Pigmented Potatoes: A Potential Panacea for Food and Nutrition Security and Health?
Abstract
:1. Introduction
2. Pigmented Potato Cultivars
3. Antioxidant Activity in Pigmented Potatoes
4. Carotenoids
5. Anthocyanins in Pigmented Cultivars
6. Pigmented Potato Biological Activity
7. The Impact of Environmental, Genotypic, and Soil Types on Pigmented Potato Characteristics
8. The Effect of Processing on Phytochemical Contents in Pigmented Potatoes
9. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gehrels, R.; Garrett, E. Chapter 11—Rising sea levels as an indicator of global change. In Climate Change, 3rd ed.; Observed Impacts on Planet Earth; Elsevier: Amsterdam, The Netherlands, 2021; pp. 205–217. [Google Scholar]
- FAO/IFAD/UNICEF/WFP/WHO. The State of Food Security and Nutrition in the World 2021. Transforming Food Systems for Food Security, Improved Nutrition and Affordable Healthy Diets for All; FAO: Rome, Italy, 2021. [Google Scholar] [CrossRef]
- WHO. Noncommunicable Diseases: Key Facts. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases (accessed on 1 November 2021).
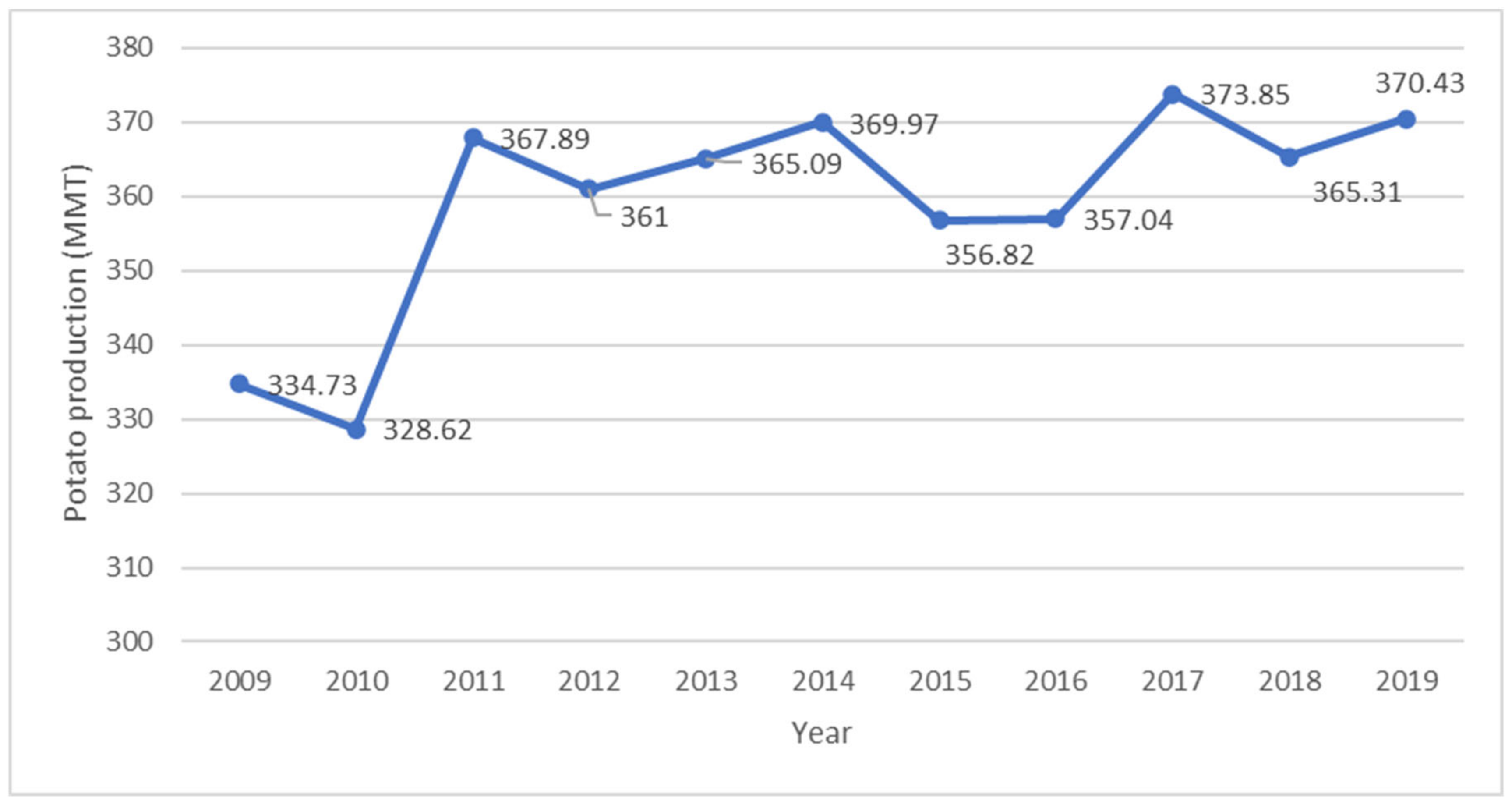
- FAO. Potato Production Worldwide from 2002 to 2019 (in Million Metric Tons). Statista Inc. 2021. Available online: https://www-statista-com.libproxy.cput.ac.za/statistics/382174/global-potato-production (accessed on 9 June 2021).
