Antimicrobial Resistance in the Food Chain: Trends, Mechanisms, Pathways, and Possible Regulation Strategies
Abstract
1. Introduction
2. Trends in AMR
3. Mechanisms in AMR
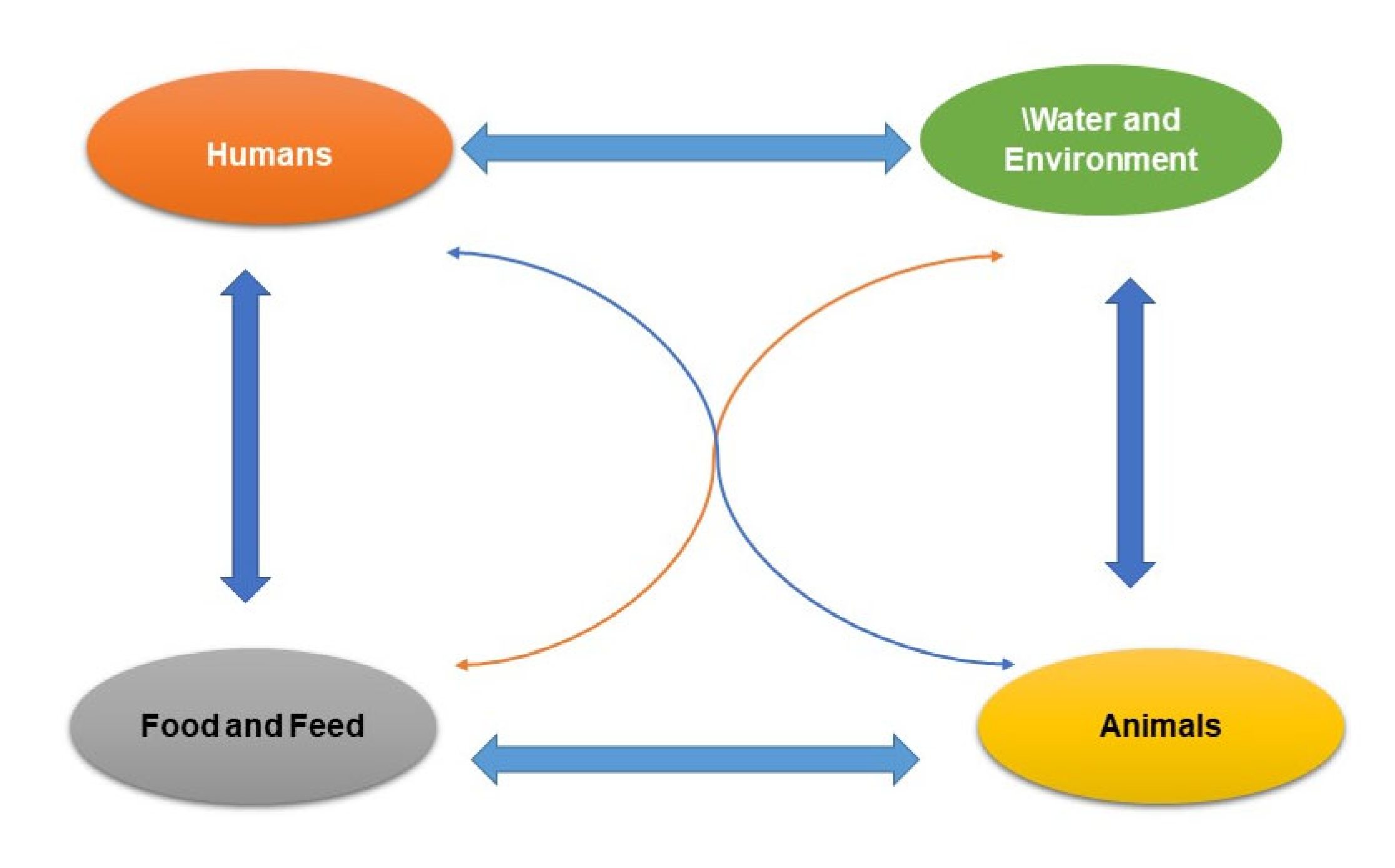
4. Pathways of AMR in the Food Chain
4.1. Spread through Foods of Animal Origin
4.2. Spread Associated with Non-Animal Origin Foods
4.3. Environment and Water Spread of AMRB
4.4. Spread-Associated Food Handlers and Food Contact Workers
5. Correlation between AMR and Usage of Antimicrobials
6. Status of AMR in the Food Chain
7. Microbes Displaying Resistance
7.1. Carbapenem-Resistant Acinetobacter (CRA)
7.2. Carbapenem-Resistant Enterobacteriaceae (CRE)
7.3. Drug-Resistant Campylobacter
7.4. ESBL Producing Enterobacteriaceae
7.5. Vancomycin-Resistant Enterococcus (VRE)
7.6. Methicillin Resistant S. aureus
7.7. Drug-Resistant Non-Typhoidal Salmonella
8. Strategies to Regulate AMR
8.1. Increase Awareness of AMR via Active Communication, Education, and Training
8.2. Support Knowledge through Observation
8.3. Decrease the Occurrence of Infectious Diseases through Cleanliness, Hygiene, and Preventive Measures
8.4. Regulate the Use of Antimicrobials in Human and Animal Health
8.5. Improve Economic Situation for Sustainable and Increased Investment in Novel Drugs, Diagnostic Tools, and Vaccines
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMR | Antimicrobial resistance |
| AMRB | AMR bacteria |
| LMICs | low- and middle-income countries |
| GLASS | Global Antimicrobial Resistance Surveillance System |
| NARMS | National Antimicrobial Resistance Monitoring System |
| OIE | World Organization for Animal Health |
| FAO | Food and Agriculture Organization |
| WHO | World Health Organization |
| HGT | Horizontal gene transfer |
| VGT | Vertical gene transfer |
| MRSA | Methicillin-resistant S. aureus |
| ESβL | Extended spectrum-β-lactamase |
| MCR-1 | Mobilized colistin resistance-1 |
| CRA | Carbapenem-resistant Acinetobacter |
| CRE | Carbapenem-resistant Enterobacteriaceae |
| CDC | Centers for Disease Control and Prevention |
| COIPARS | Colombian Integrated Program for Antimicrobial Resistance Surveillance |
References
- Nathan, C.; Cars, O. Antibiotic resistance—Problems, progress, and prospects. N. Engl. J. Med. 2014, 371, 1761–1763. [Google Scholar] [CrossRef] [PubMed]
- Nathan, C. Antibiotics at the crossroads. Nature 2004, 431, 899–902. [Google Scholar] [CrossRef]
- World Health Organization (WHO). The Evolving Threat of Antimicrobial Resistance: Options for Action. Geneva. 2012. Available online: http://apps.who.int/iris/bitstream/10665/44812/1/9789241503181_eng.pdf (accessed on 20 August 2020).
- Swann, M.M. Use of Antibiotics in Animal Husbandry and Veterinary Medicine; Stationery Office: London, UK, 1969; Volume 791, pp. cc1525–1513. Available online: https://api.parliament.uk/historic-hansard/commons/1969/nov/20/use-of-antibiotics-in-animal-husbandry (accessed on 21 August 2020).
- Vikesland, P.; Garner, E.; Gupta, S.; Kang, S.; Maile-Moskowitz, A.; Zhu, N. Differential Drivers of Antimicrobial Resistance across the World. Accounts Chem. Res. 2019, 52, 916–924. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Antimicrobial Resistance; WHO: Geneva, Switzerland, 2020; Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 20 April 2020).
