Inulin from Globe Artichoke Roots: A Promising Ingredient for the Production of Functional Fresh Pasta
Abstract
1. Introduction
2. Materials and Methods
2.1. Extraction and Characterzsation of Artichoke Root Inulin
2.1.1. Extraction Process
2.1.2. Moisture and Water Activity of AR Inulin Powder
2.1.3. Identification and Quantification of AR Inulin
2.1.4. Polymerization Degree and Molecular Weight
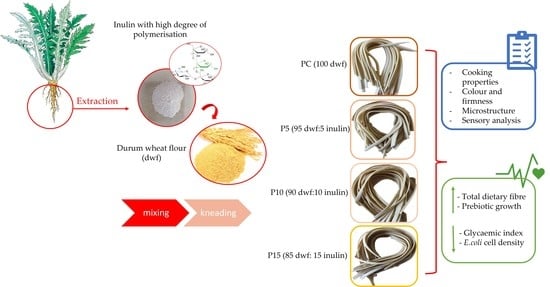
2.2. Fresh Pasta Preparation and Characterization
2.2.1. Experimental Design
2.2.2. Cooking Properties
2.2.3. Inulin Loss in Cooking Water
2.2.4. Color and Firmness Evaluation
2.2.5. Microstructure Determination
2.2.6. Quantitative Descriptive Sensory Analysis
2.3. Functional Properties Determination
2.3.1. Proximate Composition of Fresh Pasta
2.3.2. In Vitro Starch Hydrolysis
2.3.3. Prebiotic Activity Assay
2.4. Statistical Analysis
3. Results and Discussion
3.1. Characteristic of AR Inulin Powder
3.2. Quality Characteristics of Fresh Pasta
3.2.1. Cooking Properties of Fresh Pasta
3.2.2. Color and Firmness of Fresh Pasta
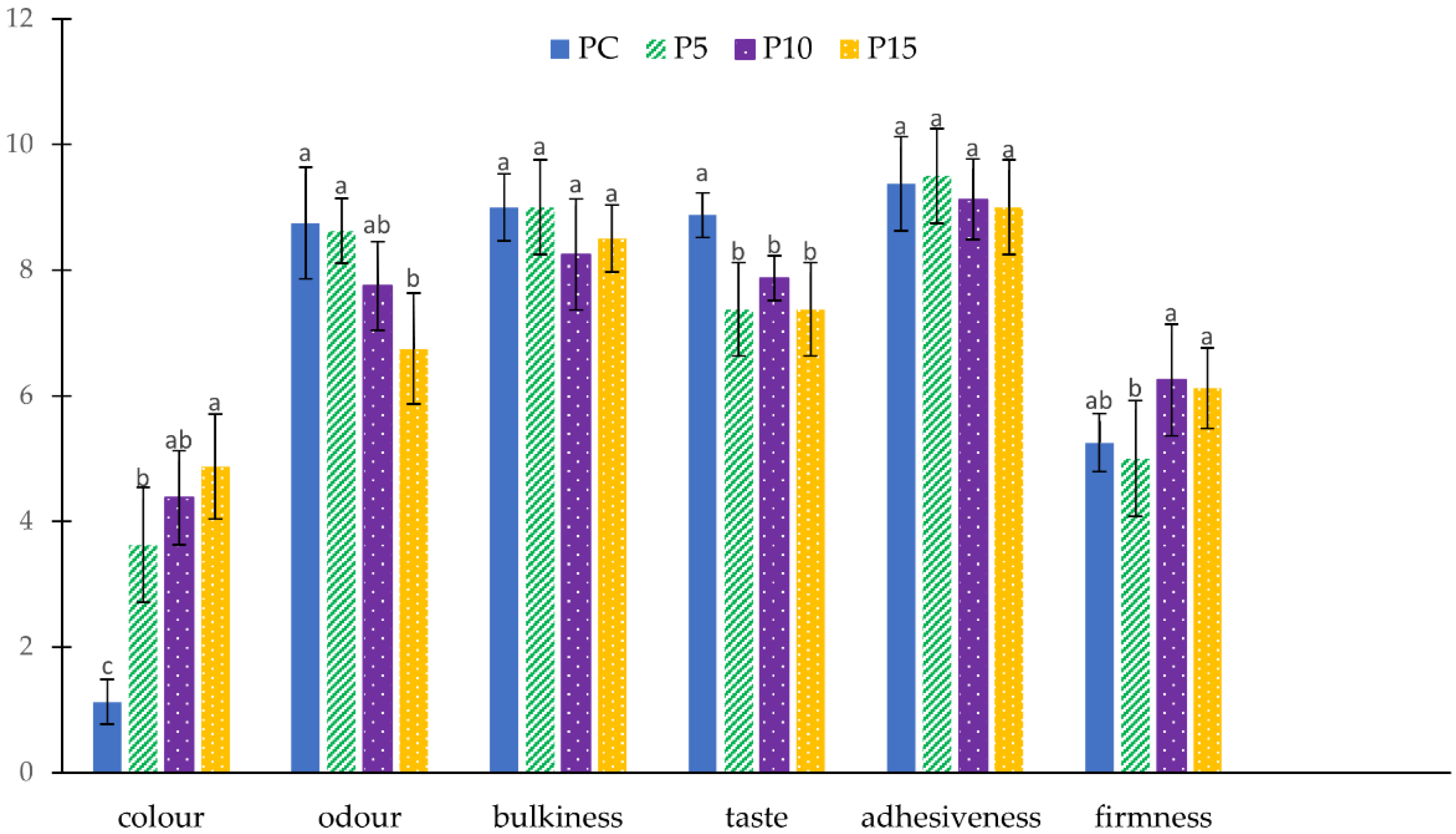
3.2.3. Sensory Evaluation of Fresh Pasta
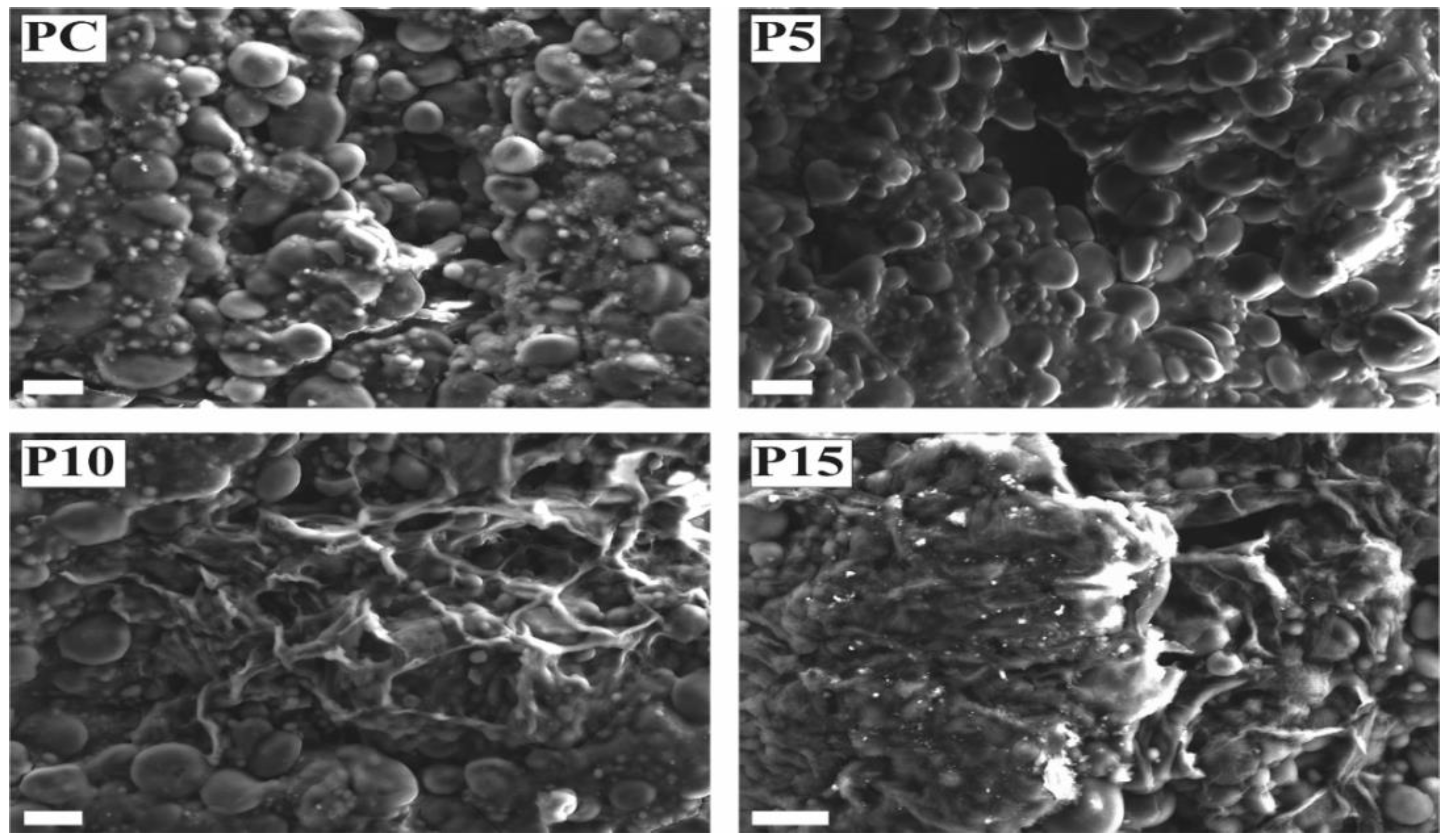
3.2.4. Microstructure
3.3. Functional Properties of Fresh Pasta
3.3.1. Proximate Composition of Fresh Pasta
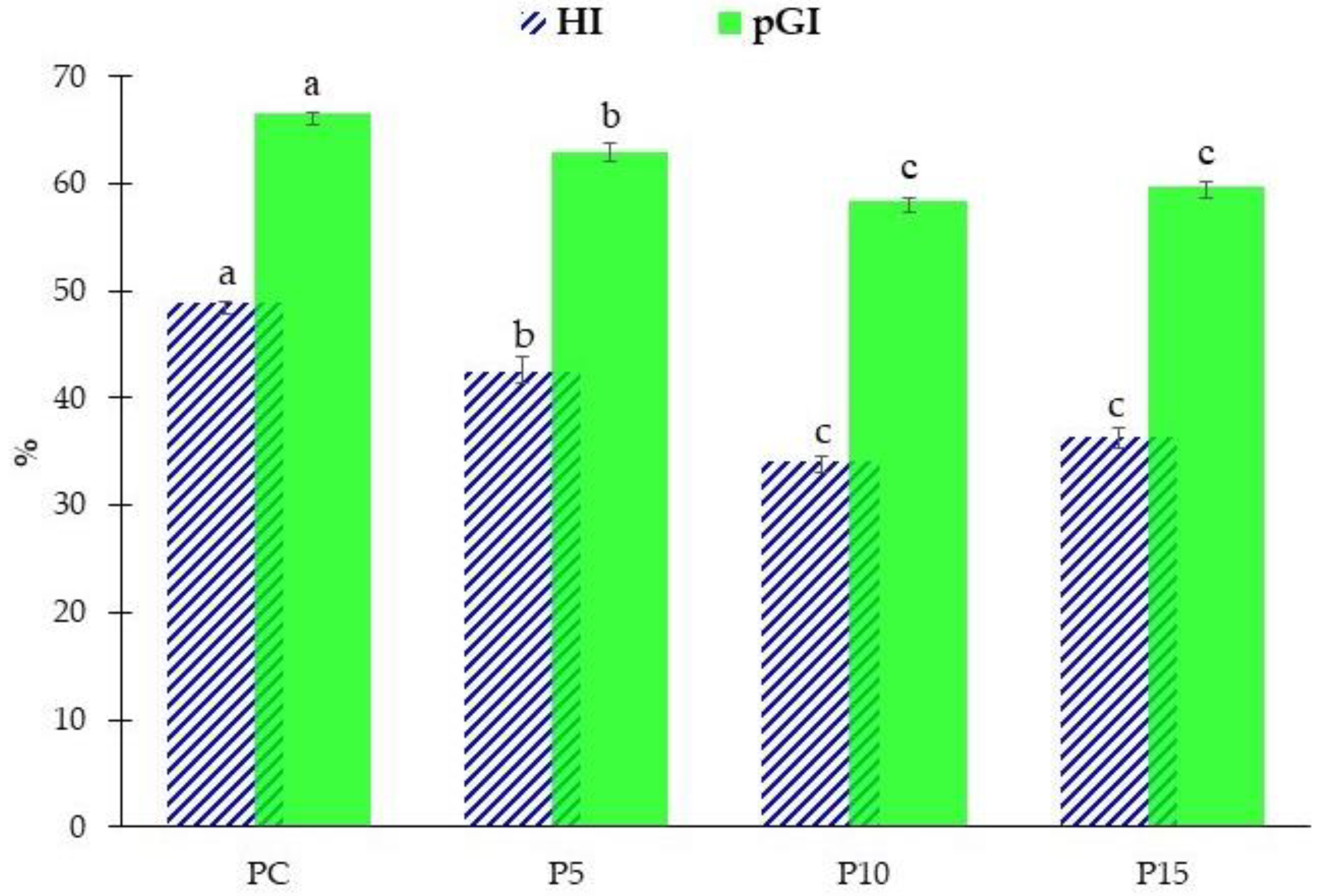
3.3.2. In Vitro Starch Hydrolysis
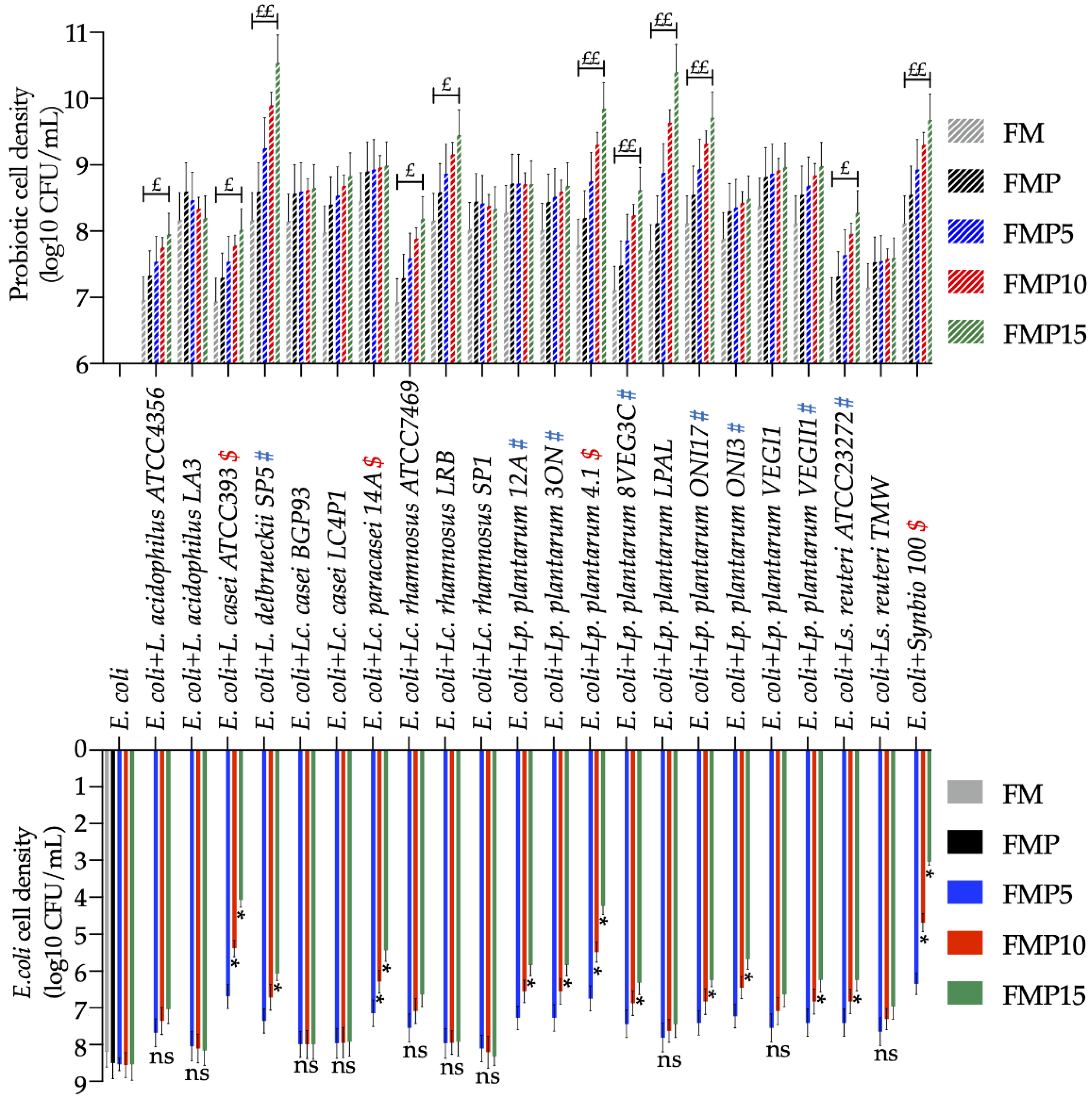
3.3.3. Evaluation of Effects Exerted by AR Inulin, Prebiotics, and Pathogen on Probiotic Growth
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Francavilla, M.; Marone, M.; Marasco, P.; Contillo, F.; Monteleone, M. Artichoke Biorefinery: From Food to Advanced Technological Applications. Foods 2021, 10, 112. [Google Scholar] [CrossRef]
- Domingo, C.S.; Rojas, A.M.; Fissore, E.N.; Gerschenson, L.N. Rheological behavior of soluble dietary fiber fractions isolated from artichoke residues. Eur. Food Res. Technol. 2019, 245, 1239–1249. [Google Scholar] [CrossRef]
- Villanueva-Suárez, M.J.; Mateos-Aparicio, I.; Pérez-Cózar, M.L.; Yokoyama, W.; Redondo-Cuenca, A. Hypolipidemic effects of dietary fibre from an artichoke by-product in Syrian hamsters. J. Funct. Foods 2019, 56, 156–162. [Google Scholar] [CrossRef]
- Zeaiter, Z.; Regonesi, M.E.; Cavini, S.; Labra, M.; Sello, G.; Di Gennaro, P. Extraction and Characterization of Inulin-Type Fructans from Artichoke Wastes and Their Effect on the Growth of Intestinal Bacteria Associated with Health. Biomed Res. Int. 2019, 2019, 1083952. [Google Scholar] [CrossRef]
- Lattanzio, V.; Kroon, P.A.; Linsalata, V.; Cardinali, A. Globe artichoke: A functional food and source of nutraceutical ingredients. J. Funct. Foods 2009, 1, 131–144. [Google Scholar] [CrossRef]
- Borsini, A.A.; Llavata, B.; Umaña, M.; Cárcel, J.A. Artichoke by Products as a Source of Antioxidant and Fiber: How It Can Be Affected by Drying Temperature. Foods 2021, 10, 459. [Google Scholar] [CrossRef]
- Pasqualone, A.; Punzi, R.; Trani, A.; Summo, C.; Paradiso, V.M.; Caponio, F.; Gambacorta, G. Enrichment of fresh pasta with antioxidant extracts obtained from artichoke canning by-products by ultrasound-assisted technology and quality characterisation of the end product. Int. J. Food Sci. Technol. 2017, 52, 2078–2087. [Google Scholar] [CrossRef]
