Microbiological Quality of Red Meat Offal Produced at Australian Export Establishments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Indicator Bacteria
2.3. Data Analysis
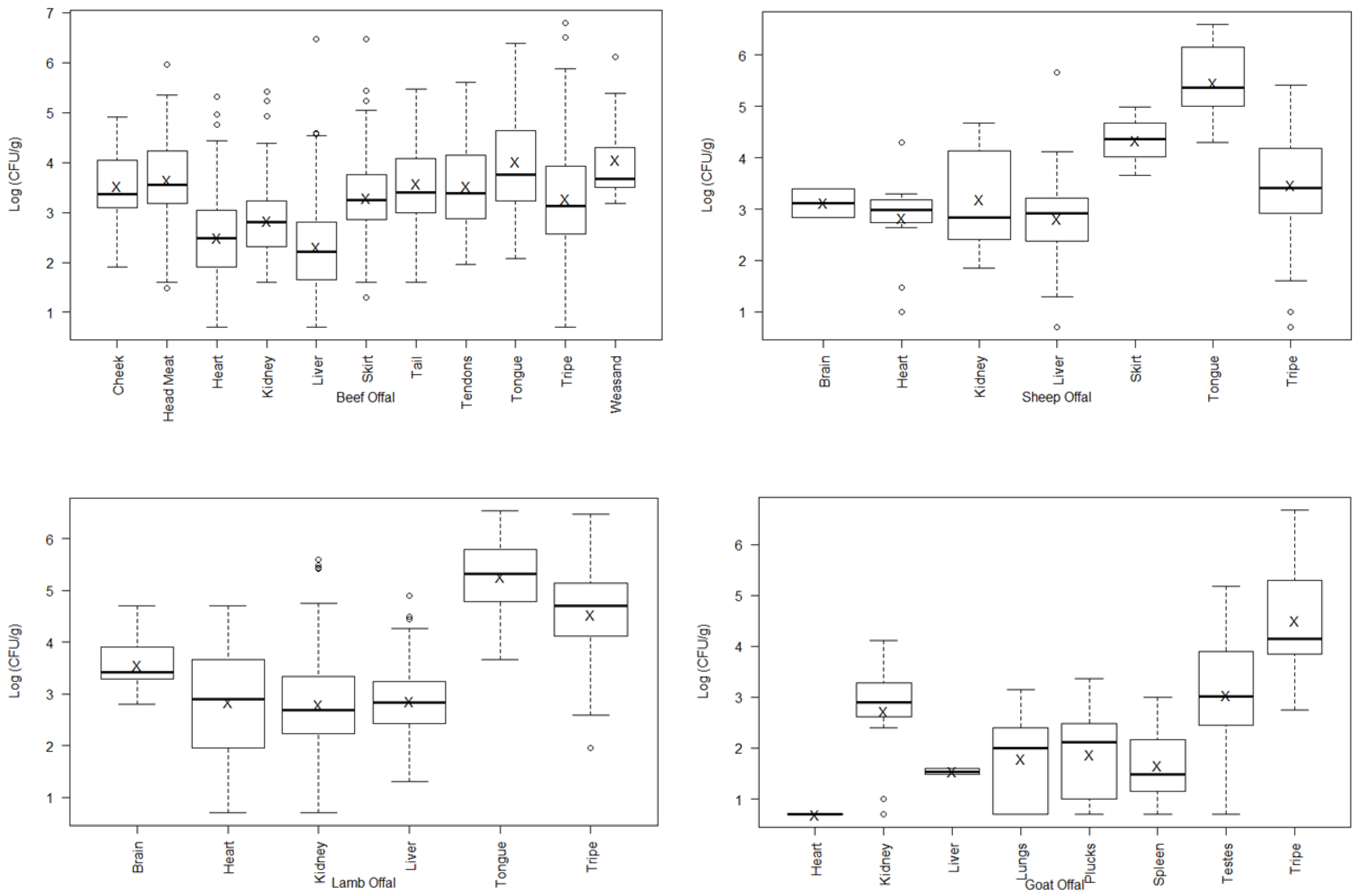
3. Results
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Department of Agriculture Water and the Environment. Red Meat Export Statistics. 2020. Available online: https://www.agriculture.gov.au/export/controlled-goods/meat/statistics (accessed on 15 September 2021).