- Aslam, B.; Wang, W.; Arshad, M.I.; Khurshid, M.; Muzammil, S.; Nisar, M.A.; Alvi, R.F.; Aslam, M.A.; Qamar, M.U.; Salamat, M.K.F.; et al. Antibiotic resistance: A rundown of a global crisis. Infect. Drug Resist. 2018, 11, 1645–1658. [Google Scholar] [CrossRef] [PubMed]
- Depoorter, P.; Persoons, D.; Uyttendaele, M.; Butaye, P.; De Zutter, L.; Dierick, K.; Herman, L.; Imberechts, H.; Van Huffel, X.; Dewulf, J. Assessment of human exposure to 3rd generation cephalosporin resistant E. coli (CREC) through consumption of broiler meat in Belgium. Int. J. Food Microbiol. 2012, 159, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Van Boxstael, S.; Dierick, K.; Van Huffel, X.; Uyttendaele, M.; Berkvens, D.; Herman, L.; Bertrand, S.; Wildemauwe, C.; Catry, B.; Butaye, P.; et al. Comparison of antimicrobial resistance patterns and phage types of Salmonella Typhimurium isolated from pigs, pork and humans in Belgium between 2001 and 2006. Food Res. Int. 2012, 45, 913–918. [Google Scholar] [CrossRef]
- Van Boeckel, T.P.; Brower, C.; Gilbert, M.; Grenfell, B.T.; Levin, S.A.; Robinson, T.P.; Teillant, A.; Laxminarayan, R. Global trends in antimicrobial use in food animals. Proc. Natl. Acad. Sci. USA 2015, 112, 5649–5654. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Lack of New Antibiotics Threatens Global Efforts to Contain Drug-Resistant Infections. 2020. Available online: https://www.who.int/news-room/detail/17-01-2020-lack-of-new-antibiotics-threatens-global-efforts-to-contain-drug-resistant-infections (accessed on 21 April 2020).
- World Health Organization (WHO). Antimicrobial Resistance—Global Report on Surveillance; WHO: Geneva, Switzerland, 2014. [Google Scholar]
- Carattoli, A. Animal reservoirs for extended spectrum β-lactamase producers. Clin. Microbiol. Infect. 2008, 14, 117–123. [Google Scholar] [CrossRef]
- Mayrhofer, S.; Paulsen, P.; Smulders, F.J.; Hilbert, F. Short Communication: Antimicrobial Resistance in Commensal Escherichia coli Isolated from Muscle Foods as Related to the Veterinary Use of Antimicrobial Agents in Food-Producing Animals in Austria. Microb. Drug Resist. 2006, 12, 278–283. [Google Scholar] [CrossRef]
- Silbergeld, E.K.; Graham, J.; Price, L.B. Industrial Food Animal Production, Antimicrobial Resistance, and Human Health. Annu. Rev. Public Health 2008, 29, 151–169. [Google Scholar] [CrossRef]
- Srinivasan, V.; Nam, H.-M.; Sawant, A.A.; Headrick, S.I.; Nguyen, L.T.; Oliver, S.P. Distribution of Tetracycline and Streptomycin Resistance Genes and Class 1 Integrons in Enterobacteriaceae Isolated from Dairy and Nondairy Farm Soils. Microb. Ecol. 2007, 55, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Stine, O.C.; Johnson, J.A.; Keefer-Norris, A.; Perry, K.L.; Tigno, J.; Qaiyumi, S.; Stine, M.S.; Morris, J.G., Jr. Widespread distribution of tetracycline resistance genes in a confined animal feeding facility. Int. J. Antimicrob. Agents 2007, 29, 348–352. [Google Scholar] [CrossRef]
- Zirakzadeh, A.; Patel, R. Epidemiology and mechanisms of glycopeptide resistance in enterococci. Curr. Opin. Infect. Dis. 2005, 18, 507–512. [Google Scholar] [CrossRef]
- Chang, Q.; Wang, W.; Regev-Yochay, G.; Lipsitch, M.; Hanage, W.P. Antibiotics in agriculture and the risk to human health: How worried should we be? Evol. Appl. 2014, 8, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Walsh, C.; Duffy, G.; Nally, P.; O’Mahony, R.; McDowell, D.; Fanning, S. Transfer of ampicillin resistance from Salmonella Typhimurium DT104 to Escherichia coli K12 in food. Lett. Appl. Microbiol. 2007, 46, 210–215. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO). The Fao Action Plan on Antimicrobial Resistance 2016–2020. 2016. Available online: http://www.fao.org/3/a-i5996e.pdf (accessed on 25 April 2020).
- World Health Organization (WHO). Global Action Plan on Antimicrobial Resistance; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Holmes, A.H.; Moore, L.S.P.; Sundsfjord, A.; Steinbakk, M.; Regmi, S.; Karkey, A.; Guerin, P.J.; Piddock, L.J.V. Understanding the mechanisms and drivers of antimicrobial resistance. Lancet 2016, 387, 176–187. [Google Scholar] [CrossRef]
- Molnar, A. Antimicrobial Resistance Awareness and Games. Trends Microbiol. 2018, 27, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Apata, D. Antibiotic Resistance in Poultry. Int. J. Poult. Sci. 2009, 8, 404–408. [Google Scholar] [CrossRef]
- Theuretzbacher, U. Global antibacterial resistance: The never-ending story. J. Glob. Antimicrob. Resist. 2013, 1, 63–69. [Google Scholar] [CrossRef]
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations; Review on Antimicrobial Resistance: London, UK, 2016; Volume 10, pp. 1–84. Available online: https://amr-review.org/sites/default/files/160518_Final%20paper_with%20cover.pdf (accessed on 5 June 2020).
- UNEP. United Nations Environment Programme. Environmental Dimensions of Antimicrobial Resistance: Summary for Policymakers. 2022. Available online: https://wedocs.unep.org/bitstream/handle/20.500.11822/38373/antimicrobial_R.pdf (accessed on 6 September 2022).
- Quadri, F.; Mazer-Amirshahi, M.; Fox, E.R.; Hawley, K.L.; Pines, J.M.; Zocchi, M.S.; May, L. Antibacterial Drug Shortages From 2001 to 2013: Implications for Clinical Practice. Clin. Infect. Dis. 2015, 60, 1737–1742. [Google Scholar] [CrossRef]
- Laxminarayan, R.; Duse, A.; Wattal, C.; Zaidi, A.K.M.; Wertheim, H.F.L.; Sumpradit, N.; Vlieghe, E.; Hara, G.L.; Gould, I.M.; Goossens, H.; et al. Antibiotic resistance—the need for global solutions. Lancet Infect. Dis. 2013, 13, 1057–1098. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Prioritization of Pathogens to Guide Discovery, Research and Development of New Antibiotics for Drug-Resistant Bacterial Infections Including Tuberculosis; World Health Organization: Geneva, Switzerland, 2017; Available online: https://www.who.int/medicines/areas/rational_use/PPLreport_2017_09_19.pdf?ua=1 (accessed on 25 May 2020).
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- The World Bank. Drug Resistant Infections: A Threat to Our Economic Future; The World Bank: Washington, DC, USA, 2017; Available online: http://documents1.worldbank.org/curated/en/323311493396993758/pdf/final-report.pdf (accessed on 30 May 2020).