- Birch, P.R.J.; Bryan, G.; Fenton, B.; Gilroy, E.M.; Hein, I.; Jones, J.T.; Prashar, A.; Taylor, M.A.; Torrance, L.; Toth, I.K. Crops that feed the world 8: Potato: Are the trends of increased global production sustainable? Food Secur. 2012, 4, 477–508. [Google Scholar] [CrossRef]
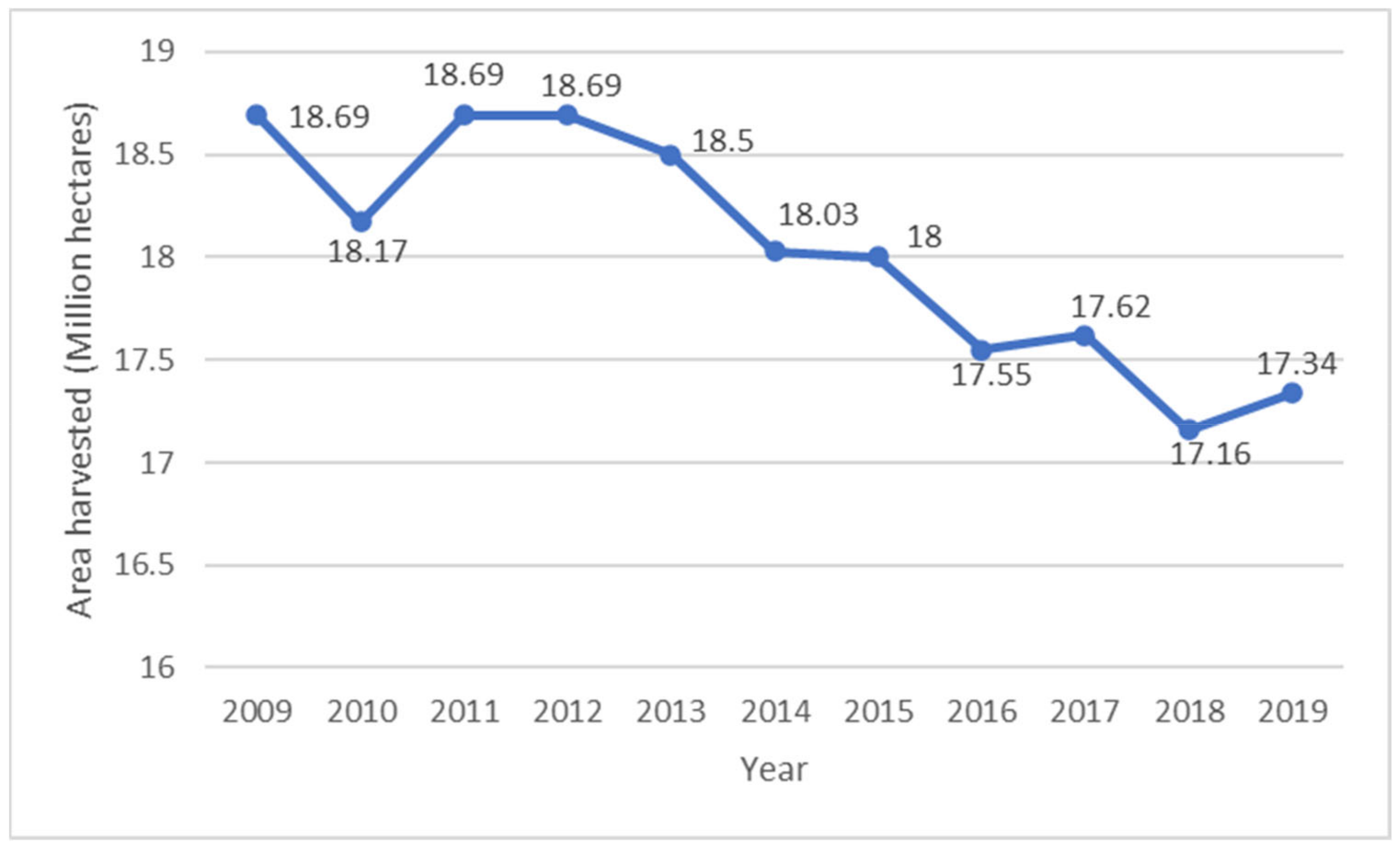
- FAO. Potato Area Harvested Worldwide from 2002 to 2019 (in Million Hectares): Statista Inc. 2021. Available online: https://www-statista-com.libproxy.cput.ac.za/statistics/625120/global-potato-area-harvested (accessed on 9 June 2021).
- FAOSTAT. Potato Consumption Per Capita. 2021. Available online: https://www.helgilibrary.com/indicators/potato-consumption-per-capita (accessed on 1 November 2021).
- Devaux, A.; Kromann, P.; Ortiz, O. Potatoes for sustainable global food security. Potato Res. 2014, 57, 185–199. [Google Scholar] [CrossRef]
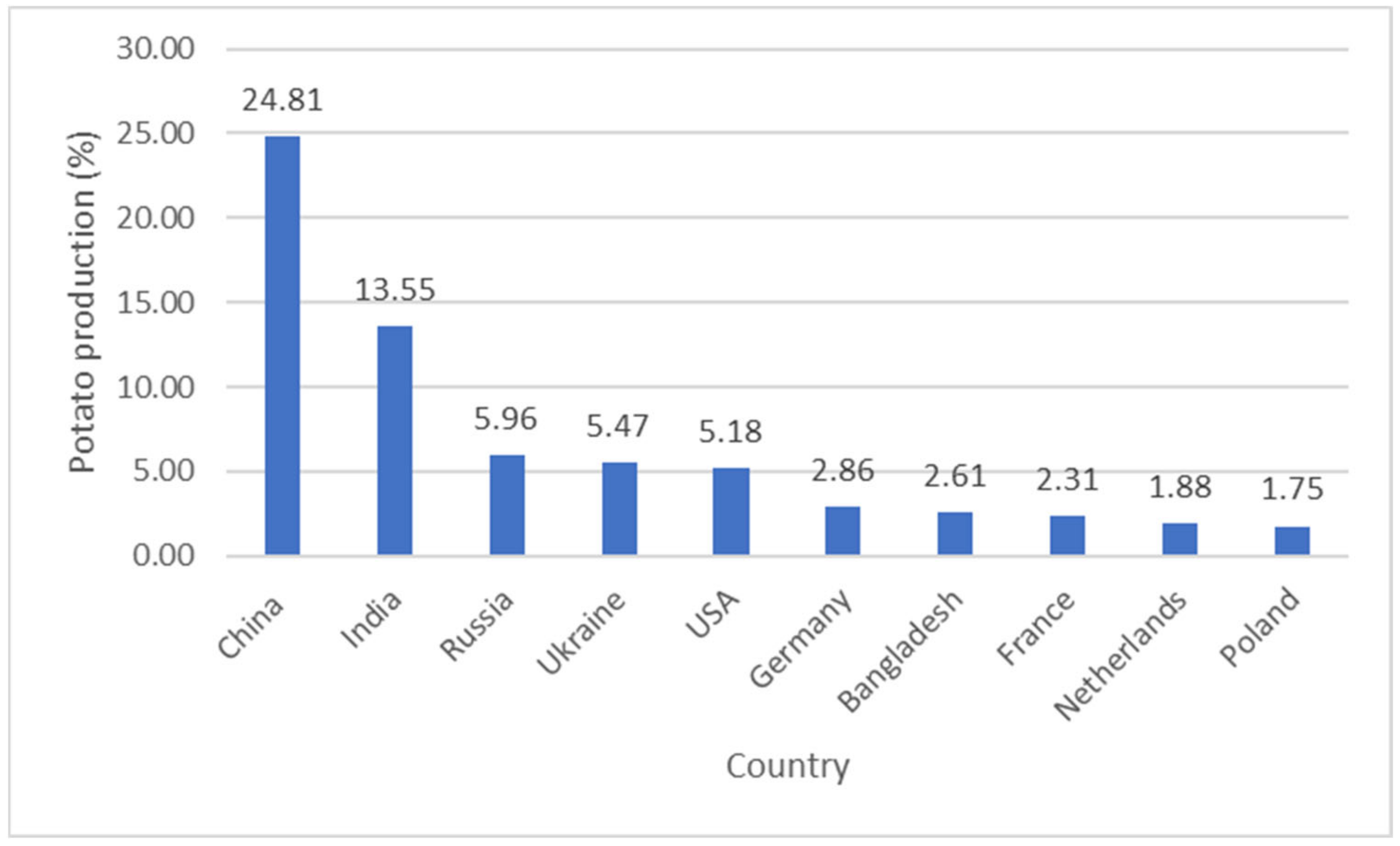
- FAO. Potato Production Worldwide in 2019, by Leading Country (in Million Metric Tons). Statista Inc. 2021. Available online: https://www-statista-com.libproxy.cput.ac.za/statistics/382192/global-potato-production-by-country (accessed on 9 June 2021).