- Holgado, F.; Campos-Monfort, G.; de las Heras, C.; Rupérez, P. In vitro fermentability of globe artichoke by-product by Lactobacillus acidophilus and Bifidobacterium bifidum. Bioact. Carbohydr. Diet. Fibre 2021, 26, 100286. [Google Scholar] [CrossRef]
- Raccuia, S.A.; Melilli, M.G. Seasonal dynamics of biomass, inulin, and water-soluble sugars in roots of Cynara cardunculus L. Field Crop. Res. 2010, 116, 147–153. [Google Scholar] [CrossRef]
- Castellino, M.; Renna, M.; Leoni, B.; Calasso, M.; Difonzo, G.; Santamaria, P.; Gambacorta, G.; Caponio, F.; De Angelis, M.; Paradiso, V.M. Conventional and unconventional recovery of inulin rich extracts for food use from the roots of globe artichoke. Food Hydrocoll. 2020, 107, 105975. [Google Scholar] [CrossRef]
- Redondo-Cuenca, A.; Herrera-Vázquez, S.E.; Condezo-Hoyos, L.; Gómez-Ordóñez, E.; Rupérez, P. Inulin extraction from common inulin-containing plant sources. Ind. Crops Prod. 2021, 170, 113726. [Google Scholar] [CrossRef]
- López-Molina, D.; Navarro-Martínez, M.D.; Rojas-Melgarejo, F.; Hiner, A.N.P.; Chazarra, S.; Rodríguez-López, J.N. Molecular properties and prebiotic effect of inulin obtained from artichoke (Cynara scolymus L.). Phytochemistry 2005, 66, 1476–1484. [Google Scholar] [CrossRef]
- Lopes, S.M.S.; Krausová, G.; Rada, V.; Gonçalves, J.E.; Gonçalves, R.A.C.; de Oliveira, A.J.B. Isolation and characterization of inulin with a high degree of polymerization from roots of Stevia rebaudiana (Bert.) Bertoni. Carbohydr. Res. 2015, 411, 15–21. [Google Scholar] [CrossRef]
- Gominho, J.; Curt, M.D.; Lourenço, A.; Fernández, J.; Pereira, H. Cynara cardunculus L. as a biomass and multi-purpose crop: A review of 30 years of research. Biomass Bioenergy 2018, 109, 257–275. [Google Scholar] [CrossRef]
- Giarnetti, M.; Paradiso, V.M.; Caponio, F.; Summo, C.; Pasqualone, A. Fat replacement in shortbread cookies using an emulsion filled gel based on inulin and extra virgin olive oil. LWT Food Sci. Technol. 2015, 63, 339–345. [Google Scholar] [CrossRef]
- Puchkova, T.S.; Pikhalo, D.M.; Karasyova, O.M. About the universal technology of processing Jerusalem artichoke and chicory for inulin. Food Syst. 2019, 2, 36–43. [Google Scholar] [CrossRef]
- El-Kholy, W.M.; Aamer, R.A.; Ali, A.N.A. Utilization of inulin extracted from chicory (Cichorium intybus L.) roots to improve the properties of low-fat synbiotic yoghurt. Ann. Agric. Sci. 2020, 65, 59–67. [Google Scholar] [CrossRef]
- Mitchell, C.M.; Davy, B.M.; Halliday, T.M.; Hulver, M.W.; Neilson, A.P.; Ponder, M.A.; Davy, K.P. The effect of prebiotic supplementation with inulin on cardiometabolic health: Rationale, design, and methods of a controlled feeding efficacy trial in adults at risk of type 2 diabetes. Contemp. Clin. Trials 2015, 45, 328–337. [Google Scholar] [CrossRef]
- Caponio, G.R.; Lippolis, T.; Tutino, V.; Gigante, I.; De Nunzio, V.; Milella, R.A.; Gasparro, M.; Notarnicola, M. Nutraceuticals: Focus on Anti-Inflammatory, Anti-Cancer, Antioxidant Properties in Gastrointestinal Tract. Antioxidants 2022, 11, 1274. [Google Scholar] [CrossRef]
- Ivkov, M.; Kosutic, M.; Filipovic, J.; Filipovic, V. Spelt pasta with addition of inulin as a functional food: Sensory evaluation and consumer attitudes. Rom. Biotechnol. Lett. 2018, 23, 13615–13624. [Google Scholar]
