Effects of 405 ± 5-nm LED Illumination on Environmental Stress Tolerance of Salmonella Typhimurium in Sliced Beef
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strain and Culture Conditions
2.2. Bacterial Inoculation and Preparation of Contaminated Beef Samples
2.3. LED Illumination Device
2.4. The Inactivation Effect of 405-nm LED on S. Typhimurium SL1344 in PBS and on Beef Slices
2.4.1. Time-Kill Assay in PBS
2.4.2. Time-Kill Assay in Beef Slices
2.5. Determination of the Resistance of S. Typhimurium in Beef Slices to Environmental Stress
2.5.1. Resistance of S. Typhimurium to Thermal Stress
2.5.2. Resistance of S. Typhimurium to Osmotic Pressure
2.5.3. Resistance of S. Typhimurium to Oxidative Stress
2.5.4. Simulated Gastric Fluid-Resistance of S. Typhimurium
2.5.5. Bile Salt-Resistance of S. Typhimurium
2.6. Effect of 405 nm LED-Illumination on Gene Transcription in S. Typhimurium
2.7. Statistical Analysis
3. Results
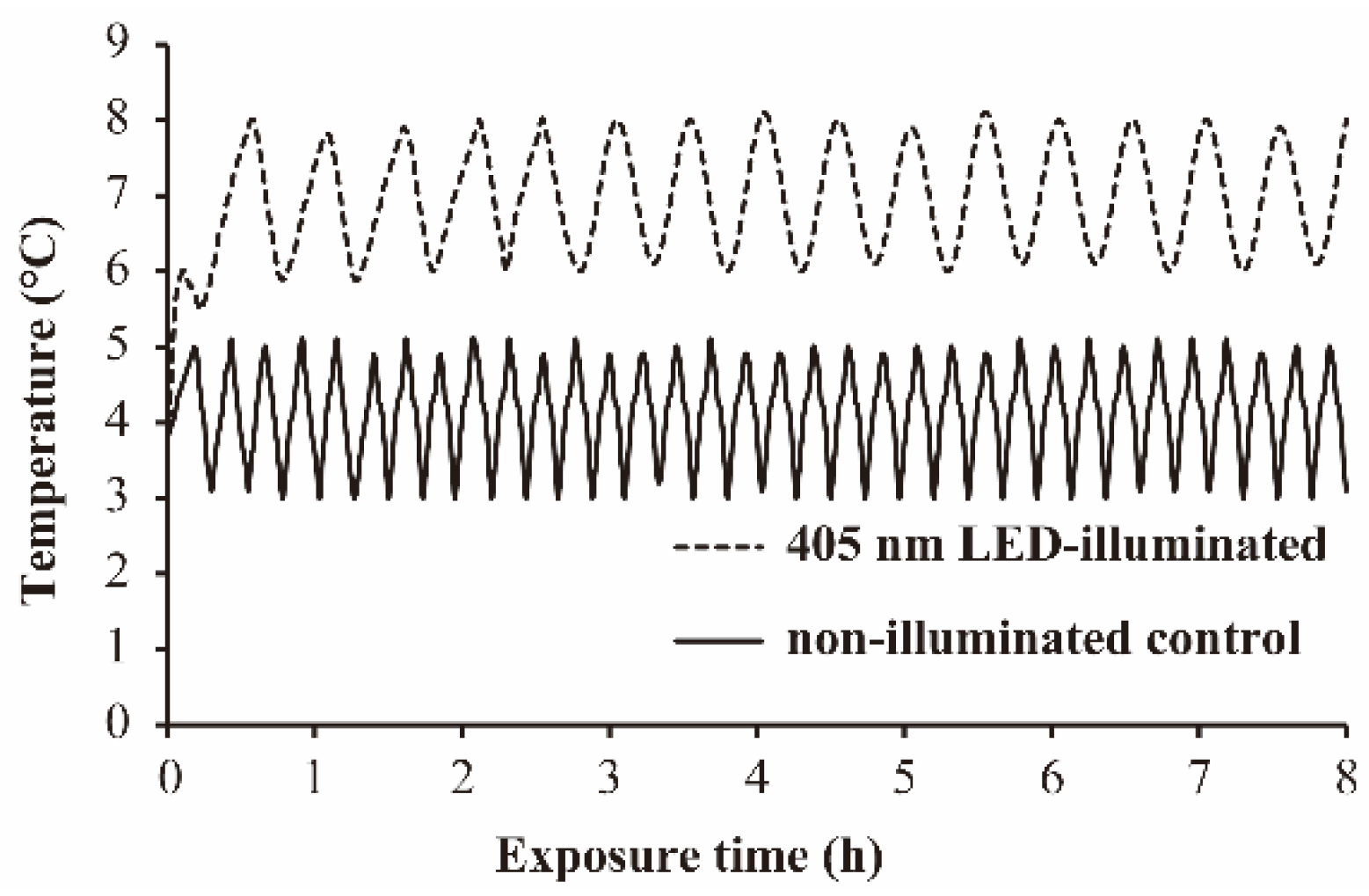
3.1. The Temperature Profile during 405 nm LED-Illumination
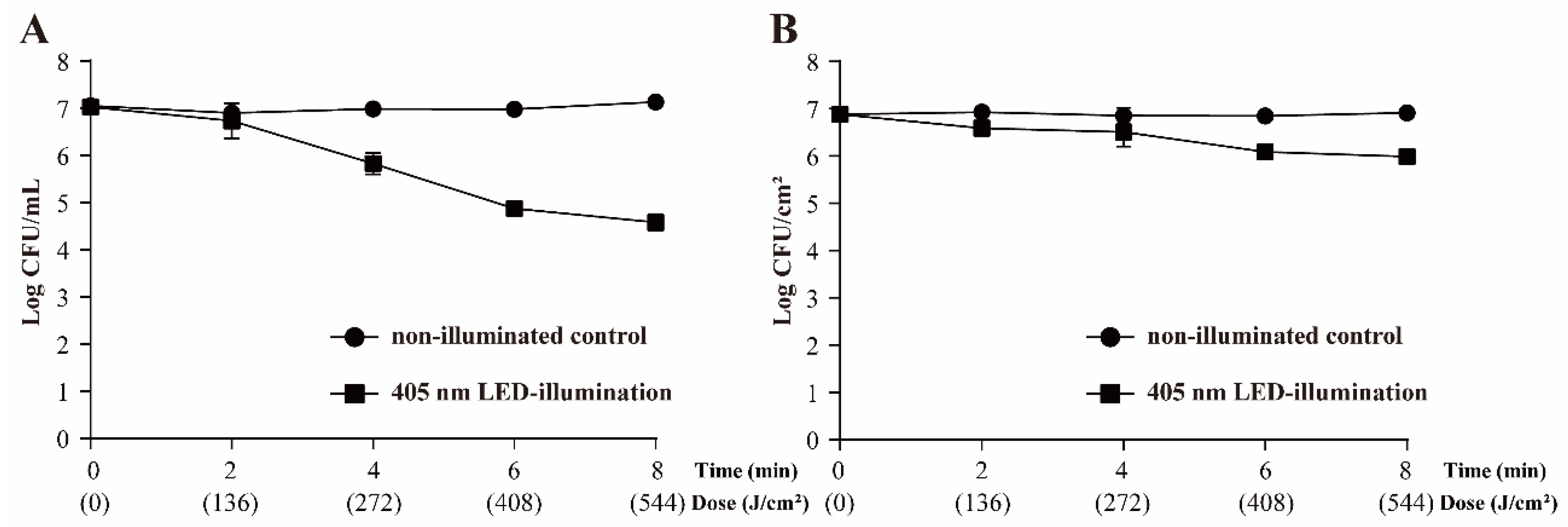
3.2. Survival of S. Typhimurium following 405 nm LED Treatment
3.3. Environmental Stress-Resistance of S. Typhimurium in Sliced Beef
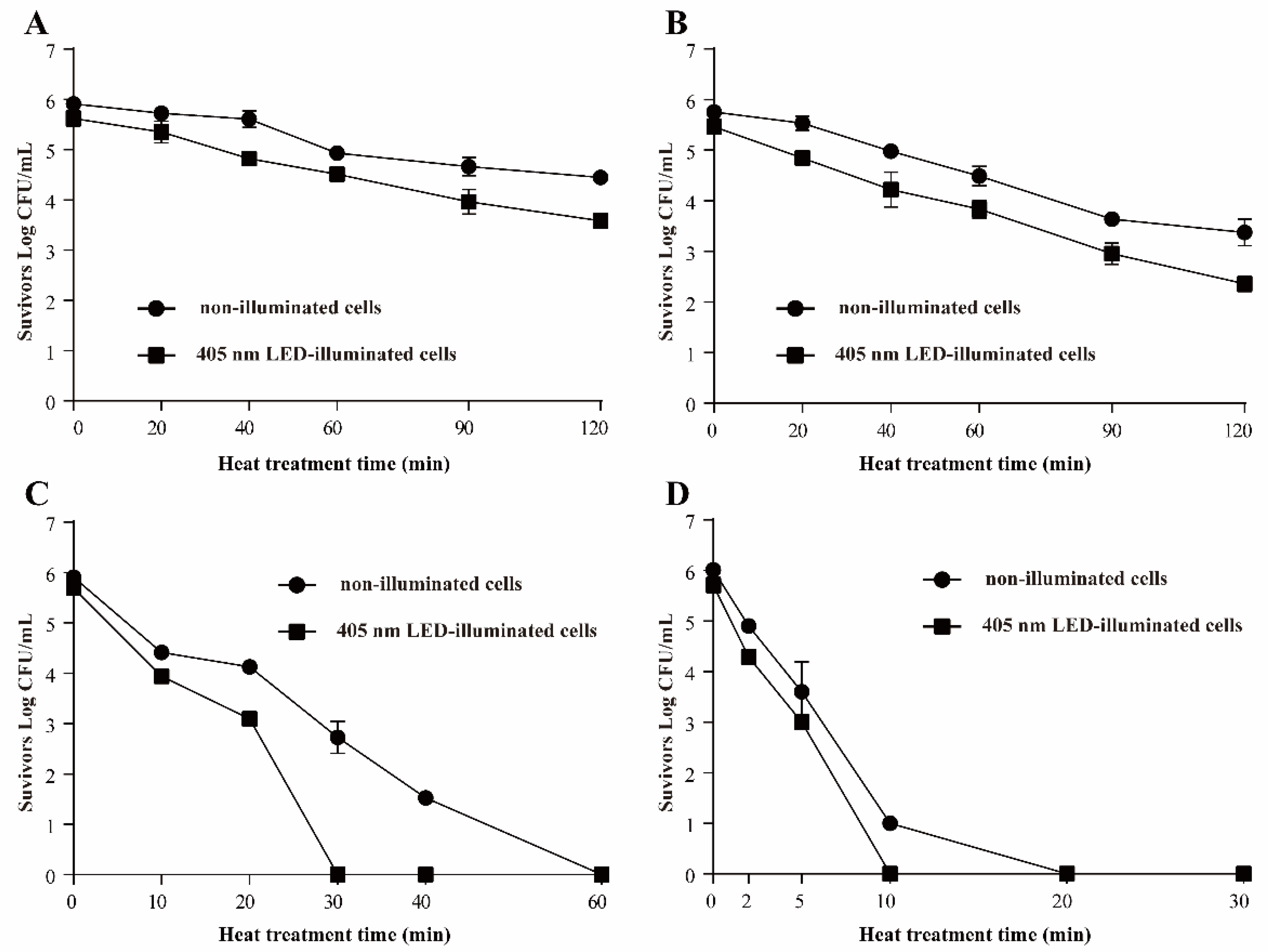
3.3.1. Resistance of S. Typhimurium to Heat Stress
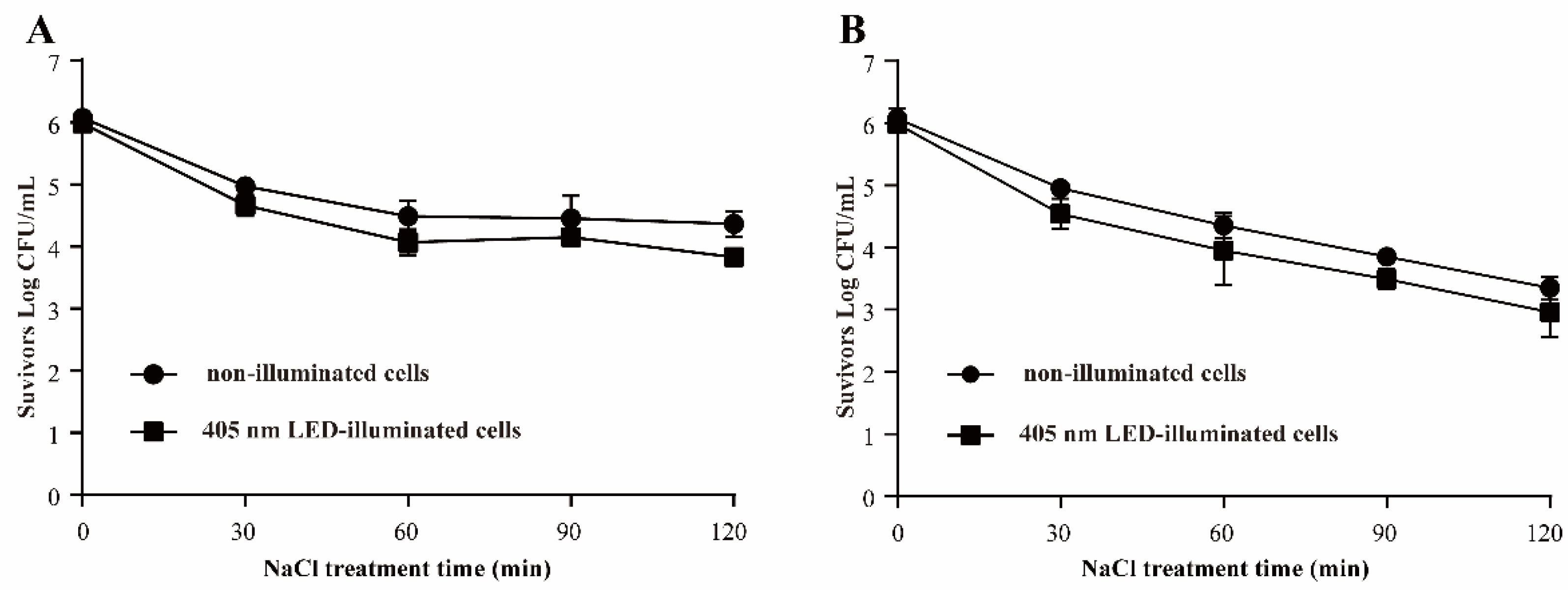
3.3.2. Resistance of S. Typhimurium to Osmotic Pressure
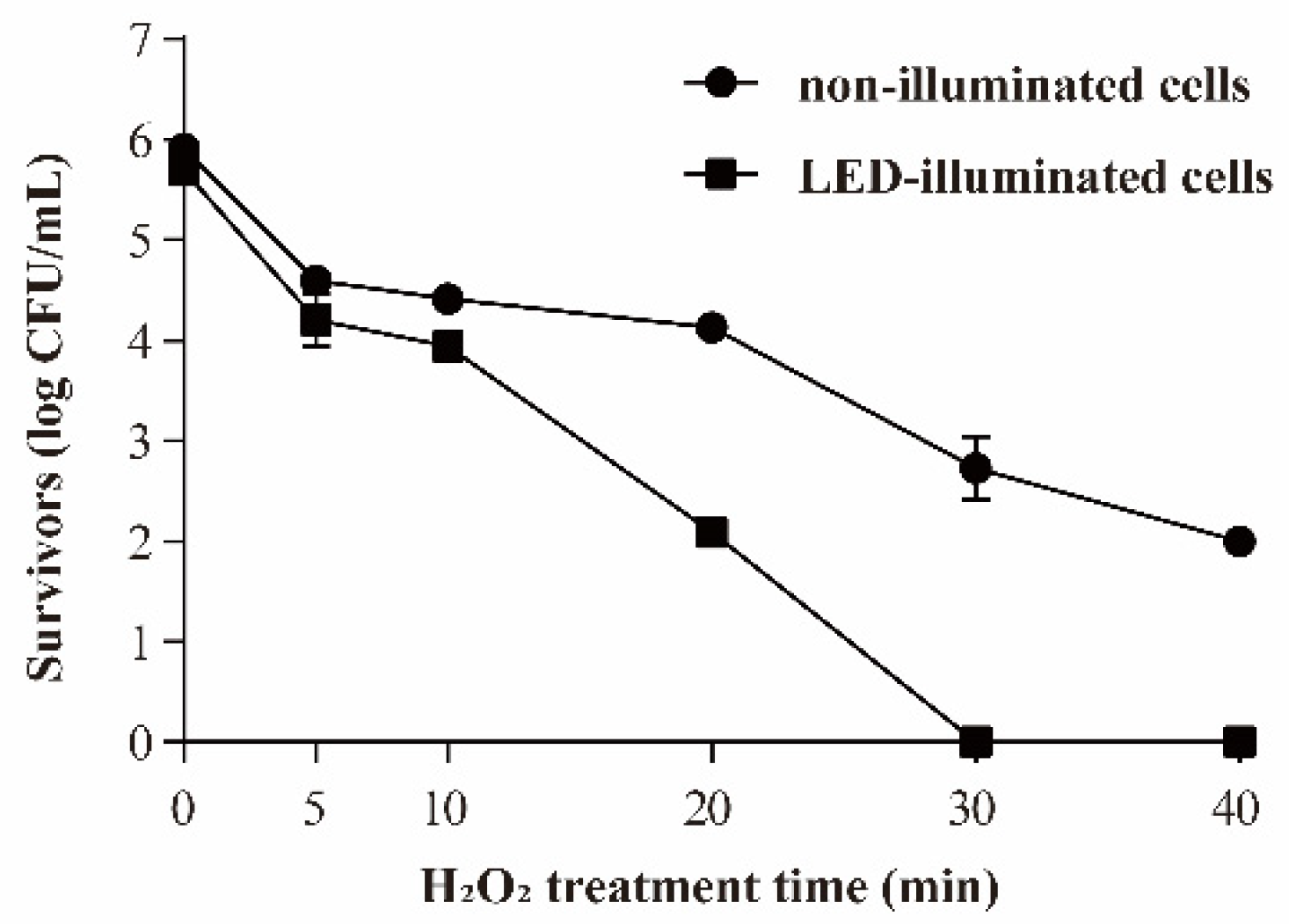
3.3.3. Resistance of S. Typhimurium to Oxidative Stress
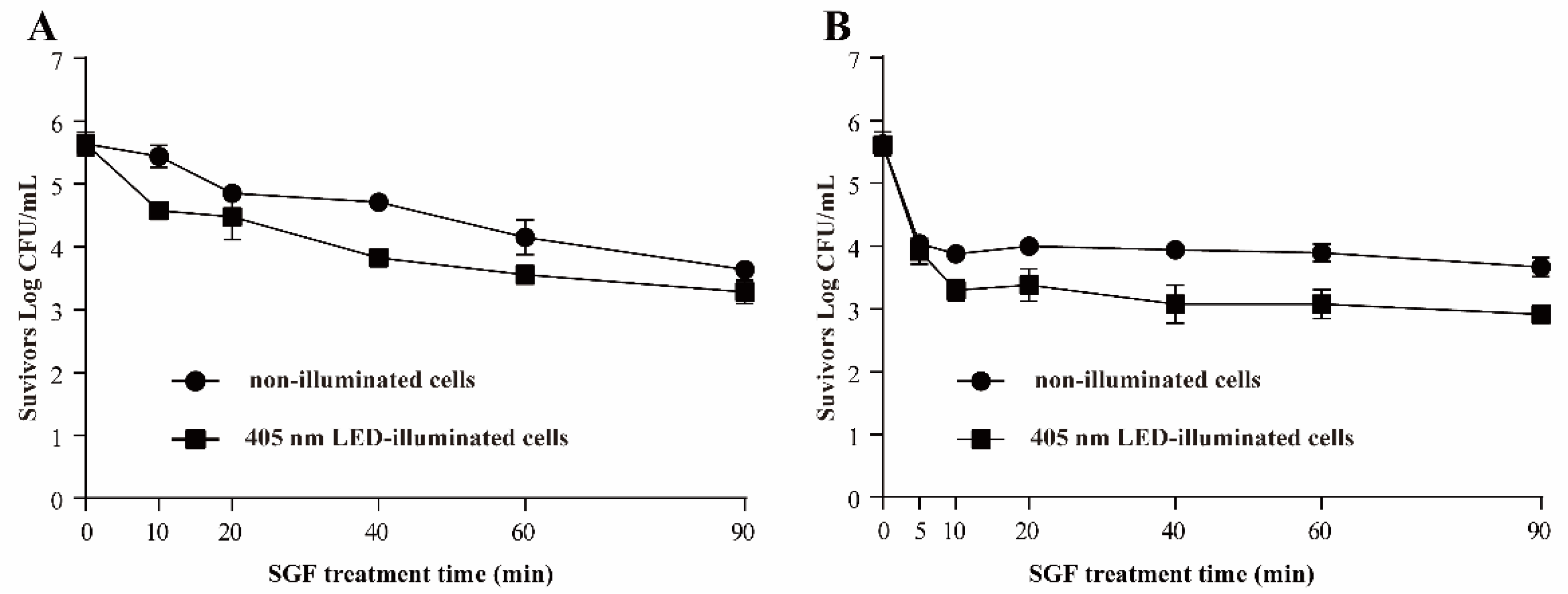
3.3.4. Resistance of S. Typhimurium to SGF
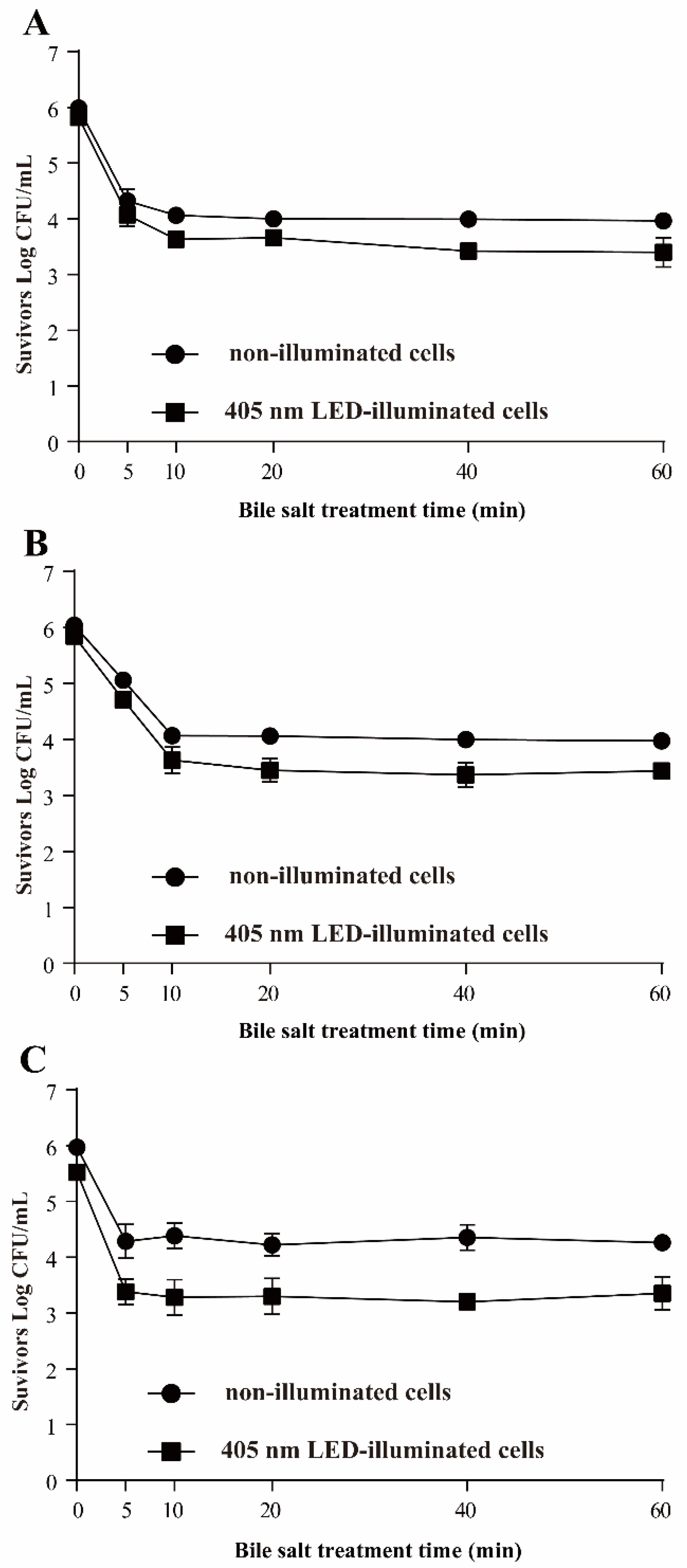
