Quality of Protein Isolates and Hydrolysates from Baltic Herring (Clupea harengus membras) and Roach (Rutilus rutilus) Produced by pH-Shift Processes and Enzymatic Hydrolysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Raw Materials and Processing
2.1.2. Reagents and Standards
2.2. Amino Acid Analysis
2.3. Extraction and Analysis of Lipids
2.4. Determination of Peroxide Values, Protein Carbonyls and Volatiles
2.5. Statistical Analysis
3. Results
3.1. Amino Acid Composition
3.2. Fatty Acid Composition
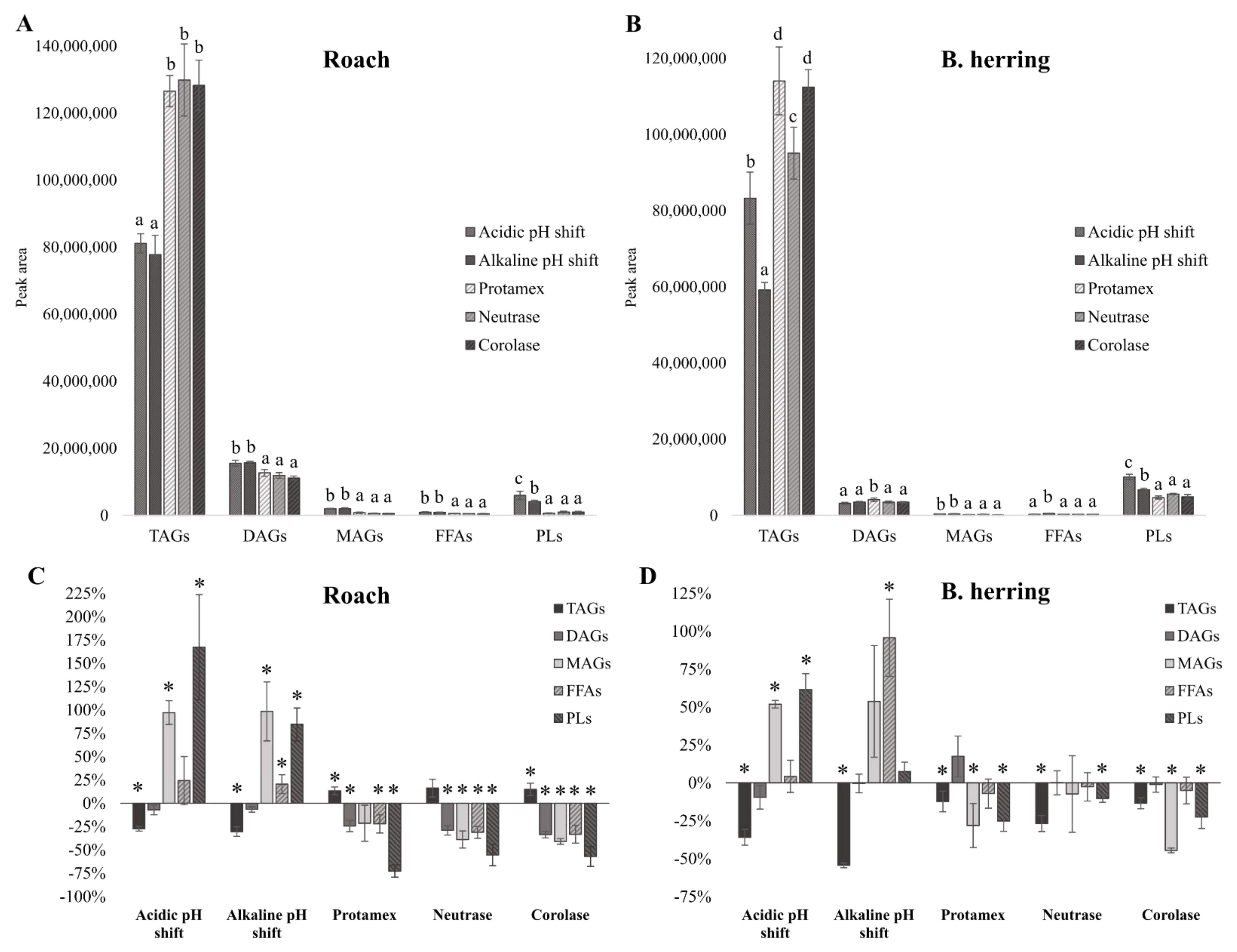
3.3. Lipid Classes
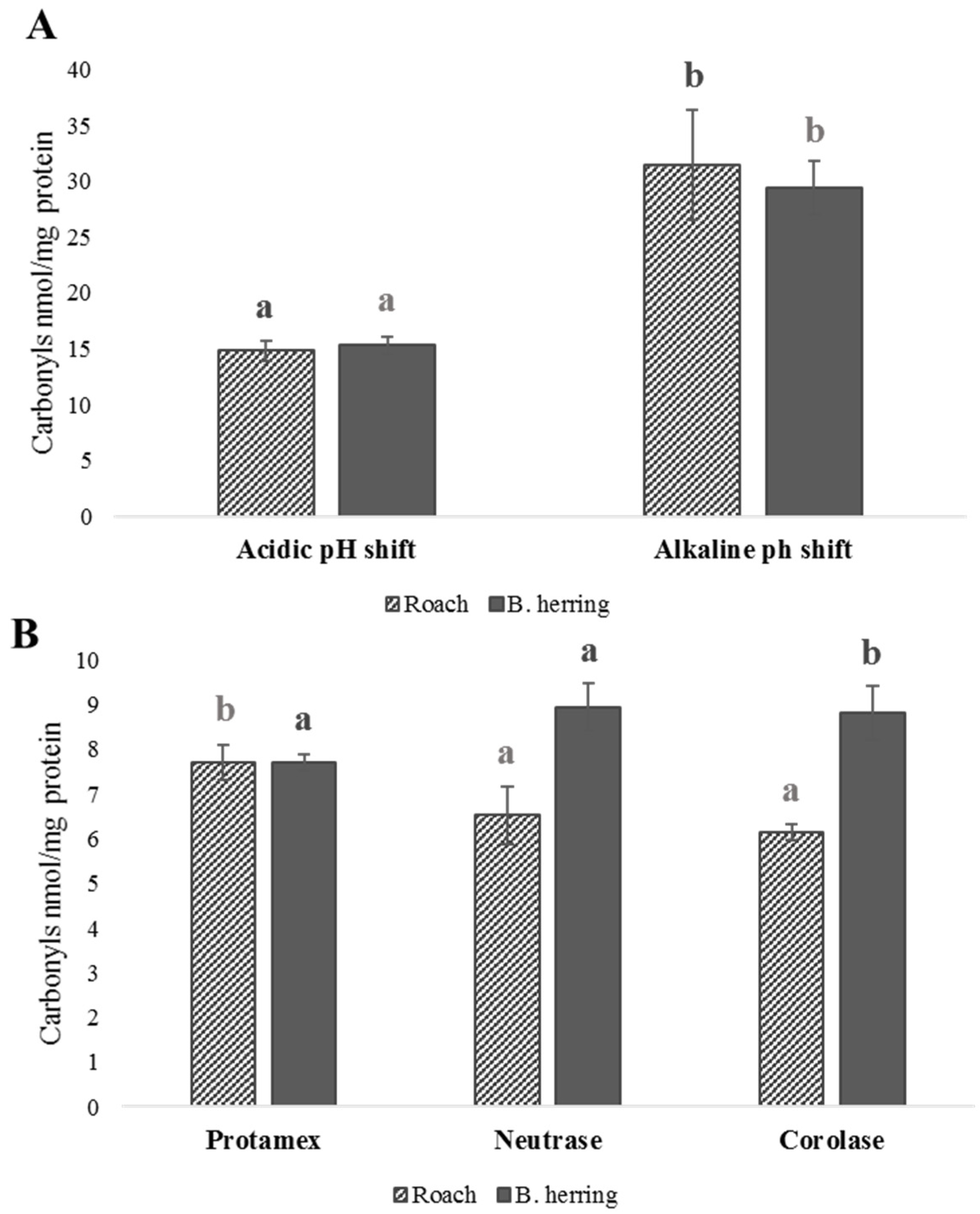