- Yang, Y.; Achaerandio, I.; Pujolà, M. Classification of potato cultivars to establish their processing aptitude. J. Sci. Food Agric. 2016, 96, 413–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Witbooi, H.; Bvenura, C.; Oguntibeju, O.O.; Kambizi, L. The role of root zone temperature on physiological and phytochemical compositions of some pigmented potato (Solanum tuberosum L.) cultivars. Cogent Food Agric. 2021, 7, 1905300. [Google Scholar] [CrossRef]
- Camire, M.E.; Kubow, S.; Donnelly, D.J. Potatoes and human health. Crit. Rev. Food Sci. Nutr. 2009, 49, 823–840. [Google Scholar] [CrossRef] [PubMed]
- Andre, C.M.; Ghislain, M.; Bertin, P.; Oufir, M.; Herrera, M.; Hoffmann, L.; Hausman, J.F.; Larondelle, Y.; Evers, D. Andean potato cultivars (Solanum tuberosum L.) as a source of antioxidant and mineral micronutrients. J. Agric. Food Chem. 2007, 55, 366–378. [Google Scholar] [CrossRef]
- Reyes, L.F.; Cisneros-Zevallos, L. Degradation kinetics and colour of anthocyanins in aqueous extracts of purple- and red-flesh potatoes (Solanum tuberosum L.). Food Chem. 2007, 100, 885–894. [Google Scholar] [CrossRef]
- Cho, K.; Cho, K.; Sohn, H.; Ha, I.J.; Hong, S. Network analysis of the metabolome and transcriptome reveals novel regulation of potato pigmentation. J. Exp. Bot. 2016, 67, 1519–1533. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Jung, C.S.; De Jong, W.S. Genetic analysis of pigmented tuber flesh in potato. Theor. Appl. Genet. 2009, 119, 143–150. [Google Scholar] [CrossRef] [Green Version]
- Ah-Hen, K.S.; Fuenzalida, C.; Hess, S.; Contreras, A.; Vega-Gálvez, A.; Lemus-Mondaca, R. Antioxidant capacity and total phenolic compounds of twelve selected potato landrace clones grown in southern Chile. Chilean J. Agric. Res. 2012, 72, 3–9. [Google Scholar] [CrossRef] [Green Version]
- Andre, C.M.; Oufir, M.; Guignard, C.; Hoffmann, L.; Hausman, J.F.; Evers, D.; Larondelle, Y. Antioxidant profiling of native Andean potato tubers (Solanum tuberosum L.) reveals cultivars with high levels of beta-carotene, alpha-tocopherol, chlorogenic acid, and petanin. J. Agric. Food Chem. 2007, 55, 10839–10849. [Google Scholar] [CrossRef]
- Andre, C.M.; Schafleitner, R.; Guignard, C.; Oufir, M.; Aliaga, C.A.; Nomberto, G.; Hoffmann, L.; Hausman, J.F.; Evers, D.; Larondelle, Y. Modification of the health-promoting value of potato tubers field grown under drought stress: Emphasis on dietary antioxidant and glycoalkaloid contents in five native andean cultivars (Solanum tuberosum L.). J. Agric. Food Chem. 2009, 57, 599–609. [Google Scholar] [CrossRef]
- Balbina, A.A.; Julia, M.M.; Luciana, B. Chemical characterization of polyphenol extracts from Andean and industrial Solanum tuberosum tubers and their cytotoxic activity on human hepatocarcinoma cells. J. Food Sci. Technol. 2017, 2, 205–217. [Google Scholar]
- Ben Jeddou, K.; Kammoun, M.; Hellström, J.K.; Gutiérrez-Quequezana, L.; Rokka, V.; Gargouri-Bouzid, R.; Ellouze-Chaâbouni, S.; Nouri-Ellouz, O. Profiling beneficial phytochemicals in a potato somatic hybrid for tuber peels processing: Phenolic acids and anthocyanins composition. Food Sci. Nutr. 2021, 9, 1388–1398. [Google Scholar] [CrossRef]
- Brown, C.R. Antioxidants in potato. Am. J. Potato Res. 2005, 62, 163–172. [Google Scholar] [CrossRef]
- Brown, C.R.; Wrolstad, R.E.; Durst, R.W.; Yang, C.P.; Clevidence, B.A. Breeding studies in potatoes containing high concentrations of anthocyanins. Am. J. Potato Res. 2003, 80, 241–250. [Google Scholar] [CrossRef]
- Brown, C.R.; Culley, D.E.; Yang, C.P.; Durst, R.W.; Wrolstad, R.E. Variation of anthocyanin and carotenoid contents and associated antioxidant values in potato breeding lines. J. Am. Soc. Hortic. Sci. 2005, 130, 174–180. [Google Scholar] [CrossRef] [Green Version]
- Brown, C.R.; Culley, D.E.; Bonierbale, M.W.; Amorós, W. Anthocyanin, carotenoid content, and antioxidant values in native South American potato cultivars. Hortscience 2007, 42, 1733–1736. [Google Scholar] [CrossRef] [Green Version]
- Brown, C.R.; Durst, R.W.; Wrolstad, R.E.; Jong, W.S. Variability of phytonutrient content of potato in relation to growing location and cooking method. Potato Res. 2008, 51, 259–270. [Google Scholar] [CrossRef]
- Chaparro, J.M.; Holm, D.G.; Broeckling, C.D.; Prenni, J.E.; Heuberger, A.L. Metabolomics and ionomics of potato tuber reveals an influence of cultivar and market class on human nutrients and bioactive compounds. Front. Nutr. 2018, 5, 36. [Google Scholar] [CrossRef]
- Yamane, T. Beneficial Effects of Anthocyanin from Natural Products on Lifestyle-Related Diseases Through Inhibition of Protease Activities. Stud. Nat. Prod. Chem. 2018, 58, 245–264. [Google Scholar]
- Fernandez-Orozco, R.; Gallardo-Guerrero, L.; Hornero-Méndez, D. Carotenoid profiling in tubers of different potato (Solanum sp.) cultivars: Accumulation of carotenoids mediated by xanthophyll esterification. Food Chem. 2013, 141, 2864–2872. [Google Scholar] [CrossRef] [PubMed]
- Fossen, T.; Andersen, Ø.M. Anthocyanins from tubers and shoots of the purple potato, Solanum tuberosum. J. Hortic. Sci. Biotechnol. 2000, 75, 360–363. [Google Scholar] [CrossRef]
- Furrer, A.; Cladis, D.P.; Kurilich, A.C.; Manoharan, R.; Ferruzzi, M.G. Changes in phenolic content of commercial potato varieties through industrial processing and fresh preparation. Food Chem. 2017, 218, 47–55. [Google Scholar] [CrossRef]
- Giusti, M.M.; Polit, M.F.; Ayvaz, H.; Tay, D.B.; Manrique, I. Characterization and quantitation of anthocyanins and other phenolics in native Andean potatoes. J. Agric. Food Chem. 2014, 62, 4408–4416. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Quequezana, L.; Vuorinen, A.L.; Kallio, H.P.; Yang, B. Impact of cultivar, growth temperature and developmental stage on phenolic compounds and ascorbic acid in purple and yellow potato tubers. Food Chem. 2020, 326, 126966. [Google Scholar] [CrossRef]