3.3.5. Resistance of S. Typhimurium to Bile Salts
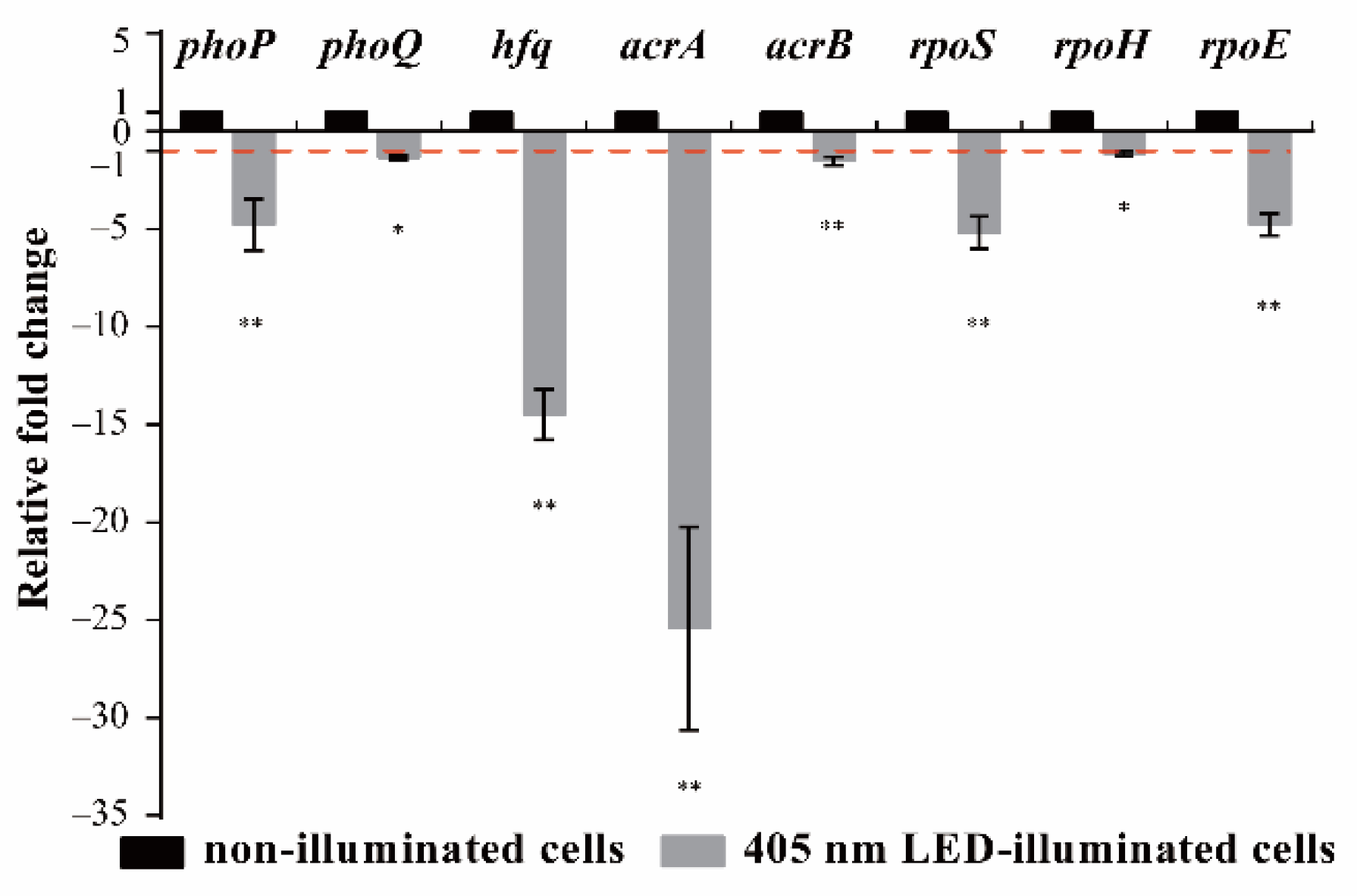
3.4. Effects of 405 nm LED Illumination on Gene Transcription of S. Typhimurium
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- López, F.E.; de las Mercedes Pescaretti, M.; Morero, R.; Delgado, M.A. Salmonella typhimurium general virulence factors: A battle of david against goliath? Food Res. Int. 2012, 45, 842–851. [Google Scholar] [CrossRef]
- Brenner, F.W.; Villar, R.G.; Angulo, F.J.; Tauxe, R.; Swaminathan, B. Salmonella nomenclature. J. Clin. Microbiol. 2000, 38, 2465–2467. [Google Scholar] [CrossRef]
- de Melo, A.N.F.; de Souza, G.T.; Schaffner, D.; de Oliveira, T.C.M.; Maciel, J.F.; de Souza, E.L.; Magnani, M. Changes in thermo-tolerance and survival under simulated gastrointestinal conditions of Salmonella Enteritidis PT4 and Salmonella Typhimurium PT4 in chicken breast meat after exposure to sequential stresses. Int. J. Food Microbiol. 2017, 251, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Pui, C.F.; Wong, W.C.; Chai, L.C.; Tunung, R.; Jeyaletchumi, P.; Hidayah, M.S.N.; Ubong, A.; Farinazleen, M.G.; Cheah, Y.K. Salmonella: A foodborne pathogen. Int. Food Res. J. 2011, 18, 465–473. [Google Scholar]
- Yuan, W.; Goston, R.; Lee, D.; Lee, S.C.; Yuk, H.G. Influence of lactate and acetate salt adaptation on Salmonella Typhimurium acid and heat resistance. Food Microbiol. 2012, 30, 448–452. [Google Scholar] [CrossRef]
