Impact of Hydro-Alcoholic Solvents on the Oil and Phenolics Extraction from Walnut (Juglans regia L.) Press-Cake and the Self-Emulsification of Extracts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Walnut Cake
2.2. Solvents and Reagents
2.3. Semi-Continuous Extraction
2.4. Characterization
2.5. Emulsification
2.6. Statistical Analysis
3. Results
3.1. Extraction Yields
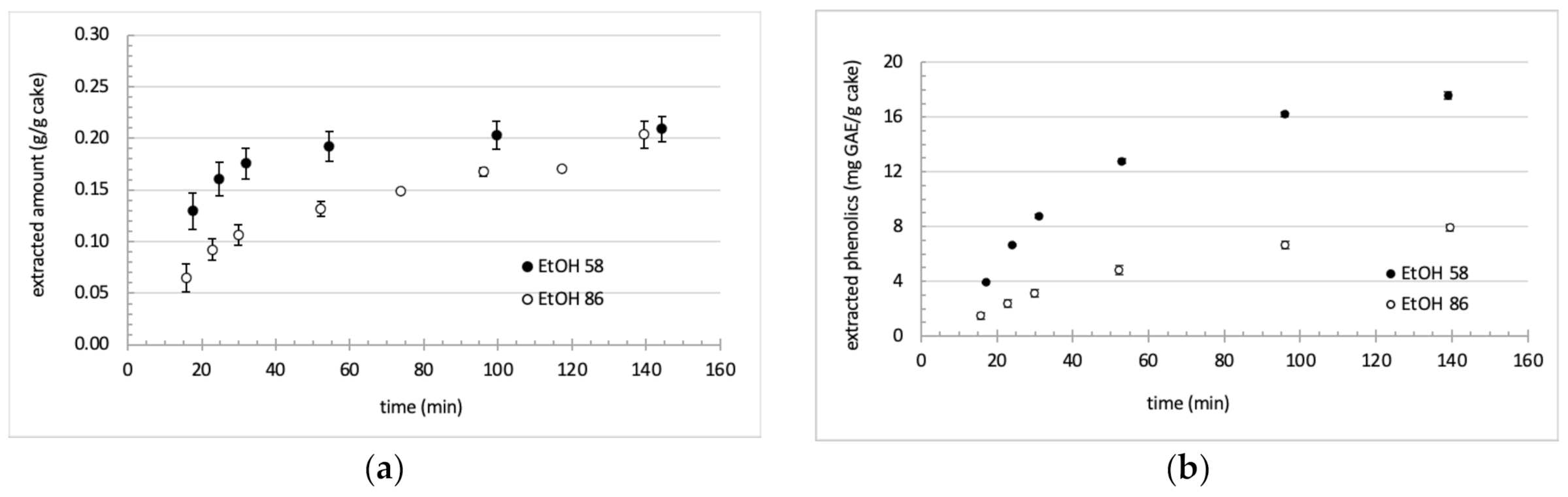
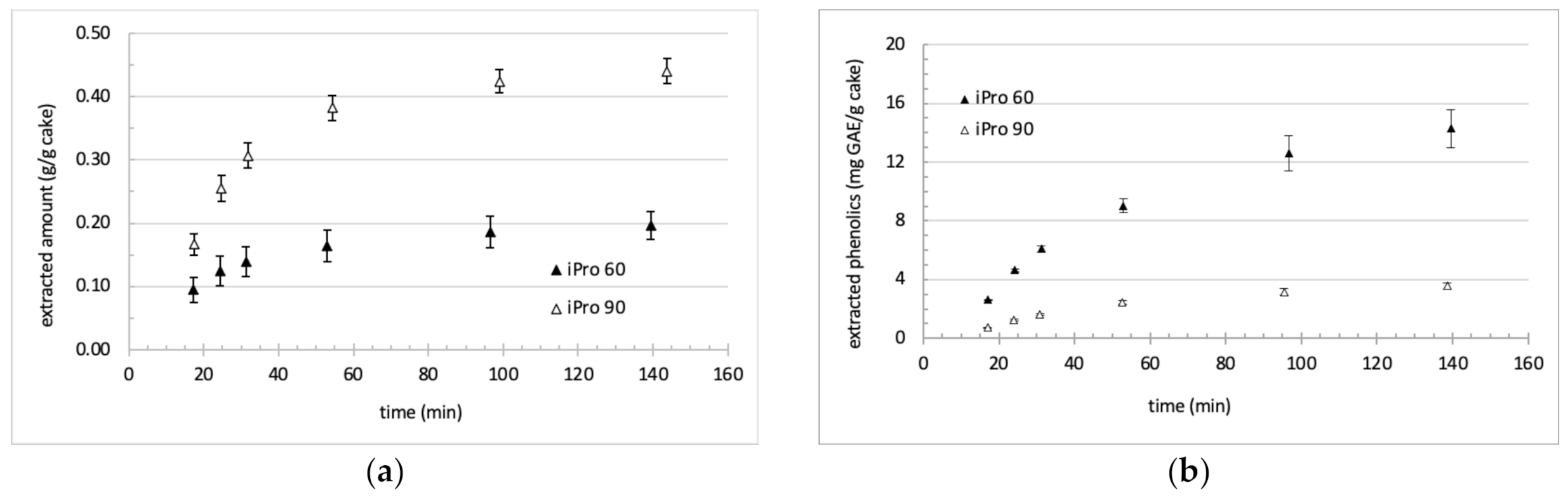
3.2. Extraction Kinetics and Aspect of Collected Fractions
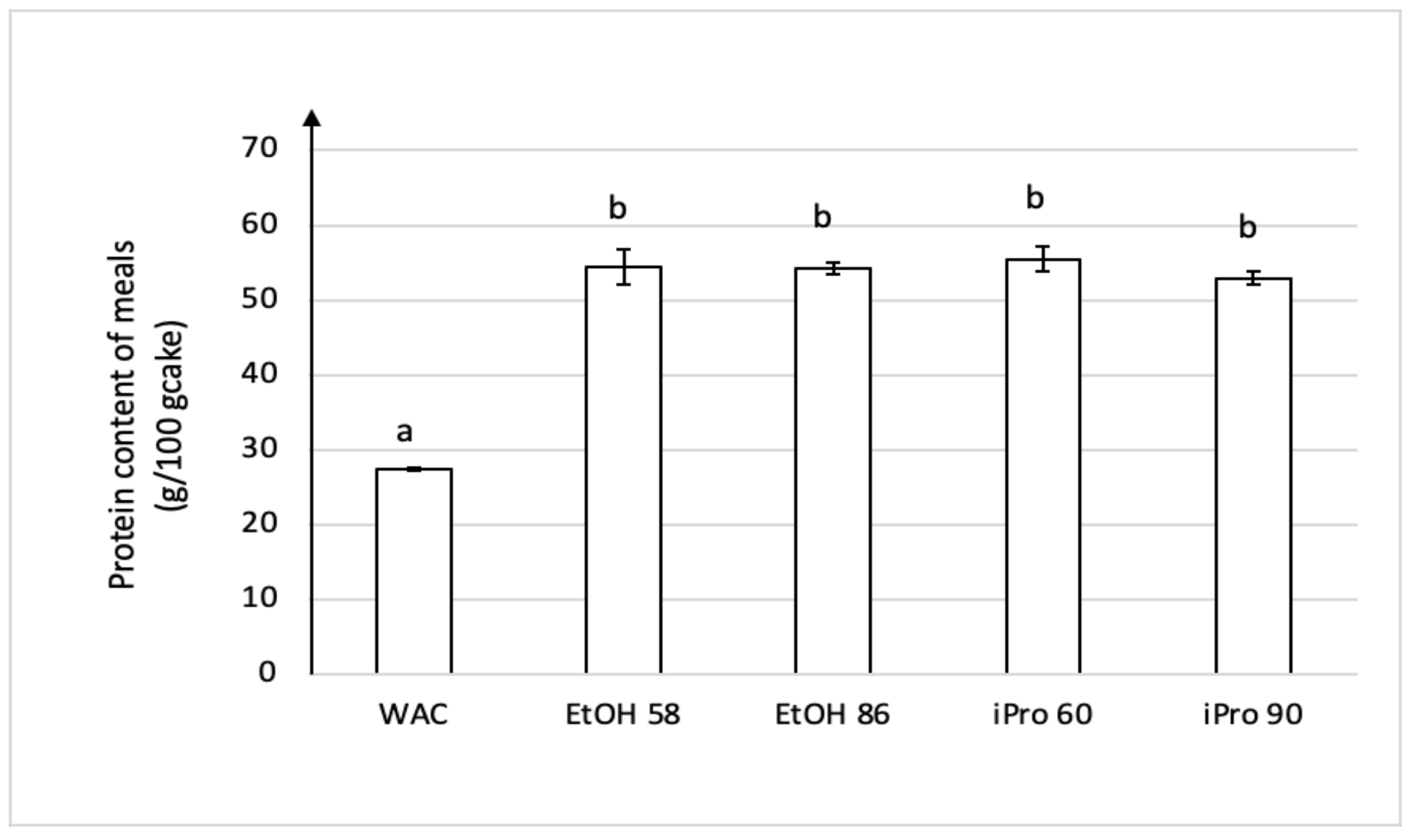
3.3. Self-Emulsification of Extracts
4. Discussion and Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jahanban-Esfahlan, A.; Ostadrahimi, A.; Tabibiazar, M.; Amarowicz, R. A comparative review on the extraction, antioxidant content and antioxidant potential of different parts of walnut (Juglans regia L.) fruit and tree. Molecules 2019, 24, 2133. [Google Scholar] [CrossRef] [Green Version]
- Gao, P.; Liu, R.; Jin, Q.; Wang, X. Effects of processing methods on the chemical composition and antioxidant capacity of walnut (Juglans regia L.) oil. LWT—Food Sci. Technol. 2021, 135, 100958. [Google Scholar] [CrossRef]
- Martinez, M.; Labuckas, D.; Lamarque, A.; Maestri, D. Walnut (Juglans regia L.): Genetic resources, chemistry, by-products. J. Sci. Food Agric. 2010, 90, 1959–1967. [Google Scholar] [CrossRef] [PubMed]
- Bakkalbasi, E.; Meral, R.; Dogan, I. Bioactive compounds, physical and sensory properties of cake made with walnut press-cake. J. Food Qual. 2015, 38, 422–430. [Google Scholar] [CrossRef]
- Santos, J.; Alvarez-Orti, M.; Sena-Moreno, E.; Rabadan, A.; Pardo, J.; Oliveira, M.B. Effect of roasting conditions on the composition and antioxidant properties of defatted walnut flour. J. Sci. Food Agric. 2018, 98, 1813–1820. [Google Scholar] [CrossRef]
- Bakkalbasi, E. Oxidative stability of enriched walnut oil with phenolic extracts from walnut press-cake under accelerated oxidation conditions and the effect of ultrasound treatment. J. Food Meas. Charact. 2019, 13, 43–50. [Google Scholar] [CrossRef]
