Lipozyme TL IM is inexpensive (approximately 100 USD/kg). In previous 2-MAG synthesis reactions, most studies used catalytic efficiency as a measure and lipase B from Candida antarctica as the optimum lipase to catalyze the alcoholysis reaction [17,19,20,29]. However, in this study, the same catalytic efficiency was obtained with 8% Lipozyme 435 and 15% Lipozyme TL IM. With this in mind, the cost of the reaction was considered; 15% Lipozyme TL IM was used for subsequent optimization.
3.2. Optimization of Reaction Conditions for Enzymatic Alcoholysis
3.2.1. Effect of Solvent Type on Enzymatic Alcoholysis
Five solvents with log P ranging from −0.24 to 3.5 were selected to investigate the effect of different solvents on enzymatic alcoholysis. The log P values for ethanol, acetone, t-butanol, dichloromethane, and hexane are in descending order. In the reaction of enzymatic preparation of 2-MAG, organic solvent, as a reaction medium, usually affects the catalytic activity [35] and the acyl migration of partial glycerides in the reaction, which affects the 2-MAG yield. Thus, the effect of solvent type was optimized in this study. The optimized reaction conditions are presented in Figure 2a.
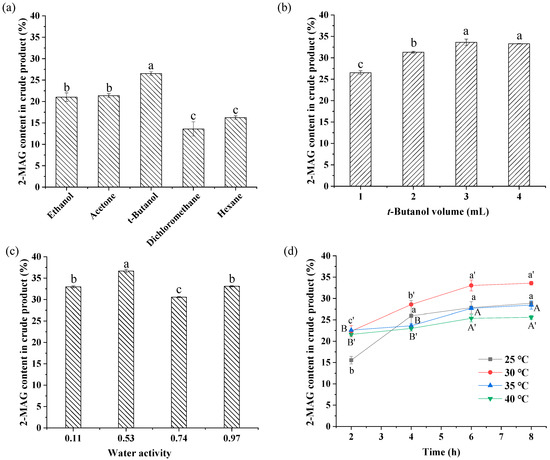
Figure 2.
Optimization of rea ction conditions of enzymatic alcoholysis. Reaction conditions: (a) molar ratio of 1:40 (fungal oil/ethanol), 15% (relative to the mass of oil) Lipozyme TL IM, solvent volume of 1 mL, aw of 0.53, 35 °C, 8 h. (b) molar ratio of 1:40 (fungal oil/ethanol), 15% (relative to the mass of oil) Lipozyme TL IM, t-butanol as solvent, aw of 0.53, 35 °C, 8 h. (c) molar ratio of 1:40 (fungal oil/ethanol), 15% (relative to the mass of oil) Lipozyme TL IM, 3 mL t-butanol as solvent, 35 °C, 8 h. (d) molar ratio of 1:40 (fungal oil/ethanol), 15% (relative to the mass of oil) Lipozyme TL IM, 3 mL t-butanol as solvent, aw of 0.53. Different letters in the figure indicated significant differences at the p < 0.05 level.
Figure 2a shows that solvent type had a significant effect on the content of 2-MAG. In the t-butanol system with moderate polarity, the 2-MAG content reached 26.53%, considerably higher than contents obtained in other solvent systems. The lowest 2-MAG contents in the crude product were observed in non-polar media (dichloromethane and hexane). This result is consistent with results reported by Damstrup et al. [36] in synthesizing MAG by glycerolysis with Lipozyme TL IM as biocatalyst in 13 solvent systems. In that study, t-butanol effectively improved the poor miscibility of reactants between glycerol and oil, leading to an increased MAG yield in the t-butanol medium. However, in this study, improvement of reactant miscibility was not the only cause of increased 2-MAG content in the t-butanol system; hexane and dichloromethane also improved reactant miscibility between ethanol and oil. The effects of solvent polarity on acyl migration of 2-MAG and lipase activity also play a crucial role in enzymatic alcoholysis [17,37]. Based on the results, t-butanol was chosen as the best solvent for further optimization.