- Martínez-Chávez, L.; Cabrera-Diaz, E.; Pérez-Montaño, J.A.; Garay-Martínez, L.E.; Varela-Hernández, J.J.; Castillo, A.; Lucia, L.; Ávila-Novoa, M.G.; Cardona-López, M.A.; Gutiérrez-González, P.; et al. Quantitative distribution of Salmonella spp. and Escherichia coli on beef carcasses and raw beef at retail establishments. Int. J. Food Microbiol. 2015, 210, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Vial, S.; Doerscher, D.; Schroeder, C.; Strickland, A.; Hedberg, C. Confounding role of Salmonella serotype Dublin testing results of boneless and ground beef purchased for the National School Lunch Program, October 2013 to July 2017. J. Food Prot. 2020, 83, 628–636. [Google Scholar] [CrossRef]
- Guillén, S.; Marcén, M.; Mañas, P.; Cebrián, G. Differences in resistance to different environmental stresses and non-thermal food preservation technologies among Salmonella enterica subsp. enterica strains. Food Res. Int. 2020, 132, 109042. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Kim, M.J.; Yuk, H.G. Influence of 405 nm light-emitting diode illumination on the inactivation of Listeria monocytogenes and Salmonella spp. on ready-to-eat fresh salmon surface at chilling storage for 8 h and their susceptibility to simulated gastric fluid. Food Control 2018, 88, 61–68. [Google Scholar] [CrossRef]
- Kim, M.J.; Bao, X.; Ye, H.Z.; Yuk, H.G. Photodynamic inactivation of Salmonella enterica Enteritidis by 405 ± 5 nm light-emitting diode and its application to control salmonellosis on cooked chicken. Food Control 2017, 82, 305–315. [Google Scholar] [CrossRef]