- Filipovic, J.; Pezo, L.; Filipovic, V.; Ludajic, G. Spelt pasta with inulin as a functional food. Acta Period. Technol. 2015, 46, 37–44. [Google Scholar] [CrossRef]
- Martín-Esparza, M.; Raga, A.; González-Martínez, C.; Albors, A. Micronised bran-enriched fresh egg tagliatelle: Significance of gums addition on pasta technological features. Food Sci. Technol. Int. 2018, 24, 309–320. [Google Scholar] [CrossRef]
- Makhlouf, S.; Jones, S.; Ye, S.-H.; Sancho-Madriz, M.; Burns-Whitmore, B.; Li, Y.O. Effect of selected dietary fibre sources and addition levels on physical and cooking quality attributes of fibre-enhanced pasta. Food Qual. Saf. 2019, 3, 117–127. [Google Scholar] [CrossRef]
- Bianchi, F.; Tolve, R.; Rainero, G.; Bordiga, M.; Brennan, C.S.; Simonato, B. Technological, nutritional and sensory properties of pasta fortified with agro-industrial by-products: A review. Int. J. Food Sci. Technol. 2021, 56, 4356–4366. [Google Scholar] [CrossRef]
- Sobota, A.; Rzedzicki, Z.; Zarzycki, P.; Kuzawińska, E. Application of common wheat bran for the industrial production of high-fibre pasta. Int. J. Food Sci. Technol. 2015, 50, 111–119. [Google Scholar] [CrossRef]
- Costantini, M.; Summo, C.; Faccia, M.; Caponio, F.; Pasqualone, A. Kabuli and Apulian black Chickpea Milling By-Products as Innovative Ingredients to Provide High Levels of Dietary Fibre and Bioactive Compounds in Gluten-Free Fresh Pasta. Molecules 2021, 26, 4442. [Google Scholar] [CrossRef]
- Fradinho, P.; Oliveira, A.; Domínguez, H.; Torres, M.D.; Sousa, I.; Raymundo, A. Improving the nutritional performance of gluten-free pasta with potato peel autohydrolysis extract. Innov. Food Sci. Emerg. Technol. 2020, 63, 102374. [Google Scholar] [CrossRef]
- Garbetta, A.; D’Antuono, I.; Melilli, M.G.; Sillitti, C.; Linsalata, V.; Scandurra, S.; Cardinali, A. Inulin enriched durum wheat spaghetti: Effect of polymerization degree on technological and nutritional characteristics. J. Funct. Foods 2020, 71, 104004. [Google Scholar] [CrossRef]
- Krawęcka, A.; Sobota, A.; Sykut-Domańska, E. Physicochemical, Sensory, and Cooking Qualities of Pasta Enriched with Oat β-Glucans, Xanthan Gum, and Vital Gluten. Foods 2020, 9, 1412. [Google Scholar] [CrossRef]
- Peressini, D.; Cavarape, A.; Brennan, M.A.; Gao, J.; Brennan, C.S. Viscoelastic properties of durum wheat doughs enriched with soluble dietary fibres in relation to pasta-making performance and glycaemic response of spaghetti. Food Hydrocoll. 2020, 102, 105613. [Google Scholar] [CrossRef]
- Foschia, M.; Peressini, D.; Sensidoni, A.; Brennan, M.A.; Brennan, C.S. How combinations of dietary fibres can affect physicochemical characteristics of pasta. LWT Food Sci. Technol. 2015, 61, 41–46. [Google Scholar] [CrossRef]
- Aravind, N.; Sissons, M.J.; Fellows, C.M.; Blazek, J.; Gilbert, E.P. Effect of inulin soluble dietary fibre addition on technological, sensory, and structural properties of durum wheat spaghetti. Food Chem. 2012, 132, 993–1002. [Google Scholar] [CrossRef]
- Padalino, L.; Costa, C.; Conte, A.; Melilli, M.G.; Sillitti, C.; Bognanni, R.; Raccuia, S.A.; Del Nobile, M.A. The quality of functional whole-meal durum wheat spaghetti as affected by inulin polymerization degree. Carbohydr. Polym. 2017, 173, 84–90. [Google Scholar] [CrossRef]