- Gill, C.O.; Jones, S.D.M. Evaluation of a commercial process for collection and cooling of beef offals by a temperature function integration technique. Int. J. Food Microbiol. 1992, 15, 131–143. [Google Scholar] [CrossRef]
- Gill, C.O.; Jeremiah, L.E. The storage life of non-muscle offals packaged under vacuum or carbon dioxide. Food Microbiol. 1991, 8, 339–353. [Google Scholar] [CrossRef]
- Abd-El-Malek, A.M.; El-Khateib, T. Microbiological evaluation of some edible bovine by-products. Int. J. Curr. Microbiol. App. Sci. 2018, 7, 3449–3458. [Google Scholar] [CrossRef]
- Khalil, A.; Mousa, M.; El-Bahy, E. Sanitary condition of some raw edible beef offal. Alexandria J. Vet. Sci. 2018, 59, 165–172. [Google Scholar] [CrossRef]
- Cohen, N.; Ennaji, H.; Hassar, M.; Karib, H. The bacterial quality of red meat and offal in Casablanca (Morocco). Mol. Nutr. Food Res. 2006, 50, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Rivas, T.; Herrera, A.; Yanguela, J. Microbial and organoleptic qualities of lamb liver during storage at 0 or 3 °C. J. Food Protect. 1992, 55, 874–879. [Google Scholar] [CrossRef] [PubMed]
- Gill, C.O.; Harrison, J.C.L. Evaluation of the hygienic efficiency of offal cooling procedures. Food Microbiol. 1985, 2, 63–69. [Google Scholar] [CrossRef]
- Im, M.C.; Seo, K.W.; Bae, D.H.; Lee, Y.J. Bacterial quality and prevalence of foodborne pathogens in edible offal from slaughterhouses in Korea. J. Food Protect. 2016, 79, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Codex Alimentarius Commission. Principles and Guidelines for the Establishment and Application of Microbiological Criteria Related to Foods. Guideline CAC/GL 21-1997. 2013. Available online: https://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXG%2B21-1997%252FCXG_021e.pdf (accessed on 15 September 2021).
- Phillips, D.; Bridger, K.; Jenson, I.; Sumner, J. An Australian national survey of the microbiological quality of frozen boneless beef and beef primal cuts. J. Food Protect. 2012, 75, 1862–1866. [Google Scholar] [CrossRef] [PubMed]
- Phillips, D.; Tholath, S.; Jenson, I.; Sumner, J. Microbiological quality of Australian sheep meat in 2011. Food Control 2013, 31, 291–294. [Google Scholar] [CrossRef]
- ISO/IEC 17025:2005; General Requirements for the Competence of Testing and Calibration Laboratories. International Standards Organization: Geneva, Switzerland, 2005.
- Official Method 990.12, Aerobic Plate Count in Foods. Dry Rehydratable Film Method; Official Methods of Analysis of AOAC International; AOAC: Rockville, MD, USA, 2016.
- Official Method 998.08. Confirmed Escherichia coli Counts in Poultry, Meats, and Seafood. Dry Rehydratable Film Method; Official Methods of Analysis of AOAC International; AOAC: Rockville, MD, USA, 2002.
- Official Method 991.14, Coliform and Escherichia coli Counts in Foods. Dry Rehydratable Film Method; Official Methods of Analysis of AOAC International; AOAC: Rockville, MD, USA, 2002.
- ISO 6887-1:1999; Microbiology of Food and Animal Feeding Stuffs—Preparation of Test Samples, Initial Suspension and Decimal Dilutions for Microbiological Examination—Part 1: General Rules for the Preparation of the Initial Suspension and Decimal Dilutions. International Standards Organization: Geneva, Switzerland, 1999.
