Food Applications and Potential Health Benefits of Hawthorn
Abstract
1. Introduction
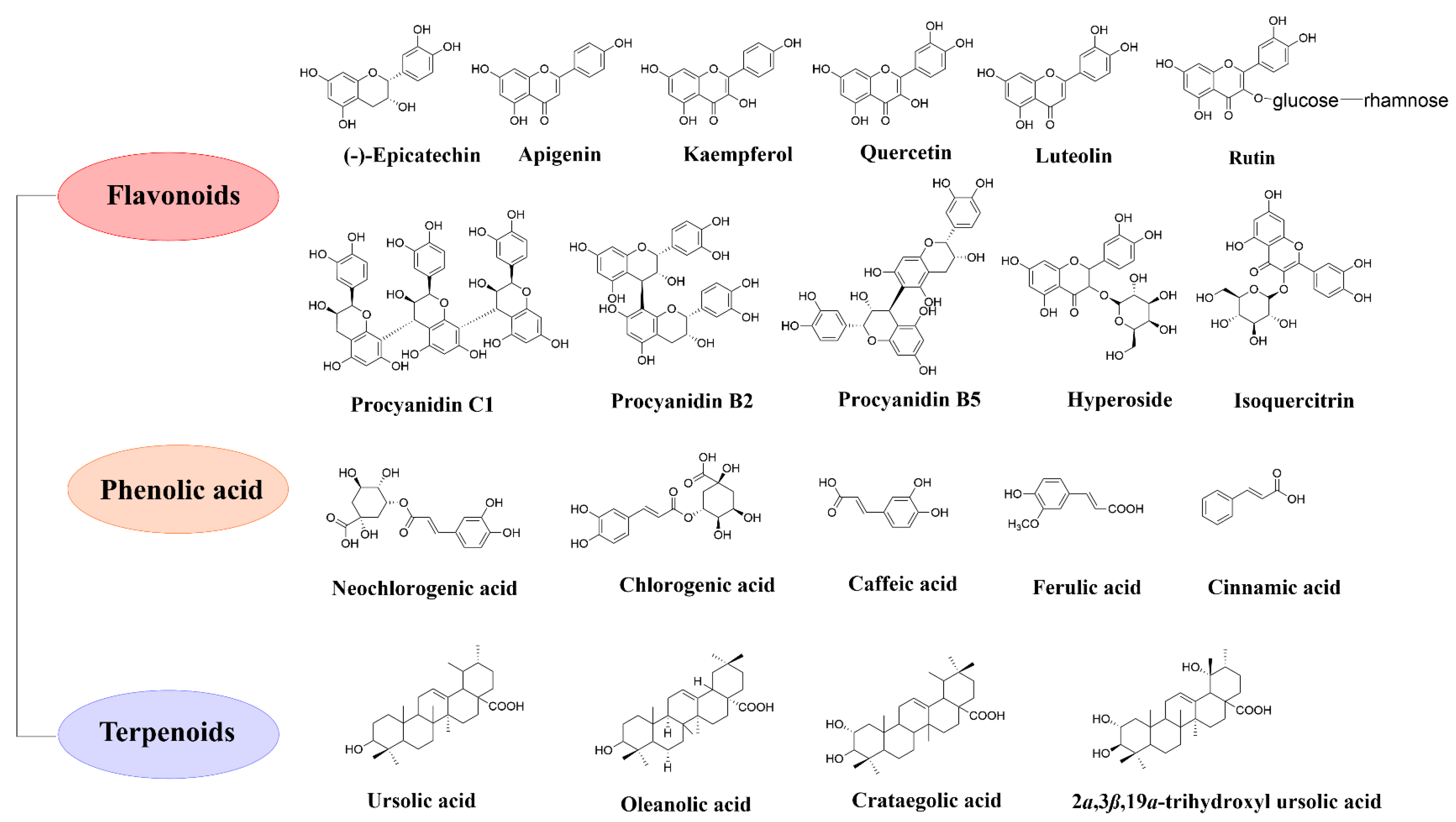
2. Nutrients and Phytochemical Compositions of Hawthorn
2.1. Nutrient Composition of Hawthorn
2.2. Phytochemical Compositions of Hawthorn
| Name | Species and Organ | Methodological and Analytical Approach | Extraction Solvent | References |
|---|---|---|---|---|
| Quercetin | C. Almaatensis; Flowers, fruits and leaves | HPLC-ESI-Q-TOF-MS and HRMS/MS | 96% Ethanol or 50% ethanol | [20] |
| Quercitrin | ||||
| Cyanidin 3-glucoside | ||||
| Catechin | ||||
| Epigallocatechin | ||||
| Rutin | ||||
| Quercetin 3-glucoside (Isoquercetin) | ||||
| Vitexin 2″-O-rhamnoside | ||||
| Vitexin 4″-O-rhamnoside | ||||
| Vitexin | ||||
| Vitexin 4″-O-glucoside | ||||
| Quercetin glucoside | ||||
| Hyperoside | C. Meyeri; Fruits | HPLC | Methanol/water (80:20, 25 mL) | [12] |
| Epicatechin | NM | HPLC | 80% Aqueous ethanol | [28] |
| Cyanidin chloride | C. pinnatifida var. Major; Fruits | HPLC-ESI-MS/MS | 50% (v/v) Aqueous ethanol | [54] |
| Luteolin | ||||
| Apigenin | ||||
| Kaempferol | ||||
| Naringenin | ||||
| Phloretin | ||||
| Quercetin dirhamnosyl hexoside | C. pinnatifida Bge. var. Major; Fruits | HPLC-UV | 80% Aqueous ethanol | [27] |
| Quercetin rhamnosyl hexoside | ||||
| Monoacetyl vitexin rhamnoside | ||||
| Luteolin-7-glycoside | ||||
| C-glycosides vitexin | ||||
| Sexangularetin-3-glucoside | ||||
| Sexangularetin-3-neohesperidoside | ||||
| Kaempferol-3-neohesperidoside | ||||
| Vitexin-2″-O-α-L-rhamnoside | C. Oxyacantha; Leaves and flowers | NM | Hydroalcoholic or water-based | [9] |
| Eriodictyol | C. azarolus Leaves | HPLC | Water/acetone mixture (1v/2v) | [55] |
| Hesperidin | C. pinnatifida Bge. or C. pimiatificia Bge. var. major N, E. Br; Fruits | UPLC/Q-TOF-MS | 50% Ethanol | [56] |
| Rutoside | C. oxyacantha L; Fruits | HPLC, TLC, and UV | Hydroalcoholic or water-based | [26] |
| Isoquercitrin | C. pinnatifida Bge; Fruits | HPLC | 80% Acetone | [38] |
| Hispertin | C. azarolus var. eu-azarolus; Leaves | RP-HPLC, UV, TLC, 1H NMR, and 13C NMR | Acetone, ethyl acetate, methanol and 70% ethanol | [23] |
| Chrysin | ||||
| Procyanidin trimers | C. orientalis, C. szovitsii and C. tanacetifolia; Leaves and twigs | VIP marker from OPLS-DA and UHPLC-ESI-QTOF-MS | Methanol | [37] |
| Isoxanthohumo | ||||
| Apigenin 7-O-glucoronide | ||||
| Chrysoeriol 7-O-(6″-malonyl-apiosyl-glucoside) | ||||
| Tetramethylscutellarein | ||||
| Myricetin 3-O-arabinoside | ||||
| Hydroxycaffeic acid | ||||
| p-Coumaric acid 4-O-glucoside | ||||
| Glycitin | ||||
| Quercetin 3-O-β-D-galactopyranoside | C. Dahurica; Fruits | HPLC, UV, 1H NMR, and 13C NMR | Methanol | [39] |
| Isorhamnetin 3-O-α-L-rhamnopyranosyl-7-O-β-D-glucopyranoside | ||||
| 2″-O-rhamnoside | C. pinnatifida Bge; Leaves | HPLC-QTOF-MS | 75% Ethanol | [53] |
| Orientin | ||||
| Iso-orientin | ||||
| Crataequinone A-B | C. Pinnatifida; Fruits | NM | NM | [24] |
| Pinnatifinosides A-D | C. Pinnatifida; Leaves | |||
| Pinnatifins C-D,I | ||||
| 1β, 9α-Dihydroxyeudesm-3-en-5β, 6α, 7α, 11α H-12, 6-olide | C. Cuneata; Fruits | |||
| Proanthocyanidin A2 | NM; Leaves and flower | HPLC | Acetone–water (7v/3v) | [29] |
| Proanthocyanidin B2 | ||||
| Proanthocyanidin B4 | ||||
| Proanthocyanidin B5 | ||||
| Proanthocyanidin C1 | ||||
| Proanthocyanidin D1 | ||||
| Proanthocyanidin E1 | ||||
| Epicatechin-(4β→6)-Epicatechin-(4β→8)-epicatechin | ||||
| Epicatechin-(4β→8)-epicatechin-(4β→6)-epicatechin | ||||
| Pinnatifinosides I | C. pinnatifida Bge. var. major N.E.Br; Leaves | UV, IR, MS, and 1D, 2D NMR | 80% Ethanol | [30] |
| (+)-Taxifolin | C. Sinaica; Leaves | 1H NMR and 13C NMR | 70% Acetone | [31] |