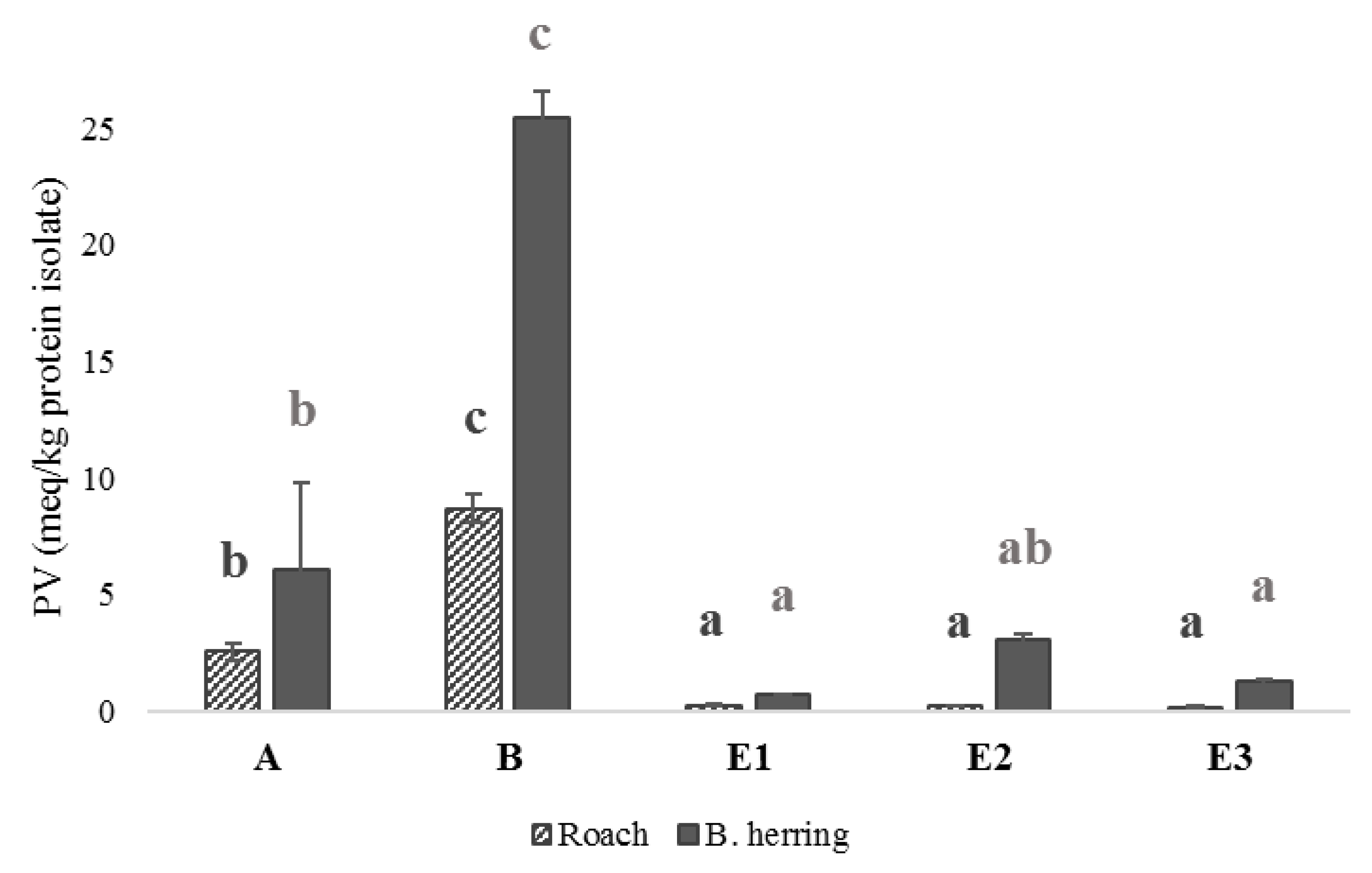
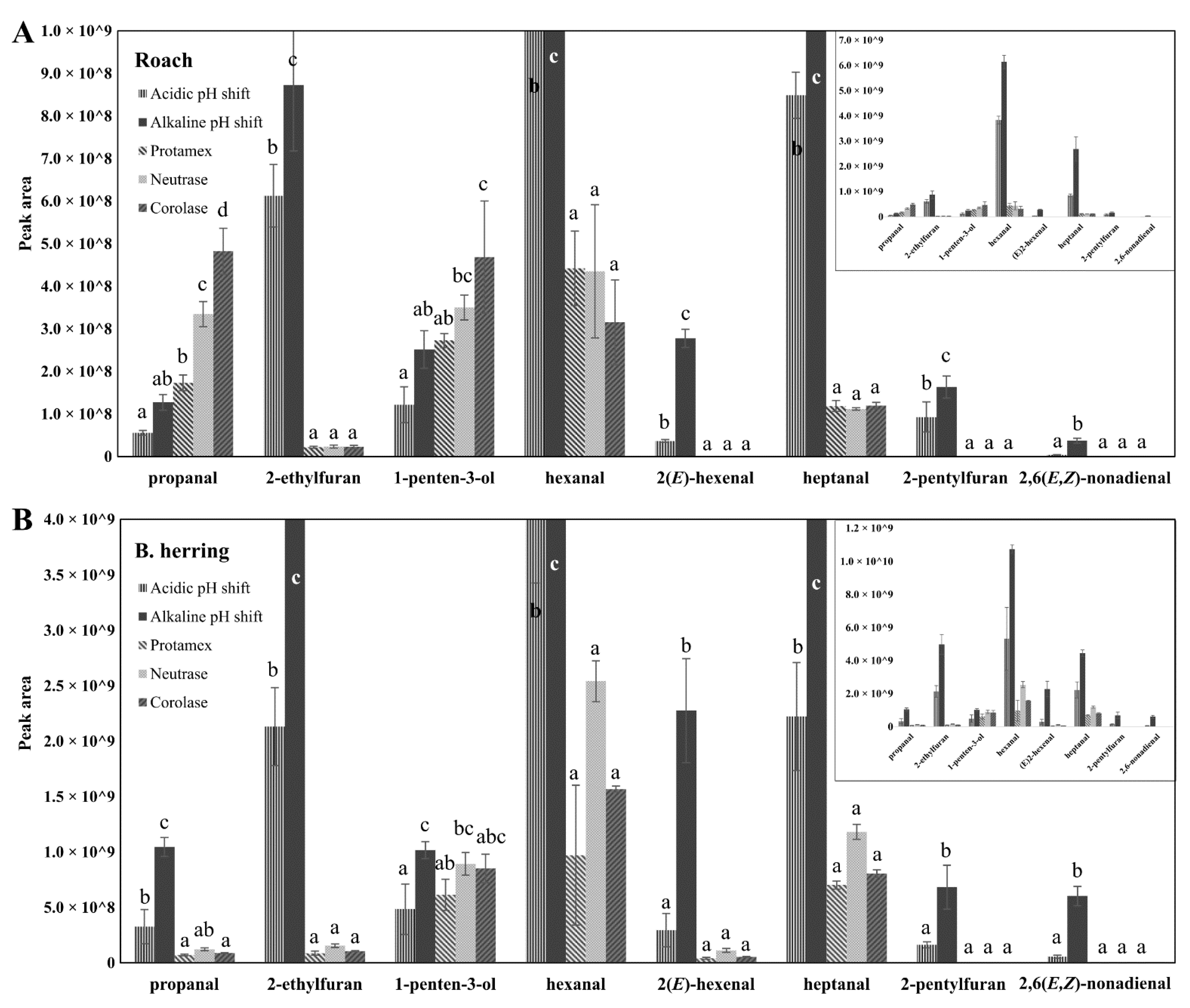
3.4. Peroxide Values, Protein Carbonyls and Oxidation-Related Volatile Compounds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- FAO Commission on Genetic Resources for Food and Agriculture. The State of the World’s Aquatic Genetic Resources for Food and Agriculture Assessments; FAO: Rome, Italy, 2019. [Google Scholar]
- Natural Resources Institute Finland Fish Market and Fish Consumption. Available online: https://www.luke.fi/en/natural-resources/fish-and-the-fishing-industry/fish-market-and-fish-consumption/ (accessed on 9 November 2020).