3.2.2. Effect of Added Amount of t-Butanol on Enzymatic Alcoholysis
In the alcoholysis system, ethanol acts as a reaction substrate and the medium. Addition of t-butanol was conducive to improving the 2-MAG yield from alcoholysis. Thus, it was necessary to determine the optimal amount of added t-butanol. It is observed in Figure 2b that the 2-MAG content increased with an increase in t-butanol volume ranging from 1 mL to 3 mL. Overall, the addition of t-butanol increased the yield. A large amount of added t-butanol may be beneficial for inhibition of acyl migration of 2-MAG or for enhancing the solubility of reactant and product [17]. However, no significant differences in 2-MAG content were found with 3 mL and 4 mL of solvent. For environmental protection, use of solvents should be minimized. Thus, 3 mL of t-butanol was considered as the optimum added amount.
3.2.3. Effect of aw on Enzymatic Alcoholysis
Generally, aw has a large impact on acyl migration of partial glycerides and enzyme activity [22]. However, few studies have reported the effect of aw on the enzymatic reaction. Several studies mentioned the effect of aw on the acidolysis and esterification reactions [38,39]. The effect on the alcoholysis reaction is still uncertain. This study is the first to consider the effect of aw on the enzymatic alcoholysis reaction. Figure 2c compares the 2-MAG content in crude alcoholysis products with aw ranging from 0.11 to 0.97. It is observed that the 2-MAG content is related to the aw of the system. When aw was increased from 0.11 to 0.53, the 2-MAG content increased from 32.98% to 36.68%. As aw was increased to 0.74, the 2-MAG content decreased to 30.57%. Increasing the aw to 0.97 increased the 2-MAG content to 33.11%, lower than that at 0.53. Thus, 0.53 was selected as the most appropriate aw for alcoholysis.
3.2.4. Effect of Temperature and Time on Enzymatic Alcoholysis
Figure 2d shows the change in 2-MAG content of the crude product at different temperatures over time. Increasing the reaction temperature generally increases the mass transfer, improves the reaction rate, and shortens the reaction time. However, excessive temperature may reduce the activity and stability of the lipase. In Figure 2d, when the reaction temperature was increased from 25 °C to 30 °C, the 2-MAG content increased from 28.89% to 33.58%. Increasing temperature further from 30 °C to 40 °C resulted in a continuous decrease in 2-MAG content, possibly attributed to an increased rate of acyl migration caused by a relatively high temperature [21,22]. Moreover, ARA-rich fungal oil with many unsaturated double bonds is vulnerable to temperature. MAG synthesis must be conducted at a relatively low temperature; 30 °C was the ideal temperature for 2-MAG synthesis.
At 30 °C, with prolonged reaction time, the sn-1(3) fatty acids of TAG were cleaved by lipase, leading to formation of 2-MAG. After reaction for 6 h, the alcoholysis reached equilibrium. There was no significant difference in 2-MAG content at 6 h and 8 h. According to Figure 2d, 6 h was considered as the optimum reaction time. In these conditions, 33.05% 2-MAG was formed in the crude product. After purification by solvent extraction, impurities including FAEE, DAG, and TAG were removed and the 2-MAG purity reached 98.5%. The purified 2-MAG was used as substrate for the subsequent acyl migration study.
3.3. Effect of Solvent Type and aw on 2-MAG Isomerization in Catalyst-Free System
From the results, solvent type and aw had strong effects on Lipozyme TL IM-catalyzed alcoholysis, possibly attributed to their effects on acyl migration of 2-MAG and lipase activity during the reaction [24,27]. With the involvement of acyl migration, the enzymatic alcoholysis has multiple reactions occurring simultaneously, and it is difficult to directly evaluate the intensity of several acyl migration reactions. To confirm our speculation and to study how solvent type and aw affect 2-MAG isomerization, the acyl migration of 2-MAG was chosen as a representative and a catalyst-free system was established by totally inactivating the lipase. The catalyst-free acyl migration system consisted of inactivated Lipozyme TL IM, solvent, and purified MAG with concentrations similar to those of the crude product. The acyl migration was conducted at 30 °C, consistent with the optimum conditions of the alcoholysis reaction, to simulate as much as possible the environment of the enzymatic alcoholysis. The acyl migration of 2-MAG in this system is described in Figure 3. The change in the ratio of 2-MAG to total MAG with time was investigated in the lipase-inactivated system, as shown in Figure 3. A lower ratio of 2-MAG to total MAG indicates more 2-MAG migrating to 1-MAG and a faster acyl migration rate.