- Hamouz, K.; Lachman, J.; Pazderu, K.; Tomášek, J.; Hejtmánková, K.; Pivec, V. Differences in anthocyanin content and antioxidant activity of potato tubers with different flesh colour. Plant Soil Environ. 2018, 57, 478–485. [Google Scholar] [CrossRef] [Green Version]
- Han, K.; Sekikawa, M.; Shimada, K.; Hashimoto, M.; Hashimoto, N.; Noda, T.; Tanaka, H.; Fukushima, M. Anthocyanin-rich purple potato flake extract has antioxidant capacity and improves antioxidant potential in rats. Br. J. Nutr. 2006, 96, 1125–1134. [Google Scholar] [CrossRef] [Green Version]
- Hejtmánková, K.; Kotikova, Z.; Hamouz, K.; Pivec, V.; Vacek, J.; Lachman, J. Influence of flesh colour, year and growing area on carotenoid and anthocyanin content in potato tubers. J. Food Compos. Anal. 2013, 32, 20–27. [Google Scholar] [CrossRef]
- Hejtmánková, K.; Pivec, V.; Trnková, E.; Hamouz, K.; Lachman, J. Quality of coloured varieties of potatoes. Czech J. Food Sci. 2018, 27, S310–S313. [Google Scholar] [CrossRef] [Green Version]
- Hillebrand, S.; Heike, B.; Kitzinski, N.; Köhler, N.; Winterhalter, P. Isolation and characterization of anthocyanins from blue-fleshed potatoes (Solanum tuberosum L.). Food 2009, 3, 96–101. [Google Scholar]
- Jansen, G.; Flamme, W. Coloured potatoes (Solanum tuberosum L.)—Anthocyanin content and tuber quality. Genet. Resour. Crop Evol. 2006, 53, 1321. [Google Scholar] [CrossRef]
- Jaromír, L.; Karel, H.; Matyáš, O. Chapter 9—Colored potatoes. In Advances in Potato Chemistry and Technology, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 249–281. [Google Scholar]
- Jeong, J.; Kim, S.; Hong, S.; Nam, J.; Sohn, H.; Kim, Y.; Mekapogu, M. Growing environment influence the anthocyanin content in purple- and red-fleshed potatoes during tuber development. Korean J. Crop Sci. 2015, 60, 231–238. [Google Scholar] [CrossRef] [Green Version]
- Kaspar, K.L.; Park, J.S.; Brown, C.R.; Mathison, B.D.; Navarre, D.A.; Chew, B.P. Pigmented potato consumption alters oxidative stress and inflammatory damage in men. J. Nutr. 2011, 141, 108–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaspar, K.; Park, J.; Brown, C.R.; Weller, K.; Ross, C.; Mathison, B.; Chew, B. Sensory evaluation of pigmented flesh potatoes (Solanum tuberosum L.). Food Nutr. Sci. 2013, 4, 77–81. [Google Scholar]
- Kim, H.; Kim, S.R.; Lee, Y.M.; Jang, H.; Kim, J.B. Analysis of variation in anthocyanin composition in Korean coloured potato cultivars by LC-DAD-ESI-MS and PLS-DA. Potato Res. 2017, 61, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Kita, A.; Bakowska-Barczak, A.M.; Hamouz, K.; Kułakowska, K.; Lisińska, G. The effect of frying on anthocyanin stability and antioxidant activity of crisps from red- and purple-fleshed potatoes (Solanum tuberosum L.). J. Food Compos. Anal. 2013, 32, 169–175. [Google Scholar] [CrossRef]
- Kita, A.; Bakowska-Barczak, A.M.; Lisińska, G.; Hamouz, K.; Kułakowska, K. Antioxidant activity and quality of red and purple flesh potato chips. LWT-Food Sci. Technol. 2015, 62, 525–531. [Google Scholar] [CrossRef]
- Kotíková, Z.; Šulc, M.; Lachman, J.; Pivec, V.; Orsák, M.; Hamouz, K. Carotenoid profile and retention in yellow-, purple- and red-fleshed potatoes after thermal processing. Food Chem. 2016, 197, 992–1001. [Google Scholar] [CrossRef]
- Külen, O.; Stushnoff, C.; Holm, D.G. Effect of cold storage on total phenolics content, antioxidant activity and vitamin C level of selected potato clones. J. Sci. Food Agric. 2013, 93, 2437–2444. [Google Scholar] [CrossRef]
- Lachman, J.; Hamouz, K.; Šulc, M.; Orsák, M.; Pivec, V.; Hejtmánková, A.; Dvořák, P.; Čepl, J.J. Cultivar differences of total anthocyanins and anthocyanidins in red and purple-fleshed potatoes and their relation to antioxidant activity. Food Chem. 2009, 114, 836–843. [Google Scholar] [CrossRef]
- Lachman, J.; Hamouz, K.; Musilová, J.; Hejtmánková, K.; Kotíková, Z.; Pazderů, K.; Domkářová, J.; Pivec, V.; Cimr, J. Effect of peeling and three cooking methods on the content of selected phytochemicals in potato tubers with various colour of flesh. Food Chem. 2013, 138, 1189–1197. [Google Scholar] [CrossRef] [PubMed]
- Mulinacci, N.; Ieri, F.; Giaccherini, C.; Innocenti, M.; Andrenelli, L.; Canova, G.; Saracchi, M.; Casiraghi, M.C. Effect of cooking on the anthocyanins, phenolic acids, glycoalkaloids, and resistant starch content in two pigmented cultivars of Solanum tuberosum L. J. Agric. Food Chem. 2008, 56, 11830–11837. [Google Scholar] [CrossRef]
- Murniece, I.; Tomsone, L.; Skrabule, I.; Vaivode, A. Carotenoids and total phenolic content In potatoes with different flesh colour. Foodbalt 2014, 206–211. [Google Scholar]
- Nayak, B.; Berrios, J.D.; Powers, J.R.; Tang, J.; Ji, Y. Colored potatoes (Solanum tuberosum L.) dried for antioxidant-rich value-added foods. J. Food Process. Preserv. 2011, 35, 571–580. [Google Scholar] [CrossRef]
- Nemś, A.; Pęksa, A.; Kucharska, A.Z.; Sokół-Łętowska, A.; Kita, A.; Drożdż, W.; Hamouz, K. Anthocyanin and antioxidant activity of snacks with coloured potato. Food Chem. 2015, 172, 175–182. [Google Scholar] [CrossRef]
- Oertel, A.; Matros, A.; Hartmann, A.; Arapitsas, P.; Dehmer, K.J.; Martens, S.; Mock, H.P. Metabolite profiling of red and blue potatoes revealed cultivar and tissue specific patterns for anthocyanins and other polyphenols. Planta 2017, 246, 281–297. [Google Scholar] [CrossRef]