- Rajasilta, M.; Hänninen, J.; Laaksonen, L.; Laine, P.; Suomela, J.-P.; Vuorinen, I.; Mäkinen, K. Influence of Environmental Conditions, Population Density, and Prey Type on the Lipid Content in Baltic Herring (Clupea harengus membras) from the Northern Baltic Sea. Can. J. Fish. Aquat. Sci. 2019, 76, 576–585. [Google Scholar] [CrossRef]
- Uusitalo, V.; Panapanaan, V.; Vallas, P.; Kuokkanen, A.; Koistinen, K. Environmental Impacts and Sustainability of Low-Value Roach Fish When Used as Food. J. Clean. Prod. 2018, 204, 693–701. [Google Scholar] [CrossRef]
- Centenaro, G.S.; Salas-Mellado, M.; Pires, C.; Batista, I.; Nunes, M.L.; Prentice, C. Fractionation of Protein Hydrolysates of Fish and Chicken Using Membrane Ultrafiltration: Investigation of Antioxidant Activity. Appl. Biochem. Biotechnol. 2014, 172, 2877–2893. [Google Scholar] [CrossRef] [PubMed]
- Chalamaiah, M.; Dinesh Kumar, B.; Hemalatha, R.; Jyothirmayi, T. Fish Protein Hydrolysates: Proximate Composition, Amino Acid Composition, Antioxidant Activities and Applications: A Review. Food Chem. 2012, 135, 3020–3038. [Google Scholar] [CrossRef] [PubMed]
- Idowu, A.T.; Benjakul, S.; Sinthusamran, S.; Sookchoo, P.; Kishimura, H. Protein Hydrolysate from Salmon Frames: Production, Characteristics and Antioxidative Activity. J. Food Biochem. 2019, 43, e12734. [Google Scholar] [CrossRef] [PubMed]
- Pacheco-Aguilar, R.; Mazorra-Manzano, M.A.; Ramírez-Suárez, J.C. Functional Properties of Fish Protein Hydrolysates from Pacific Whiting(Merluccius productus) Muscle Produced by a Commercial Protease. Food Chem. 2008, 109, 782–789. [Google Scholar] [CrossRef]
- Benjakul, S.; Morrissey, M.T. Protein Hydrolysates from Pacific Whiting Solid Wastes. J. Agric. Food Chem. 1997, 45, 3423–3430. [Google Scholar] [CrossRef]
- Klompong, V.; Benjakul, S.; Yachai, M.; Visessanguan, W.; Shahidi, F.; Hayes, K.D. Amino Acid Composition and Antioxidative Peptides from Protein Hydrolysates of Yellow Stripe Trevally (Selaroides leptolepis). J. Food Sci. 2009, 74, C126–C133. [Google Scholar] [CrossRef] [PubMed]
- Khantaphant, S.; Benjakul, S.; Ghomi, M.R. The Effects of Pretreatments on Antioxidative Activities of Protein Hydrolysate from the Muscle of Brownstripe Red Snapper (Lutjanus vitta). LWT Food Sci. Technol. 2011, 44, 1139–1148. [Google Scholar] [CrossRef]
- Vázquez, J.A.; Rodríguez-Amado, I.; Sotelo, C.G.; Sanz, N.; Pérez-Martín, R.I.; Valcárcel, J. Production, Characterization, and Bioactivity of Fish Protein Hydrolysates from Aquaculture Turbot (Scophthalmus maximus) Wastes. Biomolecules 2020, 10, 310. [Google Scholar] [CrossRef]
- Rocha Camargo, T.; Ramos, P.; Monserrat, J.M.; Prentice, C.; Fernandes, C.J.C.; Zambuzzi, W.F.; Valenti, W.C. Biological Activities of the Protein Hydrolysate Obtained from Two Fishes Common in the Fisheries Bycatch. Food Chem. 2021, 342, 128361. [Google Scholar] [CrossRef] [PubMed]
- Marmon, S.K.; Undeland, I. Protein Isolation from Gutted Herring (Clupea harengus) Using PH-Shift Processes. J. Agric. Food Chem. 2010, 58, 10480–10486. [Google Scholar] [CrossRef]
- Undeland, I.; Kelleher, S.D.; Hultin, H.O. Recovery of Functional Proteins from Herring (Clupea harengus) Light Muscle by an Acid or Alkaline Solubilization Process. J. Agric. Food Chem. 2002, 50, 7371–7379. [Google Scholar] [CrossRef]
- Panpipat, W.; Chaijan, M. Functional Properties of PH-Shifted Protein Isolates from Bigeye Snapper (Priacanthus tayenus) Head by-Product. Int. J. Food Prop. 2017, 20, 596–610. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Jaczynski, J. Protein Recovery from Rainbow Trout (Oncorhynchus mykiss) Processing Byproducts via Isoelectric Solubilization/Precipitation and Its Gelation Properties as Affected by Functional Additives. J. Agric. Food Chem. 2007, 55, 9079–9088. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.; Liu, S.; Cao, J.; Chen, S.; Wang, W.; Qin, X. Fish Protein Isolates Recovered from Silver Carp (Hypophthalmichthys molitrix) By-Products Using Alkaline PH Solubilization and Precipitation. J. Aquat. Food Prod. Technol. 2016, 25, 400–413. [Google Scholar] [CrossRef]
- Abdollahi, M.; Undeland, I. Structural, Functional, and Sensorial Properties of Protein Isolate Produced from Salmon, Cod, and Herring by-Products. Food Bioprocess Technol. 2018, 11, 1733–1749. [Google Scholar] [CrossRef]
- Joint FAO/WHO/UNU Expert Consultation on Protein and Amino Acid Requirements in Human Nutrition. Protein and Amino Acid Requirements in Human Nutrition: Report of a Joint FAO/WHO/UNU Expert Consultation; World Health Organization: Geneva, Switzerland, 2007; ISBN 978-92-4-120935-9. [Google Scholar]
- Abdollahi, M.; Olofsson, E.; Zhang, J.; Alminger, M.; Undeland, I. Minimizing Lipid Oxidation during PH-Shift Processing of Fish by-Products by Cross-Processing with Lingonberry Press Cake, Shrimp Shells or Brown Seaweed. Food Chem. 2020, 327, 127078. [Google Scholar] [CrossRef]