| (+)-Taxifolin 3-O-xylopyranoside | ||||
| (+)-Taxifolin 3-O-arabinopyranoside 3-O-arabinopyranoside | ||||
| Crateside | C. monogyna and C. pentagyna; Leaves | UV | 20% Ethanol | [32] |
| Neoschaftoside | C. Monogyna; Leaves | UV and TLC | Chloroform and butanol. | [33] |
| Neoisoschaftoside | ||||
| Cratenacin | C. Curvisepala; Leaves | UV and IV | NM | [34] |
| Name | Species and Organ | Methodological and Analytical Approach | Extraction Solvent | References |
|---|---|---|---|---|
| Ursolic aldehyde | C. dahurica; Fruits | HPLC, 1H NMR and 13C NMR, UV | Methanol | [39] |
| Uvaol | ||||
| Ursolic acid | ||||
| Pomolic acid | ||||
| Euscaphic acid | ||||
| Tormentic acid | ||||
| 3-Epi-2-oxopomolic acid | ||||
| 2α, 19α-Dihydroxy-3-oxo-urs-12-en-28-oic acid | ||||
| Fupenzic acid | ||||
| 2α-Hydroxy oleanolic acid | ||||
| Oleanolic acid | ||||
| Pinnatifidanoside A-D | C. pinnatifida; Leaves | HRESIMS, 1H NMR, and 13C NMR | 75% Ethanol | [57] |
| Byzantionoside B | ||||
| (3S, 5R, 6R, 7E, 9R)-3,6-Epoxy-7-megastig men-5,9-diol-9-O-β-D-glucopyranoside | ||||
| (6S, 7Z, 9R)-Roseoside | ||||
| Icariside B6 | ||||
| Linalool oxide β-D-glucoside | ||||
| Shanyenoside A | ||||
| Dihydrocharcone-2′-β-D-glucopyranoside | ||||
| Eriodectyol | ||||
| Shanyeside C,D,F | C. pinnatifida; Leave | HPLC-QTOF-MS | 75% Ethanol | [53] |
| Euodionosides D | ||||
| Linarionoside A,B | ||||
| (6S, 7E, 9R)-6,9-Dihydroxy-4,7-megastiymadien-3-one-9-O-[β-D-xylopyranosy-(1→6)-β-D-glucopyranoside] | ||||
| Linalool oxide β-D-glucoside | ||||
| (6R, 9R)-3-Oxo-α-ionol-9-O-β-D-glucopyranoside | ||||
| Pisumionoside | ||||
| (3S, 5R, 6R, 7E, 9S)-Megastiman-7-ene-3,5,6,9-tetrol | ||||
| Pinnatifidanoside G | ||||
| Norhawthornoid B | ||||
| Corosolic acid | C.Pinnatifida; Fruits | HPLC | 80% Acetone | [44] |
| Maslinic acid | ||||
| (3R,5S,6S,7E,9S)-Megastigman-7-ene-3,5,6,9-tetrol 9-O-β-D-glucopyranoside | C. Pinnatifida; Leaves | 1H NMR, 13C NMR, HSQC, HMBC, and NOESY | 70% Ethanol | [42] |
| (6S,7E,9R)-6,9-Dihydroxy-4,7-megastigmadien-3-one 9-O-[β-D-xylopyranosyl-(1″→6′)-β-D-glucopyranoside] | ||||
| Linarionoside A-C | C. Pinnatifida; Leaves | 1H NMR and 13C NMR | 70% Ethanol | [43] |
| 3β-D-Glucopyranosyloxy-β-ionone | ||||
| Icariside B6 | ||||
| Pisumionoside | ||||
| (3S,5R,6R,7E,9R)-3,6-Epoxy-7-megastigmen-5,9-diol-9-O-β-Dglucopyranoside | ||||
| (6S,7E,9R)-Roseoside | ||||
| (6R,9R)-3-Oxo-α-ionol-9-O-β-D-glucopyranoside | ||||
| 4-[4β-O-β-D-Xylopyranosyl-(1″→6′)-β-D-glucopyranosyl-2,6,6- trimethyl-1-cyclohexen-1-yl]-butan-2-one | C. Pinnatifida; Leaves | 1H NMR, 13C NMR, HSQC, HMBC, and NOESY | 70% Ethanol | [42] |
| (3S,9R)-3,9-Dihydroxy-megastigman-5-ene 3-O-primeveroside | ||||
| (3R,5S,6S,7E,9S)-Megastiman-7-ene-3,5,6,9-tetrol | ||||
| (5Z)-6-[5-(2-Hydroxypropan-2-yl)-2-methyltetrahydrofuran-2-yl] -3-methylhexa-1,5-dien-3-ol | ||||
| (5Z)-6-[5-(2-O-β-D-Glucopyranosyl-propan-2-yl)-2-methyl tetrahydrofur-an-2-yl]-3-methylhexa-1,5-dien-3-ol | ||||
| 5-Ethenyl-2-[2-O-β-D-glucopyranosyl-(1′′→6′)-β-D-glucopyranosyl-propan-2-yl]-5-methyltetrahydrofuran-2-ol |
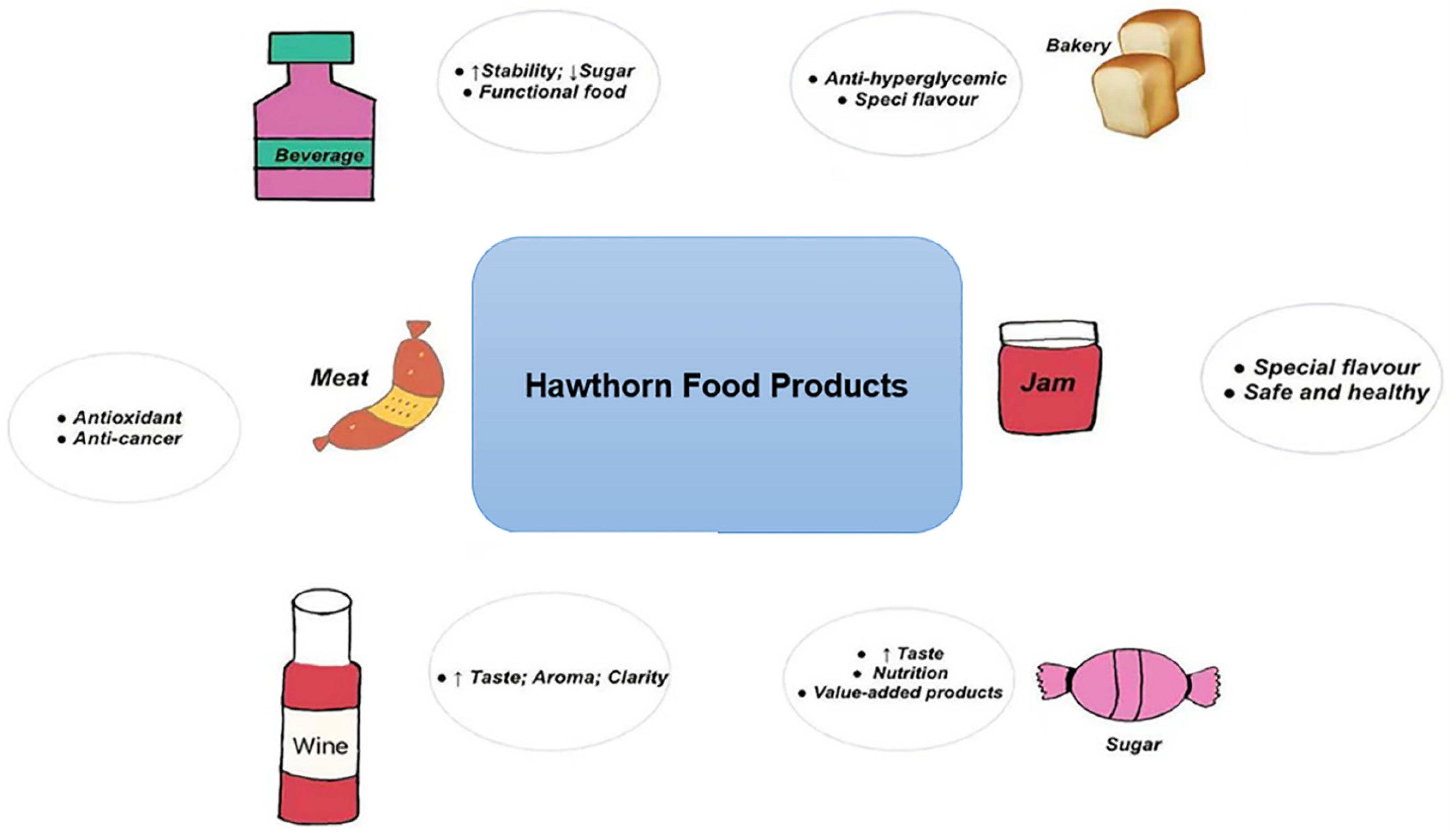
3. Applications in Food Products
3.1. Traditional Hawthorn Products
3.2. Bakery Products
3.3. Brewing Products
3.4. Beverages
3.5. Meat Products
3.6. Jams
3.7. Sugar Products
4. Ethnomedicinal and Biotechnological Uses
5. Health Benefits
5.1. Anticancer
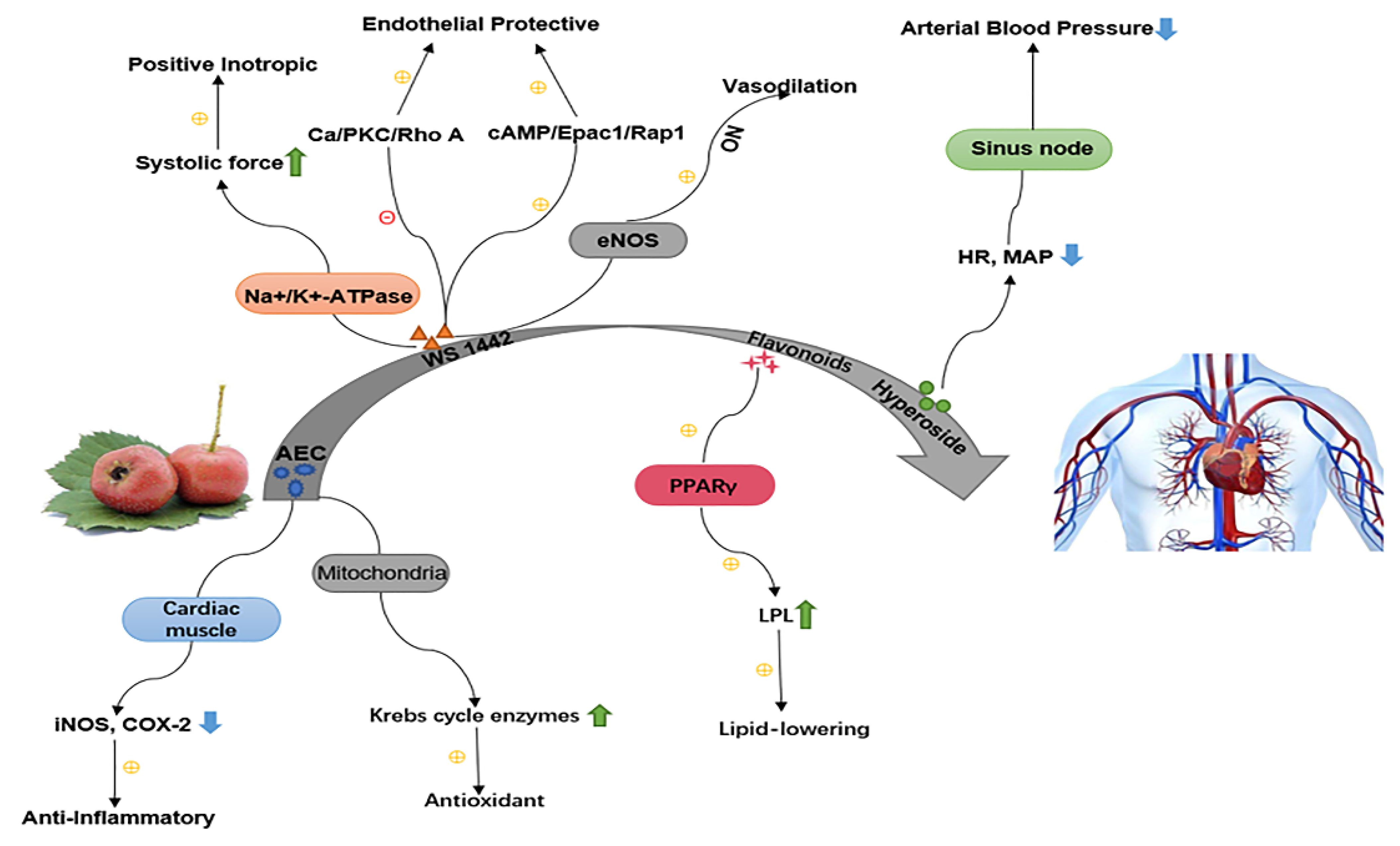
5.2. Cardiovascular System
5.2.1. Anti-Hypertensive
5.2.2. Lipid Regulation and Anti-Atherosclerosis
5.2.3. Cardioprotective Effect
5.3. Anti-Hyperglycemic
5.4. Antibacterial and Anti-Inflammatory
5.5. Antioxidant
5.6. Anti-Digestion
5.7. Others