- Perla, V.; Holm, D.G.; Jayanty, S.S. Effects of cooking methods on polyphenols, pigments and antioxidant activity in potato tubers. LWT-Food Sci. Technol. 2012, 45, 161–171. [Google Scholar] [CrossRef]
- Reyes, L.F.; Miller, J.C.; Cisneros-Zevallos, L. Antioxidant capacity, anthocyanins and total phenolics in purple-and red-fleshed potato (Solanum tuberosum L.) genotypes. Am. J. Potato Res. 2005, 82, 271–277. [Google Scholar] [CrossRef]
- Reyes, L.F.; Miller, J.C.; Cisneros-Zevallos, L. Environmental conditions influence the content and yield of anthocyanins and total phenolics in purple- and red-flesh potatoes during tuber development. Am. J. Potato Res. 2008, 81, 187–193. [Google Scholar] [CrossRef]
- Rodriguez-Saona, L.; Giusti, M.; Wrolstad, R. Anthocyanin pigment composition of red-fleshed potatoes. J. Food Sci. 1998, 63, 458–465. [Google Scholar] [CrossRef]
- Rodriguez-Saona, L.E.; Giusti, M.M.; Wrolstad, R.E. Color and pigment stability of red radish and red-fleshed potato anthocyanins in juice model systems. Food Sci. 2006, 64, 451–456. [Google Scholar] [CrossRef]
- Rodriguez-Saona, L.E.; Wrolstad, R.E.; Pereira, C.B. Glycoalkaloid content and anthocyanin stability to alkaline treatment of red-fleshed potato extracts. J. Food Sci. 2006, 64, 445–450. [Google Scholar] [CrossRef]
- Rytel, E.; Tajner-Czopek, A.; Kita, A.; Aniołowska, M.; Kucharska, A.Z.; Sokół-Łętowska, A.; Hamouz, K. Content of polyphenols in coloured and yellow fleshed potatoes during dices processing. Food Chem. 2014, 161, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Rytel, E.; Tajner-Czopek, A.; Kita, A.; Soko, A.B.; Towska Kucharska, A.Z.; Hamouz, K. Effect of the production process on the content of anthocyanins in dried red-fleshed potato cubes. Ital. J. Food Sci. 2019, 31, 150–160. [Google Scholar]
- Sampaio, S.L.; Lonchamp, J.; Dias, M.I.; Liddle, C.; Petropoulos, S.A.; Glamočlija, J.; Alexopoulos, A.A.; Santos-Buelga, C.; Ferreira, I.C.; Barros, L. Anthocyanin-rich extracts from purple and red potatoes as natural colourants: Bioactive properties, application in a soft drink formulation and sensory analysis. Food Chem. 2020, 342, 128526. [Google Scholar] [CrossRef] [PubMed]
- Silveyra, M.X.; Lanteri, M.L.; Damiano, R.B.; Andreu, A.B. Bactericidal and cytotoxic activities of polyphenol extracts from Solanum tuberosum spp. tuberosum and spp. andigena cultivars on Escherichia coli and human Neuroblastoma SH-SY5Y Cells In Vitro. J. Nutr. Metab. 2018, 2018, 8073679. [Google Scholar]
- Stushnoff, C.; Holm, D.; Thompson, M.D.; Jiang, W.; Thompson, H.J.; Joyce, N.I.; Wilson, P. Antioxidant properties of cultivars and selections from the Colorado potato breeding program. Am. J. Potato Res. 2008, 85, 267–276. [Google Scholar] [CrossRef]
- Tatarowska, B.; Milczarek, D.; Wszelaczyńska, E.; Pobereżny, J.; Keutgen, N.; Keutgen, A.J.; Flis, B. Carotenoids variability of potato tubers in relation to genotype, growing location and year. Am. J. Potato Res. 2019, 96, 493–504. [Google Scholar] [CrossRef] [Green Version]
- Thompson, M.D.; Thompson, H.J.; McGinley, J.N.; Neil, E.S.; Rush, D.K.; Holm, D.G.; Stushnoff, C. Functional food characteristics of potato cultivars (Solanum tuberosum L.): Phytochemical composition and inhibition of 1-methyl-1-nitrosourea induced breast cancer in rats. J. Food Compos. Anal. 2009, 22, 571–576. [Google Scholar] [CrossRef]
- Tong, C.; Ru, W.; Wu, L.; Wu, W.; Bao, J. Fine structure and relationships with functional properties of pigmented sweet potato starches. Food Chem. 2020, 311, 126011. [Google Scholar] [CrossRef] [PubMed]
- Vaitkevičienė, N.; Kulaitienė, J.; Jarienė, E.; Levickienė, D.; Danillčenko, H.; Średnicka-Tober, D.; Rembiałkowska, E.; Hallmann, E. Characterization of bioactive compounds in colored potato (Solanum tuberosum L.) cultivars grown with conventional, organic, and biodynamic methods. Sustainability 2020, 12, 2701. [Google Scholar] [CrossRef] [Green Version]
- Valiñas, M.A.; Lanteri, M.L.; Ten Have, A.; Andreu, A.B. Chlorogenic acid, anthocyanin and flavan-3-ol biosynthesis in flesh and skin of Andean potato tubers (Solanum tuberosum subsp. andigena). Food Chem. 2017, 229, 837–846. [Google Scholar] [CrossRef]
- Witbooi, H.; Bvenura, C.; Reid, A.-M.; Lall, N.; Oguntibeju, O.O.; Kambizi, L. The potential of elevated root zone temperature on the concentration of chlorogenic, caffeic, ferulic acids and biological activity of some pigmented Solanum tuberosum L. cultivar extracts. Appl. Sci. 2021, 11, 6971. [Google Scholar] [CrossRef]
- Yin, L.; Chen, T.; Li, Y.; Fu, S.; Li, L.; Xu, M.; Niu, Y. A comparative study on total anthocyanin content, composition of anthocyanidin, total phenolic content and antioxidant activity of pigmented potato peel and flesh. Food Sci. Technol. Res. 2016, 22, 219–226. [Google Scholar] [CrossRef] [Green Version]
- Mamta Misra, K.; Dhillon, G.S.; Brar, S.K.; Verma, M. Antioxidants. In Biotransformation of Waste Biomass into High Value Biochemicals; Brar, S., Dhillon, G., Soccol, C., Eds.; Springer: New York, NY, USA, 2014. [Google Scholar]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [Green Version]
- Prakash, D.; Gupta, C. Carotenoids: Chemistry and Health Benefits. In Phytochemicals of Nutraceutical Importance; Prakash, D., Sharma, G., Eds.; CAB International: Wallingford, UK, 2014; pp. 181–195. [Google Scholar]
- Marhuenda-Muñoz, M.; Hurtado-Barroso, S.; Tresserra-Rimbau, A.; Lamuela-Raventós, R.S. A review of factors that affect carotenoid concentrations in human plasma: Differences between Mediterranean and Northern diets. Eur. J. Clin. Nutr. 2019, 72, 18–25. [Google Scholar] [CrossRef]
- ChemSpider. β-Carotene. Available online: https://www.chemspider.com/Chemical-Structure.4444129.html (accessed on 5 November 2021).