- Nikoo, M.; Benjakul, S.; Yasemi, M.; Ahmadi Gavlighi, H.; Xu, X. Hydrolysates from Rainbow Trout (Oncorhynchus mykiss) Processing by-Product with Different Pretreatments: Antioxidant Activity and Their Effect on Lipid and Protein Oxidation of Raw Fish Emulsion. LWT 2019, 108, 120–128. [Google Scholar] [CrossRef]
- Shahidi, F.; Zhong, Y. Lipid Oxidation and Improving the Oxidative Stability. Chem. Soc. Rev. 2010, 39, 4067–4079. [Google Scholar] [CrossRef]
- Yarnpakdee, S.; Benjakul, S.; Nalinanon, S.; Kristinsson, H.G. Lipid Oxidation and Fishy Odour Development in Protein Hydrolysate from Nile Tilapia (Oreochromis niloticus) Muscle as Affected by Freshness and Antioxidants. Food Chem. 2012, 132, 1781–1788. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, Y.; Wang, Z.; Cai, S.; Zhu, B.; Dong, X. Recent Advances in Fishy Odour in Aquatic Fish Products, from Formation to Control. Int. J. Food Sci. Technol. 2021, 56, 4959–4969. [Google Scholar] [CrossRef]
- Yarnpakdee, S.; Benjakul, S.; Kristinsson, H.G. Effect of Pretreatments on Chemical Compositions of Mince from Nile Tilapia (Oreochromis niloticus) and Fishy Odor Development in Protein Hydrolysate. Int. Aquat. Res. 2012, 4, 7. [Google Scholar] [CrossRef][Green Version]
- Erickson, M.C. Lipid Oxidation of Muscle Foods. In Food Lipids; Marcel Dekker, Inc.: New York, NY, USA, 2002; pp. 321–364. [Google Scholar]
- Yarnpakdee, S.; Benjakul, S.; Kristinsson, H.G.; Maqsood, S. Effect of Pretreatment on Lipid Oxidation and Fishy Odour Development in Protein Hydrolysates from the Muscle of Indian Mackerel. Food Chem. 2012, 135, 2474–2482. [Google Scholar] [CrossRef]
- Nisov, A.; Kakko, T.; Alakomi, H.-L.; Lantto, R.; Honkapää, K. Comparison of Enzymatic and PH Shift Methods to Extract Protein from Whole Baltic Herring (Clupea harengus membras) and Roach (Rutilus rutilus). Food Chem. 2021, 373, 131524. [Google Scholar] [CrossRef]
- Lansdowne, L.R.; Beamer, S.; Jaczynski, J.; Matak, K.E. Survival of Listeria Innocua after Isoelectric Solubilization and Precipitation of Fish Protein. J. Food Sci. 2009, 74, M201–M205. [Google Scholar] [CrossRef] [PubMed]
- Lansdowne, L.R.; Beamer, S.; Jaczynski, J.; Matak, K.E. Survival of Escherichia Coli after Isoelectric Solubilization and Precipitation of Fish Protein. J. Food Prot. 2009, 72, 1398–1403. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Wu, Z.; Jia, S.; Wu, G. Analysis of Amino Acid Composition in Proteins of Animal Tissues and Foods as Pre-Column o-Phthaldialdehyde Derivatives by HPLC with Fluorescence Detection. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2014, 964, 116–127. [Google Scholar] [CrossRef]
- Henderson, J.W.; Ricker, R.D.; Bidlingmeyer, B.A.; Woodward, C. Rapid, Accurate, Sensitive, and Reproducible HPLC Analysis of Amino Acids. Amino Acid Analysis Using Zorbax Eclipse-AAA Columns and the Agilent 1100 HPLC. Available online: https://www.agilent.com/cs/library/chromatograms/59801193.pdf (accessed on 15 November 2021).
- Pripis-Nicolau, L.; de Revel, G.; Marchand, S.; Beloqui, A.A.; Bertrand, A. Automated HPLC Method for the Measurement of Free Amino Acids Including Cysteine in Musts and Wines; First Applications. J. Sci. Food Agric. 2001, 81, 731–738. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Stanley, G.H.S. A Simple Method for the Isolation and Purification of Total Lipides from Animal Tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Christie, W.W. Lipid Analysis: Isolation, Separation, Identification and Structural Analysis of Lipids, 3rd ed.; Bridgewater: Oily, PA, USA, 2003. [Google Scholar]
- Damerau, A.; Kakko, T.; Tian, Y.; Tuomasjukka, S.; Sandell, M.; Hopia, A.; Yang, B. Effect of Supercritical CO2 Plant Extract and Berry Press Cakes on Stability and Consumer Acceptance of Frozen Baltic Herring (Clupea harengus membras) Mince. Food Chem. 2020, 332, 127385. [Google Scholar] [CrossRef]
- Damerau, A.; Ahonen, E.; Kortesniemi, M.; Puganen, A.; Tarvainen, M.; Linderborg, K.M. Evaluation of the Composition and Oxidative Status of Omega-3 Fatty Acid Supplements on the Finnish Market Using NMR and SPME-GC–MS in Comparison with Conventional Methods. Food Chem. 2020, 330, 127194. [Google Scholar] [CrossRef] [PubMed]
- Lehtonen, M.; Kemmo, S.; Lampi, A.-M.; Piironen, V. Effects of Esterification on the Formation and Decomposition of Steryl Hydroperoxides. Eur. Food Res. Technol. 2011, 232, 255–264. [Google Scholar] [CrossRef]
- Levine, R.L.; Williams, J.A.; Stadtman, E.P.; Shacter, E. Carbonyl Assays for Determination of Oxidatively Modified Proteins. In Methods in Enzymology; Oxygen Radicals in Biological Systems Part C; Academic Press: Cambridge, MA, USA, 1994; Volume 233, pp. 346–357. [Google Scholar]
- Mesquita, C.S.; Oliveira, R.; Bento, F.; Geraldo, D.; Rodrigues, J.V.; Marcos, J.C. Simplified 2,4-Dinitrophenylhydrazine Spectrophotometric Assay for Quantification of Carbonyls in Oxidized Proteins. Anal. Biochem. 2014, 458, 69–71. [Google Scholar] [CrossRef] [PubMed]