Figure 3.
The migration of 2-MAG to 1-MAG.
3.3.1. Effect of Solvent Type on 2-MAG Isomerization
With the lipase totally inactivated and aw maintained at 0.53, only the solvent affects 2-MAG isomerization. According to Figure 4a, after 20 days, the 2-MAG/MAG ratios in the ethanol, acetone, t-butanol, dichloromethane, and hexane systems were 73.14%, 68.76%, 79.98%, 64.62%, and 34.01%, respectively, indicating that t-butanol exhibited the best inhibitory effect on acyl migration with time, followed by ethanol and acetone. Non-polar dichloromethane and hexane, especially hexane with the lowest polarity, strongly promoted acyl migration. These results suggest that acyl migration of 2-MAG was closely related to solvent polarity (log P). However, the log P of the solvent is not the only parameter determining the acyl migration rate; acyl migration of 2-MAG in ethanol (log P of −0.24) and acetone (log P of −0.23) were faster than in t-butanol (log P of 0.6) (Figure 4a). Generally, the more polar the solvent, the stronger the inhibition of acyl migration.
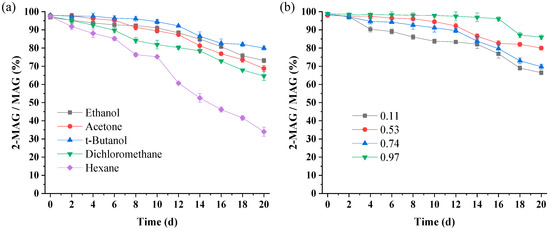
Figure 4.
Acyl migration of 2-MAG in various solvents (a) and aw (b) at 30 °C for 20 days. Acyl migration conditions: (a) 0.7 g of purified 2-MAG, 7 mL of solvent, 0.3 g inactivated Lipozyme TL IM, aw of 0.53; (b) 0.7 g of purified 2-MAG, solvent of 7 mL t-butanol, 0.3 g inactivated Lipozyme TL IM.
Acyl migration is a nucleophilic substitution reaction [40]. Negatively charged hydroxyl oxygen at the sn-1 position of 2-MAG has lone pair electrons, which nucleophilic attack on the positively charged carbon, leading to the formation of a five-membered ring intermediate [40,41,42]. Subsequently, the hydroxyl oxygen again makes a nucleophilic attack on the carbon, the strained ring opens and 1-MAG is formed [24]. The high-polarity systems are not conducive to charge dispersion in the transition state and the energy of the transition state increased, leading to a reduction in the acyl migration rate [20,24]. While slower acyl migration occurred in t-butanol with higher log P value than ethanol and acetone (Figure 4a). Damstrup et al. [36] have pointed out that both polarity and functional groups play an important role in the performance of solvents. Thus, the inhibition of t-butanol may be due to its special functional groups, which increase the activation energy of acyl migration [24].
The effect of solvent type on the other lipid components of the crude product is shown in Figure 5a. The main impurity in the alcoholysis product was FAEE, followed by DAG and TAG. TAG contents of crude product in the dichloromethane and hexane systems were 12.84% and 11.96%, respectively, several times greater than those in the ethanol, acetone, and t-butanol systems. Correspondingly, FAEE levels in nonpolar systems were lower, indicating that the alcoholysis of glycerides progressed slowly in these two systems. Theoretically, the miscibility of oil with a low-polar solvent is better than with a polar solvent; the contact areas between enzyme and substrate and between substrate and substrate are larger [43], which usually leads to improved catalytic efficiency of the enzyme. Surprisingly, increasing the miscibility of the oil with ethanol in a non-polar system led to a decreased 2-MAG yield in lipase-catalyzed alcoholysis. There are two explanations for this observation. First, the low-polar solvents strongly promoted acyl migration of 2-MAG to 1-MAG, which was subsequently converted to glycerol and FAEE by Lipozyme TL IM. In addition, in organic solvents, the substrate must be separated from the solvent before binding to the active center of the lipase (desolvation) [44]. The hydrophobic matrix oil binds more closely to more nonpolar solvents, making it difficult to “squeeze out” of the solvents, hindering interaction between the substrate and the lipase, reducing the catalytic efficiency of the lipase. Thus, only approximately 15% 2-MAG was produced in dichloromethane and hexane systems in Lipozyme TL IM-catalyzed alcoholysis after 8 h (Figure 2a).