- Faraloni, C.; Torzillo, G. Synthesis of antioxidant carotenoids in microalgae in response to physiological stress. In Carotenoids; Cvetkovic, D.J., Nikolic, G.S., Eds.; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef] [Green Version]
- Kiokias, S.; Proestos, C.; Oreopoulou, V. Effect of natural food antioxidants against LDL and DNA oxidative changes. Antioxidants 2018, 7, 133. [Google Scholar] [CrossRef] [Green Version]
- Stahl, W.; Sies, H. Carotenoids and flavonoids contribute to nutritional protection against skin damage from sunlight. Mol. Biotechnol. 2007, 37, 26–30. [Google Scholar] [CrossRef]
- Kambizi, L.; Bvenura, C. Medicinal plants for strong immune system and traditional skin therapy in South Africa. In Medicinal Plants and the Human Immune System; Husen, A., Ed.; CRC/Taylor & Francis: Boca Raton, FL, USA, 2021; pp. 123–172. [Google Scholar]
- Bvenura, C.; Ngemakwe Nitcheu, P.H.; Chen, L.; Sivakumar, D. Nutritional and health benefits of temperate fruits. In Postharvest Biology and Technology of Temperate Fruits; Mir, S.A., Shah, M.A., Mir, M.M., Eds.; Springer: Cham, Switzerland, 2018; pp. 51–75. [Google Scholar]
- Reyes, B.A.; Dufourt, E.C.; Ross, J.A.; Warner, M.J.; Tanquilut, N.C.; Leung, A.B. Selected Phyto and Marine Bioactive Compounds: Alternatives for the Treatment of Type 2 Diabetes. Stud. Nat. Prod. Chem. 2018, 55, 111–143. [Google Scholar]
- Valavanidis, A.; Vlachogianni, T. Chapter 8: Plant Polyphenols. Recent Advances in Epidemiological Research and Other Studies on Cancer Prevention. Stud. Nat. Prod. Chem. Bioact. Nat. Prod. 2013, 39, 269–295. [Google Scholar]
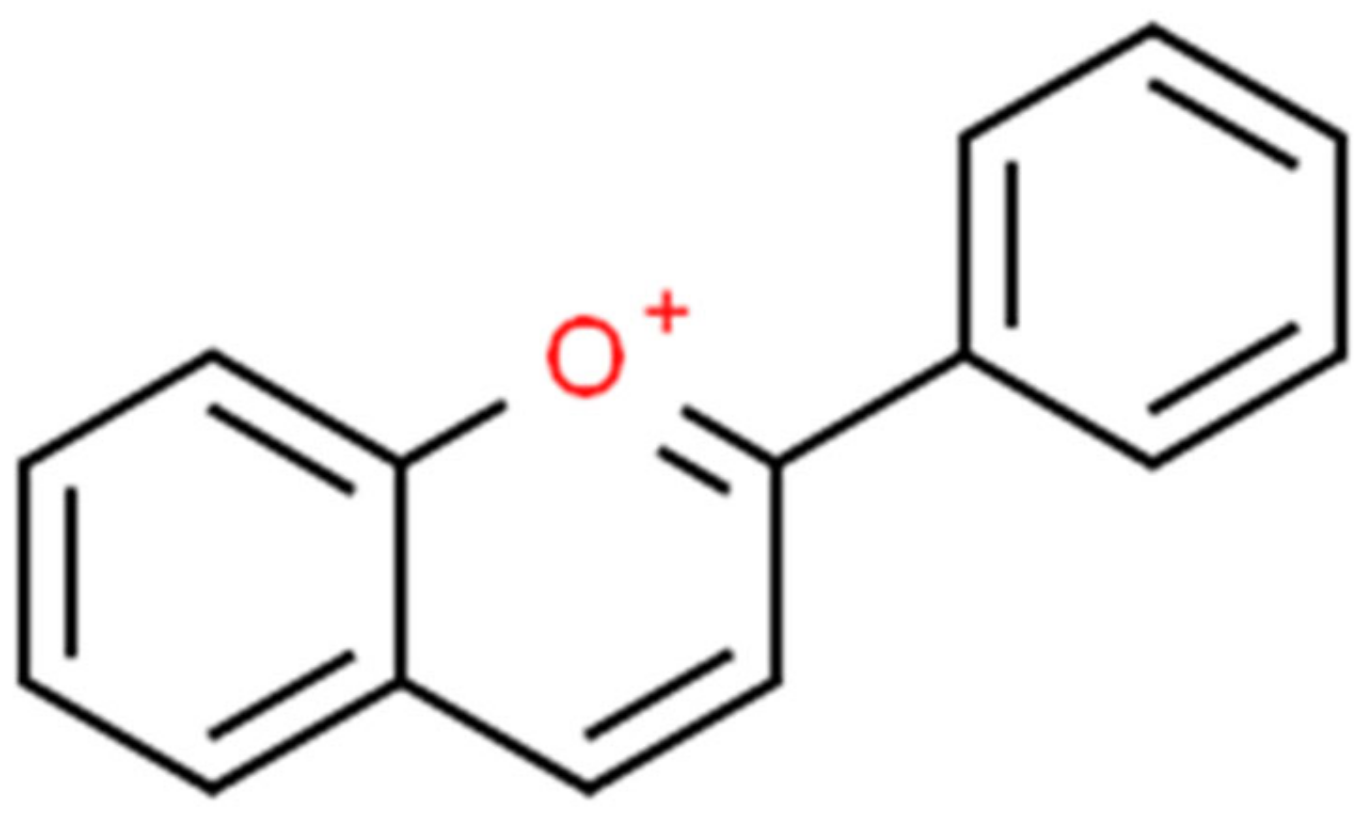
- ChemSpider. Flavylium. Available online: https://www.chemspider.com/Chemical-Structure.128674.html?rid=18e11ee5-0ecf-4264-bc49-ed7446fbe36e (accessed on 4 November 2021).