- Abdollahi, M.; Rezaei, M.; Jafarpour, A.; Undeland, I. Sequential Extraction of Gel-Forming Proteins, Collagen and Collagen Hydrolysate from Gutted Silver Carp (Hypophthalmichthys molitrix), a Biorefinery Approach. Food Chem. 2018, 242, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Amini Sarteshnizi, R.; Sahari, M.A.; Ahmadi Gavlighi, H.; Regenstein, J.M.; Nikoo, M.; Udenigwe, C.C. Influence of Fish Protein Hydrolysate-Pistachio Green Hull Extract Interactions on Antioxidant Activity and Inhibition of α-Glucosidase, α-Amylase, and DPP-IV Enzymes. LWT 2021, 142, 111019. [Google Scholar] [CrossRef]
- Shahidi, F.; Han, X.-Q.; Synowiecki, J. Production and Characteristics of Protein Hydrolysates from Capelin (Mallotus villosus). Food Chem. 1995, 53, 285–293. [Google Scholar] [CrossRef]
- Wu, H.; Abdollahi, M.; Undeland, I. Effect of Recovery Technique, Antioxidant Addition and Compositional Features on Lipid Oxidation in Protein Enriched Products from Cod- Salmon and Herring Backbones. Food Chem. 2021, 360, 129973. [Google Scholar] [CrossRef]
- Abdollahi, M.; Wu, H.; Undeland, I. Impact of Processing Technology on Macro- and Micronutrient Profile of Protein-Enriched Products from Fish Backbones. Foods 2021, 10, 950. [Google Scholar] [CrossRef] [PubMed]
- Linko, R.R.; Kaitaranta, J.K.; Vuorela, R. Comparison of the Fatty Acids in Baltic Herring and Available Plankton Feed. Comp. Biochem. Physiol. Part B Comp. Biochem. 1985, 82, 699–705. [Google Scholar] [CrossRef]
- Kim, J.Y.; Yi, B.; Lee, C.; Gim, S.-Y.; Kim, M.-J.; Lee, J. Effects of PH on the Rates of Lipid Oxidation in Oil–Water System. Appl. Biol. Chem. 2016, 59, 157–161. [Google Scholar] [CrossRef]
- Tatiyaborworntham, N.; Yin, J.; Richards, M.P. Factors Influencing the Antioxidant Effect of Phospholipase A2 against Lipid Oxidation Promoted by Trout Hemoglobin and Hemin in Washed Muscle. Food Chem. 2021, 343, 128428. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Xiao, S.; Yin, J.; Zhang, J.; Richards, M.P. Mechanisms Involved in the Inhibitory Effects of Free Fatty Acids on Lipid Peroxidation in Turkey Muscle. Food Chem. 2021, 342, 128333. [Google Scholar] [CrossRef]
- Richards, M.P.; Hultin, H.O. Rancidity Development in a Fish Model System as Affected by Phospholipids. J. Food Lipids 2001, 8, 215–230. [Google Scholar] [CrossRef]
- Marmon, S.K.; Undeland, I. Effect of Alkaline PH-Shift Processing on in Vitro Gastrointestinal Digestion of Herring (Clupea harengus) Fillets. Food Chem. 2013, 138, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Kristinsson, H.G.; Hultin, H.O. The Effect of Acid and Alkali Unfolding and Subsequent Refolding on the Pro-Oxidative Activity of Trout Hemoglobin. J. Agric. Food Chem. 2004, 52, 5482–5490. [Google Scholar] [CrossRef]
- Phetsang, H.; Panpipat, W.; Undeland, I.; Panya, A.; Phonsatta, N.; Chaijan, M. Comparative Quality and Volatilomic Characterisation of Unwashed Mince, Surimi, and PH-Shift-Processed Protein Isolates from Farm-Raised Hybrid Catfish (Clarias macrocephalus × Clarias gariepinus). Food Chem. 2021, 364, 130365. [Google Scholar] [CrossRef] [PubMed]
- Kristinsson, H.G.; Hultin, H.O. Changes in Trout Hemoglobin Conformations and Solubility after Exposure to Acid and Alkali PH. J. Agric. Food Chem. 2004, 52, 3633–3643. [Google Scholar] [CrossRef]
- Maqsood, S.; Benjakul, S. Comparative Studies on Molecular Changes and Pro-Oxidative Activity of Haemoglobin from Different Fish Species as Influenced by PH. Food Chem. 2011, 124, 875–883. [Google Scholar] [CrossRef]
- Richards, M.P.; Modra, A.M.; Li, R. Role of Deoxyhemoglobin in Lipid Oxidation of Washed Cod Muscle Mediated by Trout, Poultry and Beef Hemoglobins. Meat Sci. 2002, 62, 157–163. [Google Scholar] [CrossRef]
- Pazos, M.; Medina, I.; Hultin, H.O. Effect of PH on Hemoglobin-Catalyzed Lipid Oxidation in Cod Muscle Membranes in Vitro and in Situ. J. Agric. Food Chem. 2005, 53, 3605–3612. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Cortés, P.; Sacks, G.L.; Brenna, J.T. Quantitative Analysis of Volatiles in Edible Oils Following Accelerated Oxidation Using Broad Spectrum Isotope Standards. Food Chem. 2015, 174, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Sajib, M.; Undeland, I. Towards Valorization of Herring Filleting By-Products to Silage 2.0: Effect of Temperature and Time on Lipid Oxidation and Non-Enzymatic Browning Reactions. LWT 2020, 127, 109441. [Google Scholar] [CrossRef]
- Joaquin, H.J.F.; Tolasa, S.; Oliveira, A.C.M.; Lee, C.M.; Lee, K.H. Effect of Milk Protein Concentrate on Lipid Oxidation and Formation of Fishy Volatiles in Herring Mince (Clupea harengus) during Frozen Storage. J. Agric. Food Chem. 2008, 56, 166–172. [Google Scholar] [CrossRef]