5.7.1. Immune Regulation
5.7.2. Anticoagulant
5.7.3. Neuroprotective
| Extracts or Compounds | Observation or Methods | Effects | References | |
|---|---|---|---|---|
| Anticancer activity | ||||
| Triterpenoids isolated from hawthorn berries | In vitro, MTT assay. | All 15 triterpenoids showed effective antiproliferative activity against human HepG2, MCF-7 and MDA-MB- 231 tumor cells showed potent anti-proliferative activity(compound 2–4 EC50 < 5 µM). | [111] | |
| Ursolic acid, oleanolic acid, corosolic acid, and maslinic acid | In vitro. | CA showed the highest antiproliferative activity against human HepG2 (EC50 = 9.44 μM), MCF-7 (EC50 = 22.01 µM) and MDA-MB-231 (EC50 =26.83 μM) tumor cells among the four triterpenoids, followed by UA, MA, and OA. | [44] | |
| Phenylpropanoids isolated from hawthorn fruit | In vitro, MTT assay. | Five compounds (1a/1b, 2–4) were used in the treatment of human HepG2 and Hep3B cells with better cytotoxicity(1a IC50: 59.57, >100 µM; 1b IC50: 35.37, 70.42 µM; 2 IC50: 27.36, 39.40 µM; 3 IC50: 18.68, 38.96 µM;4 IC50: 17.50, 43.58 µM;). | [112] | |
| Homogeneous polysaccharide (HPS) | In vitro, WST-1 colorimetric method. | Treatments with 500 and 1000 μg/mL of HPS for 12 h resulted in more than 74% of growth inhibition against human HCT116 cell. | [49] | |
| An extract enriched with TOF | In vivo. | TOF extract from hawthorn leaves exerts an antitumor effect by decreasing the melanoma tumor growth in vivo (6 times less weight). | [55] | |
| Maslinic acid | In vitro, AO/EB staining assay and Annexin V/PI dual staining. | It can cause human neuroblastoma SHSY-5Y cells increase the percentage of apoptotic cells from 9% in the control group to 54% at higher drug doses. | [18] | |
| Cardiovascular system activities | ||||
| Hawthorn Leaf Flavonoids(HLF) | In vivo. | HLF protect against diabetes-induced cardiomyopathy in rats via PKC-α signaling pathway. | [25] | |
| Hawthorn Leafs Extract | In vitro, MTS. | Hydroalcoholic extracts of hawthorn leaves at 300 and 1000 mg/mL significantly reduced the frequency of arrhythmias induced by adrenaline stimulation. | [82] | |
| Hawthorn Fruit Extract (HFE) | In vivo. | HFE could dose-dependently reduce the TMAO-aggravated atherosclerosis. | [113] | |
| Flavonoids | In vivo, carrageenan-induced tail thrombosis model. | Inhibiting TXA2 release, decreasing the level of Ca2+ in platelets or blocking glycoprotein IIb/IIIa receptors may be the mechanism of the antithrombotic effects of flavonoids. | [81] | |
| Hawthorn Fruit Extract | In vivo, Western Blot. | The hepatic triglyceride (TG) and malondialdehyde (MDA) levels were significantly reduced in the hawthorn groups compared with the ovariectomized group (p < 0.05). | [83] | |
| Hawthorn Fruit Extract | In vivo, spectrophotometry. | Compared with the blood TC levels of rats in the type 2 diabetic group, the blood TC levels of rats in the high, medium and low dose of Hawthorn extract decreased by 162.54%, 122.68% and 92.13% respectively. | [86] | |
| Hawthorn Fruit Extract | In vivo. | Echocardiographic parameters (LVESD, LVEDD) were reduced in rats with chronic heart failure treated with hawthorn extract (p < 0.01) | [84] | |
| Hawthorn Extract | In vivo. | Hawthorn extract groups suppressed the high-fat diet-induced increases in the concentrations of LDL (p < 0.05). | [85] | |
| Anti-hyperglycemic activity | ||||
| Hawthorn Fruit Extract | In vivo. | Hawthorn extract in high, middle and low dose could significantly reduce the fasting blood glucose levels of type II diabetic rats from 20.25 ± 1.9 mmol L−1 to 10.5 ± 0.87 mmol L −1, 15.13 ± 0.55 mmol L −1 and 17.9 ± 0.87 mmol L−1 (p < 0.01 and p < 0.05). | [86] | |