- Zou, S.; Xu, W.; Zhang, R.; Tang, J.; Chen, Y.; Zhang, G. Occurrence and distribution of antibiotics in coastal water of the Bohai Bay, China: Impacts of river discharge and aquaculture activities. Environ. Pollut. 2011, 159, 2913–2920. [Google Scholar] [CrossRef] [PubMed]
- Miranda, C.D.; Kehrenberg, C.; Ulep, C.; Schwarz, S.; Roberts, M.C. Diversity of Tetracycline Resistance Genes in Bacteria from Chilean Salmon Farms. Antimicrob. Agents Chemother. 2003, 47, 883–888. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.; Davies, D. Origins and Evolution of Antibiotic Resistance. Microbiol. Mol. Biol. Rev. 2010, 74, 417–433. [Google Scholar] [CrossRef] [PubMed]
- González-Zorn, B.; Escudero, J.A. Ecology of antimicrobial resistance: Humans, animals, food and environment. Int. Microbiol. 2012, 15, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Verraes, C.; Van Boxstael, S.; Van Meervenne, E.; Van Coillie, E.; Butaye, P.; Catry, B.; de Schaetzen, M.-A.; Van Huffel, X.; Imberechts, H.; Dierick, K.; et al. Antimicrobial Resistance in the Food Chain: A Review. Int. J. Environ. Res. Public Health 2013, 10, 2643–2669. [Google Scholar] [CrossRef]
- Carattoli, A. Importance of integrons in the diffusion of resistance. Veter. Res. 2001, 32, 243–259. [Google Scholar] [CrossRef]
- Wright, G.D. Aminoglycoside-modifying enzymes. Curr Opin Microbiol. 1999, 2, 499–503. [Google Scholar] [CrossRef]
- Aminov, R.I. The role of antibiotics and antibiotic resistance in nature. Environ. Microbiol. 2009, 11, 2970–2988. [Google Scholar] [CrossRef]
- Kothari, C.; Gaind, R.; Singh, L.C.; Sinha, A.; Kumari, V.; Arya, S.; Chellani, H.; Saxena, S.; Deb, M. Community acquisition of β-lactamase producing Enterobacteriaceae in neonatal gut. BMC Microbiol. 2013, 13, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Andersson, D.I.; Hughes, D. Microbiological effects of sublethal levels of antibiotics. Nat. Rev. Microbiol. 2014, 12, 465–478. [Google Scholar] [CrossRef] [PubMed]
- Drlica, K.; Zhao, X. DNA gyrase, topoisomerase IV, and the 4-quinolones. Microbiol. Mol. Biol. Rev. 1997, 61, 377–392. [Google Scholar] [PubMed]
- Santiago-Rodriguez, T.M.; Fornaciari, G.; Luciani, S.; Dowd, S.; Toranzos, G.A.; Marota, I.; Cano, R.J. Gut Microbiome of an 11th Century A.D. Pre-Columbian Andean Mummy. PLoS ONE 2015, 10, e0138135. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control (ECDC). Antimicrobial resistance surveillance in Europe 2012. Annual Report of the European Antimicrobial Resistance Surveillance Network (EARS-Net). Stockholm. 2012. Available online: https://www.ecdc.europa.eu/sites/default/files/media/en/publications/Publications/antimicrobial-resistance-surveillance-europe-2012.pdf (accessed on 30 May 2020).
- Redgrave, L.S.; Sutton, S.B.; Webber, M.A.; Piddock, L.J. Fluoroquinolone resistance: Mechanisms, impact on bacteria, and role in evolutionary success. Trends Microbiol. 2014, 22, 438–445. [Google Scholar] [CrossRef]
- Bell, B.G.; Schellevis, F.; Stobberingh, E.; Goossens, H.; Pringle, M. A systematic review and meta-analysis of the effects of antibiotic consumption on antibiotic resistance. BMC Infect. Dis. 2014, 14, 13. [Google Scholar] [CrossRef]
- McMurry, L.; Petrucci Jr, R.E.; Levy, S.B. Active efflux of tetracycline encoded by four genetically different tetracycline resistance determinants in Escherichia coli. Proc. Natl. Acad. Sci. USA 1980, 77, 3974–3977. [Google Scholar] [CrossRef]
- Viseur, N.; Lambert, M.L.; Delmée, M.; Van Broeck, J.; Catry, B. Nosocomial and non-nosocomial Clostridium difficile infections in hospitalised patients in Belgium—Compulsory surveillance data from 2008 to 2010. Eurosurveillance 2011, 16, 20000. [Google Scholar] [CrossRef]
- Catry, B.; Croubels, S.; Schwarz, S.; Deprez, P.; Cox, B.; Kehrenberg, C.; Opsomer, G.; Decostere, A.; Haesebrouck, F. Influence of systemic fluoroquinolone administration on the presence of Pasteurella multocida in the upper respiratory tract of clinically healthy calves. Acta Veter-Scand. 2008, 50, 36. [Google Scholar] [CrossRef]
- Acar, J.F.; Moulin, G. Antimicrobial resistance at farm level. Rev. Sci. Tech. (Int. Off. Epizoot.) 2006, 25, 775–792. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Foodborne antimicrobial resistance as a biological hazard-Scientific Opinion of the Panel on Biological Hazards. EFSA J. 2008, 6, 765. [Google Scholar] [CrossRef]
- Rosenquist, H.; Smidt, L.; Andersen, S.R.; Jensen, G.B.; Wilcks, A. Occurrence and significance of Bacillus cereus and Bacillus thuringiensis in ready-to-eat food. FEMS Microbiol. Lett. 2005, 250, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Meldrum, R.J.; Ribeiro, C.D.; Smith, R.M.M.; Walker, A.M.; Simmons, M.; Worthington, D.; Edwards, C. Microbiological Quality of Ready-to-Eat Foods: Results from a Long-Term Surveillance Program (1995 through 2003). J. Food Prot. 2005, 68, 1654–1658. [Google Scholar] [CrossRef] [PubMed]
- Luber, P.; Brynestad, S.; Topsch, D.; Scherer, K.; Bartelt, E. Quantification of Campylobacter Species Cross-Contamination during Handling of Contaminated Fresh Chicken Parts in Kitchens. Appl. Environ. Microbiol. 2006, 72, 66–70. [Google Scholar] [CrossRef]
- Bohaychuk, V.M.; Bradbury, R.W.; Dimock, R.; Fehr, M.; Gensler, G.E.; King, R.K.; Rieve, R.; Barrios, P.R. A Microbiological Survey of Selected Alberta-Grown Fresh Produce from Farmers’ Markets in Alberta, Canada. J. Food Prot. 2009, 72, 415–420. [Google Scholar] [CrossRef]
- Hansen, T.B.; Christensen, B.B.; Aabo, S. Salmonella in Pork Cuttings in Supermarkets and Butchers’ Shops in Denmark in 2002 and 2006. Zoonoses Public Health 2010, 57, 23–29. [Google Scholar] [CrossRef]
- Losio, M.; Pavoni, E.; Bilei, S.; Bertasi, B.; Bove, D.; Capuano, F.; Farneti, S.; Blasi, G.; Comin, D.; Cardamone, C.; et al. Microbiological survey of raw and ready-to-eat leafy green vegetables marketed in Italy. Int. J. Food Microbiol. 2015, 210, 88–91. [Google Scholar] [CrossRef]
- European Food Safety Authority, European Centre for Disease Prevention and Control. Multi-country outbreak of Salmonella Stanley infections Update. EFSA J. 2012, 10, 2893. [Google Scholar] [CrossRef]
- Joint Ecdc–Efsa Rapid Outbreak Assessment. Multi-country outbreak of Salmonella Stanley infections. 2014. Available online: https://www.ecdc.europa.eu/sites/default/files/media/en/publications/Publications/salmonella-stanley-multi-country-outbreak-assessment-8-May-2014.pdf (accessed on 30 May 2020).