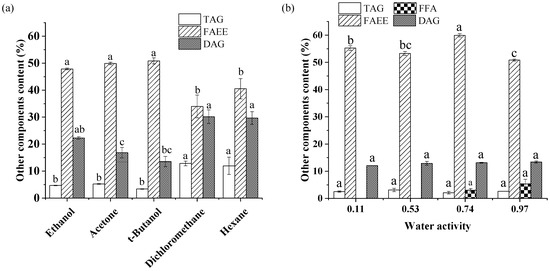
Figure 5.
Effect of solvent type (a) and aw (b) on the contents of other lipid components in the crude product. Reaction conditions: (a) molar ratio of 1:40 (fungal oil/ethanol), 15% (relative to the mass of oil) Lipozyme TL IM, solvent volume of 1 mL, aw of 0.53, 35 °C, 8 h; (b) molar ratio of 1:40 (fungal oil/ethanol), 15% (relative to the mass of oil) Lipozyme TL IM, 3 mL t-butanol as solvent, 35 °C, 8 h. Different letters in the figure indicated significant differences at the p < 0.05 level.
3.3.2. Effect of aw on 2-MAG Isomerization
The effect of aw on the 2-MAG yield in Lipozyme-catalyzed alcoholysis may also be attributed to its effect on acyl migration. When the lipase was totally inactivated and the solvent was t-butanol, only aw affected 2-MAG isomerization. According to the change trend of the 2-MAG/MAG ratio over 20 days shown in Figure 4b, the rate of acyl migration for different aw values can be ranked corresponding to 0.11 > 0.74 > 0.53 > 0.97. The highest acyl migration rate occurred in the 0.11 aw system. Increasing aw led to a decrease in acyl migration in the catalyst-free system. Many studies have shown that the polarity of the system increases with an increase in aw, which is not conducive to charge dispersion of the acyl migration transition state, inhibiting acyl migration [17,27,39] With aw ranging from 0.53 to 0.97, a free fatty acid hydrolysis product was formed in the crude product, indicating that hydrolysis occurred at a high aw level.
In the enzymatic preparation of 2-MAG, the lipase must maintain a certain degree of flexibility to reach the required conformation in the state of the greatest fit with the substrate [28]. Duan et al. [28] indicated that the presence of water could make the stable closed structure of the enzyme “loose”, enhancing the flexibility of the enzyme molecule and improving the catalytic activity. However, as shown in Figure 5b, the FAEE content in the product with an aw of 0.11 was considerably higher than that in the system with an aw of 0.53, indicating that the main reason for the change in 2-MAG content in different aw conditions may not be the change in enzyme activity. With a continuous increase in aw, excessive water pushed the enzyme molecules past the optimal state; the structure became too soft and loose, weakening the catalytic activity of the enzyme [44]. When aw was increased to 0.97, inhibition of acyl migration of 2-MAG was beneficial to the increase in 2-MAG yield in enzymatic alcoholysis. However, the lowest FAEE level indicated that an excessively high aw decreased enzyme activity, producing a less favorable environment than with an aw of 0.53. In the 0.53 aw system, the enzyme was in the best hydration state and the acyl migration was relatively slow; the highest 2-MAG content was observed in Lipozyme TL IM-catalyzed alcoholysis.