- Cardinal, M.; Chaussy, M.; Donnay-Moreno, C.; Cornet, J.; Rannou, C.; Fillonneau, C.; Prost, C.; Baron, R.; Courcoux, P. Use of Random Forest Methodology to Link Aroma Profiles to Volatile Compounds: Application to Enzymatic Hydrolysis of Atlantic Salmon (Salmo salar) by-Products Combined with Maillard Reactions. Food Res. Int. 2020, 134, 109254. [Google Scholar] [CrossRef] [PubMed]
- Halldorsdottir, S.M.; Sveinsdottir, H.; Gudmundsdottir, A.; Thorkelsson, G.; Kristinsson, H.G. High Quality Fish Protein Hydrolysates Prepared from By-Product Material with Fucus Vesiculosus Extract. J. Funct. Foods 2014, 9, 10–17. [Google Scholar] [CrossRef]
| Fish | Process | Protein (%, as is) | Protein, (%, d.w.) | Lipids (%, as is) | Lipids (%, d.w.) |
|---|---|---|---|---|---|
| Roach | Raw material | 16.1 ± 0.1 | 64.9 ± 0.3 | 4.2 ± 0.0 | 17.0 ± 0.1 |
| Acidic pH shift | 80.1 ± 1.0 | 83.0 ± 1.6 | 10.7 ± 0.5 | 11.1 ± 0.4 | |
| Alkaline pH shift | 70.8 ± 1.7 | 73.0 ± 2.0 | 18.8 ± 0.9 | 19.4 ± 0.9 | |
| Protamex | 80.4 ± 2.2 | 84.0 ± 1.5 | 5.5 ± 0.1 | 5.8 ± 0.1 | |
| Neutrase | 81.3 ± 1.6 | 85.1 ± 1.5 | 5.7 ± 0.2 | 6.0 ± 0.2 | |
| Corolase | 79.9 ± 1.6 | 83.6 ± 1.5 | 6.0 ± 0.1 | 6.3 ± 0.1 | |
| B. herring | Raw material | 15.0 ± 0.0 | 63.1 ± 0.0 | 7.2 ± 0.1 | 29.9 ± 0.4 |
| Acidic pH shift | 79.4 ± 0.7 | 80.4 ± 0.8 | 16.5 ± 1.0 | 16.7 ± 1.0 | |
| Alkaline pH shift | 76.0 ± 0.9 | 77.9 ± 0.7 | 16.4 ± 1.8 | 16.8 ± 1.8 | |
| Protamex | 87.2 ± 1.0 | 91.1 ± 1.2 | 3.1 ± 0.1 | 3.2 ± 0.1 | |
| Neutrase | 84.1 ± 1.3 | 87.9 ± 0.7 | 3.1 ± 0.2 | 3.3 ± 0.2 | |
| Corolase | 85.0 ± 1.4 | 88.7 ± 1.2 | 3.4 ± 0.2 | 3.5 ± 0.2 |
| Roach | B. Herring | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Raw Material | Acidic pH Shift | Alkaline pH Shift | Protamex | Neutrase | Corolase | Raw Material | Acidic pH Shift | Alkaline pH Shift | Protamex | Neutrase | Corolase | FAO/WHO/UNU (2007) | |
| Essential | |||||||||||||
| His | 27.11 ± 2.72 b | 22.47 ± 2.77 a | 21.60 ± 2.78 a | 23.99 ± 1.93 ab | 24.26 ± 1.72 ab | 22.93 ± 2.15 a | 21.10 ± 0.42 c | 24.35 ± 1.33 d | 24.59 ± 0.76 d | 18.37 ± 0.65 a | 19.86 ± 0.48 bc | 19.38 ± 0.38 ab | 15 |
| Ile | 39.95 ± 2.47 b | 46.20 ± 0.71 c | 47.11 ± 1.58 c | 32.54 ± 0.62 a | 32.90 ± 1.02 a | 31.12 ± 0.74 a | 35.77 ± 0.81 b | 46.03 ± 1.74 c | 45.03 ± 0.77 c | 24.43 ± 0.63 a | 25.08 ± 0.32 a | 25.10 ± 0.26 a | 30 |
| Leu | 70.55 ± 3.48 b | 79.43 ± 0.74 c | 80.91 ± 2.53 c | 60.67 ± 1.00 a | 61.00 ± 1.43 a | 59.86 ± 1.42 a | 63.53 ± 1.11 b | 81.66 ± 2.97 c | 79.75 ± 2.15 c | 53.46 ± 0.82 a | 55.54 ± 0.49 a | 55.32 ± 0.66 a | 59 |
| Lys | 78.58 ± 14.00 | 85.99 ± 5.95 | 82.22 ± 12.93 | 77.61 ± 6.77 | 81.71 ± 5.54 | 73.94 ± 13.92 | 74.05 ± 4.93 ab | 79.92 ± 5.18 ab | 83.87 ± 3.86 b | 70.67 ± 7.81 a | 74.04 ± 5.71 ab | 74.68 ± 5.08 ab | 45 |
| Met | 25.76 ± 1.24 b | 28.28 ± 0.45 c | 29.01 ± 0.98 c | 21.66 ± 0.43 a | 21.93 ± 0.48 a | 21.25 ± 0.50 a | 24.62 ± 0.51 b | 29.94 ± 1.01 c | 30.01 ± 0.86 c | 20.71 ± 0.41 a | 21.02 ± 0.11 a | 21.31 ± 0.20 a | 22 * |
| Phe | 36.63 ± 2.95 b | 40.51 ± 0.61 c | 41.81 ± 1.78 c | 29.62 ± 0.79 a | 29.84 ± 1.12 a | 29.19 ± 1.06 a | 32.73 ± 0.91 b | 37.99 ± 1.56 c | 38.27 ± 0.76 c | 22.91 ± 0.59 a | 23.14 ± 0.50 a | 23.75 ± 0.38 a | 38 ** |
| Thr | 38.14 ± 1.80 b | 41.28 ± 0.44 c | 42.36 ± 1.47 c | 33.93 ± 0.81 a | 34.01 ± 0.90 a | 33.71 ± 0.97 a | 36.24 ± 0.64 b | 42.14 ± 1.59 c | 42.20 ± 1.06 c | 31.25 ± 0.70 a | 31.91 ± 0.68 a | 31.83 ± 0.46 a | 23 |
| Trp | 10.03 ± 0.24 b | 11.71 ± 0.33 c | 13.51 ± 0.35 d | 7.20 ± 0.14 a | 7.42 ± 0.27 a | 6.73 ± 0.29 a | 9.04 ± 0.34 b | 11.84 ± 0.16 c | 12.30 ± 0.33 c | 6.06 ± 0.42 a | 5.96 ± 0.27 a | 6.35 ± 0.02 a | 6 |
| Val | 39.49 ± 1.88 b | 43.64 ± 0.34 c | 43.74 ± 1.54 c | 32.07 ± 0.78 a | 32.73 ± 0.67 a | 31.78 ± 0.76 a | 39.39 ± 0.72 b | 45.46 ± 1.77 c | 46.25 ± 1.22 c | 30.05 ± 0.70 a | 30.66 ± 0.41 a | 30.72 ± 0.51 a | 39 |
| Non-essential | |||||||||||||
| Ala | 48.70 ± 1.91 a | 50.12 ± 1.00 ab | 48.77 ± 1.71 a | 50.03 ± 1.38 ab | 49.32 ± 1.44 ab | 51.18 ± 1.40 b | 47.59 ± 0.43 a | 54.22 ± 2.11 c | 53.90 ± 1.51 c | 47.45 ± 0.72 a | 50.03 ± 0.72 b | 49.00 ± 0.81 ab | |
| Arg | 55.06 ± 2.82 a | 57.69 ± 0.73 b | 55.02 ± 2.38 a | 54.00 ± 1.41 a | 53.86 ± 0.96 a | 55.03 ± 1.18 a | 59.32 ± 0.33 b | 59.74 ± 2.51 b | 63.79 ± 1.96 c | 49.28 ± 1.24 a | 50.55 ± 1.03 a | 51.32 ± 1.07 a | |
| Asn + Asp | 85.34 ± 3.86 b | 91.71 ± 1.67 c | 92.25 ± 3.68 c | 79.01 ± 1.87 a | 79.29 ± 1.87 a | 78.87 ± 1.99 a | 72.24 ± 1.33 b | 86.84 ± 2.78 c | 87.96 ± 3.09 c | 67.77 ± 1.35 a | 69.40 ± 0.70 ab | 69.37 ± 1.17 ab | |
| Cys *** | 2.28 ± 1.10 c | 3.25 ± 0.46 d | 2.11 ± 0.43 bc | 1.25 ± 0.51 a | 1.44 ± 0.31 ab | 1.05 ± 0.15 a | 2.77 ± 0.19 c | 1.95 ± 0.82 b | 1.97 ± 0.39 b | 0.50 ± 0.20 a | 0.36 ± 0.07 a | 0.47 ± 0.21 a | |
| Gln + Glu | 124.56 ± 5.34 a | 134.62 ± 2.46 b | 133.14 ± 5.63 b | 123.09 ± 3.15 a | 122.62 ± 3.18 a | 125.50 ± 3.26 a | 104.98 ± 1.56 a | 131.52 ± 4.16 d | 125.50 ± 3.78 c | 114.16 ± 1.63 b | 119.02 ± 1.07 b | 117.60 ± 1.80 b | |