- Mao, X.; Hua, Y.; Chen, G. Amino Acid Composition, Molecular Weight Distribution and Gel Electrophoresis of Walnut (Juglans regia L.) Proteins and Protein Fractionations. Int. J. Mol. Sci. 2014, 15, 2003–2014. [Google Scholar] [CrossRef] [Green Version]
- Sze-Tao, K.W.C.; Sathe, S.K. Walnuts (Juglans regia L): Proximate composition, protein solubility, protein amino acid composition and protein in vitro digestibility. J. Sci. Food Agric. 2000, 80, 1393–1401. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R. Plant Phenolics: Extraction, Analysis and Their Antioxidant and Anticancer Properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- Arranz, S.; Perez-Jiménez, J.; Saura-Calixto, F. Antioxidant capacity of walnut (Juglans regia L.): Contribution of oil and defatted matter. Eur. Food Res. Technol. 2008, 227, 425–431. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.; Wu, W.; Chen, D.; Lin, Y.; Ma, Y.; Chen, C.; Zhao, S. Optimization of simultaneous microwave/ultrasonic-assisted extraction of phenolic compounds from walnut flour using response surface methodology. Pharm. Biol. 2017, 55, 1999–2004. [Google Scholar] [CrossRef]
- Ojeda-Amador, R.; Desamparados, M.; Gomez-Alonso, S.; Fregapane, G. Characterization of virgin walnut oils and their residual cakes produced from different varieties. Food Res. Int. 2018, 108, 396–404. [Google Scholar] [CrossRef]
- Fregapane, G.; Guisantes-Batan, E.; Ojeda-Amador, R.; Desamparados Salvador, M. Development of functional edible oils enriched with pistachio and walnut phenolic extracts. Food Chem. 2020, 310, 125917. [Google Scholar] [CrossRef]
- Garcia-Mendoza, M.P.; Espinosa-Pardo, F.A.; Savoire, R.; Etchegoyen, C.; Harscoat-Schiavo, C.; Subra-Paternault, P. Recovery and antioxidant activity of phenolic compounds extracted from walnut press-cake using various methods and conditions. Ind. Crops Prod. 2021, 167, 113546. [Google Scholar] [CrossRef]