- Josewin, S.W.; Kim, M.J.; Yuk, H.G. Inactivation of Listeria monocytogenes and Salmonella spp. on cantaloupe rinds by blue light emitting diodes (LEDs). Food Microbiol. 2018, 76, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Kim, M.J.; Bang, W.S.; Yuk, H.G. Anti-biofilm effect of 405 nm LEDs against Listeria monocytogenes in simulated ready-to-eat fresh salmon storage conditions. Food Control 2018, 84, 513–521. [Google Scholar] [CrossRef]
- Hyun, J.-E.; Lee, S.-Y. Antibacterial effect and mechanisms of action of 460–470 nm light-emitting diode against Listeria monocytogenes and Pseudomonas fluorescens on the surface of packaged sliced cheese. Food Microbiol. 2020, 86, 103314. [Google Scholar] [CrossRef]
- Kang, S.; Meng, Y.; Cheng, X.; Tu, J.; Guo, D.; Xu, Y.; Liang, S.; Xia, X.; Shi, C. Effects of 405-nm LED Treatment on the resistance of Listeria monocytogenes to subsequent environmental stresses. Front. Microbiol. 2019, 10, 1097. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Yu, H.; Guo, D.; Fei, S.; Shi, C. Survival and environmental stress resistance of Cronobacter sakazakii exposed to vacuum or air packaging and stored at different temperatures. Front. Microbiol. 2019, 10, 303. [Google Scholar] [CrossRef]
- Guo, D.; Yang, Z.; Zheng, X.; Kang, S.; Yang, Z.; Xu, Y.; Shi, C.; Tian, H.; Xia, X. Thymoquinone inhibits biofilm formation and attachment-invasion in host cells of Vibrio parahaemolyticus. Foodborne Pathog. Dis. 2019, 16, 671–678. [Google Scholar] [CrossRef]
- Spector, M.P.; Kenyon, W.J. Resistance and survival strategies of Salmonella enterica to environmental stresses. Food Res. Int. 2012, 45, 455–481. [Google Scholar] [CrossRef]