- Mensink, M.A.; Frijlink, H.W.; van der Voort Maarschalk, K.; Hinrichs, W.L.J. Inulin, a flexible oligosaccharide I: Review of its physicochemical characteristics. Carbohydr. Polym. 2015, 130, 405–419. [Google Scholar] [CrossRef]
- Pasqualone, A.; Gambacorta, G.; Summo, C.; Caponio, F.; Di Miceli, G.; Flagella, Z.; Marrese, P.P.; Piro, G.; Perrotta, C.; De Bellis, L.; et al. Functional, textural and sensory properties of dry pasta supplemented with lyophilized tomato matrix or with durum wheat bran extracts produced by supercritical carbon dioxide or ultrasound. Food Chem. 2016, 213, 545–553. [Google Scholar] [CrossRef]
- AACC, American Association of Cereal Chemists. Approved Methods of Analysis, 11th ed.; AACC International: St. Paul, MN, USA, 2000. [Google Scholar]
- Bustos, M.C.; Pérez, G.T.; León, A.E. Effect of Four Types of Dietary Fiber on the Technological Quality of Pasta. Food Sci. Technol. Int. 2011, 17, 213–221. [Google Scholar] [CrossRef]
- Pasqualone, A.; De Angelis, D.; Squeo, G.; Difonzo, G.; Caponio, F.; Summo, C. The effect of the addition of apulian black chickpea flour on the nutritional and qualitative properties of durum wheat-based bakery products. Foods 2019, 8, 504. [Google Scholar] [CrossRef]
- AOAC International, Association of Official Analytical Chemists. Official Method of Analyisis, 17th ed.; AOAC International: Gaithersburg, MD, USA, 2006. [Google Scholar]
- Liljeberg, H.; Åkerberg, A.; Björck, I. Resistant starch formation in bread as influenced by choice of ingredients or baking conditions. Food Chem. 1996, 56, 389–394. [Google Scholar] [CrossRef]
- Capriles, V.D.; Arêas, J.A.G. Effects of prebiotic inulin-type fructans on structure, quality, sensory acceptance and glycemic response of gluten-free breads. Food Funct. 2013, 4, 104–110. [Google Scholar] [CrossRef]
- Kamiloglu, S.; Capanoglu, E. In vitro gastrointestinal digestion of polyphenols from different molasses (pekmez) and leather (pestil) varieties. Int. J. Food Sci. Technol. 2014, 49, 1027–1039. [Google Scholar] [CrossRef]
- Caponio, G.R.; Noviello, M.; Calabrese, F.M.; Gambacorta, G.; Giannelli, G.; De Angelis, M. Effects of Grape Pomace Polyphenols and In Vitro Gastrointestinal Digestion on Antimicrobial Activity: Recovery of Bioactive Compounds. Antioxidants 2022, 11, 567. [Google Scholar] [CrossRef]
- Vacca, M.; Celano, G.; Lenucci, M.S.; Fontana, S.; la Forgia, F.M.; Minervini, F.; Scarano, A.; Santino, A.; Dalfino, G.; Gesualdo, L.; et al. In Vitro Selection of Probiotics, Prebiotics, and Antioxidants to Develop an Innovative Synbiotic (NatuREN G) and Testing Its Effect in Reducing Uremic Toxins in Fecal Batches from CKD Patients. Microorganisms 2021, 9, 1316. [Google Scholar] [CrossRef] [PubMed]
- Maumela, P.; van Rensburg, E.; Chimphango, A.F.A.; Görgens, J.F. Sequential extraction of protein and inulin from the tubers of Jerusalem artichoke (Helianthus tuberosus L.). J. Food Sci. Technol. 2020, 57, 775–786. [Google Scholar] [CrossRef] [PubMed]
- Rubel, I.A.; Iraporda, C.; Novosad, R.; Cabrera, F.A.; Genovese, D.B.; Manrique, G.D. Inulin rich carbohydrates extraction from Jerusalem artichoke (Helianthus tuberosus L.) tubers and application of different drying methods. Food Res. Int. 2018, 103, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Apolinário, A.C.; de Carvalho, E.M.; de Lima Damasceno, B.P.G.; da Silva, P.C.D.; Converti, A.; Pessoa, A.; da Silva, J.A. Extraction, isolation and characterization of inulin from Agave sisalana boles. Ind. Crops Prod. 2017, 108, 355–362. [Google Scholar] [CrossRef]