- Grosso, A.; Asensio, C.; Nepote, V.; Grosso, N. Antioxidant activity displayed by phenolic compounds obtained from walnut oil cake used for walnut oil preservation. J. Am. Oil Chem. Soc. 2018, 95, 1409–1419. [Google Scholar] [CrossRef]
- Boidora, R.; Maestri, D. Phenolic Compounds from Nuts: Extraction, Chemical Profiles, and Bioactivity. J. Agric. Food Chem. 2020, 68, 927–942. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, T.; Ito, H.; Yoshida, T. Antioxidative polyphenols from walnuts (Juglans regia L.). Phytochemistry 2003, 63, 795–801. [Google Scholar] [CrossRef]
- Labuckas, D.; Maestri, D.; Perello, M.; Martinez, M.; Lamarque, A. Phenolics from walnut (Juglans regia L.) kernels: Antioxidant activity and interactions with proteins. Food Chem. 2008, 107, 607–612. [Google Scholar] [CrossRef]
- Carré, P. About solvents used in the preparation of oils for cosmetic products complying with the Cosmos standard. OCL 2021, 28, 16. [Google Scholar] [CrossRef]
- Citeau, M.; Slabi, A.S.; Joffre, F.; Carré, P. Improved rapeseed oil extraction yield and quality via cold separation of ethanol miscella. OCL 2018, 25, D207. [Google Scholar] [CrossRef] [Green Version]
- Citeau, M.; Regis, J.; Carré, P.; Fine, F. Value of hydroalcoholic treatment of rapeseed for oil extraction and protein enrichment. OCL 2019, 26, 1. [Google Scholar] [CrossRef] [Green Version]
- Dagostin, A.; Carpiné, D.; Corazza, M. Extraction of soybean oil using ethanol and mixtures with alkyl esters (biodiesel) as co-solvent: Kinetics and thermodynamics. Ind. Crop. Prod. 2015, 74, 69–75. [Google Scholar] [CrossRef]
- Baümler, E.; Carrin, M.; Carelli, A. Extraction of sunflower oil using ethanol as solvent. J. Food Eng. 2016, 178, 190–197. [Google Scholar] [CrossRef]