- Kim, M.J.; Mikš-Krajnik, M.; Kumar, A.; Ghate, V.; Yuk, H.G. Antibacterial effect and mechanism of high-intensity 405 ± 5 nm light emitting diode on Bacillus cereus, Listeria monocytogenes, and Staphylococcus aureus under refrigerated condition. J. Photochem. Photobiol. B Biol. 2015, 153, 33–39. [Google Scholar] [CrossRef]
- Cui, X.; Hu, C.; Ou, L.; Kuramitsu, Y.; Masuda, Y.; Honjoh, K.-I.; Miyamoto, T. Transcriptional analysis on heat resistance and recovery from thermal damage in Salmonella under high salt condition. LWT-Food Sci. Technol. 2019, 106, 194–200. [Google Scholar] [CrossRef]
- Doyle, M.E.; Mazzotta, A.S. Review of studies on the thermal resistance of Salmonellae. J. Food Prot. 2000, 63, 779–795. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Alrefaei, R.; Bushlaibi, M.; Ndegwa, E.; Wynn, C. Influence of growth temperature on thermal tolerance of leading foodborne pathogens. Food Sci. Nutr. 2019, 7, 4027–4036. [Google Scholar] [CrossRef]
- Chung, H.J.; Bang, W.; Drake, M.A. Stress response of Escherichia coli. Compr. Rev. Food Sci. Food Saf. 2006, 5, 52–64. [Google Scholar] [CrossRef]
- Sleator, R.D.; Hill, C. Bacterial osmoadaptation: The role of osmolytes in bacterial stress and virulence. FEMS Microbiol. Rev. 2002, 26, 49–71. [Google Scholar] [CrossRef] [PubMed]
- Ghate, V.S.; Ng, K.S.; Zhou, W.; Yang, H.; Khoo, G.H.; Yoon, W.-B.; Yuk, H.-G. Antibacterial effect of light emitting diodes of visible wavelengths on selected foodborne pathogens at different illumination temperatures. Int. J. Food Microbiol. 2013, 166, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Mikš-Krajnik, M.; Kumar, A.; Yuk, H.G. Inactivation by 405 ± 5 nm light emitting diode on Escherichia coli O157:H7, Salmonella Typhimurium, and Shigella sonnei under refrigerated condition might be due to the loss of membrane integrity. Food Control 2016, 59, 99–107. [Google Scholar] [CrossRef]