- R Core Team. R 3.5.0: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Spooncer, W.F.; Caballero, B. “Offal—Types of offal”. In Encyclopedia of Food Science and Nutrition, 2nd ed.; Caballero, B., Trugo, L.C., Finglas, P.M., Eds.; Academic Press: Oxford, UK, 2003; pp. 442–455. [Google Scholar]
- Delmore, R.J.; Sofos, J.N.; Belk, K.E.; Lloyd, W.R.; Bellinger, G.L.; Schmidt, G.R.; Smith, G.C. Good manufacturing practices for improving the microbiological quality of beef variety meats. Dairy Food Environ. Sanit. 1999, 19, 742–752. [Google Scholar]
- Yang, X. Microbial ecology of beef carcasses and beef products. In Quantitative Microbiology in Food Processing; Wiley: Hoboken, NJ, USA, 2017; pp. 442–462. [Google Scholar]
- Dykes, G.A. Laboratory-based simulation of freezing profiles of beef trim for Escherichia coli O157 survival determinations. J. Microbiol. Methods 2006, 64, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Sheridan, J.J.; Lynch, B. The influence of processing and refrigeration on the bacterial numbers on beef and sheep offals. Meat Sci. 1988, 24, 143–150. [Google Scholar] [CrossRef]
- Department of Agriculture Water and the Environment. Guideline for the Application of the Refrigeration Index to Refrigeration of Meat and Meat Products. 20-06. Meat Notice. 2020. Available online: https://www.agriculture.gov.au/export/controlled-goods/meat/elmer-3/notices/2020 (accessed on 15 September 2021).
- Pointon, A.; Hamilton, D.; Kiermeier, A. Assessment of the post-mortem inspection of beef, sheep, goats and pigs in Australia: Approach and qualitative risk-based results. Food Control 2018, 90, 222–232. [Google Scholar] [CrossRef]
- Bell, R.G.; Mills, J.; Moorhead, S.M.; Haines, J.M.; Withers, K.M.; Forkert, G.D. Hygienic Status of Seven Internationally Traded Beef Offals; Meat Industry Research Institute of New Zealand: Palmerston North, New Zealand, 2000. [Google Scholar]
- Jolley, J.; Kiermeier, A.; Sumner, J. Process Monitoring for the Australian Meat Industry—A Comparative Industry Trial; Australian Meat Processor Corporation: Sydney, Australia, 2019. [Google Scholar]
- Hernandez-Jover, M.; Culley, F.; Heller, J.; Ward, M.P.; Jenson, I. Semi-quantitative food safety risk profile of the Australian red meat industry. Int. J. Food Microbiol. 2021, 353, 109294. [Google Scholar] [CrossRef] [PubMed]
| Species | ||||
|---|---|---|---|---|
| Offal Type | Beef | Sheep | Lamb | Goat |
| Brain | - a | 2 | 25 | - |
| Cheek | 75 | - | - | - |
| Head Meat | 89 | - | - | - |
| Heart | 101 | 13 | 26 | 3 |
| Kidney | 60 | 20 | 133 | 23 |
| Liver | 107 | 32 | 110 | 2 |
| Lungs | - | - | - | 13 |
| Pluck | - | - | - | 14 |
| Skirt | 115 | 3 | - | - |
| Spleen | - | - | - | 16 |
| Tail | 102 | - | - | - |
| Tendons | 54 | - | - | - |
| Testes | - | - | - | 22 |
| Tongue | 103 | 10 | 107 | - |
| Tripe | 156 | 80 | 85 | 42 |
| Weasand | 13 | - | - | - |
| Indicator/ Species | Prevalence | Average log10 CFU/g 1 | |||
|---|---|---|---|---|---|
| Coliforms | |||||
| Beef | 30.5% | a | 1.54 ± 0.64 | ||
| Sheep | 38.1% | a, b | 1.65 ± 0.63 | ||
| Lamb | 37.4% | b | 1.67 ± 0.71 | ||
| Goat | 43.0% | b | 2.05 ± 0.78 | ||
| E. coli | |||||
| Beef | 15.4% | a | 1.42 ± 0.63 | ||
| Sheep | 28.1% | b | 1.52 ± 0.57 | ||