- Capellini, M.; Chiavoloni, L.; Giacomini, V.; Rodrigues, C. Alcoholic extraction of sesame seed cake oil: Influence of the process conditions on the physicochemical characteristics of the oil and defatted meal proteins. J. Food Eng. 2019, 240, 145–152. [Google Scholar] [CrossRef]
- Capellini, M.; Giacomini, V.; Cuevas, M.; Rodrigues, C. Rice bran oil extraction using alcoholic solvents: Physicochemical characterization of oil and protein fraction functionality. Ind. Crop. Prod. 2017, 104, 133–143. [Google Scholar] [CrossRef]
- Navarro, S.; Capellini, M.; Aracava, K.; Rodrigues, C. Corn germ-bran oils extracted with alcoholic solvents: Extraction yield, oil composition and evaluation of protein solubility of defatted meal. Food Bioprod. Process 2016, 100, 185–194. [Google Scholar] [CrossRef]
- Rodriguez, L.; Fernandez, M.; Pérez, E.; Crapiste, G. Performance of Green Solvents in the Extraction of Sunflower Oil from Enzyme-Treated Collets. Eur. J. Lipid Sci. Technol. 2021, 123, 2000132. [Google Scholar] [CrossRef]
- Seth, S.; Agrawal, Y.C.; Ghosh, P.K.; Jayas, D.S.; Singh, B.P.N. Oil extraction rates of soya bean using isopropyl alcohol as solvent. Biosyst. Eng. 2007, 97, 209–217. [Google Scholar] [CrossRef]
- Zhang, F.; Rhee, K.C.; Koseoglu, S.S. Isopropyl alcohol extraction of cottonseed collets: Efficiency and performance. J. Food Lipids 2002, 9, 147–160. [Google Scholar] [CrossRef]
- Sinichi, S.; Diosady, L. Isopropyl Alcohol Extraction of De-hulled Yellow Mustard Flour. J. Am. Oil Chem. Soc. 2014, 91, 2143–2153. [Google Scholar] [CrossRef]
- McClements, D.; Gumus, C. Natural emulsifiers—Biosurfactants, phospholipids, biopolymers, and colloidal particles: Molecular and physicochemical basis of functional performance. Adv. Colloid Interface Sci. 2016, 234, 3–26. [Google Scholar] [CrossRef] [Green Version]
- Dammak, I.; do Amaral Sobral, P.J.; Aquino, A.; das Neves, M.A.; Conte-Junior, C.A. Nanoemulsions: Using emulsifiers from natural sources replacing synthetic ones—A review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2721–2746. [Google Scholar] [CrossRef] [PubMed]