- Li, W.; Zhang, J.; Yu, C.; Li, Q.; Dong, F.; Wang, G.; Gu, G.; Guo, Z. Extraction, degree of polymerization determination and prebiotic effect evaluation of inulin from Jerusalem artichoke. Carbohydr. Polym. 2015, 121, 315–319. [Google Scholar] [CrossRef]
- Petkova, N.T.; Sherova, G.; Denev, P.P. Characterization of inulin from dahlia tubers isolated by microwave and ultrasound-assisted extractions. Int. Food Res. J. 2018, 25, 1876–1884. [Google Scholar]
- Xu, H.; Gunenc, A.; Hosseinian, F. Ultrasound affects physical and chemical properties of Jerusalem artichoke and chicory inulin. J. Food Biochem. 2022, 46, 1–10. [Google Scholar] [CrossRef]
- Jirayucharoensak, R.; Khuenpet, K.; Jittanit, W.; Sirisansaneeyakul, S. Physical and chemical properties of powder produced from spray drying of inulin component extracted from Jerusalem artichoke tuber powder. Dry. Technol. 2019, 37, 1215–1227. [Google Scholar] [CrossRef]
- Simonato, B.; Tolve, R.; Rainero, G.; Rizzi, C.; Sega, D.; Rocchetti, G.; Lucini, L.; Giuberti, G. Technological, nutritional, and sensory properties of durum wheat fresh pasta fortified with Moringa oleifera L. leaf powder. J. Sci. Food Agric. 2021, 101, 1920–1925. [Google Scholar] [CrossRef]
- Neylon, E.; Arendt, E.K.; Zannini, E.; Sahin, A.W. Fundamental study of the application of brewers spent grain and fermented brewers spent grain on the quality of pasta. Food Struct. 2021, 30, 100225. [Google Scholar] [CrossRef]
- Simonato, B.; Trevisan, S.; Tolve, R.; Favati, F.; Pasini, G. Pasta fortification with olive pomace: Effects on the technological characteristics and nutritional properties. LWT 2019, 114, 108368. [Google Scholar] [CrossRef]
- Naji-Tabasi, S.; Niazmand, R.; Modiri-Dovom, A. Application of mucilaginous seeds (Alyssum homolocarpum and Salvia macrosiphon Boiss) and wheat bran in improving technological and nutritional properties of pasta. J. Food Sci. 2021, 86, 2288–2299. [Google Scholar] [CrossRef] [PubMed]
- Attanzio, A.; Diana, P.; Barraja, P.; Carbone, A.; Spanò, V.; Parrino, B.; Cascioferro, S.M.; Allegra, M.; Cirrincione, G.; Tesoriere, L.; et al. Quality, functional and sensory evaluation of pasta fortified with extracts from Opuntia ficus-indica cladodes. J. Sci. Food Agric. 2019, 99, 4242–4247. [Google Scholar] [CrossRef] [PubMed]
- Renoldi, N.; Brennan, C.S.; Lagazio, C.; Peressini, D. Evaluation of technological properties, microstructure and predictive glycaemic response of durum wheat pasta enriched with psyllium seed husk. LWT 2021, 151, 112203. [Google Scholar] [CrossRef]
- Brennan, C.S.; Tudorica, C.M. Fresh Pasta Quality as affected by enrichment of nonstarch polysaccharides. J. Food Sci. 2007, 72, S659–S665. [Google Scholar] [CrossRef]
- Xu, F.; Li, X.; Li, J.; Chen, J. The interaction between inulin and wheat starch and effects of inulin on frozen storage quality of noodles. Int. J. Food Sci. Technol. 2021, 56, 2423–2431. [Google Scholar] [CrossRef]
- Zarroug, Y.; Djebali, K.; Sfayhi, D.; Khemakhem, M.; Boulares, M.; El Felah, M.; Mnasser, H.; Kharrat, M. Optimization of barley flour and inulin addition for pasta formulation using mixture design approach. J. Food Sci. 2022, 87, 68–79. [Google Scholar] [CrossRef]