| Lamb | 17.5% | a | 1.44 ± 0.59 | ||
| Goat | 39.3% | c | 1.82 ± 0.62 | ||
| APC | |||||
| Beef | 99.0% | a | 3.25 ± 1.06 | ||
| Sheep | 98.8% | a | 3.38 ± 1.09 | ||
| Lamb | 99.0% | a | 3.70 ± 1.34 | ||
| Goat | 85.9% | b | 2.97 ± 1.53 |
| Offal Type | log10 APC 1 | |||||||
|---|---|---|---|---|---|---|---|---|
| Beef | Lamb | Sheep | Goat | |||||
| Liver | 2.30 ± 1.01 | b | 2.86 ± 0.62 | a | 2.81 ± 0.90 | b | ||
| Heart | 2.50 ± 0.91 | b, c | 2.83 ± 1.02 | a | ||||
| Kidney | 2.83 ± 0.82 | c | 2.80 ± 1.01 | a | 3.18 ± 0.93 | a, b | 2.73 ± 1.02 | a |
| Tripe | 3.28 ± 1.14 | a | 4.54 ± 0.95 | 3.46 ± 0.97 | a | 4.51 ± 1.03 | ||
| Skirt | 3.29 ± 0.83 | a | ||||||
| Cheek | 3.53 ± 0.62 | a | ||||||
| Tendons | 3.53 ± 0.95 | a | ||||||
| Tail | 3.57 ± 0.78 | a | ||||||
| Head Meat | 3.65 ± 0.81 | a, d | ||||||
| Tongue | 4.01 ± 1.05 | d | 5.26 ± 0.74 | |||||
| Weasand | 4.06 ± 0.84 | a, d | ||||||
| Brain | 3.55 ± 0.40 | |||||||
| Pluck | 1.83 ± 0.91 | b | ||||||
| Testes | 3.05 ± 1.26 | a | ||||||
| Other | 3.95 ± 1.42 | a | 1.51 ± 0.73 | b | ||||
| Offal Type | Beef | Lamb | Sheep | Goat | ||||
|---|---|---|---|---|---|---|---|---|
| Prevalence | Count 1 | Prevalence | Count 1 | Prevalence | Count 1 | Prevalence | Count 1 | |
| Liver | 9.3% | 1.69 ± 0.87 | 17.3% | 1.31 ± 0.46 | 9.4% | 1.91 ±0.82 | ||
| Heart | 5.9% | 1.61 ± 0.99 | 0% | - 2 | ||||
| Kidney | 8.3% | 1.45 ± 0.62 | 4.5% | 1.40 ± 0.44 | 45% | 1.36 ± 0.51 | 26.1% | 1.35 ± 0.33 |
| Tripe | 10.9% | 1.77 ± 0.99 | 29.4% | 1.52 ± 0.73 | 30% | 1.57 ± 0.61 | 76.2% | 1.87 ± 0.61 |
| Skirt | 19.1% | 1.39 ± 0.70 | ||||||
| Cheek | 17.3% | 1.23 ± 0.36 | ||||||
| Tendons | 9.3% | 1.37 ± 0.67 | ||||||
| Tail | 20.6% | 1.26 ± 0.28 | ||||||
| Head Meat | 32.6% | 1.42 ± 0.52 | ||||||
| Tongue | 15.5% | 1.24 ± 0.34 | 29% | 1.48 ± 0.59 | ||||
| Weasand | 46.2% | 1.41 ± 0.40 | ||||||
| Brain | 16% | 1.27 ± 0.32 | ||||||
| Pluck | 3.7% | 2.76 3 | ||||||
| Testes | 60.9% | 1.85 ± 0.66 | ||||||
| Other | 32.1% | 1.42 ± 0.43 | 0% | - 2 | ||||
| Offal Type | Average log10 CFU/g 1 | ||
|---|---|---|---|
| Chilled | Frozen | Difference | |
| Tripe | 4.49 ± 0.71 | 3.69 ± 1.20 | 0.80 |
| Tongue | 5.05 ± 1.09 | 4.46 ± 1.04 | 0.58 |
| Kidney | 3.19 ± 0.94 | 2.63 ± 0.91 | 0.56 |
| Liver | 2.90 ± 0.77 | 2.47 ± 0.91 | 0.43 |
| Skirt | 3.29 ± 1.03 | 3.33 ± 0.73 | −0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vanderlinde, P.; Horchner, P.; Huynh, L.; Jenson, I. Microbiological Quality of Red Meat Offal Produced at Australian Export Establishments. Foods 2022, 11, 3007. https://doi.org/10.3390/foods11193007
Vanderlinde P, Horchner P, Huynh L, Jenson I. Microbiological Quality of Red Meat Offal Produced at Australian Export Establishments. Foods. 2022; 11(19):3007. https://doi.org/10.3390/foods11193007
Chicago/Turabian StyleVanderlinde, Paul, Peter Horchner, Long Huynh, and Ian Jenson. 2022. "Microbiological Quality of Red Meat Offal Produced at Australian Export Establishments" Foods 11, no. 19: 3007. https://doi.org/10.3390/foods11193007
APA StyleVanderlinde, P., Horchner, P., Huynh, L., & Jenson, I. (2022). Microbiological Quality of Red Meat Offal Produced at Australian Export Establishments. Foods, 11(19), 3007. https://doi.org/10.3390/foods11193007