- Marzocchi, S.; Anankabil, S.; Caboni, M.F.; Guo, Z. Enzymatic alkylsuccinylation of tyrosol: Synthesis, characterization and property evaluation as a dual-functional antioxidant. Food Chem. 2018, 246, 108–114. [Google Scholar] [CrossRef]
- Rayner, M.; Marku, D.; Eriksson, M.; Sjöö, M.; Dejmek, P.; Wahlgren, M. Biomass-based particles for the formulation of Pickering type emulsions in food and topical applications. Colloids Surf. A: Physicochem. Eng. Asp. 2014, 458, 48–62. [Google Scholar] [CrossRef]
- Joseph, C.; Savoire, R.; Harscoat-Schiavo, C.; Pintori, D.; Monteil, J.; Faure, C.; Leal-Calderon, F. Pickering emulsions stabilized by various plant materials: Cocoa, rapeseed press cake and lupin hulls. LWT—Food Sci. Technol. 2020, 130, 109621. [Google Scholar] [CrossRef]
- Espinosa-Pardo, F.A.; Savoire, R.; Subra-Paternault, P.; Harscoat-Schiavo, C. Oil and protein recovery from corn germ: Extraction yield, composition and protein functionality. Food Bioprod. Process 2020, 120, 131–142. [Google Scholar] [CrossRef]
- Lahtinen, M.; Valoppi, F.; Juntti, V.; Heikkinen, S.; Kilpeläinen, P.; Maina, N.; Mikkonen, K. Lignin-Rich PHWE Hemicellulose Extracts Responsible for Extended Emulsion Stabilization. Front. Chem. 2019, 7, 871. [Google Scholar] [CrossRef]
- Ralla, T.; Herz, E.; Salminen, H.; Edelmann, M.; Dawid, C.; Hofman, T.; Weiss, J. Emulsifying Properties of Natural Extracts from Panax ginseng L. Food Biophys. 2017, 12, 479–490. [Google Scholar] [CrossRef]
- Jarzebski, M.; Siejak, P.; Smułek, W.; Fathordoobady, F.; Guo, Y.; Pawlicz, J.; Trzeciak, T.; Łukasz Kowalczewski, P.; Kitts, D.; Singh, A.; et al. Plant Extracts Containing Saponins Affects the Stability and Biological Activity of Hempseed Oil Emulsion System. Molecules 2020, 25, 2696. [Google Scholar] [CrossRef]
- Filotheou, A.; Ritzoulis, C.; Avgidou, M.; Kalogianni, E.; Pavlou, A.; Panayiotou, C. Novel emulsifiers from olive processing solid waste. Food Hydrocoll. 2015, 48, 274–281. [Google Scholar] [CrossRef]