- Lucisano, M.; Cappa, C.; Fongaro, L.; Mariotti, M. Characterisation of gluten-free pasta through conventional and innovative methods: Evaluation of the cooking behaviour. J. Cereal Sci. 2012, 56, 667–675. [Google Scholar] [CrossRef]
- Li, Y.; Ma, X.; Liu, X. Physicochemical and rheological properties of cross-linked inulin with different degree of polymerization. Food Hydrocoll. 2019, 95, 318–325. [Google Scholar] [CrossRef]
- Liu, J.; Luo, D.; Li, X.; Xu, B.; Zhang, X.; Liu, J. Effects of inulin on the structure and emulsifying properties of protein components in dough. Food Chem. 2016, 210, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Brennan, M.A.; Brennan, C.S.; Serventi, L. Effect of Vegetable Juice, Puree, and Pomace on Chemical and Technological Quality of Fresh Pasta. Foods 2021, 10, 1931. [Google Scholar] [CrossRef] [PubMed]
- Cárdenas-Hernández, A.; Beta, T.; Loarca-Piña, G.; Castaño-Tostado, E.; Nieto-Barrera, J.O.; Mendoza, S. Improved functional properties of pasta: Enrichment with amaranth seed flour and dried amaranth leaves. J. Cereal Sci. 2016, 72, 84–90. [Google Scholar] [CrossRef]
- Minarovičová, L.; Lauková, M.; Kohajdová, Z.; Karovičová, J.; Dobrovická, D.; Kuchtová, V. Qualitative properties of pasta enriched with celery root and sugar beet by-products. Czech J. Food Sci. 2018, 36, 66–72. [Google Scholar] [CrossRef]
- Bustos, M.C.; Perez, G.T.; Leon, A.E. Structure and quality of pasta enriched with functional ingredients. RSC Adv. 2015, 5, 30780–30792. [Google Scholar] [CrossRef]
- Aravind, N.; Sissons, M.; Egan, N.; Fellows, C. Effect of insoluble dietary fibre addition on technological, sensory, and structural properties of durum wheat spaghetti. Food Chem. 2012, 130, 299–309. [Google Scholar] [CrossRef]
- Chillo, S.; Civica, V.; Iannetti, M.; Suriano, N.; Mastromatteo, M.; Del Nobile, M.A. Properties of quinoa and oat spaghetti loaded with carboxymethylcellulose sodium salt and pregelatinized starch as structuring agents. Carbohydr. Polym. 2009, 78, 932–937. [Google Scholar] [CrossRef]
- Aravind, N.; Sissons, M.; Egan, N.; Fellows, C.M.; Blazek, J.; Gilbert, E.P. Effect of β-Glucan on Technological, Sensory, and Structural Properties of Durum Wheat Pasta. Cereal Chem. J. 2012, 89, 84–93. [Google Scholar] [CrossRef]
- Padalino, L.; Amalia, C.; Lucia, L.; Desislava, L.; Vincenzo, S.; Teresa Maria, P.; Marco, P.; Matteo Alessandro Del, N. Functional pasta with tomato by-product as a source of antioxidant compounds and dietary fibre. Czech J. Food Sci. 2017, 35, 48–56. [Google Scholar] [CrossRef]
- European Parliament. Regulation (EC) No. 1924/2006 of the European Parliament and of the Council of 20 December 2006 on Nutrition and Health Claims Made on Foods. Off. J. Eur. Union 2006, 404, 9. Available online: https://eur-lex.europa.eu/legal-content/en/ALL/?uri=CELEX%3A32006R1924 (accessed on 30 July 2022).
- Alongi, M.; Melchior, S.; Anese, M. Reducing the glycemic index of short dough biscuits by using apple pomace as a functional ingredient. LWT 2019, 100, 300–305. [Google Scholar] [CrossRef]