- Price, L.B.; Stegger, M.; Hasman, H.; Aziz, M.; Larsen, J.; Andersen, P.S.; Pearson, T.; Waters, A.E.; Foster, J.T.; Schupp, J.; et al. Staphylococcus aureus CC398: Host Adaptation and Emergence of Methicillin Resistance in Livestock. MBio 2012, 3, e00305-11. [Google Scholar] [CrossRef]
- Coetzee, J.; Corcoran, C.; Prentice, E.; Moodley, M.; Mendelson, M.; Poirel, L.; Nordmann, P.; Brink, A.J. Emergence of plasmid-mediated colistin resistance (MCR-1) among Escherichia coli isolated from South African patients. S. Afr. Med. J. 2016, 106, 449–450. [Google Scholar] [CrossRef]
- Liu, Y.-Y.; Wang, Y.; Walsh, T.R.; Yi, L.-X.; Zhang, R.; Spencer, J.; Doi, Y.; Tian, G.; Dong, B.; Huang, X.; et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: A microbiological and molecular biological study. Lancet Infect. Dis. 2016, 16, 161–168. [Google Scholar] [CrossRef]
- Tate, H.; Ayers, S.; Nyirabahizi, E.; Li, C.; Borenstein, S.; Young, S.; Rice-Trujillo, C.; Saint Fleurant, S.; Bodeis-Jones, S.; Li, X.; et al. Prevalence of Antimicrobial Resistance in Select Bacteria from Retail Seafood—United States, 2019. Front Microbiol. 2022, 13, 928509. [Google Scholar] [CrossRef] [PubMed]
- Watts, J.E.M.; Schreier, H.J.; Lanska, L.; Hale, M.S. The Rising Tide of Antimicrobial Resistance in Aquaculture: Sources, Sinks and Solutions. Mar. Drugs 2017, 15, 158. [Google Scholar] [CrossRef] [PubMed]
- Marshall, B.M.; Levy, S.B. Food Animals and Antimicrobials: Impacts on Human Health. Clin. Microbiol. Rev. 2011, 24, 718–733. [Google Scholar] [CrossRef]
- European Food Safety Authority, European Centre for Disease Prevention and Control. EU Summary Report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2013. EFSA J. 2015, 13, 4036. [Google Scholar]
- Duse, A.; Waller, K.P.; Emanuelson, U.; Unnerstad, H.E.; Persson, Y.; Bengtsson, B. Occurrence and Spread of Quinolone-Resistant Escherichia coli on Dairy Farms. Appl. Environ. Microbiol. 2016, 82, 3765–3773. [Google Scholar] [CrossRef]
- Directorate, V.M. UK One Health Report. Joint Report on Antibiotic Use and Antibiotic Resistance, 2013–2017. New Haw, Addlestone. 2019. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/775075/One_Health_Report_2019_v45.pdf (accessed on 30 May 2020).
- Catry, B.; Dewulf, J.; Maes, D.; Pardon, B.; Callens, B.; Vanrobaeys, M.; Opsomer, G.; De Kruif, A.; Haesebrouck, F. Effect of Antimicrobial Consumption and Production Type on Antibacterial Resistance in the Bovine Respiratory and Digestive Tract. PLoS ONE 2016, 11, e0146488. [Google Scholar] [CrossRef]
- EFSA Panel on Biological Hazards (BIOHAZ). Scientific Opinion on the risk posed by pathogens in food of non-animal origin. Part 1 (outbreak data analysis and risk ranking of food/pathogen combinations). EFSA J. 2013, 11, 3025. [Google Scholar] [CrossRef]
- Young, J.S.; Gormley, E.; Wellington, E.M.H. Molecular Detection of Mycobacterium bovis and Mycobacterium bovis BCG (Pasteur) in Soil. Appl. Environ. Microbiol. 2005, 71, 1946–1952. [Google Scholar] [CrossRef]
- Mannion, C.; Leonard, F.C.; Lynch, P.B.; Egan, J. Efficacy of cleaning and disinfection on pig farms in Ireland. Vet. Rec. 2007, 161, 371–375. [Google Scholar] [CrossRef]
- Wellington, E.M.H.; Boxall, A.B.A.; Cross, P.; Feil, E.J.; Gaze, W.H.; Hawkey, P.M.; Johnson-Rollings, A.S.; Jones, D.L.; Lee, N.M.; Otten, W.; et al. The role of the natural environment in the emergence of antibiotic resistance in Gram-negative bacteria. Lancet Infect. Dis. 2013, 13, 155–165. [Google Scholar] [CrossRef]
- Hartung, J.; Seedorf, J.; Trickl, T.; Gronauer, H. Emission of particulates from a pig farm with central air exhaust in the pig stall. DTW. Dtsch. Tierarztl. Wochenschr. 1998, 105, 244–245. [Google Scholar] [PubMed]
- Schmithausen, R.M.; Schulze-Geisthoevel, S.V.; Stemmer, F.; El-Jade, M.; Reif, M.; Hack, S.; Meilaender, A.; Montabauer, G.; Fimmers, R.; Parčina, M.; et al. Analysis of Transmission of MRSA and ESBL-E among Pigs and Farm Personnel. PLoS ONE 2015, 10, e0138173. [Google Scholar] [CrossRef] [PubMed]
- Schmithausen, R.M.; Kellner, S.R.; Schulze-Geisthoevel, S.V.; Hack, S.; Engelhart, S.; Bodenstein, I.; Al-Sabti, N.; Reif, M.; Fimmers, R.; Körber-Irrgang, B.; et al. Eradication of Methicillin-Resistant Staphylococcus aureus and of Enterobacteriaceae Expressing Extended-Spectrum Beta-Lactamases on a Model Pig Farm. Appl. Environ. Microbiol. 2015, 81, 7633–7643. [Google Scholar] [CrossRef]
- Hartung, J.; Whyte, R.T. Erfassung und Bewertung von Luftverunreinigungen in der Nutztierhaltung. Atemwegs-Lungenkr 1994, 20, 17–25. [Google Scholar]
- Weese, J.S.; DaCosta, T.; Button, L.; Goth, K.; Ethier, M.; Boehnke, K. Isolation of methicillin-resistant Staphylococcus aureus from the environment in a veterinary teaching hospital. J. Vet. Int. Med. 2004, 18, 468–470. [Google Scholar] [CrossRef]
- Friese, A.; Schulz, J.; Hoehle, L.; Fetsch, A.; Tenhagen, B.-A.; Hartung, J.; Roesler, U. Occurrence of MRSA in air and housing environment of pig barns. Veter. Microbiol. 2012, 158, 129–135. [Google Scholar] [CrossRef]
- Gibbs, S.G.; Green, C.F.; Tarwater, P.M.; Scarpino, P.V. Airborne Antibiotic Resistant and Nonresistant Bacteria and Fungi Recovered from Two Swine Herd Confined Animal Feeding Operations. J. Occup. Environ. Hyg. 2004, 1, 699–706. [Google Scholar] [CrossRef]
- Broens, E.; Graat, E.; Van Der Wolf, P.; Van De Giessen, A.; De Jong, M. Prevalence and risk factor analysis of livestock associated MRSA-positive pig herds in The Netherlands. Prev. Veter. Med. 2011, 102, 41–49. [Google Scholar] [CrossRef]
- Lupo, A.; Coyne, S.; Berendonk, T.U. Origin and Evolution of Antibiotic Resistance: The Common Mechanisms of Emergence and Spread in Water Bodies. Front. Microbiol. 2012, 3, 18. [Google Scholar] [CrossRef]