3.3.3. Acyl Migration of 2-MAG in Lipase-Catalyzed and Lipase-Inactivated Systems
In the lipase-catalyzed system, when 2-MAG is migrated to 1-MAG, the produced 1-MAG is continuously converted to FAEE and glycerol by the 1,3-specific lipase (Lipozyme TL IM), resulting in a decreased level of 1-MAG or total MAG. Thus, acyl migration of 2-MAG in lipase-catalyzed system leads to the formation of glycerol and FAEE rather than 1-MAG. The ratio of 2-MAG to MAG cannot be an indicator to reflect the degree of acyl migration in enzymatic alcoholysis, whereas a high level of FAEE in the product may be attributed to enhanced alcoholysis of TAG by the sn-1,3 lipase rather than acyl migration. Additionally, sn-1,3 selectivity of the lipase in lipase-catalyzed system also varies with the solvent type and aw [44,45]. When solvent type or aw may make the sn-1,3 specific lipase has higher catalytic activity toward the fatty acid at the sn-2 position (becoming non-specific), the non-specific lipase can convert 2-MAG directly to FAEE in the presence of ethanol. Therefore, a low 2-MAG content in the product in enzymatic alcoholysis may be attributed to increased non-specificity of the lipase rather than acyl migration of 2-MAG. For these reasons, the influences of solvent type and aw on the enzymatic alcoholysis were discussed by separately observing the effects on the acyl migration of 2-MAG in the catalyst-free system. The degree of migration can be evaluated by calculating the ratio of 2-MAG to MAG in the catalyst-free system. However, the actual acyl migration in lipase-catalyzed system is more complicated than that in the catalyst-free system. In non-enzymatic system, the effects of solvent type and aw on acyl migration can be observed more visually and accurately, but the role of lipase was ignored. Based on the study in the lipase-inactivated system, we can conclude that different solvents and aw significantly affect the acyl migration of 2-MAG in lipase-mediated alcoholysis, but influencing degree is still unknown. In further study, the influencing mechanism of the synergistic effect of lipase and acyl migration should be investigated by other evaluative measures such as kinetic parameters rather than product concentration.
4. Conclusions
2-AG was successfully prepared by efficient enzymatic alcoholysis. After optimization of the alcoholysis conditions, the maximum 2-MAG content in the crude product was 33.58%, and the 2-MAG purity reached 98.5% after purification. Solvent type and aw had considerable effects on alcoholysis. They also affected the acyl migration of 2-MAG in the catalyst-free system. 2-MAG migrated much faster in non-polar solvents than in polar solvents. Aw had multiple effects on enzymatic alcoholysis. Overall, a high aw is beneficial for inhibiting acyl migration of 2-MAG.
Author Contributions
Conceptualization, X.W. (Xiaosan Wang); methodology, X.W. (Xiaohan Wang); formal analysis, X.W. (Xiaohan Wang); investigation, X.W. (Xiaohan Wang), K.L. and Y.W.; data curation, X.W. (Xiaohan Wang), K.L. and Z.H.; writing—original draft preparation, X.W. (Xiaohan Wang); writing—review and editing, X.W. (Xiaosan Wang); funding acquisition, X.W. (Xiaosan Wang). All authors have read and agreed to the published version of the manuscript.
Funding
This study was financially supported by National Natural Science Foundation of China (grant nos. 31972035).