- Sabri, N.; Moulai-Mostefa, N. Formulation and Characterization of Oil-in-Water Emulsions Stabilized by Saponins Extracted from Hedera Helix Algeriensis Using Response Surface Method. Biointerface Res. 2020, 10, 6282–6292. [Google Scholar] [CrossRef]
- Jin, F.; Wang, Y.; Tang, H.; Regenstein, J.; Wang, F. Limited hydrolysis of dehulled walnut (Juglans regia L.) proteins using trypsin: Functional properties and structural characteristics. LWT—Food Sci. Technol. 2020, 33, 110035. [Google Scholar] [CrossRef]
- Moghadam, M.; Salami, M.; Mohammadian, M.; Emam-Djomeh, Z.; Jahanbani, R.; Moosavi-Movahedi, A. Physicochemical and bio-functional properties of walnut proteins as affected by trypsin-mediated hydrolysis. Food Biosci. 2020, 36, 100611. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhu, W.; Yi, J.; Liu, N.; Cao, Y.; Lu, J.; Decker, E.; McClements, D. Effects of sonication on the physicochemical and functional properties of walnut protein isolate. Food Res. Int. 2018, 106, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yao, L.; Lee, S.-L.; Wang, T. Extraction of Phospholipids from Egg Yolk Flakes Using Aqueous Alcohols. J. Am. Oil Chem. Soc. 2017, 94, 309–314. [Google Scholar] [CrossRef]
- Price, N.; Fei, T.; Clark, S.; Wang, T. Extraction of phospholipids from a dairy by-product (whey protein phospholipid concentrate) using ethanol. J. Dairy Sci. 2018, 101, 8778–8787. [Google Scholar] [CrossRef]
- Figueroa-Espinoza, M.C.; Zafimahova, A.; Maldonado Alvarado, P.; Dubreucq, E.; Poncet-Legrand, C. Grape seed and apple tannins: Emulsifying and antioxidant properties. Food Chem. 2015, 178, 38–44. [Google Scholar] [CrossRef]
- Zhang, H.; Shin, J.A.; Hong, S.T.; Lee, K.T. Stability and antioxidant effect of rapeseed extract in oil-in-water emulsion. Korean J. Agricult. Sci. 2016, 43, 249–257. [Google Scholar] [CrossRef] [Green Version]