- Baquero, F.; Martinez, J.L.; Cantón, R. Antibiotics and antibiotic resistance in water environments. Curr. Opin. Biotechnol. 2008, 19, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Coleman, B.L.; Salvadori, M.I.; McGEER, A.J.; Sibley, K.A.; Neumann, N.F.; Bondy, S.J.; Gutmanis, I.A.; McEWEN, S.A.; Lavoie, M.; Strong, D.; et al. The role of drinking water in the transmission of antimicrobial-resistant E. coli. Epidemiol. Infect. 2011, 140, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Hölzel, C.S.; Schwaiger, K.; Harms, K.; Küchenhoff, H.; Kunz, A.; Meyer, K.; Müller, C.; Bauer, J. Sewage sludge and liquid pig manure as possible sources of antibiotic resistant bacteria. Environ. Res. 2010, 110, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Vital, P.G.; Zara, E.S.; Paraoan, C.E.M.; Dimasupil, M.A.Z.; Abello, J.J.M.; Santos, I.T.G.; Rivera, W.L. Antibiotic Resistance and Extended-Spectrum Beta-Lactamase Production of Escherichia coli Isolated from Irrigation Waters in Selected Urban Farms in Metro Manila, Philippines. Water 2018, 10, 548. [Google Scholar] [CrossRef]
- Hammerum, A.M.; Sandvang, D.; Andersen, S.R.; Seyfarth, A.M.; Porsbo, L.J.; Frimodt-Møller, N.; Heuer, O.E. Detection of sul1, sul2 and sul3 in sulphonamide resistant Escherichia coli isolates obtained from healthy humans, pork and pigs in Denmark. Int. J. Food Microbiol. 2006, 106, 235–237. [Google Scholar] [CrossRef]
- Price, L.B.; Graham, J.P.; Lackey, L.G.; Roess, A.; Vailes, R.; Silbergeld, E. Elevated risk of carrying gentamicin-resistant Escherichia coli among US poultry workers. Environ. Health Perspect. 2007, 115, 1738–1742. [Google Scholar] [CrossRef]
- McMahon, M.A.S.; Xu, J.; Moore, J.E.; Blair, I.S.; McDowell, D.A. Environmental Stress and Antibiotic Resistance in Food-Related Pathogens. Appl. Environ. Microbiol. 2007, 73, 211–217. [Google Scholar] [CrossRef]
- Whitehead, R.N.; Overton, T.W.; Kemp, C.L.; Webber, M.A. Exposure of Salmonella enterica Serovar Typhimurium to High Level Biocide Challenge Can Select Multidrug Resistant Mutants in a Single Step. PLoS ONE 2011, 6, e22833. [Google Scholar] [CrossRef]
- Teuber, M.; Meile, L.; Schwarz, F. Acquired antibiotic resistance in lactic acid bacteria from food. Antonie Van Leeuwenhoek 1999, 76, 115–137. [Google Scholar] [CrossRef]
- Masco, L.; Van Hoorde, K.; De Brandt, E.; Swings, J.; Huys, G. Antimicrobial susceptibility of Bifidobacterium strains from humans, animals and probiotic products. J. Antimicrob. Chemother. 2006, 58, 85–94. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Opinion of the Scientific Panel on Genetically Modified Organisms on the use of antibiotic resistance genes as marker genes in genetically modified plants. EFSA J. 2004, 2, 48. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Statement on the safe use of the nptII antibiotic resistance marker gene in genetically modified plants by the Scientific Panel on genetically modified organisms (GMO). EFSA J. 2007, 5, 742. [Google Scholar]
- Centres for Disease Control and Prevention (US). Antibiotic Resistance Threats in the United States, 2013. In Centres for Disease Control and Prevention, US Department of Health and Human Services.; 2013. Available online: https://www.cdc.gov/drugresistance/Threat-Report-2013/pdf/ar-Threats-2013-508.pdf (accessed on 30 May 2020).
- Zellweger, R.M.; Basnyat, B.; Shrestha, P.; Prajapati, K.G.; Dongol, S.; Sharma, P.K.; Koirala, S.; Darton, T.C.; Boinett, C.; Thompson, C.N.; et al. Changing Antimicrobial Resistance Trends in Kathmandu, Nepal: A 23-Year Retrospective Analysis of Bacteraemia. Front. Med. 2018, 5, 262. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Report on Surveillance of Antibiotic Consumption: 2016–2018 Early Implementation. 2018. Available online: https://www.who.int/medicines/areas/rational_use/who-amr-amc-report-20181109.pdf (accessed on 30 May 2020).
- Goossens, H.; Ferech, M.; Vander Stichele, R.; Elseviers, M.; ESAC Project Group. Outpatient antibiotic use in Europe and association with resistance: A cross-national database study. Lancet 2005, 365, 579–587. [Google Scholar] [CrossRef]
- Malhotra-Kumar, S.; Lammens, C.; Coenen, S.; Van Herck, K.; Goossens, H. Effect of azithromycin and clarithromycin therapy on pharyngeal carriage of macrolide-resistant streptococci in healthy volunteers: A randomised, double-blind, placebo-controlled study. Lancet 2007, 369, 482–490. [Google Scholar] [CrossRef]
- Fridkin, S.K.; Edwards, J.R.; Courval, J.M.; Hill, H.; Tenover, F.C.; Lawton, R.; Gaynes, R.P.; McGowan, J.E. Intensive Care Antimicrobial Resistance Epidemiology (ICARE) Project and the National Nosocomial Infections Surveillance (NNIS) System Hospitals. The effect of vancomycin and third-generation cephalosporins on prevalence of vancomycin-resistant enterococci in 126 US adult intensive care units. Ann. Int. Med. 2001, 135, 175–183. [Google Scholar]
- Costelloe, C.; Metcalfe, C.; Lovering, A.; Mant, D.; Hay, A. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: Systematic review and meta-analysis. BMJ 2010, 340, c2096. [Google Scholar] [CrossRef]
- Van Boeckel, T.P.; Gandra, S.; Ashok, A.; Caudron, Q.; Grenfell, B.T.; Levin, S.; Laxminarayan, R. Global antibiotic consumption 2000 to 2010: An analysis of national pharmaceutical sales data. Lancet Infect. Dis. 2014, 14, 742–750. [Google Scholar] [CrossRef]
- Klein, E.Y.; Van Boeckel, T.P.; Martinez, E.M.; Pant, S.; Gandra, S.; Levin, S.A.; Goossens, H.; Laxminarayan, R. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc. Natl. Acad. Sci. USA 2018, 115, E3463–E3470. [Google Scholar] [CrossRef]
- Cecchini, M.; Langer, J.; Slawomirski, L. Antimicrobial Resistance in G7 Countries and Beyond: Economic Issues. Policies and Options for Action. 2015. Available online: https://www.oecd.org/els/health-systems/Antimicrobial-Resistance-in-G7-Countries-and-Beyond.pdf (accessed on 30 May 2020).
- Food and Agriculture Organization of the United Nations (FAO). Status Report on Antimicrobial Resistance. Rome: Food and Agriculture Organization of the United Nations. 2015. Available online: http://www.fao.org/3/a-mm736e.pdf (accessed on 5 June 2020).