- Dong, R.; Qin, X.; He, S.; Zhou, X.; Cui, Y.; Shi, C.; He, Y.; Shi, X. DsrA confers resistance to oxidative stress in Salmonella enterica serovar Typhimurium. Food Control 2021, 121, 107571. [Google Scholar] [CrossRef]
- Alvarez-Ordóñez, A.; Fernández, A.; Bernardo, A.; López, M. Acid adaptation sensitizes Salmonella enterica serovar Typhimurium to osmotic and oxidative stresses. Arch. Lebensm. 2010, 61, 148–152. [Google Scholar] [CrossRef]
- Coral, P.-E.; Hidalgo, A.A.; Camila, A.; Briones, A.C.; Cabezas, C.E.; Juan, C.S.; Fuentes, J.A.; Opazo, C.M.; Riedel, C.A.; Carolina, O. The ArcAB two-component regulatory system promotes resistance to reactive oxygen species and systemic infection by Salmonella Typhimurium. PLoS ONE 2018, 13, e0203497. [Google Scholar] [CrossRef]
- Morales, E.H.; Calderon, I.L.; Collao, B.; Gil, F.; Porwollik, S.; Mcclelland, M.; Saavedra, C.P. Hypochlorous acid and hydrogen peroxide-induced negative regulation of Salmonella enterica serovar Typhimurium ompW by the response regulator ArcA. BMC Microbiol. 2012, 12, 63. [Google Scholar] [CrossRef]
- Bang, I.-S.; Frye, J.G.; Mcclelland, M.; Velayudhan, J.; Fang, F.C. Alternative sigma factor interactions in Salmonella: σE and σH promote antioxidant defences by enhancing σS levels. Mol. Microbiol. 2005, 56, 811–823. [Google Scholar] [CrossRef]