| Gly | 38.03 ± 1.46 b | 30.95 ± 1.27 a | 30.31 ± 2.44 a | 49.72 ± 2.02 c | 50.14 ± 2.25 c | 51.89 ± 2.11 c | 42.43 ± 2.43 b | 28.52 ± 1.20 a | 30.97 ± 0.73 a | 44.78 ± 1.86 bc | 46.71 ± 1.54 c | 47.02 ± 2.89 c | |
| Hyp | 5.29 ± 0.41 b | 1.31 ± 0.02 a | 1.27 ± 0.04 a | 10.87 ± 0.93 c | 10.63 ± 0.58 c | 11.91 ± 0.68 c | 6.74 ± 0.85 b | 1.95 ± 0.04 a | 2.05 ± 0.01 a | 8.70 ± 0.50 c | 9.46 ± 0.54 c | 9.07 ± 0.31 c | |
| Pro | 35.33 ± 1.53 b | 31.77 ± 0.58 a | 32.23 ± 1.26 a | 38.44 ± 1.45 c | 39.08 ± 0.82 c | 40.20 ± 0.73 c | 37.54 ± 1.34 b | 33.61 ± 1.78 a | 34.78 ± 1.18 a | 33.66 ± 1.12 a | 33.19 ± 0.86 a | 35.20 ± 1.15 ab | |
| Ser | 36.88 ± 1.74 bc | 37.35 ± 0.49 c | 37.23 ± 1.47 bc | 34.43 ± 0.89 a | 35.16 ± 0.82 a | 35.69 ± 0.98 ab | 33.74 ± 0.30 b | 37.04 ± 1.54 c | 37.80 ± 1.02 c | 31.61 ± 0.61 a | 32.46 ± 0.38 ab | 32.42 ± 0.57 ab | |
| Tyr | 32.55 ± 1.96 c | 37.14 ± 1.14 d | 38.40 ± 1.90 d | 24.40 ± 0.52 ab | 25.26 ± 0.78 b | 23.26 ± 0.64 a | 29.59 ± 0.49 b | 36.41 ± 1.41 c | 35.10 ± 1.38 c | 18.41 ± 0.43 a | 17.99 ± 0.41 a | 18.54 ± 0.29 a | |
| essential: non-essential | 0.79 ± 0.03 c | 0.84 ± 0.02 d | 0.85 ± 0.03 d | 0.69 ± 0.02 ab | 0.70 ± 0.01 b | 0.65 ± 0.03 a | 0.77 ± 0.02 b | 0.85 ± 0.01 c | 0.85 ± 0.01 c | 0.67 ± 0.01 a | 0.67 ± 0.01 a | 0.67 ± 0.01 a | |
| Roach | B. Herring | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Raw Material | Acidic pH Shift | Alkaline pH Shift | Protamex | Neutrase | Corolase | Raw Material | Acidic pH Shift | Alkaline pH Shift | Protamex | Neutrase | Corolase | |
| Saturated | ||||||||||||
| 14:0 | 2.26 ± 0.02 b | 1.94 ± 0.03 a | 2.32 ± 0.13 bc | 2.69 ± 0.01 e | 2.39 ± 0.03 cd | 2.44 ± 0.03 d | 5.37 ± 0.02 a | 5.51 ± 0.18 ab | 6.01 ± 0.21 d | 5.70 ± 0.03 bc | 5.89 ± 0.04 cd | 5.71 ± 0.05 c |
| 16:0 | 16.54 ± 0.11 a | 18.28 ± 0.02 b | 17.99 ± 0.24 b | 17.84 ± 0.16 b | 19.59 ± 0.49 c | 20.02 ± 0.46 c | 17.39 ± 0.06 a | 24.80 ± 0.13 e | 25.43 ± 0.27 f | 18.02 ± 0.26 b | 20.36 ± 0.11 d | 18.87 ± 0.21 c |
| 18:0 | 3.23 ± 0.06 a | 4.00 ± 0.04 c | 3.76 ± 0.14 b | 3.23 ± 0.04 a | 3.86 ± 0.17 bc | 3.99 ± 0.11 c | 1.65 ± 0.01 a | 2.69 ± 0.03 e | 2.72 ± 0.01 e | 1.78 ± 0.07 b | 2.14 ± 0.04 d | 1.93 ± 0.04 c |
| others | 1.13 ± 0.02 bc | 1.08 ± 0.02 ab | 1.06 ± 0.04 a | 1.12 ± 0.01 b | 1.17 ± 0.05 c | 1.17 ± 0.03 c | 1.13 ± 0.01 ab | 1.15 ± 0.02 bc | 1.19 ± 0.05 c | 1.10 ± 0.02 a | 1.16 ± 0.03 bc | 1.13 ± 0.01 ab |
| Σ saturated | 23.16 ± 0.15 a | 25.30 ± 0.05 b | 25.14 ± 0.27 b | 24.88 ± 0.21 b | 27.02 ± 0.73 c | 27.62 ± 0.63 c | 25.54 ± 0.07 a | 34.15 ± 0.28 e | 35.34 ± 0.52 f | 26.60 ± 0.35 b | 29.56 ± 0.12 d | 27.64 ± 0.24 c |
| Monounsaturated | ||||||||||||
| 16:1(n-7) | 13.25 ± 0.10 a | 12.30 ± 0.06 a | 17.96 ± 1.59 b | 17.85 ± 0.12 b | 17.14 ± 0.59 b | 17.88 ± 0.52 b | 9.45 ± 0.11 e | 6.16 ± 0.27 a | 7.50 ± 0.21 b | 8.86 ± 0.10 d | 8.54 ± 0.04 c | 8.33 ± 0.06 c |
| 18:1(n-7) | 5.22 ± 0.07 a | 5.49 ± 0.04 b | 5.53 ± 0.10 b | 5.39 ± 0.02 ab | 5.19 ± 0.16 a | 5.21 ± 0.27 a | 2.85 ± 0.04 a | 3.23 ± 0.04 c | 3.69 ± 0.04 d | 2.80 ± 0.05 a | 3.06 ± 0.06 b | 2.79 ± 0.07 a |
| 18:1(n-9) | 22.88 ± 0.22 d | 21.72 ± 0.20 b | 20.67 ± 0.38 a | 21.75 ± 0.18 b | 22.42 ± 0.14 c | 22.38 ± 0.23 c | 25.62 ± 0.14 c | 19.73 ± 0.79 a | 22.84 ± 0.75 b | 26.34 ± 0.22 c | 26.17 ± 0.23 c | 25.98 ± 0.18 c |
| 20:1(n-9) | 0.87 ± 0.02 ab | 0.97 ± 0.01 d | 0.87 ± 0.05 a | 0.91 ± 0.01 bc | 0.95 ± 0.02 cd | 0.93 ± 0.01 cd | 1.92 ± 0.02 c | 1.36 ± 0.07 a | 1.60 ± 0.08 b | 2.02 ± 0.01 d | 1.93 ± 0.02 c | 1.93 ± 0.03 c |
| 24:1(n-9) | 0.17 ± 0.01 c | 0.23 ± 0.00 d | 0.22 ± 0.01 d | 0.11 ± 0.01 b | 0.09 ± 0.01 ab | 0.08 ± 0.02 a | 0.96 ± 0.01 a | 1.12 ± 0.01 d | 1.26 ± 0.02 e | 1.00 ± 0.02 b | 1.08 ± 0.00 c | 0.99 ± 0.02 b |
| others | 0.43 ± 0.02 a | 0.48 ± 0.02 ab | 0.56 ± 0.03 c | 0.45 ± 0.01 ab | 0.51 ± 0.07 bc | 0.46 ± 0.01 ab | 0.54 ± 0.01 ab | 0.50 ± 0.02 a | 0.56 ± 0.03 abc | 0.59 ± 0.01 bc | 0.62 ± 0.04 c | 0.61 ± 0.07 bc |
| Σ monounsaturated | 42.83 ± 0.17 b | 41.20 ± 0.25 a | 45.81 ± 1.10 c | 46.47 ± 0.11 cd | 46.29 ± 0.39 cd | 46.94 ± 0.42 d | 41.35 ± 0.25 c | 32.10 ± 1.15 a | 37.45 ± 1.07 b | 41.62 ± 0.27 c | 41.39 ± 0.24 c | 40.63 ± 0.28 c |
| Polyunsaturated | ||||||||||||
| n-3 | ||||||||||||
| 18:3(n-3) | 1.87 ± 0.05 d | 1.37 ± 0.03 c | 1.03 ± 0.11 ab | 1.15 ± 0.03 ab | 1.18 ± 0.14 b | 1.01 ± 0.11 a | 1.47 ± 0.02 c | 1.26 ± 0.02 b | 1.02 ± 0.03 a | 1.54 ± 0.01 d | 1.47 ± 0.03 c | 1.66 ± 0.03 e |
| 18:4(n-3) | 0.61 ± 0.02 b | 0.48 ± 0.01 a | 0.60 ± 0.04 b | 0.68 ± 0.02 c | 0.63 ± 0.02 b | 0.62 ± 0.02 b | 1.63 ± 0.00 d | 0.96 ± 0.01 b | 0.74 ± 0.03 a | 1.66 ± 0.02 de | 1.40 ± 0.04 c | 1.69 ± 0.03 e |
| 20:3(n-3) | 0.45 ± 0.01 c | 0.43 ± 0.01 c | 0.30 ± 0.04 ab | 0.34 ± 0.00 b | 0.31 ± 0.03 ab | 0.28 ± 0.03 a | 0.79 ± 0.01 c | 0.73 ± 0.01 b | 0.60 ± 0.01 a | 0.89 ± 0.01 e | 0.84 ± 0.01 d | 0.88 ± 0.01 e |
| 20:4(n-3) | 0.72 ± 0.02 b | 0.66 ± 0.01 a | 0.73 ± 0.01 b | 0.83 ± 0.01 c | 0.75 ± 0.04 b | 0.73 ± 0.03 b | 1.16 ± 0.01 d | 0.93 ± 0.02 b | 0.70 ± 0.04 a | 1.21 ± 0.01 e | 1.03 ± 0.02 c | 1.15 ± 0.01 d |