| Hawthorn polyphenols, D- chiro- inositol (DCI), and epigallocatechin gallate (EGCG) | In vitro. | Three ingredients exerted the synergistic hypoglycemic effect to enhance glucose consumption and glycogen levels and inhibit hepatic gluconeogenesis in IR-HepG2 cells. | [95] | |
| Hawthorn Extract | In vivo. | Hawthorn treated groups (0.5 g/kg/day, 1.0 g/kg/day) showed a significant reduction in insulin resistance compared with the HF group (p < 0.05, p < 0.01). | [85] | |
| Antibacterial and anti-inflammatory activities | ||||
| Hawthorn Fruit Extract | In vivo. | The hawthorn treatment group reduced the levels of IL-6, IL-8, IL-1β and TNF-α in cardiomyocytes due to doxorubicin treatment for heart failure (p < 0.01). | [93] | |
| Water fraction from hawthorn fruit | In vitro, ELISA. | Water fraction from hawthorn fruit at 200, 400 and 600 µg/mL increased the survival rate of RAW264.7 cells to 61.8%, 72.7% and 83.4% respectively. | [99] | |
| Hawthorn Methanolic Extract (ME) | In vitro. | ME from hawthorn had a minimum MIC and MBC value of 1.25 µg/mL against S. aureus and S. typhimurium. | [38] | |
| Hawthorn polysaccharide (HAW1-2) | In vivo. | The relative expression of IL-1β, IL-6 and TNF-α were suppressed after HAW1–2 treatment. | [50] | |
| Hawthorn phenolic extract | In vivo. | The extract decreased the percent-age of CD4−CD8− and CD4+ thymocytes but elevated the percentage of CD4+CD8+ and CD8+ thymic cells, increased the total number, percentage, and absolute count of T and B splenocytes. | [75] | |
| Pectin oligosaccharide (POS) | In vivo, ELISA. | Higher dose (0.75, 1.5 g/kg) of POS significantly (p < 0.01) decreased the contents of hepatic TNF-α and IL-6, while significantly (p < 0.05–0.01) increased the level of IL-10, compared with the high fat control group. | [98] | |
| Total Flavonoid Extract from Hawthorn (TFH) | In vitro. | TFH (50–200 µg/mL) treatment inhibited the increase of inflammatory cytokines IL-6, IL-1β, MCP-1 and IL-8 in Caco-2 cells in a dose-dependent manner. | [76] | |
| Anti-digestion activty | ||||
| Hawthorn Seed Eextract (HSEAE) | In vivo. ELISA. | Different doses of HSEAE effectively promoted the gastric emptying and small intestinal propulsion (p < 0.05 or p < 0.01). In addition, HSEAE increased SOD and GSH-Px in the rats’ stomachs while decreasing MDA, and increased plasma ghrelin while decreasing MTL and GAS (p < 0.05 or p < 0.01). | [104] | |
| Ethyl acetate part of hawthorn | In vivo, LC-MS. | The effect of ethyl acetate extract of hawthorn on gastric emptying rate and intestinal propulsion rate in a rat model of atropine sulfate-induced gastrointestinal motility retardation was significant (p < 0.05, p < 0.001). | [92] | |
| Charred hawthorn | In vivo. | Hawthorn decoction coupled with the odor of charred hawthorn effectively alleviate high-calorie-diet-induced dys-pepsia in rats by regulating the “Brain-Gut” axis and gut flora. | [91] | |
| Antioxidant activity | ||||
| Triterpenoids isolated from hawthorn berries | In vitro, PSC and superoxide anion free radical assay. | In PSC assay, compounds 1, 10 and 12 had pronounced antioxidant activity with an EC50 of 0.2 ± 0.01, 0.5 ± 0.01, and 0.7 ± 0.01 µM. | [111] | |
| Phenolic composition of Kazakh Crataegus | LC-MS | In the free radical scavenging activity assay (DPPH), the most potent extract was the phenolic compound from hawthorn leaves (IC50 48 ± 2 µg/mL). | [20] | |
| Hawthorn polyphenol extract (HPE) | In vivo and vitro, MTT. | After UVB irradiation, the cell viability significantly decreased (p < 0.05). HPE at 5 and 10 µg/mL significantly increased cell survival (p < 0.05). | [102] | |