- Bvenura, C.; Sivakumar, D. The role of wild fruits and vegetables in delivering a healthy and balanced diet. Food Res. Int. 2017, 99, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Patras, A.; Brunton, N.P.; O’Donnell, C.; Tiwari, B.K. Effect of thermal processing on anthocyanin stability in foods; mechanisms and kinetics of degradation. Trends Food Sci. Technol. 2010, 21, 3–11. [Google Scholar] [CrossRef]



| Cultivar and Colour | Country | Ref. | |
|---|---|---|---|
| 1 | Shepody, Desirée, 457-CON-1157, Corazón de buey, 302-UA-1634A, 304-UA-1135, (Boyo de chancho, Michuñeroja, Meca de gato, Cacho azu, Corazón azul, 239-UA-1388, Chona negra, and Bruja | Chiloe Island and Valdivia, Chile | [17] |
| 2 | NA | Louvain-La-Neuve, Belgium | [13] |
| 3 | NA | Louvain-La-Neuve, Belgium | [18] |
| 4 | 704,429-Guincho Negra, 700,347-SS-2613, 702,535-Sipancachi, 701,997-Sullu, and 703,905-Huata Colorada | Louvain-La-Neuve, Belgium | [19] |
| 5 | Waicha—Reddish | Argentina | [20] |
| Moradita—Purple | |||
| 6 | Spunta, CN1—Pink | Sfax, Tunisia | [21] |
| 7 | No data | Global | [22] |
| 8 | Purple (PA97B29-2, PA97B29-4, PA97B29-5, and PA97B29-6) | Washington, DC, USA | [23] |
| Red (PA97B29-3, PA97B35-1, PA97B35-2, PA97B36-3, PA97B37-2, PA97B37-3, PA97B37-7, PA97B39-2, and NDOP5847-1) | |||
| 9 | Light Yellow (Adora, Divina, Fabula, Ilona, Morning gold, Provento, Satino, Yukon Gold, and POR00PG4-2 | Washington, DC, USA | [24] |
| Dark yellow (91E22, PA99P11-2, PA99P1-2, PA99P2-1, and POR00PG4-1) | |||
| Red and yellow (PO00PG9-1, PO00PG9-2, PO00PG9-3, PO00PG9-5, and PO00PG9-6) | |||
| 10 | 38 Cultivars | Washington, DC, USA | [25] |
| 11 | Purple (PA97B29-2, PA97B29-4, PA97B29-5, and PA97B29-6) | Washington, DC, USA | [26] |
| Red (PA97B29-3, PA97B35-1, PA97B35-2, PA97B36-3, PA97B37-2, PA97B37-3, PA97B37-7, PA97B39-2, and NDOP5847-1) | |||
| 12 | NA | Colorado, CO, USA | [27] |
| 13 | Hongyoung, Jayoung, and Atlantic | Dae-GwalLyeong, Korea | [15] |
| 14 | Hermanns Blaue, Highland Burgundy Red, Shetland Black, and Vitelotte | Barum, Germany 0.6–46 mg | [28] |
| 15 | NA | Sevilla, Spain | [29] |
| 16 | Congo—Purple | Bergen, Norway | [30] |
| 17 | Yellow (Innovator, Bintje, Challenger, Yukon, AR2009-10) | New Brunswick, Canada | [31] |
| Purple (AR2, ADB) | |||
| White flesh, red skin (Norland) | |||
| Blue (Adirondack Blue) | |||
| Red (Adirondack Red, ADR), | |||
| 18 | Purple (705,534, 703,640, 706,726, 704,733, 703,862, and 704,133) | Lima, Peru | [32] |
| Red (702,556, 706,630, 700,234, 705,841, 703,752, 703,695, 705,820, 704,537, 705,946, 705,500, 702,464, and 703,782) | |||
| Yellow (706,884 and 704,481) | |||
| 19 | Purple (Blue Congo, Blaue Veltlin, Blaue Schweden, Synkeä Sakari). | Finland | [33] |
| 20 | Purple (Blaue Elise, Blaue St. Galler, Blue Congo, Valfi, Violette, and Vitelotte) Red (Herbie 26, Highland B. Red, Rosalinde, and Rote Emma) | Prague, Czech Republic | [34] |
| 21 | Light purple—KM | Obihiro, Hokkaido, Japan | [35] |
| Medium-dark purple—H92 | |||
| 22 | Yellow (Agria, Russet Burbank, Lady Balfour, and Mayan Gold) | Czech Republic | [36] |
| Purple (Violette, Vitelotte, Violetta, Valfi, Blue Congo, Blaue St. Galler, Olivia, and Blaue Anneliese) | |||
| Red (Rosemarie, Rote Emmalie, Highland Burgundy Red, and Herbie 26) | |||
| Yellow with red spots (Mayan Queen) | |||
| 23 | Salad Blue, Shetland Black, Blue Congo, Blaue St. Galler, Highland Burgundy Red, Violette, and Valfi, Vitelotte | Přerov nad Labem, Suchdol, Valečov and Stachy, Czech Republic | [37] |
| 24 | Hermanns Blaue, Vitelotte, Shetland Black, and Valfi | Braunschweig, Germany | [38] |
| 25 | Blue violet (Blaue Schweden, British Columbia Blue, Violettfleischige, 1.81.203–92N, Blaue Mauritius, Bleu, Blaue Utwill, Peru Purple, Mesabi Purple Smith’s Purple, UAC NEG 61, UAC CON 917, Weinberger Blaue, Mesabi Purple, Bells Purple, Magdeburger Blaue, Shetland Black, Caribe, Purple and White, Mrs. Moerles Purple Baker, Long Blue, Edzell Blue, Odenwa¨lder Blaue, Schwarze Ungarin, Arran Victory, UAC 1258, Purple Fiesta, Viola, and Blaue Zimmerli). Red (Sangre, Kefermarkter Zuchtstamm, and Red Cardinal). | Lenzen, Germany 0.01–1.57 g/kg | [39] |
| 26 | Review | Review | [40] |
| 27 | Purple—Hongyoung, Red—Jayoung | Korea | [41] |
| 28 | WP (Ranger Russet), YP (PORO3PG6–3), and PP (PORO4PG82–1) | Washington, USA | [42] |
| 29 | White (Russet Burbank), yellow (PORO3PG6-3), and purple-flesh (PORO4PG82-1) | Toppenish, Washington, USA | [43] |
| 30 | Red—Hongyoung | Korea | [44] |
| Purple—Jayoung, clones Jje08-11, | |||
| DJ12X-5, and Jje08-43 | |||
| 31 | Purple (Salad Blue, Vitelotte, Valfi, Blue Congo) | Prerov nad Labem, Czech Republic | [45] |
| Red (Rosalinde, Herbie 26, Highland Burgundy Red) | |||
| 32 | Purple (Blaue Elise, Blaue St. Galler, Blue Congo, Valfi, and Vitelotte) | Přerov nad Labem, Czech Republic | [46] |
| Red (Highland Burgundy Red, Herbie 26, Rosalinde, and Rote Emma) | |||
| 33 | Yellow (Agria, Russet Burbank, Valy, Salome, Bohemia, Axa, Jelly, Ditta, Bionta, Kerˇkovsky’ rohlícˇek, Dali, and Mayan Gold) | Valečov, Czech Republic | [47] |