| 20:5(n-3) | 8.30 ± 0.12 c | 7.40 ± 0.04 b | 6.46 ± 0.16 a | 7.07 ± 0.06 b | 6.67 ± 0.31 a | 6.41 ± 0.27 a | 7.22 ± 0.04 c | 7.03 ± 0.31 c | 5.50 ± 0.46 a | 6.46 ± 0.04 b | 5.74 ± 0.05 a | 6.56 ± 0.02 b |
| 22:4(n-3) | 0.03 ± 0.00 c | 0.03 ± 0.00 bc | 0.03 ± 0.00 bc | 0.04 ± 0.00 d | 0.03 ± 0.00 ab | 0.02 ± 0.00 a | 1.14 ± 0.02 e | 0.73 ± 0.02 b | 0.52 ± 0.02 a | 1.22 ± 0.01 f | 0.99 ± 0.02 c | 1.10 ± 0.02 d |
| 22:5(n-3) | 2.31 ± 0.04 e | 1.98 ± 0.03 c | 1.95 ± 0.01 bc | 2.17 ± 0.03 d | 1.91 ± 0.01 ab | 1,88 ± 0,02 | 0.86 ± 0.03 d | 0.73 ± 0.03 b | 0.51 ± 0.04 a | 0.83 ± 0.01 cd | 0.70 ± 0.02 b | 0.81 ± 0.01 c |
| 22:6(n-3) | 8.63 ± 0.17 c | 10.89 ± 0.23 d | 8.92 ± 0.14 c | 7.47 ± 0.07 b | 6.62 ± 0.26 a | 6.38 ± 0,31 a | 10.45 ± 0.05 b | 14.39 ± 1.08 c | 11.03 ± 1.02 b | 9.13 ± 0.14 a | 8.63 ± 0.25 a | 9.19 ± 0.21 a |
| 24:4(n-3) | 0.02 ± 0.01 abc | 0.02 ± 0.01 c | 0.02 ± 0.00 bc | 0.01 ± 0.00 ab | 0.01 ± 0.00 ab | 0.01 ± 0.00 a | 1.10 ± 0.01 d | 0.72 ± 0.02 b | 0.52 ± 0.01 a | 1.17 ± 0.01 e | 0.95 ± 0.03 c | 1.07 ± 0.02 d |
| 24:5(n-3) | 0.23 ± 0.00 c | 0.22 ± 0.00 b | 0.20 ± 0.01 a | 0.26 ± 0.00 d | 0.21 ± 0.01 a | 0.20 ± 0.00 a | 0.61 ± 0.06 b | 0.37 ± 0.09 a | 0.28 ± 0.12 a | 0.71 ± 0.11 b | 0.58 ± 0.12 b | 0.73 ± 0.07 b |
| Σ n-3 | 23.15 ± 0.07 c | 23.50 ± 0.26 c | 20.25 ± 0.38 b | 20.01 ± 0.03 b | 18.31 ± 0.80 a | 17.55 ± 0.76 a | 26.44 ± 0.17 c | 27.87 ± 1.45 c | 21.44 ± 1.52 a | 24.82 ± 0.19 b | 22.34 ± 0.17 a | 24.83 ± 0.07 b |
| n-6 | ||||||||||||
| 18:2(n-6) | 4.85 ± 0.10 d | 3.70 ± 0.04 c | 3.32 ± 0.12 b | 3.72 ± 0.05 c | 3.24 ± 0.28 b | 2.93 ± 0.26 a | 3.80 ± 0.02 c | 3.26 ± 0.05 a | 3.39 ± 0.03 b | 4.01 ± 0.03 e | 3.92 ± 0.03 d | 4.05 ± 0.02 e |
| 20:2(n-6) | 1.12 ± 0.02 cd | 1.17 ± 0.00 d | 0.95 ± 0.10 a | 1.02 ± 0.02 ab | 1.07 ± 0.02 bc | 1.03 ± 0.01 ab | 1.29 ± 0.01 c | 1.06 ± 0.03 b | 1.02 ± 0.01 a | 1.39 ± 0.01 e | 1.32 ± 0.01 d | 1.33 ± 0.01 d |
| 20:4(n-6) | 4.11 ± 0.07 c | 4.37 ± 0.07 d | 3.77 ± 0.23 b | 3.09 ± 0.02 a | 3.21 ± 0.05 a | 3.10 ± 0.05 a | 0.74 ± 0.00 b | 0.89 ± 0.05 c | 0.76 ± 0.07 b | 0.66 ± 0.01 a | 0.67 ± 0.01 a | 0.68 ± 0.01 a |
| 22:2(n-6) | 0.04 ± 0.00 b | 0.04 ± 0.00 b | 0.03 ± 0.00 a | 0.04 ± 0.00 b | 0.03 ± 0.00 ab | 0.03 ± 0.00 a | 0.71 ± 0.01 c | 0.56 ± 0.04 b | 0.52 ± 0.02 a | 0.76 ± 0.01 d | 0.69 ± 0.02 c | 0.71 ± 0.01 c |
| others | 0.74 ± 0.01 a | 0.73 ± 0.00 a | 0.73 ± 0.05 a | 0.77 ± 0.02 ab | 0.81 ± 0.03 b | 0.81 ± 0.04 b | 0.13 ± 0.00 d | 0.11 ± 0.00 b | 0.08 ± 0.00 a | 0.13 ± 0.01 d | 0.12 ± 0.00 c | 0.13 ± 0.00 d |
| Σ n-6 | 10.85 ± 0.14 d | 10.01 ± 0.04 c | 8.80 ± 0.48 b | 8.64 ± 0.11 b | 8.37 ± 0.32 ab | 7.90 ± 0.29 a | 6.67 ± 0.04 c | 5.89 ± 0.06 b | 5.77 ± 0.08 a | 6.95 ± 0.04 d | 6.71 ± 0.03 c | 6.90 ± 0.03 d |
| Σ polyunsaturated | 34.00 ± 0.09 c | 33.50 ± 0.29 c | 29.05 ± 0.86 b | 28.65 ± 0.11 b | 26.69 ± 1.11 a | 25.44 ± 1.05 a | 33.10 ± 0.20 cd | 33.75 ± 1.39 d | 27.20 ± 1.59 a | 31.78 ± 0.19 c | 29.05 ± 0.14 b | 31.73 ± 0.05 c |
| Ratios | ||||||||||||
| unsaturated:saturated | 3.32 ± 0.03 c | 2.95 ± 0.01 b | 2.98 ± 0.04 b | 3.02 ± 0.03 b | 2.70 ± 0.10 a | 2.62 ± 0.08 a | 2.91 ± 0.01 f | 1.93 ± 0.02 b | 1.83 ± 0.04 a | 2.76 ± 0.05 e | 2.38 ± 0.01 c | 2.62 ± 0.03 d |
| n-3:n-6 | 2.13 ± 0.03 a | 2.35 ± 0.02 c | 2.30 ± 0.08 c | 2.32 ± 0.03 c | 2.19 ± 0.03 ab | 2.22 ± 0.03 b | 3.97 ± 0.02 c | 4.74 ± 0.29 d | 3.71 ± 0.21 bc | 3.57 ± 0.04 ab | 3.33 ± 0.04 a | 3.60 ± 0.03 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kakko, T.; Damerau, A.; Nisov, A.; Puganen, A.; Tuomasjukka, S.; Honkapää, K.; Tarvainen, M.; Yang, B. Quality of Protein Isolates and Hydrolysates from Baltic Herring (Clupea harengus membras) and Roach (Rutilus rutilus) Produced by pH-Shift Processes and Enzymatic Hydrolysis. Foods 2022, 11, 230. https://doi.org/10.3390/foods11020230
Kakko T, Damerau A, Nisov A, Puganen A, Tuomasjukka S, Honkapää K, Tarvainen M, Yang B. Quality of Protein Isolates and Hydrolysates from Baltic Herring (Clupea harengus membras) and Roach (Rutilus rutilus) Produced by pH-Shift Processes and Enzymatic Hydrolysis. Foods. 2022; 11(2):230. https://doi.org/10.3390/foods11020230
Chicago/Turabian StyleKakko, Tanja, Annelie Damerau, Anni Nisov, Anna Puganen, Saska Tuomasjukka, Kaisu Honkapää, Marko Tarvainen, and Baoru Yang. 2022. "Quality of Protein Isolates and Hydrolysates from Baltic Herring (Clupea harengus membras) and Roach (Rutilus rutilus) Produced by pH-Shift Processes and Enzymatic Hydrolysis" Foods 11, no. 2: 230. https://doi.org/10.3390/foods11020230
APA StyleKakko, T., Damerau, A., Nisov, A., Puganen, A., Tuomasjukka, S., Honkapää, K., Tarvainen, M., & Yang, B. (2022). Quality of Protein Isolates and Hydrolysates from Baltic Herring (Clupea harengus membras) and Roach (Rutilus rutilus) Produced by pH-Shift Processes and Enzymatic Hydrolysis. Foods, 11(2), 230. https://doi.org/10.3390/foods11020230