- Berk, P.A.; Jonge, R.D.; Zwietering, M.H.; Abee, T.; Kieboom, J. Acid resistance variability among isolates of Salmonella enterica serovar Typhimurium DT104. J. Appl. Microbiol. 2010, 99, 859–866. [Google Scholar] [CrossRef]
- Fang, F.C.; Frawley, E.R.; Tapscott, T.; Vázquez-Torres, A. Bacterial stress responses during host infection. Cell Host Microbe 2016, 20, 133–143. [Google Scholar] [CrossRef]
- Oliveira, A.R.; Domingues, F.C.; Ferreira, S. The influence of resveratrol adaptation on resistance to antibiotics, benzalkonium chloride, heat and acid stresses of Staphylococcus aureus and Listeria monocytogenes. Food Control 2017, 73, 1420–1425. [Google Scholar] [CrossRef]
- Lehrke, G.; Hernaez, L.; Mugliaroli, S.L.; Staszewski, M.V.; Jagus, R.J. Sensitization of Listeria innocua to inorganic and organic acids by natural antimicrobials. LWT—Food Sci. Technol. 2011, 44, 984–991. [Google Scholar] [CrossRef]
- Lang, C.; Zhang, Y.; Mao, Y.; Yang, X.; Zhu, L. Acid tolerance response of Salmonella during simulated chilled beef storage and its regulatory mechanism based on the PhoP/Q system. Food Microbiol. 2021, 95, 103716. [Google Scholar] [CrossRef]
- Prouty, A.M.; Van Velkinburgh, J.C.; Gunn, J.S. Salmonella enterica serovar typhimurium resistance to bile: Identification and characterization of the tolQRA cluster. J. Bacteriol. 2002, 184, 1270–1276. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ruiz, L.; Margolles, A.; Sánchez, B. Bile resistance mechanisms in Lactobacillus and Bifidobacterium. Front. Microbiol. 2013, 4, 396. [Google Scholar] [CrossRef] [PubMed]