- Da Costa, P.M.; Loureiro, L.; Matos, A.J.F. Transfer of Multidrug-Resistant Bacteria Between Intermingled Ecological Niches: The Interface Between Humans, Animals and the Environment. Int. J. Environ. Res. Public Health 2013, 10, 278–294. [Google Scholar] [CrossRef]
- Ewnetu, D.; Mihret, A. Prevalence and Antimicrobial Resistance ofCampylobacterIsolates from Humans and Chickens in Bahir Dar, Ethiopia. Foodborne Pathog. Dis. 2010, 7, 667–670. [Google Scholar] [CrossRef]
- Fischer, J.; Rodríguez, I.; Schmoger, S.; Friese, A.; Roesler, U.; Helmuth, R.; Guerra, B. Escherichia coli producing VIM-1 carbapenemase isolated on a pig farm. J. Antimicrob. Chemother. 2012, 67, 1793–1795. [Google Scholar] [CrossRef] [PubMed]
- Al Bayssari, C.; Dabboussi, F.; Hamze, M.; Rolain, J.-M. Emergence of carbapenemase-producing Pseudomonas aeruginosa and Acinetobacter baumannii in livestock animals in Lebanon. J. Antimicrob. Chemother. 2014, 70, 950–951. [Google Scholar] [CrossRef] [PubMed]
- European Commission, Guidelines on the Prudent Use of Antimicrobials in Veterinary Medicine. 2015. Available online: https://ec.europa.eu/health/sites/health/files/antimicrobial_resistance/docs/2015_prudent_use_guidelines_en.pdf (accessed on 5 June 2020).
- Hao, H.; Cheng, G.; Iqbal, Z.; Ai, X.; Hussain, H.I.; Huang, L.; Dai, M.; Wang, Y.; Liu, Z.; Yuan, Z.-H. Benefits and risks of antimicrobial use in food-producing animals. Front. Microbiol. 2014, 5, 288. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, J. Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations. Review on Antimicrobial Resistance. Review on Antimicrobial Resistance, London, UK. 2014. Available online: https://amr-review.org/sites/default/files/AMR%20Review%20Paper%20-%20Tackling%20a%20crisis%20for%20the%20health%20and%20wealth%20of%20nations_1.pdf (accessed on 5 June 2020).
- Antimicrobial Resistance Empirical and Statistical Evidence-Base. A Report from the Department of Health Antimicrobial Resistance Strategy Analytical Working Group. 2016. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/553267/AMR_EBO_2016.pdf (accessed on 5 June 2020).
- Pires, D.; de Kraker, M.E.; Tartari, E.; Abbas, M.; Pittet, D. ‘Fight antibiotic resistance—It’s in your hands’: Call from the World Health Organization for 5th may 2017. Clin. Infect. Dis. 2017, 64, 1780–1783. [Google Scholar] [CrossRef]
- Abat, C.; Rolain, J.-M.; Dubourg, G.; Fournier, P.-E.; Chaudet, H.; Raoult, D. Evaluating the Clinical Burden and Mortality Attributable to Antibiotic Resistance: The Disparity of Empirical Data and Simple Model Estimations. Clin. Infect. Dis. 2017, 65, S58–S63. [Google Scholar] [CrossRef]
- Gandra, S.; Joshi, J.; Trett, A.; Lamkang, A.S.; Laxminarayan, R. Scoping Report on Antimicrobial Resistance in India; Center for Disease Dynamics, Economics & Policy: Washington, DC, USA, 2017; Volume 2017, pp. 1–146. Available online: https://cddep.org/wp-content/uploads/2017/11/AMR-INDIA-SCOPING-REPORT.pdf (accessed on 5 June 2020).
- Laxminarayan, R.; Matsoso, P.; Pant, S.; Brower, C.; Røttingen, J.-A.; Klugman, K.; Davies, S. Access to effective antimicrobials: A worldwide challenge. Lancet 2015, 387, 168–175. [Google Scholar] [CrossRef]
- Robinson, T.P.; Bu, D.P.; Carrique-Mas, J.; Fevre, E.; Gilbert, M.; Grace, D.; Hay, S.; Jiwakanon, J.; Kakkar, M.; Kariuki, S.; et al. Antibiotic resistance is the quintessential One Health issue. Trans. R. Soc. Trop. Med. Hyg. 2016, 110, 377–380. [Google Scholar] [CrossRef]
- Dahal, R.; Upadhyay, A.; Ewald, B. One Health in South Asia and its challenges in implementation from stakeholder perspective. Veter. Rec. 2017, 181, 626. [Google Scholar] [CrossRef]
- Talkington, K. Superbugs Don’t Respect Borders. Combating the Growing Threat of Antibiotic Resistance Must Remain a Top Global Priority. The Pew Charitable Trusts. 2018. Available online: https://www.pewtrusts.org/en/research-and-analysis/articles/2017/10/10/superbugs-dont-respect-borders (accessed on 1 January 2021).
- Wang, R.; van Dorp, L.; Shaw, L.P.; Bradley, P.; Wang, Q.; Wang, X.; Jin, L.; Zhang, Q.; Liu, Y.; Rieux, A.; et al. The global distribution and spread of the mobilized colistin resistance gene mcr-1. Nat. Commun. 2018, 9, 1179. [Google Scholar] [CrossRef]
- Gandra, S.; Mojica, N.; Klein, E.; Ashok, A.; Nerurkar, V.; Kumari, M.; Ramesh, U.; Dey, S.; Vadwai, V.; Das, B.R.; et al. Trends in antibiotic resistance among major bacterial pathogens isolated from blood cultures tested at a large private laboratory network in India, 2008–2014. Int. J. Infect. Dis. 2016, 50, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Sifri, Z.; Chokshi, A.; Cennimo, D.; Horng, H. Global contributors to antibiotic resistance. J. Glob. Infect. Dis. 2019, 11, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Taneja, N.; Sharma, M. Antimicrobial resistance in the environment: The Indian scenario. Indian J. Med. Res. 2019, 149, 119–128. [Google Scholar] [CrossRef] [PubMed]
- CDC. Antibiotic Resistance Threats in the United States; U.S. Department of Health and Human Services: Atlanta, GA, USA, 2019. Available online: https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf (accessed on 5 June 2020).