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Moreira, D.K.T.; Gandra, R.L.P.; Zuin, J.C.; Ract, J.N.R.R.; Ribeiro, A.P.B.; Juliana Alves Macedo, J.A.; Gambero, A.; Akil, E.; Torres, A.G.; Macedo, G.A. Synthesis and characterization of structured lipid rich in behenic acid by enzymatic interesterification. Food Bioprod. Process. 2020, 122, 303–310. [Google Scholar] [CrossRef]
- Mateos, P.S.; Navas, M.B.; Morcelle, S.R.; Ruscitti, C.; Matkovic, S.R.; Briand, L.E. Insights in the biocatalyzed hydrolysis, esterification and transesterification of waste cooking oil with a vegetable lipase. Catal. Today 2021, 372, 211–219. [Google Scholar] [CrossRef]
- Abed, S.M.; Wei, W.; Ali, A.H.; Korma, S.A.; Mousa, A.H.; Hassan, H.M.; Jin, Q.; Wang, X. Synthesis of 6structured lipids enriched with medium-chain fatty acids via solvent-free acidolysis of microbial oil catalyzed by Rhizomucor miehei lipase. LWT-Food Sci. Technol. 2018, 93, 306–315. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Xie, D.; Zou, S.; Jin, Q.; Wang, X. Synthesis and concentration of 2-monoacylglycerols rich in polyunsaturated fatty acids. Food Chem. 2018, 250, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Xu, F.; Zhang, N.; Wu, Y.; Ju, X.; Wang, L. Dietary a novel structured lipid synthesized by soybean oil and coconut oil alter fatty acid metabolism in C57BL/6J mice. Food Biosci. 2021, 44, 101396. [Google Scholar] [CrossRef]
- Ledesma, R.; Martínez-Pérez, R.B.; Curiel, D.A.; Fernández, L.M.; Silva, M.L.; Canales-Aguirre, A.A.; Rodríguez, J.A.; Mateos-Díaz, J.C.; Preza y Lerma, A.M.; Madrigal, M. Potential benefits of structured lipids in bulk compound chocolate: Insights on bioavailability and effect on serum lipids. Food Chem. 2022, 375, 131824. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, J.; Kannan, S.; Govindasamy, A. Structured form of DHA prevents neurodegenerative disorders: A better insight into the pathophysiology and the mechanism of DHA transport to the brain. Nutr. Res. 2021, 85, 119–134. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Jin, Q.; Wang, X.; Akoh, C.C. High sn-2 docosahexaenoic acid lipids for brain benefits, and their enzymatic syntheses: A review. Engineering 2020, 6, 424–431. [Google Scholar] [CrossRef]
- Bedse, G.; Hill, M.N.; Patel, S. 2-Arachidonoylglycerol modulation of anxiety and stress adaptation: From grass roots to novel therapeutics. Biol. Psychiatry 2020, 88, 520–530. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.; Clapper, J.R.; Fu, J.; D’Agostino, G.; Guijarro, A.; Thongkham, D.; Avanesian, A.; Astarita, G.; DiPatrizio, N.V.; Frontini, A.; et al. 2-Arachidonoylglycerol signaling in forebrain regulates systemic energy metabolism. Cell Metab. 2012, 15, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Kokona, D.; Spyridakos, D.; Tzatzarakis, M.; Papadogkonaki, S.; Filidou, E.; Arvanitidis, K.I.; Kolios, G.; Lamani, M.; Makriyannis, A.; Malamas, M.S.; et al. The endocannabinoid 2-arachidonoylglycerol and dual ABHD6/MAGL enzyme inhibitors display neuroprotective and anti-inflammatory actions in the in vivo retinal model of AMPA excitotoxicity. Neuropharmacology 2021, 185, 108450. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Yang, L.; Wang, B.; Cui, L.; Li, C.; Zhuo, Y.; Zhang, L.; Zhang, S.; Zhang, Q.; Wang, X. The role of 2-arachidonoylglycerol in the regulation of the tumor-immune microenvironment in murine models of pancreatic cancer. Biomed. Pharmacother. 2019, 115, 108952. [Google Scholar] [CrossRef] [PubMed]
- Baggelaar, M.P.; Maccarrone, M.; van der Stelt, M. 2-Arachidonoylglycerol: A signaling lipid with manifold actions in the brain. Prog. Lipid Res. 2018, 71, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Bornscheuer, U.T. Lipase-catalyzed syntheses of monoacylglycerols. Enzym. Microb. Technol. 1995, 17, 578–586. [Google Scholar] [CrossRef]