- Begley, M.; Gahan, C.G.M.; Hill, C. The interaction between bacteria and bile. FEMS Microbiol. Rev. 2005, 29, 625–651. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Nam, D.; Kweon, D.-H.; Shin, D. ATP reduction by MgtC and Mg2+ homeostasis by MgtA and MgtB enables Salmonella to accumulate RpoS upon low cytoplasmic Mg2+ stress. Mol. Microbiol. 2018, 110, 283–295. [Google Scholar] [CrossRef]
- Sittka, A.; Pfeiffer, V.; Tedin, K.; Vogel, J. The RNA chaperone Hfq is essential for the virulence of Salmonella typhimurium. Mol. Microbiol. 2007, 63, 193–217. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, D.; Bai, Y.; Fei, S.; Yang, Y.; Li, J.; Yang, B.; Lü, X.; Xia, X.; Shi, C. Effects of 405 ± 5-nm LED Illumination on Environmental Stress Tolerance of Salmonella Typhimurium in Sliced Beef. Foods 2022, 11, 136. https://doi.org/10.3390/foods11020136
Guo D, Bai Y, Fei S, Yang Y, Li J, Yang B, Lü X, Xia X, Shi C. Effects of 405 ± 5-nm LED Illumination on Environmental Stress Tolerance of Salmonella Typhimurium in Sliced Beef. Foods. 2022; 11(2):136. https://doi.org/10.3390/foods11020136
Chicago/Turabian StyleGuo, Du, Yichen Bai, Shengyi Fei, Yanpeng Yang, Jiahui Li, Baowei Yang, Xin Lü, Xiaodong Xia, and Chao Shi. 2022. "Effects of 405 ± 5-nm LED Illumination on Environmental Stress Tolerance of Salmonella Typhimurium in Sliced Beef" Foods 11, no. 2: 136. https://doi.org/10.3390/foods11020136
APA StyleGuo, D., Bai, Y., Fei, S., Yang, Y., Li, J., Yang, B., Lü, X., Xia, X., & Shi, C. (2022). Effects of 405 ± 5-nm LED Illumination on Environmental Stress Tolerance of Salmonella Typhimurium in Sliced Beef. Foods, 11(2), 136. https://doi.org/10.3390/foods11020136