| Red (Rosara, Rosemarie, Königspurpur, Highland Burgundy Red, Herbie 26, and Red Emmalie) | |||
| Purple (Valfi, Violeta, Blaue Anneliese, and Vitelotte) | |||
| 34 | CO97226-2R/R, CO99364-3R/R, CO97215-2P/P, CO97216-3P/P, CO97227-2P/P, CO97222-1R/R, Purple Majesty, Mountain Rose, and All Blue), and yellow (Yukon Gold) | Colorado, USA | [48] |
| 35 | Blue Congo, Highland Burgundy Red, Salad Blue, Shetland Black, Valf, Vitelotte, Violette, Blaue St. Galler, Blaue Hindel Bank, Blaue Ludiano, Blaue Mauritius, Blaue Schweden, British Columbia Blue, Farbe Kartoffel, Hafija, and Salad Red | Czech Republic | [49] |
| 36 | HB Red, Rote Emma, Blaue St Galler, Valfi, Violette and Agria | Czech Republic | [50] |
| 37 | Vitelotte Noire and Highland Burgundy Red | Milan, Italy | [51] |
| 38 | Yellow (Agrie Dzeltenie, Prelma, Lenora, Brasla, Anuschka, Gundega, S04009-37) | Priekuli, Latvia | [52] |
| Light yellow (S99108-8) | |||
| Purple (Fenton, Purple Fiesta, British Columbia | |||
| Blue, Purple Peru, and Blue Congo) | |||
| 39 | Red (Red Rodeo) Yellow (Yukon Gold), and Purple Majesty (CO94165-3P/P) | Washington, USA | [53] |
| 40 | Red—Herbie 26 | Poland | [54] |
| Purple—Valfi, Blue Congo and Salad Blue | |||
| 41 | Purple (Violettfleischige x Blue Marker-B, (Violettfleischige)-A1, (Blue Marker)-B, Purple, Violettfleischige x Blue Marker-D, (Violettfleischige)-A2, Vitelotte, and Blaue Ajanhuiri) | Groß Lüsewitz, Germany | [55] |
| Yellow (Bangladesh, Desiree, Early Rose, and Shetland Blau I,) | |||
| Red (Ko¨nigspurpur, Rote Emmalie, and Rosemarie) | |||
| 42 | Red (CO99256-2R, CO98012-5R, Colorado Rose, VC0967-2R/Y, CO97222-1R/R, and CO97226-2R/R) | Colorado, USA | [56] |
| Purple (CO01399-10P/Y, AC99329-7PW/Y, and Purple Majesty) | |||
| 43 | Purple (All Blue and CO94165-3P/P) | Texas, USA | [14] |
| Red (NDC4069-4 and CO94183-1R/R) | |||
| 44 | All Blue, NDC4069-4, Russian Blue, Purple Peruvian, COl11F2-1, COl12F1-1, COl12F1-2, CO141F2-1, CO142F2-1, RC2003-2 | Colorado and Texas, USA | [57] |
| 45 | Purple (All Blue, Purple Peruvian, and RC2003-2) | Texas and Colorado, USA | [58] |
| Red (NDC4069-4, CO94183-1R/R, and CO94183-1R/) | |||
| 46 | NA | Oregon, USA | [59] |
| 47 | NA | Oregon, USA | [60] |
| 48 | NA | Oregon, USA | [61] |
| 49 | Blue—Valfi, Blaue Elise, Bore Volley, and Blue Congo | Přerov nad Labem, Czech Republic | [62] |
| 50 | Red—Rosemarie, Herbie 26, and Rote Emma | Wrocław, Poland | [63] |
| 51 | Red (Rosemary, Red Emmalie, and Red Cardinal) | Velestino, Greece | [64] |
| Purple (Purple, Violetta, and Kefermarkter Blaue) | |||
| 52 | Purple (Moradita) | Argentina | [65] |
| Yellow (Waicha) | |||
| Red (Santa Marıa) | |||
| 53 | Rio Grande, Mountain Rose, R Burbank, R Nugget, Yukon Gold, and Purple Majesty | Colorado, USA | [66] |
| 54 | Yellow (Satina and Tajfun, and Jelly) | Central Poland | [67] |
| 55 | Purple Majesty, Yukon Gold, Mountain Rose | Colorado, USA | [68] |
| 56 | Zheshu 13, 33, 75, 81, 132, 259, 6025 | An’ji, Zhejiang Province, China | [69] |
| 57 | Red (Red Emmalie, Tornado, and Laura) | Širvintos district, Lithuania | [70] |
| Dark purple (Violetta) | |||
| Dark-blue purple (Salad Blue) | |||
| 58 | 50 cultivars | Argentina | [71] |
| 59 | Salad Blue, Pink Fir Apple, and Highland Burgundy Red | Cape Town, South Africa | [11] |
| 60 | Salad Blue, Pink Fir Apple, and Highland Burgundy Red | Cape Town, South Africa | [72] |
| 61 | Agata, Cherie, Kennebec, Monalisa, Red Pontiac and Spirit | Barcelona, Spain | [10] |
| 62 | Purple Cloud No. 1, Red Cloud No. 1, Yunnan Potato 303, Yunnan Potato 603, S03-2677, S03-2685, S03-2796, S05-603, S06-277, and S06-1693 | Chengdu, China 60–294 mg | [73] |
| Anthocyanin | Ref. | |
|---|---|---|
| Blue | Petunidin, Cyanidin, Delphinidin, Petunidin, Pelargonidin, Peonidin, Malvidin | [28,32,38,73] |
| Red | Pelargonidin, Cyanidin, Delphinidin, Petunidin, Peonidin, Malvidin | [28,32,38,73] |
| Purple | Peonidin, Petunidin, Delphinidin, Petunidin, Pelargonidin, Peonidin, Malvidin | [28,32,38,73] |
| Yellow | Malvidin, petunidin, Cyanidin, Delphinidin, Petunidin, Pelargonidin, Peonidin | [28,32,38,73] |
| Pink | Cyanidin, Delphinidin, Petunidin, Pelargonidin, Peonidin, Malvidin | [32,38,73] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bvenura, C.; Witbooi, H.; Kambizi, L. Pigmented Potatoes: A Potential Panacea for Food and Nutrition Security and Health? Foods 2022, 11, 175. https://doi.org/10.3390/foods11020175
Bvenura C, Witbooi H, Kambizi L. Pigmented Potatoes: A Potential Panacea for Food and Nutrition Security and Health? Foods. 2022; 11(2):175. https://doi.org/10.3390/foods11020175
Chicago/Turabian StyleBvenura, Callistus, Hildegard Witbooi, and Learnmore Kambizi. 2022. "Pigmented Potatoes: A Potential Panacea for Food and Nutrition Security and Health?" Foods 11, no. 2: 175. https://doi.org/10.3390/foods11020175
APA StyleBvenura, C., Witbooi, H., & Kambizi, L. (2022). Pigmented Potatoes: A Potential Panacea for Food and Nutrition Security and Health? Foods, 11(2), 175. https://doi.org/10.3390/foods11020175