- Stamatov, S.D.; Stawinski, J. Novel, regioselective transformation of an oxirane system. An efficient approach to the synthesis of endocannabinoid 2-arachidonoylglycerol. Tetrahedron Lett. 2002, 43, 1759–1761. [Google Scholar] [CrossRef]
- Duclos, R.I., Jr.; Johnston, M.; Vadivel, S.K.; Makriyannis, A.; Glaser, S.T.; Gatley, S.J. A methodology for radiolabeling of the endocannabinoid 2-arachidonoylglycerol (2-AG). J. Org. Chem. 2011, 76, 2049–2055. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, M.; Wang, T.; Jin, Q.; Wang, X. An improved method for the synthesis of 2-arachidonoylglycerol. Process Biochem. 2014, 49, 1415–1421. [Google Scholar] [CrossRef]
- Kim, B.H.; Akoh, C.C. Recent research trends on the enzymatic synthesis of structured lipids. J. Food Sci. 2015, 80, 1713–1724. [Google Scholar] [CrossRef] [PubMed]
- Vadivel, S.K.; Whitten, K.M.; Makriyannis, A. Chemoenzymatic synthesis of 2-arachidonoylglycerol, an endogenous ligand forcannabinoid receptors. Tetrahedron Lett. 2011, 52, 1149–1150. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhao, X.; Yang, Z.; Wang, X.; Wang, T. Effect of solvent on acyl migration of 2-monoacylglycerols in enzymatic ethanolysis. J. Agric. Food Chem. 2020, 68, 12358–12364. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Fruekilde, M.; Xu, X. Suppression of acyl migration in enzymatic production of structured lipids through temperature programming. Food Chem. 2005, 92, 101–107. [Google Scholar] [CrossRef]
- Compton, D.L.; Vermillion, K.E.; Laszlo, J.A. Acyl migration kinetics of 2-monoacylglycerols from soybean oil via 1H NMR. J. Am. Oil Chem. Soc. 2008, 84, 343–348. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, Z.; Lee, W.J.; Xie, X.; Li, A.; Wang, Y. Acyl migration occurrence of palm olein during interesterification catalyzed by sn-1,3 specific lipase. LWT-Food Sci. Technol. 2021, 142, 111023. [Google Scholar] [CrossRef]
- Li, W.; Du, W.; Li, Q.; Sun, T.; Liu, D. Study on acyl migration kinetics of partial glycerides: Dependence on temperature and water activity. J. Mol. Catal. B Enzym. 2010, 63, 17–22. [Google Scholar] [CrossRef]
- Cao, X.; Mangas-Sánchez, J.; Feng, F.; Adlercreutz, P. Acyl migration in enzymatic interesterification of triacylglycerols: Effects of lipases from Thermomyces lanuginosus and Rhizopus oryzae, support material, and water activity. Eur. J. Lipid Sci. Technol. 2016, 118, 1579–1587. [Google Scholar] [CrossRef]
- Li, W.; Du, W.; Li, Q.; Li, R.; Liu, D. Dependence on the properties of organic solvent: Study on acyl migration kinetics of partial glycerides. Bioresour. Technol. 2010, 101, 5737–5742. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.; Chen, F.; Liu, X.; Hu, J.; Zheng, L.; Jing, L.; Deng, Z. Trace water activity could improve the formation of 1,3-oleic-2-medium chain-rich triacylglycerols by promoting acyl migration in the lipase RM IM catalyzed interesterification. Food Chem. 2020, 313, 126130. [Google Scholar] [CrossRef] [PubMed]
- Duan, Z.; Du, W.; Liu, D. The pronounced effect of water activity on the positional selectivity of Novozym 435 during 1,3-diolein synthesis by esterification. Catal. Commun. 2010, 11, 356–358. [Google Scholar] [CrossRef]
- Jin, J.; Zhang, S.; Akoh, C.C. Preparation and characterization of sn-2 polyunsaturated fatty acids-rich monoacylglycerols from menhaden oil and DHA-single cell oil. LWT-Food Sci. Technol. 2022, 156, 13012. [Google Scholar] [CrossRef]