- Caponio, G.R.; Difonzo, G.; de Gennaro, G.; Calasso, M.; De Angelis, M.; Pasqualone, A. Nutritional Improvement of Gluten-Free Breadsticks by Olive Cake Addition and Sourdough Fermentation: How Texture, Sensory, and Aromatic Profile Were Affected? Front. Nutr. 2022, 9, 830932. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Farias, C.; Slezak, K.; Fuller, Z.; Duncan, A.; Holtrop, G.; Louis, P. Effect of inulin on the human gut microbiota: Stimulation of Bifidobacterium adolescentis and Faecalibacterium prausnitzii. Br. J. Nutr. 2008, 101, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Le Bastard, Q.; Chapelet, G.; Javaudin, F.; Lepelletier, D.; Batard, E.; Montassier, E. The effects of inulin on gut microbial composition: A systematic review of evidence from human studies. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Brennan, C.S.; Kuri, V.; Tudorica, C.M. Inulin-enriched pasta: Effects on textural properties and starch degradation. Food Chem. 2004, 86, 189–193. [Google Scholar] [CrossRef]
- Shoaib, M.; Shehzad, A.; Omar, M.; Rakha, A.; Raza, H.; Sharif, H.R.; Shakeel, A.; Ansari, A.; Niazi, S. Inulin: Properties, health benefits and food applications. Carbohydr. Polym. 2016, 147, 444–454. [Google Scholar] [CrossRef]
- Romão, B.; Falcomer, A.L.; Palos, G.; Cavalcante, S.; Botelho, R.B.A.; Nakano, E.Y.; Raposo, A.; Shakeel, F.; Alshehri, S.; Mahdi, W.A.; et al. Glycemic Index of Gluten-Free Bread and Their Main Ingredients: A Systematic Review and Meta-Analysis. Foods 2021, 10, 506. [Google Scholar] [CrossRef]
- Brennan, C.S.; Tudorica, C.M. Evaluation of potential mechanisms by which dietary fibre additions reduce the predicted glycaemic index of fresh pastas. Int. J. Food Sci. Technol. 2008, 43, 2151–2162. [Google Scholar] [CrossRef]
- Kumar, M.; Nagpal, R.; Kumar, R.; Hemalatha, R.; Verma, V.; Kumar, A.; Chakraborty, C.; Singh, B.; Marotta, F.; Jain, S.; et al. Cholesterol-Lowering Probiotics as Potential Biotherapeutics for Metabolic Diseases. Exp. Diabetes Res. 2012, 2012, 902917. [Google Scholar] [CrossRef]
- Tako, E.; Glahn, R.P.; Knez, M.; Stangoulis, J.C. The effect of wheat prebiotics on the gut bacterial population and iron status of iron deficient broiler chickens. Nutr. J. 2014, 13, 58. [Google Scholar] [CrossRef]
- Caio, G.; Lungaro, L.; Segata, N.; Guarino, M.; Zoli, G.; Volta, U.; De Giorgio, R. Effect of Gluten-Free Diet on Gut Microbiota Composition in Patients with Celiac Disease and Non-Celiac Gluten/Wheat Sensitivity. Nutrients 2020, 12, 1832. [Google Scholar] [CrossRef] [PubMed]
- Vacca, M.; Porrelli, A.; Calabrese, F.M.; Lippolis, T.; Iacobellis, I.; Celano, G.; Pinto, D.; Russo, F.; Giannelli, G.; De Angelis, M. How Metabolomics Provides Novel Insights on Celiac Disease and Gluten-Free Diet: A Narrative Review. Front. Microbiol. 2022, 13, 859467. [Google Scholar] [CrossRef] [PubMed]
- Kareem, K.Y.; Hooi Ling, F.; Teck Chwen, L.; May Foong, O.; Anjas Asmara, S. Inhibitory activity of postbiotic produced by strains of Lactobacillus plantarum using reconstituted media supplemented with inulin. Gut Pathog. 2014, 6, 23. [Google Scholar] [CrossRef]
- Baxter, N.T.; Schmidt, A.W.; Venkataraman, A.; Kim, K.S.; Waldron, C.; Schmidt, T.M. Dynamics of Human Gut Microbiota and Short-Chain Fatty Acids in Response to Dietary Interventions with Three Fermentable Fibers. MBio 2019, 10, e02566-18. [Google Scholar] [CrossRef]
- Cosola, C.; De Angelis, M.; Rocchetti, M.T.; Montemurno, E.; Maranzano, V.; Dalfino, G.; Manno, C.; Zito, A.; Gesualdo, M.; Ciccone, M.M.; et al. Beta-Glucans Supplementation Associates with Reduction in P-Cresyl Sulfate Levels and Improved Endothelial Vascular Reactivity in Healthy Individuals. PLoS ONE 2017, 12, e0169635. [Google Scholar] [CrossRef]