- AOAC. Official Methods of Analysis, 17th ed.; Method 992.23; The Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Odabas, H.; Koca, I. Application of response surface methodology for optimizing the recovery of phenolic compounds from hazelnut skin using different extraction methods. Ind. Crop. Prod. 2016, 91, 114–124. [Google Scholar] [CrossRef]
- Tir, R.; Dutta, P.; Badjah-Hadj-Ahmed, A. Effect of the extraction solvent polarity on the sesame seeds oil composition. Eur. J. Lipid Sci. Technol. 2012, 114, 1427–1438. [Google Scholar] [CrossRef]
- Cornelio-Santiago, H.; Mazalli, M.; Rodrigues, C.; de Oliveira, A. Extraction of Brazil nut kernel oil using green solvents: Effects of the process variables in the oil yield and composition. J. Food Process Eng. 2019, 42, e1327. [Google Scholar] [CrossRef]
- Sampaio Neto, O.; Batista, E.; Meirelles, A. The employment of ethanol as solvent to extract Brazil nut oil. J. Cleaner Prod. 2018, 180, 866–875. [Google Scholar] [CrossRef]
- Sawada, M.; Venâncio, L.; Toda, T.; Rodrigues, C. Effects of different alcoholic extraction conditions on soybean oil yield, fatty acid composition and protein solubility of defatted meal. Food Res. Int. 2014, 62, 662–670. [Google Scholar] [CrossRef]
- Oliveira, R.; Oliveira, V.; Kazue Aracava, K.; da Costa Rodrigues, C. Effects of the extraction conditions on the yield and composition of rice bran oil extracted with ethanol—A response surface approach. Food Bioprod. Process 2012, 90, 22–31. [Google Scholar] [CrossRef]
- Kwiatkowski, J.; Cheryan, M. Extraction of Oil from Ground Corn Using Ethanol. J. Am. Oil Chem. Soc. 2002, 79, 825–830. [Google Scholar] [CrossRef]
- Malmberg, C.; Maryott, A. Dielectric Constant of Water from 0° to 100 °C. J. Res. Natl. Bur. Stand. 1956, 56, 2641. [Google Scholar] [CrossRef]
- Delvar, A.; De Satgé-Caro, P.; Candy, L.; Caro, Y.; Cheong Sing, A.-S.; Raynaud, C. Integrated process for extraction and formulation in emulsions of active molecules from fresh passion fruits (Passiflora edulis Sims). J. Food Eng. 2019, 263, 388–397. [Google Scholar] [CrossRef] [Green Version]