| Phenolic compounds | In vitro, ORAC. | The antioxidant activity of phenolic compounds in hawthorn was significant, with ORAC values for the eight phenolic compounds ranging from 5.25 ± 0.54–62.79 ± 1.46 μmol TE/μmol. | [114] | |
| Hawthorn fruit extract | FRAP. | The antioxidant activity was widely varied (p < 0.001) in species of Crataegus, ranging from 0.32–1.84 mmol Fe++/g DW. | [12] | |
| Phenolic compounds | DPPH, ABTS, and FRAP. | The total antioxidant activity of organic fresh hawthorn berry fruit determined by DPPH, FRAP and ABTS assay was up to 286 ± 4, 320 ± 5 and 328 ± 6 μmol TE/g DW. | [54] | |
| Hawthorn extract | DPPH. | The DPPH scavenging capacity of the fresh hawthorn slices was 3.48 mmol TE/100 g DW. | [101] | |
| Extract from peel of hawthorn fruit(EPHF) | DPPH and ORAC. | EPHF has the strongest oxygen radical scavenging capacity (IC50 = 11.72 μg/mL). | [115] | |
| Organic freeze-dried hawthorn berries (OFDHB) | ABTS, FRAP and DPPH. | The peel of OFDHB sample had the highest antioxidant capacity followed the decreasing order of ABTS (577.5 µmol TE g−1) > FRAP (455.84 µmol TE g−1) > DPPH (410.75 µmol TE g−1) assay. | [4] | |
| Flavonoids | FRAP. | The highest antioxidant activity was observed in the leaves of C. pentagyna as 4.65 mmol Fe++/g DW, whereas the lowest activity (0.9 mmol Fe++/g DW) was found in the leaves of C. azarolus var. aronia. | [116] | |
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Santini, A.; Novellino, E.; Armini, V.; Ritieni, A. State of the Art of Ready-to-Use Therapeutic Food: A Tool for Nutraceuticals Addition to Foodstuff. Food Chem. 2013, 140, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Sixt, M.; Strube, J. Systematic Design and Evaluation of an Extraction Process for Traditionally Used Herbal Medicine on the Example of Hawthorn (Crataegus monogyna JACQ.). Processes 2018, 6, 73. [Google Scholar] [CrossRef]
- Lund, J.A.; Brown, P.N.; Shipley, P.R. Differentiation of Crataegus spp. Guided by Nuclear Magnetic Resonance Spectrometry with Chemometric Analyses. Phytochemistry 2017, 141, 11–19. [Google Scholar] [CrossRef]
- Lou, X.; Yuan, B.; Wang, L.; Xu, H.; Hanna, M.; Yuan, L. Evaluation of Physicochemical Characteristics, Nutritional Composition and Antioxidant Capacity of Chinese Organic Hawthorn Berry (Crataegus pinnatifida). Int. J. Food Sci. Technol. 2020, 55, 1679–1688. [Google Scholar] [CrossRef]
- Chinese Medicine Treasures. Available online: http://zhongyibaodian.com/bencaogangmu/shanzha.html (accessed on 12 August 2022).
- Chinese Pharmacopoeia 2020 Edition I. Available online: https://db2.ouryao.com/yd2020/view.php?id=fbcdae7d88 (accessed on 12 August 2022).
- Ma, S.; Lu, Y. Classification and Phylogenetic Analysis of Chinese Hawthorn Assessed by Plant and Pollen Morphology. Genet. Mol. Res. 2016, 3, gmr.15038739. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Zhang, X.; Bu, H.; Zhang, T.; Lao, Y.; Dong, W. Molecular Analysis of Evolution and Origins of Cultivated Hawthorn (Crataegus spp.) and Related Species in China. Front. Plant Sci. 2019, 10, 443. [Google Scholar] [CrossRef]
- Wang, J.; Xiong, X.; Feng, B. Effect of Crataegus Usage in Cardiovascular Disease Prevention: An Evidence-Based Approach. Evid.-Based Complement. Altern. Med. 2013, 2013, 149363. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Fu, S.; Huang, X.; Zhang, X.; Cui, Y.; Zhang, Z.; Ma, Y.; Zhang, X.; Yu, Q.; Yang, S. Biological Properties and Potential Application of Hawthorn and Its Major Functional Components: A Review. J. Funct. Foods 2022, 90, 104988. [Google Scholar] [CrossRef]
- Nazhand, A.; Lucarini, M.; Durazzo, A.; Zaccardelli, M.; Cristarella, S.; Souto, S.B.; Silva, A.M.; Severino, P.; Souto, E.B.; Santini, A. Hawthorn (Crataegus spp.): An Updated Overview on Its Beneficial Properties. Forests 2020, 11, 564. [Google Scholar] [CrossRef]