- Hassani, S.; Moosavy, M.-H.; Gharajalar, S.N.; Khatibi, S.A.; Hajibemani, A.; Barabadi, Z. High prevalence of antibiotic resistance in pathogenic foodborne bacteria isolated from bovine milk. Sci. Rep. 2022, 12, 3878. [Google Scholar] [CrossRef]
- Boonyasiri, A.; Tangkoskul, T.; Seenama, C.; Saiyarin, J.; Tiengrim, S.; Thamlikitkul, V. Prevalence of antibiotic resistant bacteria in healthy adults, foods, food animals, and the environment in selected areas in Thailand. Pathog. Glob. Health 2014, 108, 235–245. [Google Scholar] [CrossRef]
- Chao, G.; Zhou, X.; Jiao, X.; Qian, X.; Xu, L. Prevalence and Antimicrobial Resistance of Foodborne Pathogens Isolated from Food Products in China. Foodborne Pathog. Dis. 2007, 4, 277–284. [Google Scholar] [CrossRef]
- Akosua, B.K.; Kwasi, O.-D.; Enoch, H.F.; Karen, A.K. Multidrug resistant Campylobacter in faecal and carcasses of commercially produced poultry. Afr. J. Microbiol. Res. 2017, 11, 271–277. [Google Scholar] [CrossRef]
- Kottawatta, K.S.A.; Van Bergen, M.A.P.; Abeynayake, P.; Wagenaar, J.A.; Veldman, K.T.; Kalupahana, R.S. Campylobacter in Broiler Chicken and Broiler Meat in Sri Lanka: Influence of Semi-Automated vs. Wet Market Processing on Campylobacter Contamination of Broiler Neck Skin Samples. Foods 2017, 6, 105. [Google Scholar] [CrossRef]
- Puig-Peña, Y.; Leyva-Castillo, V.; Tejedor-Arias, R.; Illnait-Zaragozí, M.T.; Aportela-López, N.; Camejo-Jardines, A.; Ramírez-Areces, J. Antimicrobial Resistance in Bacteria Isolated from Foods in Cuba. MEDICC Rev. 2022, 22, 40–45. [Google Scholar]
- Frana, T.S.; Beahm, A.R.; Hanson, B.M.; Kinyon, J.M.; Layman, L.L.; Karriker, L.A.; Ramirez, A.; Smith, T.C. Isolation and Characterization of Methicillin-Resistant Staphylococcus aureus from Pork Farms and Visiting Veterinary Students. PLoS ONE 2013, 8, e53738. [Google Scholar] [CrossRef]
- Penders, J.; Stobberingh, E. Antibiotic resistance of motile aeromonads in indoor catfish and eel farms in the southern part of The Netherlands. Int. J. Antimicrob. Agents 2008, 31, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Ejo, M.; Garedew, L.; Alebachew, Z.; Worku, W. Prevalence and Antimicrobial Resistance of Salmonella Isolated from Animal-Origin Food Items in Gondar, Ethiopia. BioMed Res. Int. 2016, 2016, 1–8. [Google Scholar] [CrossRef]
- Brown, J.C.; Jiang, X. Prevalence of Antibiotic-Resistant Bacteria in Herbal Products. J. Food Prot. 2008, 71, 1486–1490. [Google Scholar] [CrossRef] [PubMed]
- Schwaber, M.J.; Carmeli, Y. Carbapenem-resistant Enterobacteriaceae: A potential threat. JAMA 2008, 300, 2911–2913. [Google Scholar] [PubMed]
- Lozano, R.; Naghavi, M.; Foreman, K.; Lim, S.; Shibuya, K.; Aboyans, V.; Abraham, J.; Adair, T.; Aggarwal, R.; Ahn, S.Y.; et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2095–2128. [Google Scholar] [CrossRef]
- Doi, Y.; Iovleva, A.; Bonomo, R.A. The ecology of extended-spectrum β-lactamases (ESBLs) in the developed world. J. Travel Med. 2017, 24, S44–S51. [Google Scholar] [CrossRef]
- Bevan, E.R.; Jones, A.M.; Hawkey, P.M. Global epidemiology of CTX-M β-lactamases: Temporal and geographical shifts in genotype. J. Antimicrob. Chemother. 2017, 72, 2145–2155. [Google Scholar] [CrossRef]
- Karanika, S.; Karantanos, T.; Arvanitis, M.; Grigoras, C.; Mylonakis, E. Fecal colonization with extended-spectrum beta-lactamase–producing Enterobacteriaceae and risk factors among healthy individuals: A systematic review and metaanalysis. Rev. Infect. Dis. 2018, 63, 310–318. [Google Scholar] [CrossRef]
- Frost, I.; Van Boeckel, T.P.; Pires, J.; Craig, J.; Laxminarayan, R. Global geographic trends in antimicrobial resistance: The role of international travel. J. Travel Med. 2019, 26, taz036. [Google Scholar] [CrossRef]
- Pourmand, A.; Mazer-Amirshahi, M.; Jasani, G.; May, L. Emerging trends in antibiotic resistance: Implications for emergency medicine. Am. J. Emerg. Med. 2017, 35, 1172–1176. [Google Scholar] [CrossRef]
- VT Nair, D.; Venkitanarayanan, K.; Kollanoor Johny, A. Antibiotic-resistant Salmonella in the food supply and the potential role of antibiotic alternatives for control. Foods 2018, 7, 167. [Google Scholar] [CrossRef]
- James, S.L.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef]
- Koch, K.; Kristensen, B.; Holt, H.M.; Ethelberg, S.; Mølbak, K.; Schønheyder, H.C. International travel and the risk of hospitalization with non-typhoidal Salmonella bacteremia. A Danish population-based cohort study, 1999–2008. BMC Infect. Dis. 2011, 11, 277. [Google Scholar] [CrossRef] [PubMed]
- Harbarth, S.; Participants, F.T.W.H.-A.I.R.F.; Balkhy, H.H.; Goossens, H.; Jarlier, V.; Kluytmans, J.A.J.W.; Laxminarayan, R.; Saam, M.; Van Belkum, A.; Pittet, D. Antimicrobial resistance: One world, one fight! Antimicrob. Resist. Infect. Control 2015, 4, 49. [Google Scholar] [CrossRef]
- World Health Organization. WHO Competency Framework for Health Workers’ Education and Training on Antimicrobial Resistance; World Health Organization: Geneva, Switzerland, 2018; Available online: https://apps.who.int/iris/bitstream/handle/10665/272766/WHO-HIS-HWF-AMR-2018.1-eng.pdf?ua=1 (accessed on 30 June 2020).
- Clift, C. Review of Progress on Antimicrobial Resistance; Chatham House: London, UK, 2019; Available online: https://www.chathamhouse.org/sites/default/files/publications/research/2019-10-11-AMR-Full-Paper.pdf (accessed on 30 June 2020).
- Prabhakar, S.V.; Sano, D.; Srivastava, N.; Food safety in the Asia-Pacific region: Current status, policy perspectives, and a way forward. Sustain. Consum. Prod. Asia-Pac. Reg. Eff. Responses A Resour. Constrained World. 2010, III, 215–238. Available online: https://www.iges.or.jp/en/pub/food-safety-asia-pacific-current-status-policy/en (accessed on 25 November 2020).
- World Organisation for Animal Health (Protecting Animals, Preserving Our Future), Terrestrial Animal Health Code. 2019. Available online: https://rr-africa.oie.int/wp-content/uploads/2019/11/en_csat-vol1-2019.pdf (accessed on 30 June 2020).
- DANMAP. Use of Antimicrobial Agents and Occurrence of Antimicrobial Resistance in Bacteria from Food Animals, Food and Humans in Denmark. 2017. Available online: https://backend.orbit.dtu.dk/ws/files/161713656/Rapport_DANMAP_2017.pdf (accessed on 10 July 2020).
- FDA. National Antimicrobial Resistance Monitoring System-Enteric Bacteria (NARMS): 2015 Integrated Report. 2015. Available online: https://www.fda.gov/animal-veterinary/national-antimicrobial-resistance-monitoring-system/2015-narms-integrated-report (accessed on 10 July 2020).
- Maran, M.; Monitoring of Antimicrobial Resistance and Antibiotic Usage in Animals in the Netherlands in 2014. Lelystad: Central Veterinary Institute, Part of Wageningen University and Research Centre (CVI). 2015. Available online: https://www.wur.nl/upload_mm/c/8/f/1cc4937d-97d9-4874-82e7-eb5d6cc64a4e_NethmapMaran2015.pdf (accessed on 25 July 2020).
- Donado-Godoy, P.; Castellanos, L.R.; León, M.; Arevalo, A.; Clavijo, V.; Bernal, J.; León, D.; Tafur, M.A.; Byrne, B.A.; Smith, W.A.; et al. The Establishment of the Colombian Integrated Program for Antimicrobial Resistance Surveillance (COIPARS): A Pilot Project on Poultry Farms, Slaughterhouses and Retail Market. Zoonoses Public Health 2015, 62, 58–69. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Worldwide Country Situation Analysis: Response to Antimicrobial Resistance; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- De, K.B.; Halliday, J.; Lubroth, J. Integrating the surveillance of animal health, foodborne pathogens and foodborne diseases in developing and in-transition countries. Rev. Sci. Tech. (Int. Off. Epizoot.) 2013, 32, 539–548. [Google Scholar] [CrossRef]
- United Nations. Follow-Up to the Political Declaration of the High-Level Meeting of the General Assembly on Antimicrobial Resistance; A/73/869; United Nations: New York, NY, USA, 2019; Available online: https://digitallibrary.un.org/record/3807197?ln=en (accessed on 25 July 2020).