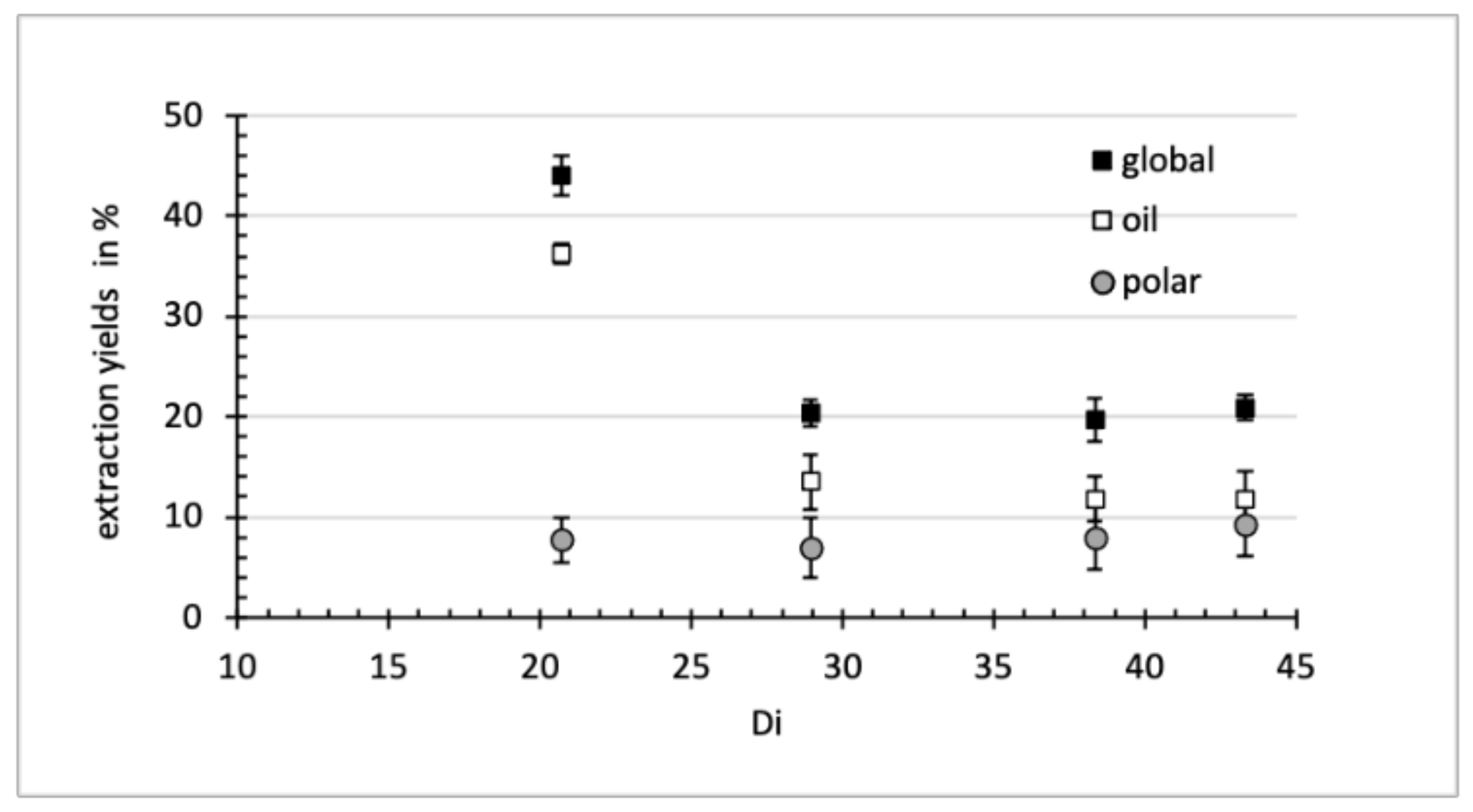
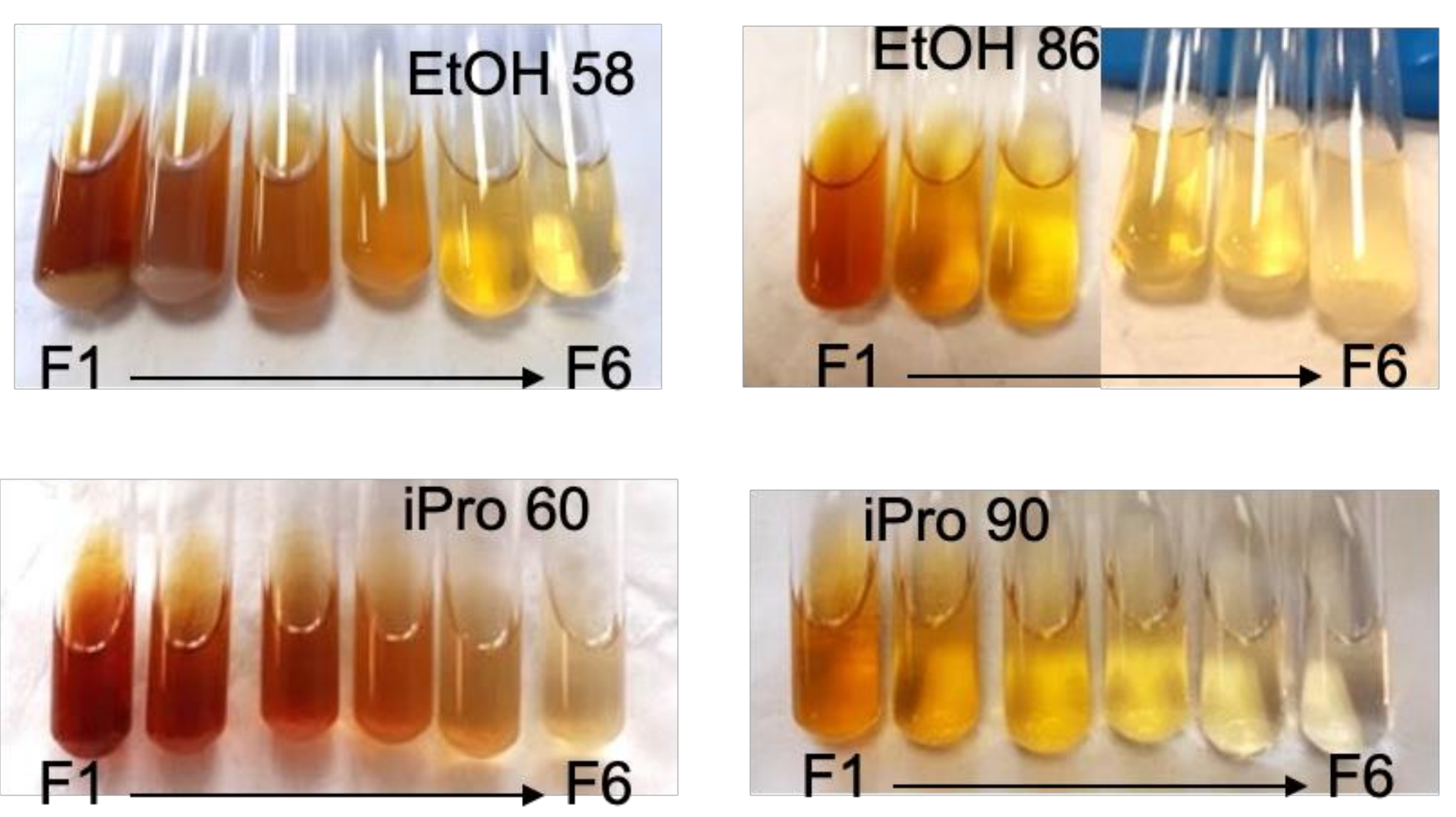

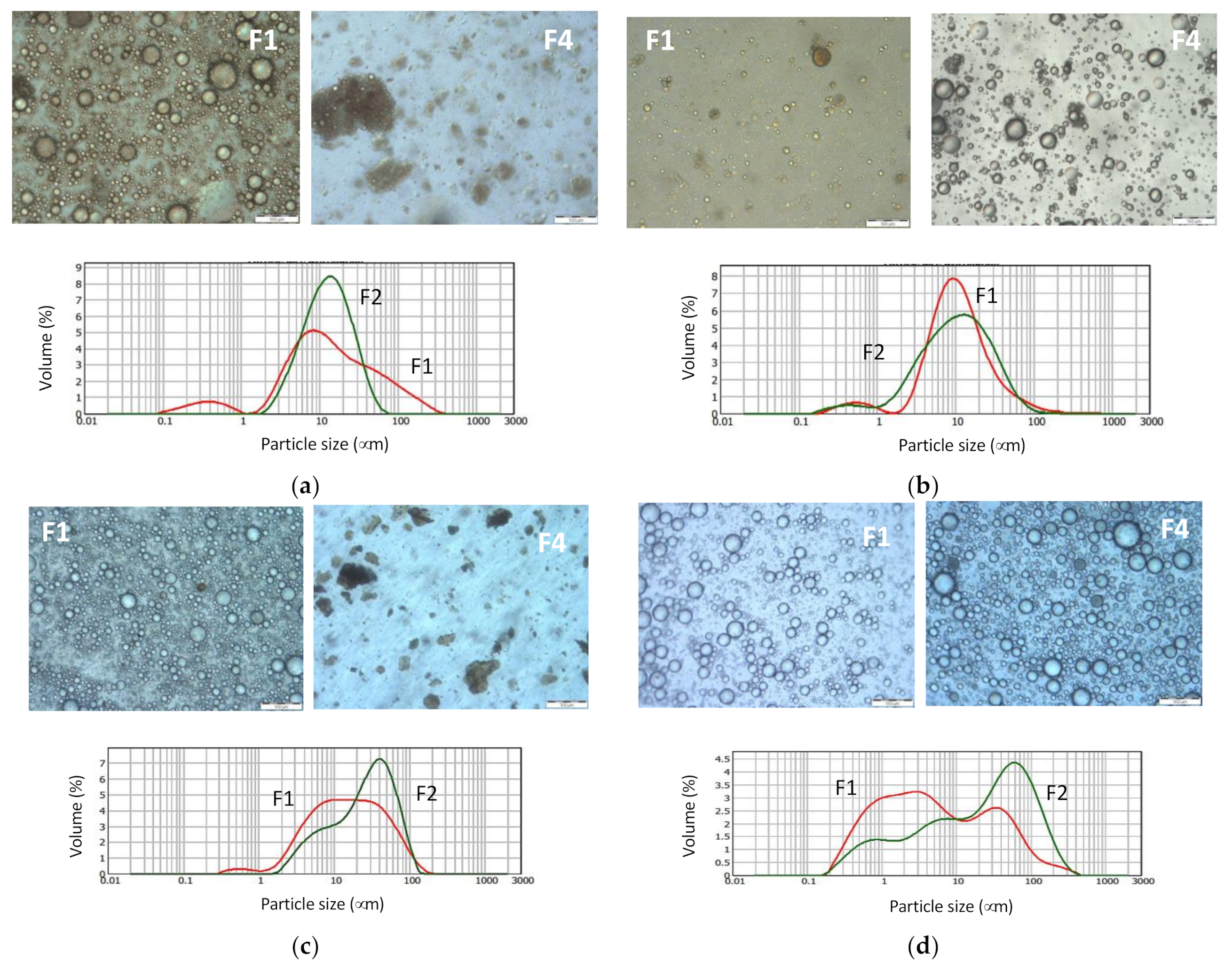
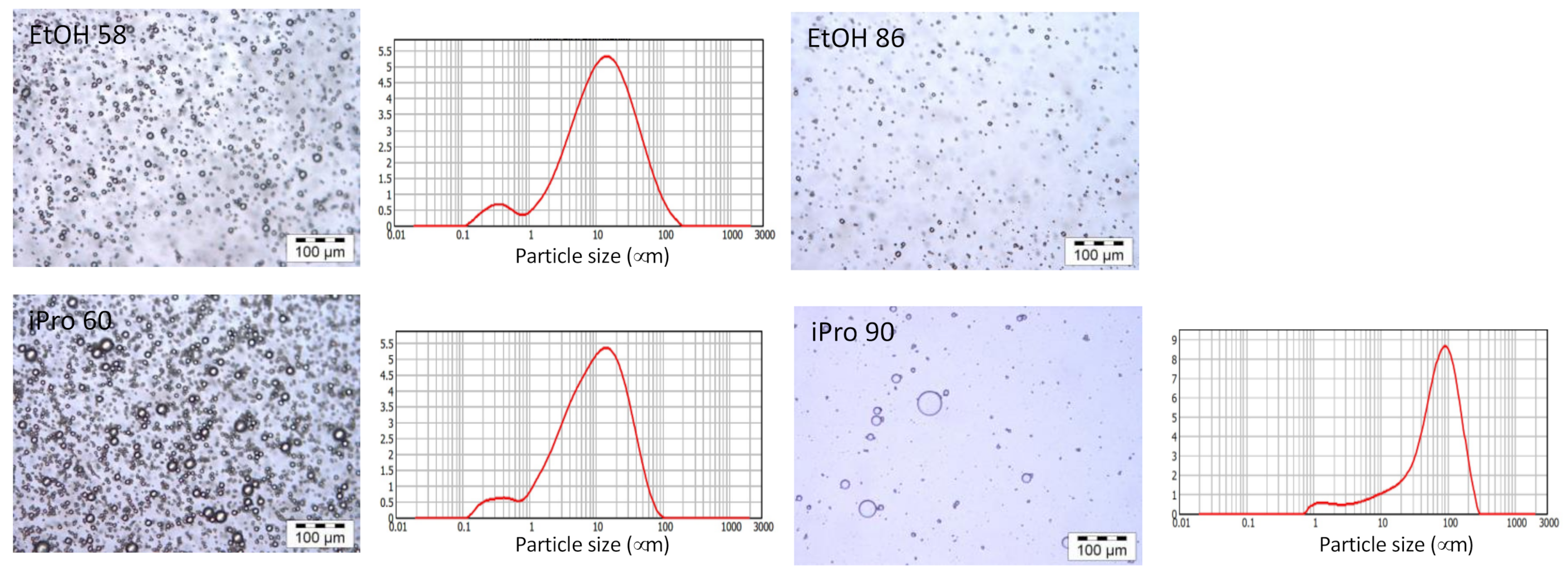
| Solvent | Total Extract (gextract/gcake) | Total Phenolics (mg GAE/gcake) | Oil Extraction (g oil/gcake) | Di |
|---|---|---|---|---|
| EtOH 58 | 0.21 ± 0.01 a | 17.6 ± 0.3 a | 0.12 ± 0.03 a | 43.3 |
| EtOH 86 | 0.20 ± 0.01 a | 7.9 ± 0.3 b | 0.14 ± 0.03 a | 28.9 |
| iPro 60 | 0.20 ± 0.02 a | 14 ± 1 c | 0.12 ± 0.02 a | 38.3 |
| iPro 90 | 0.44 ± 0.02 b | 3.6 ± 0.2 d | 0.36 ± 0.01 b | 20.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Subra-Paternault, P.; Garcia-Mendoza, M.d.P.; Savoire, R.; Harscoat-Schiavo, C. Impact of Hydro-Alcoholic Solvents on the Oil and Phenolics Extraction from Walnut (Juglans regia L.) Press-Cake and the Self-Emulsification of Extracts. Foods 2022, 11, 186. https://doi.org/10.3390/foods11020186
Subra-Paternault P, Garcia-Mendoza MdP, Savoire R, Harscoat-Schiavo C. Impact of Hydro-Alcoholic Solvents on the Oil and Phenolics Extraction from Walnut (Juglans regia L.) Press-Cake and the Self-Emulsification of Extracts. Foods. 2022; 11(2):186. https://doi.org/10.3390/foods11020186
Chicago/Turabian StyleSubra-Paternault, Pascale, Maria del Pilar Garcia-Mendoza, Raphaëlle Savoire, and Christelle Harscoat-Schiavo. 2022. "Impact of Hydro-Alcoholic Solvents on the Oil and Phenolics Extraction from Walnut (Juglans regia L.) Press-Cake and the Self-Emulsification of Extracts" Foods 11, no. 2: 186. https://doi.org/10.3390/foods11020186
APA StyleSubra-Paternault, P., Garcia-Mendoza, M. d. P., Savoire, R., & Harscoat-Schiavo, C. (2022). Impact of Hydro-Alcoholic Solvents on the Oil and Phenolics Extraction from Walnut (Juglans regia L.) Press-Cake and the Self-Emulsification of Extracts. Foods, 11(2), 186. https://doi.org/10.3390/foods11020186