- Alirezalu, A.; Ahmadi, N.; Salehi, P.; Sonboli, A.; Alirezalu, K.; Mousavi Khaneghah, A.; Barba, F.J.; Munekata, P.E.S.; Lorenzo, J.M. Physicochemical Characterization, Antioxidant Activity, and Phenolic Compounds of Hawthorn (Crataegus spp.) Fruits Species for Potential Use in Food Applications. Foods 2020, 9, 436. [Google Scholar] [CrossRef]
- Doellman, M.; Ragland, G.; Hood, G.; Meyers, P.; Egan, S.; Powell, T.; Lazorchak, P.; Glover, M.; Tait, C.; Schuler, H.; et al. Genomic Differentiation during Speciation-with-Gene-Flow: Comparing Geographic and Host-Related Variation in Divergent Life History Adaptation in Rhagoletis pomonella. Genes 2018, 9, 262. [Google Scholar] [CrossRef]
- Yalçın Dokumacı, K.; Uslu, N.; Hacıseferoğulları, H.; Örnek, M.N. Determination of Some Physical and Chemical Properties of Common Hawthorn (Crataegus monogyna Jacq. Var. Monogyna). Erwerbs-Obstbau 2021, 63, 99–106. [Google Scholar] [CrossRef]
- Zhang, J.; Deng, Y.; Tong, X.; Liang, Y.; Li, Y.; Deng, C. Nutrition and Health Function of Crataegus pinnatifida Bunge and Its Application Progres. Anhui Agric. Sci. Bull. 2021, 27, 116–118. [Google Scholar]
- Li, L.; Gao, X.; Liu, J.; Chitrakar, B.; Wang, B.; Wang, Y. Hawthorn Pectin: Extraction, Function and Utilization. Curr. Res. Food Sci. 2021, 4, 429–435. [Google Scholar] [CrossRef]
- Zhu, R.; Zhang, X.; Wang, Y.; Zhang, L.; Wang, C.; Hu, F.; Ning, C.; Chen, G. Pectin Oligosaccharides from Hawthorn (Crataegus pinnatifida Bunge. Var. Major): Molecular Characterization and Potential Antiglycation Activities. Food Chem. 2019, 286, 129–135. [Google Scholar] [CrossRef]
- Cosmulescu, S.N.; Trandafir, I.; Scrieciu, F.; Stoenescu, A.-M. Content in Organic Acids of Mespilus spp. and Crataegus spp. Genotypes. Not. Bot. Horti Agrobot. Cluj-Napoca 2020, 48, 171–176. [Google Scholar] [CrossRef]
- Sun, B.; Huo, H.; Cai, A.; Xie, Y.; Li, H.; Li, D. Determination of contents of eight phenolic acids in Malus doumeri fruit by HPLC. Guihaia 2021, 07, 1135–1144. [Google Scholar]
- Elmira, B.; Wirginia, K.-K.; Tomasz, B.; Natalia, S.; Galiya, I.; Wojciech, K.; Kazimierz, G.; Saken, T.; Zuriyadda, S.; Fabio, B. Phenolic composition and antioxidant potential of different organs of Kazakh Crataegus almaatensis Pojark: A comparison with the European Crataegus oxyacantha L. flowers. Open Chem. 2018, 16, 415–426. [Google Scholar]
- Muradoğlu, F.; Gürsoy, S.; Yıldız, K. Quantification Analysis of Biochemical and Phenolic Composition in Hawthorn (Crataegus spp.) Fruits. Erwerbs-Obstbau 2019, 61, 189–194. [Google Scholar] [CrossRef]
- Wu, X.; Song, M.; Qiu, P.; Li, F.; Wang, M.; Zheng, J.; Wang, Q.; Xu, F.; Xiao, H. A Metabolite of Nobiletin, 4′-Demethylnobiletin and Atorvastatin Synergistically Inhibits Human Colon Cancer Cell Growth by Inducing G0/G1 Cell Cycle Arrest and Apoptosis. Food Funct. 2018, 9, 87–95. [Google Scholar] [CrossRef]
- Abu-Gharbieh, E.; Shehab, N.G. Therapeutic Potentials of Crataegus azarolus Var. eu- azarolus Maire Leaves and Its Isolated Compounds. BMC Complementary Altern. Med. 2017, 17, 218. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.E.; Brown, P.N.; Talent, N.; Dickinson, T.A.; Shipley, P.R. A Review of the Chemistry of the Genus Crataegus. Phytochemistry 2012, 79, 5–26. [Google Scholar] [CrossRef]
- Min, Q.; Bai, Y.; Zhang, Y.; Yu, W.; Zhang, M.; Liu, D.; Diao, T.; Lv, W. Hawthorn Leaf Flavonoids Protect against Diabetes-Induced Cardiomyopathy in Rats via PKC-α Signaling Pathway. Evid.-Based Complementary Altern. Med. 2017, 2017, 2071952. [Google Scholar] [CrossRef] [PubMed]