- Muñío, M.M.; Esteban, L.; Robles, A.; Hita, E.; Jiménez, M.J.; González, P.A.; Camacho, B.; Molina, E. Synthesis of 2-monoacylglycerols rich in polyunsaturated fatty acids by ethanolysis of fish oil catalyzed by 1,3 specific lipases. Process Biochem. 2008, 43, 1033–1039. [Google Scholar] [CrossRef]
- Esteban, L.; Jimenez, M.J.; Hita, E.; Gonzalez, P.A.; Martin, L.; Robles, A. Production of structured triacylglycerols rich in pal mitic acid at sn-2 position and oleic acid at sn-1,3 positions as human milk fat substitutes by enzymatic acidolysis. Biochem. Eng. J. 2011, 54, 62–69. [Google Scholar] [CrossRef]
- Du, W.; Wang, L.; Liu, D. Improved methanol tolerance during Novozym 435-mediated methanolysis of SODD for biodiesel production. Green Chem. 2007, 9, 173–176. [Google Scholar] [CrossRef]
- Salihu, A.; Alam, M.Z. Solvent tolerant lipases: A review. Process Biochem. 2015, 50, 86–96. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Y.; Ma, Y.; Jin, Q.; Wang, X. Lipozyme 435-catalyzed synthesis of eicosapentaenoyl ethanolamide in a solvent-free system. J. Mol. Catal. B Enzym. 2015, 122, 233–239. [Google Scholar] [CrossRef]
- Wang, Z.; Dai, L.; Liu, D.; Liu, H.; Du, W. Kinetics and mechanism of solvent influence on the lipase-catalyzed 1,3-diolein synthesis. ACS Omega 2020, 5, 24708–24716. [Google Scholar]
- Damstrup, M.L.; Jensen, T.; Sparsø, F.V.; Kiil, S.Z.; Jensen, A.D.; Xu, X. Solvent optimization for efficient enzymatic monoacyl-glycerol production based on a glycerolysis reaction. J. Am. Oil Chem. Soc. 2005, 82, 559–564. [Google Scholar] [CrossRef]
- Banik, S.D.; Nordblad, M.; Woodley, J.M.; Peters, G.H. A correlation between the activity of Candida antarctica lipase B and differences in binding free energies of organic solvent and substrate. ACS Catal. 2016, 6, 6350–6361. [Google Scholar] [CrossRef]
- Oh, J.E.; Lee, K.W.; Park, H.K.; Kim, J.Y.; Kwon, K.I.L.; Kim, J.W.; Kim, H.R.; Kim, I.N.H. Lipase-catalyzed acidolysis of olive oil with capric acid: Effect of water activity on incorporation and acyl migration. J. Agric. Food Chem. 2009, 57, 9280–9283. [Google Scholar] [CrossRef]
- Zulkeflee, S.A.; Sata, S.A.; Rohman, F.S.; Aziz, N. Modelling of immobilized Candida rugosa lipase catalysed esterification process in batch reactor equipped with temperature and water activity control system. Biochem. Eng. J. 2020, 16, 1107669. [Google Scholar] [CrossRef]
- Fureby, A.M.; Virto, C.; Adlercreutz, P.; Mattiasson, B. Acyl group migrations in 2-monoolein. Biocatal. Biotransform. 1996, 14, 89–111. [Google Scholar] [CrossRef]
- Mao, J.; Hu, Z.; Hu, J.; Zhu, X.; Xiong, H. A Density functional theory (DFT) study of the acyl migration occurring during lipase-catalyzed transesterifications. Int. J. Mol. Sci. 2019, 20, 3438. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Lee, W.J.; Wang, Y. Evaluation of enzymatic interesterification in structured triacylglycerols preparation: A concise review and prospect. Crit. Rev. Food Sci. Nutr. 2021, 61, 3145–3159. [Google Scholar] [CrossRef] [PubMed]
- Satyawali, Y.; Cauwenberghs, L.; Maesen, M.; Dejonghe, W. Lipase catalyzed solvent free synthesis of monoacylglycerols in various reaction systems and coupling reaction with pervaporation for in situ water removal. Chem. Eng. Process.-Process Intensif. 2021, 166, 108475. [Google Scholar] [CrossRef]
- Schmitke, J.L.; Wescott, C.R.; Klibanov, A.M. The mechanistic dissection of the plunge in enzymatic activity upon transition from water to anhydrous solvents. J. Am. Oil Chem. Soc. 1996, 118, 3360–3365. [Google Scholar] [CrossRef]
- Bi, Y.; Wang, Z.; Duan, Z.; Zhao, X.; Chen, X.; Nie, L. An insight into the solvent effect on the positional selectivity of the immobilized lipase from Burkholderia cepacia in 1,3-diolein synthesis. RSC Adv. 2015, 5, 23122–23124. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).