- Saa, P.; Urrutia, A.; Silva-Andrade, C.; Martín, A.J.; Garrido, D. Modeling approaches for probing cross-feeding interactions in the human gut microbiome. Comput. Struct. Biotechnol. J. 2022, 20, 79–89. [Google Scholar] [CrossRef]

| Sample | OCT (min) | WAI (g/100 g) | SI | CL (g/100 g) | IL (g/100 g) |
|---|---|---|---|---|---|
| PC | 6.30 | 73.20 ± 0.01 a | 1.56 ± 0.03 a | 2.37 ± 0.05 d | - |
| P5 | 6.30 | 73.24 ± 0.10 a | 1.53 ± 0.04 ab | 2.70 ± 0.14 c | 1.01 ± 0.03 c |
| P10 | 6.45 | 72.51 ± 0.62 a | 1.48 ± 0.03 b | 3.11 ± 0.07 b | 1.45 ± 0.07 b |
| P15 | 6.45 | 73.24 ± 0.20 a | 1.47 ± 0.04 b | 3.62 ± 0.06 a | 2.19 ± 0.12 a |
| L* | a* | b* | Firmness | ||
|---|---|---|---|---|---|
| Raw pasta | PC | 79.65 ± 0.09 a | 2.21 ± 0.07 e | 33.57 ± 0.46 a | 18.25 ± 0.45 b |
| P5 | 74.57 ± 0.19 c | 3.21 ± 0.02 c | 30.49 ± 0.26 b | 19.63 ± 0.50 a | |
| P10 | 72.02 ± 0.38 e | 3.89 ± 0.10 b | 28.52 ± 0.19 c | 19.89 ± 0.05 a | |
| P15 | 70.72 ± 0.25 f | 4.48 ± 0.08 a | 27.62 ± 0.09 cd | 19.77 ± 0.23 a | |
| Cooked pasta | PC | 78.89 ± 0.43 b | 0.30 ± 0.02 f | 27.11 ± 0.56 d | 5.85 ± 0.36 d |
| P5 | 73.69 ± 0.18 d | 2.00 ± 0.11 e | 24.13 ± 0.41 e | 6.22 ± 0.26 d | |
| P10 | 72.24 ± 0.06 e | 2.82 ± 0.11 d | 24.18 ± 0.33 e | 7.37 ± 0.48 c | |
| P15 | 68.12 ± 012 g | 3.79 ± 0.10 b | 22.29 ± 0.43 f | 7.25 ± 0.37 c | |
| p-value | P *C | <0.0001 | <0.0001 | <0.0001 | <0.05 |
| Parameters | PC | P5 | P10 | P15 |
|---|---|---|---|---|
| Ash | 0.41 ± 0.02 b | 0.41 ± 0.01 b | 0.49 ± 0.01 a | 0.50 ± 0.01 a |
| Protein | 10.75 ± 0.03 a | 9.71 ± 0.44 b | 9.25 ± 0.02 b | 9.27 ± 0.08 b |
| Total dietary fiber | 1.47 ± 0.04 d | 3.44 ± 0.10 c | 8.16 ± 0.12 b | 12.69 ± 0.08 a |
| Lipid | 0.23 ± 0.02 a | 0.10 ± 0.01 b | 0.06 ± 0.01 c | 0.08 ± 0.01 c |
| Moisture | 28.21 ± 0.17 a | 27.00 ± 0.21 b | 26.98 ± 0.10 b | 28.24 ± 0.30 a |
| Carbohydrates | 58.94 ± 0.22 a | 59.34 ± 0.25 a | 55.07 ± 0.02 b | 49.20 ± 0.42 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Difonzo, G.; de Gennaro, G.; Caponio, G.R.; Vacca, M.; dal Poggetto, G.; Allegretta, I.; Immirzi, B.; Pasqualone, A. Inulin from Globe Artichoke Roots: A Promising Ingredient for the Production of Functional Fresh Pasta. Foods 2022, 11, 3032. https://doi.org/10.3390/foods11193032
Difonzo G, de Gennaro G, Caponio GR, Vacca M, dal Poggetto G, Allegretta I, Immirzi B, Pasqualone A. Inulin from Globe Artichoke Roots: A Promising Ingredient for the Production of Functional Fresh Pasta. Foods. 2022; 11(19):3032. https://doi.org/10.3390/foods11193032
Chicago/Turabian StyleDifonzo, Graziana, Giuditta de Gennaro, Giusy Rita Caponio, Mirco Vacca, Giovanni dal Poggetto, Ignazio Allegretta, Barbara Immirzi, and Antonella Pasqualone. 2022. "Inulin from Globe Artichoke Roots: A Promising Ingredient for the Production of Functional Fresh Pasta" Foods 11, no. 19: 3032. https://doi.org/10.3390/foods11193032
APA StyleDifonzo, G., de Gennaro, G., Caponio, G. R., Vacca, M., dal Poggetto, G., Allegretta, I., Immirzi, B., & Pasqualone, A. (2022). Inulin from Globe Artichoke Roots: A Promising Ingredient for the Production of Functional Fresh Pasta. Foods, 11(19), 3032. https://doi.org/10.3390/foods11193032