- Olah, N.-K.; Burtescu, R.; Petrescu, S.; Brasovan, A.; Chise, E.; Codruta, S.; Cobzac, A.; Hanganu, D. Phytochemical Screening of Different Crataegus oxyacantha Extracts. Studia UBB Chem. 2017, 62, 57–73. [Google Scholar] [CrossRef]
- Jurikova, T.; Sochor, J.; Rop, O.; Mlcek, J.; Balla, S.; Szekeres, L.; Adam, V.; Kizek, R. Polyphenolic Profile and Biological Activity of Chinese Hawthorn (Crataegus pinnatifida BUNGE) Fruits. Molecules 2012, 17, 14490–14509. [Google Scholar] [CrossRef]
- Han, X.; Li, W.; Huang, D.; Yang, X. Polyphenols from Hawthorn Peels and Fleshes Differently Mitigate Dyslipidemia, Inflammation and Oxidative Stress in Association with Modulation of Liver Injury in High Fructose Diet-Fed Mice. Chem.-Biol. Interact. 2016, 257, 132–140. [Google Scholar] [CrossRef]
- Hellenbrand, N.; Sendker, J.; Lechtenberg, M.; Petereit, F.; Hensel, A. Isolation and quantification of oligomeric and polymeric procyanidins in leaves and flowers of Hawthorn (Crataegus spp.). Fitoterapia 2015, 104, 14–22. [Google Scholar] [CrossRef]
- Zhang, P.; Xu, S. Flavonoid ketohexosefuranosides from the leaves of Crataegus pinnatifida Bge. var. major N.E.Br. Phytochemistry 2001, 57, 1249–1253. [Google Scholar] [CrossRef]
- Shahat, A.; Ismail, S.; Hammouda, F.; Azzam, S.; Lemiere, G.; Bruyne, T.; Swaef, S.; Pieters, L.; Vlietinck, A. Anti-HIV activity of flavonoids and proanthocyanidins from Crataegus sinaica. Phytomedicine 1998, 5, 133–136. [Google Scholar] [CrossRef]
- Nikolov, N.; Batyuk, V.; Ivanov, V. Crateside—A New Flavonol Glycoside Crataegus monogyna and C. pentagyna. Chem. Nat. Compd. 1973, 9, 150–151. [Google Scholar] [CrossRef]
- Nikolov, N.; Dwllamonica, G.; Chopin, J. Di-C-Glycosyl Flavones from Crataegus monogyna. Phytochemistry 1981, 20, 2780–2781. [Google Scholar] [CrossRef]
- Batyuk, V.; Chernobrovaya, N.; Prokopenko, A. Cratenacin—A New Flavone Glycoside from Crataegus Curvisepala. Khim. Prir. Soedin. 1966, 2, 90–93. [Google Scholar]
- Kurkin, V.A.; Pravdivtseva, O.E.; Shaikhutdinov, I.K.; Kurkina, A.V.; Volkova, N.A. Quantitative Determination of Total Flavonoids in Blood-Red Hawthorn Fruit. Pharm. Chem. J. 2020, 54, 36–39. [Google Scholar] [CrossRef]
- Sagaradze, V.A.; Babaeva, E.Y.; Kalenikova, E.I. HPLC-UV Method for Determing Flavonoids in Hawthorn Flowers and Leaves. Pharm. Chem. J. 2017, 51, 277–280. [Google Scholar] [CrossRef]
- Rocchetti, G.; Senizza, B.; Zengin, G.; Mahomodally, M.F.; Senkardes, I.; Lobine, D.; Lucini, L. Untargeted Metabolomic Profiling of Three Crataegus Species (Hawthorn) and Their in vitro Biological Activities. J. Sci. Food Agric. 2020, 100, 1998–2006. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, L.; Xu, J. Chemical Composition, Antibacterial Activity and Action Mechanism of Different Extracts from Hawthorn (Crataegus pinnatifida Bge.). Sci. Rep. 2020, 10, 8876. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, C.; Peng, Y.; Zhang, H.; Wang, Z.; Gao, Y.; Liu, Y.; Zhang, H. Chemical Constituents, Antioxidant and Gastrointestinal Transit Accelerating Activities of Dried Fruit of Crataegus dahurica. Food Chem. 2018, 246, 41–47. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, P.; Sun, J.; Zhao, C.; Song, Y.; Wu, J. Research progress on chemical constituents and pharmacological action of hawthorn. J. Northwest Pharm. 2021, 3, 521–523. [Google Scholar]
- Tohtahon, Z.; Zhang, L.; Han, J.; Xie, X.; Tu, Z.; Yuan, T. Extraction Optimization, Structural Characterization and Bioactivity Evaluation of Triterpenoids from Hawthorn (Crataegus cuneata) Fruits: Tohtahon et al. J. Food Biochem. 2017, 41, e12377. [Google Scholar] [CrossRef]