- WHO F. OIE. Monitoring Global Progress on Addressing Antimicrobial Resistance: Analysis Report of the Second Round of Results of AMR Country Self-Assessment Survey 2018. World Health Organization, Food and Agriculture Organization of the United Nations and World Organisation for Animal Health (OIE), Geneva. 2018. Available online: https://apps.who.int/iris/bitstream/handle/10665/273128/9789241514422-eng.pdf?ua=1 (accessed on 25 August 2021).
- World Health Organization and the United Nations Children’s Fund, WASH in health care facilities: Global Baseline Report 2019, WHO and UNICEF, Geneva. 2019. Available online: https://apps.who.int/iris/bitstream/handle/10665/311620/9789241515504-eng.pdf (accessed on 25 September 2021).
- Collignon, P.; Beggs, J.J.; Walsh, T.R.; Gandra, S.; Laxminarayan, R. Anthropological and socioeconomic factors contributing to global antimicrobial resistance: A univariate and multivariable analysis. Lancet Planet. Health 2018, 2, e398–e405. [Google Scholar] [CrossRef]
- Castanon, J.I. History of the use of antibiotic as growth promoters in European poultry feeds. Poult. Sci. 2007, 86, 2466–2471. [Google Scholar] [CrossRef]
- Founou, L.L.; Founou, R.C.; Essack, S. Antibiotic Resistance in the Food Chain: A Developing Country-Perspective. Front. Microbiol. 2016, 7, 1881. [Google Scholar] [CrossRef]
- O’Neill, J. Antimicrobials in Agriculture and the Environment: Reducing Unnecessary Use and Waste. In The Review on Antimicrobial Resistance; Wellcome Trust: London, UK, 2015. [Google Scholar]
- Global Antimicrobial Resistance Surveillance System (GLASS) Report: Early Implementation 2017–2018; World Health Organization: Geneva, Switzerland. 2018. Available online: https://apps.who.int/iris/bitstream/handle/10665/279656/9789241515061-eng.pdf?ua=1 (accessed on 25 July 2021).
- Woolhouse, M.; Ward, M.; Van Bunnik, B.; Farrar, J. Antimicrobial resistance in humans, livestock and the wider environment. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140083. [Google Scholar] [CrossRef]
- Zakeri, B.; Lu, T.K. Synthetic Biology of Antimicrobial Discovery. ACS Synth. Biol. 2012, 2, 358–372. [Google Scholar] [CrossRef] [PubMed]
| AMR Microorganisms | Food Samples | Country/Region | References |
|---|---|---|---|
| Escherichia coli, Listeria monocytogenes, Staphylococcus aureus, and Salmonella spp. | Raw bovine milk | Iran | [128] |
| ESBL producing E. coli | Pigs, broiler, fish, etc. | Thailand (Different selected areas) | [129] |
| Salmonella, L. monocytogenes, and S. aureus | Raw milk, cooked food products, and raw meat | China | [130] |
| Campylobacter jejuni, C. coli, C. iari | Poultry products | Africa | [131] |
| C. jejuni, C. coli | Poultry meat and neck skin | Sri Lanka | [132] |
| Escherichia coli, Salmonella, Vibrio cholerae, Staphylococcus | Meats#br#products,#br#dairy#br#products, etc. | Cuba | [133] |
| Methicillin-resistant Staphylococcus aureus (MRSA) | Pigs | - | [134] |
| Aeromonas | Catfish and eel farms (aquaculture) | Netherlands (southern) | [135] |
| Salmonella species | Animal origin food (such as cream cake, egg sandwich, raw meat, raw milk, etc.) | Ethiopia | [136] |
| Bacillus spp., Erwinia spp.,#br#Ewingella americana. Staphylococcus spp., Enterobacter cloacae, and Stenotrophomonas maltophilia | Herbal products (such ginger root, garlic powder, etc.) | - | [137] |
| Resistant Identified (Year) | Antibiotic Released (Year) |
|---|---|
| Amphotericin B-resistant Candida auris (2016) | Amphotericin B; 1959 |
| Azithromycin-resistant N. gonorrhoeae (2011) | Azithromycin; 1980 |
| Ceftazidime-avibactam-resistant KPC-producing K. pneumonia (2015) | Ceftazidime-avibactam; 2015 |
| Ciprofloxacin-resistant N. gonorrhoeae (2007) | Ciprofloxacin; 1987 |
| Daptomycin-resistant methicillin-resistant S. aureus (2004) | Daptomycin; 2003 |
| Extended-spectrum β-lactamase-producing E. coli (1983) | Extended-spectrum cephalosporins; 1980 (Cefotaxime) |
| Fluconazole-resistant Candida (1988) | Fluconazole; 1990 (FDA approved) |
| K. pneumoniae carbapenemase (KPC)-producing K. pneumonia (1996) | Imipenem; 1985 |
| Methicillin-resistant Staphylococcus aureus (1960) | Methicillin; 1960 |
| Penicillinase-producing N. gonorrhoeae (1976) | Penicillin; 1941 |
| Penicillin-resistant S. aureus (1942) | |
| Penicillin-resistant S. pneumonia (1967) | |
| Plasmid-mediated vancomycin-resistant Enterococcus faecium (1988) | Vancomycin; 1958 |
| Vancomycin-resistant S. aureus (2002) | Vancomycin; 1958 |
| Possible Strategies | Consequence |
|---|---|
| Increase awareness | Awareness programs are supported by mass, and social media repeated messaging regarding issues related to AMR, which may decrease antibiotic usage and AMR rates. |
| Support knowledge through observation | Organizations (govt/non-govt) along with industry and academia can improve the practical knowledge to combat AMR concerns |
| Cleanliness, hygiene, and preventive measures | Proper hygiene and cleanliness by following necessary guidelines can help to decrease AMR issues. |
| Regulate the use of antimicrobials | Guidelines should be compulsory, especially for antibiotics used to treat infectious diseases in animals and humans. |
| Improve economic situation | There is a need for investment in the advancement of novel antimicrobial treatments, analytical tools, and vaccines. Shortage in such investment reveals the trends of continued AMR. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samtiya, M.; Matthews, K.R.; Dhewa, T.; Puniya, A.K. Antimicrobial Resistance in the Food Chain: Trends, Mechanisms, Pathways, and Possible Regulation Strategies. Foods 2022, 11, 2966. https://doi.org/10.3390/foods11192966
Samtiya M, Matthews KR, Dhewa T, Puniya AK. Antimicrobial Resistance in the Food Chain: Trends, Mechanisms, Pathways, and Possible Regulation Strategies. Foods. 2022; 11(19):2966. https://doi.org/10.3390/foods11192966
Chicago/Turabian StyleSamtiya, Mrinal, Karl R. Matthews, Tejpal Dhewa, and Anil Kumar Puniya. 2022. "Antimicrobial Resistance in the Food Chain: Trends, Mechanisms, Pathways, and Possible Regulation Strategies" Foods 11, no. 19: 2966. https://doi.org/10.3390/foods11192966
APA StyleSamtiya, M., Matthews, K. R., Dhewa, T., & Puniya, A. K. (2022). Antimicrobial Resistance in the Food Chain: Trends, Mechanisms, Pathways, and Possible Regulation Strategies. Foods, 11(19), 2966. https://doi.org/10.3390/foods11192966