- Song, S.; Li, L.; Gao, P.; Peng, Y.; Yang, J.; Wu, C. Terpenoids and hexenes from the leaves of Crataegus pinnatifida. Food Chem. 2011, 129, 933–939. [Google Scholar] [CrossRef]
- Gao, P.; Li, L.; Peng, Y.; Li, F.; Niu, C.; Huang, X.; Ming, M.; Song, S. Monoterpene and lignan glycosides in the leaves of Crataegus pinnatifida. Biochem. Syst. Ecol. 2010, 38, 988–992. [Google Scholar] [CrossRef]
- Wen, L.; Guo, R.; You, L.; Abbasi, A.M.; Li, T.; Fu, X.; Liu, R.H. Major Triterpenoids in Chinese Hawthorn “Crataegus pinnatifida” and Their Effects on Cell Proliferation and Apoptosis Induction in MDA-MB-231 Cancer Cells. Food Chem. Toxicol. 2017, 100, 149–160. [Google Scholar] [CrossRef]
- Luan, M.; Wang, H.; Wang, J.; Zhang, X.; Zhao, F.; Liu, Z.; Meng, Q. Advances in Anti-Inflammatory Activity, Mechanism and Therapeutic Application of Ursolic Acid. MRMC 2022, 22, 422–436. [Google Scholar] [CrossRef]
- Huang, X.; Xu, Y.; Bai, M.; Zhou, L.; Song, S.; Wang, X. Lignans from the Seeds of Chinese Hawthorn (Crataegus pinnatifida Var. Major N.E.Br.) against β-Amyloid Aggregation. Nat. Prod. Res. 2018, 32, 1706–1713. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Ren, Q.; Song, X.; Zhou, L.; Yao, G.; Wang, X.; Song, S. Seven New Sesquineolignans Isolated from the Seeds of Hawthorn and Their Neuroprotective Activities. Fitoterapia 2018, 125, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Lou, L.; Liu, S.; Zhou, L.; Huang, X.; Song, S. Antioxidant and Anti-Inflammatory Neolignans from the Seeds of Hawthorn. Bioorg. Med. Chem. Lett. 2016, 26, 5501–5506. [Google Scholar] [CrossRef]
- Ma, L.; Xu, G.; Tang, X.; Zhang, C.; Zhao, W.; Wang, J.; Chen, H. Anti-Cancer Potential of Polysaccharide Extracted from Hawthorn (Crataegus) on Human Colon Cancer Cell Line HCT116 via Cell Cycle Arrest and Apoptosis. J. Funct. Foods 2020, 64, 103677. [Google Scholar] [CrossRef]
- Guo, C.; Wang, Y.; Zhang, S.; Zhang, X.; Du, Z.; Li, M.; Ding, K. Crataegus pinnatifida Polysaccharide Alleviates Colitis via Modulation of Gut Microbiota and SCFAs Metabolism. Int. J. Biol. Macromol. 2021, 181, 357–368. [Google Scholar] [CrossRef]
- Chen, J.; Song, S. Advances in hawthorn research. Res. Inf. Tradit. Chin. Med. 2005, 7, 20–23+26. [Google Scholar]
- Kowalski, R.; Kowalska, G.; Kalwa, K.; Sujka, M. Essential Oil Composition of Hawthorn Crataegus monogyna Inflorescence. Chem. Nat. Compd. 2018, 54, 995–997. [Google Scholar] [CrossRef]
- Gao, P.; Li, S.; Liu, K.; Sun, C.; Song, S.; Li, L. Antiplatelet Aggregation and Antithrombotic Benefits of Terpenes and Flavones from Hawthorn Leaf Extract Isolated Using the Activity-Guided Method. Food Funct. 2019, 10, 859–866. [Google Scholar] [CrossRef]
- Lou, X.; Xu, H.; Hanna, M.; Yuan, L. Identification and Quantification of Free, Esterified, Glycosylated and Insoluble-Bound Phenolic Compounds in Hawthorn Berry Fruit (Crataegus pinnatifida) and Antioxidant Activity Evaluation. LWT 2020, 130, 109643. [Google Scholar] [CrossRef]
- Mustapha, N.; Mokdad-Bzéouich, I.; Maatouk, M.; Ghedira, K.; Hennebelle, T.; Chekir-Ghedira, L. Antitumoral, Antioxidant, and Antimelanogenesis Potencies of Hawthorn, a Potential Natural Agent in the Treatment of Melanoma. Melanoma Res. 2016, 26, 211–222. [Google Scholar] [CrossRef]
- Ao, N.N.; Qu, Y.; Deng, Y.Y.; Cai, Q.; Suo, T.J.; Zheng, Y. Chemical basis of hawthorn processed with honey on myocardial ischaemia protective effect. Food Funct. 2020, 11, 3134–3143. [Google Scholar] [CrossRef]
- Li, L.; Gao, P.; Song, S.; Yuan, Y.; Liu, C.; Huang, X.; Liu, Q. Monoterpenes and flavones from the leaves of Crataegus pinnatifida with anticoagulant activities. J. Funct. Foods 2015, 12, 237–245. [Google Scholar] [CrossRef]
- Nie, J.; Su, J.; Zhang, C. Antioxidant and lipase activity of Hawthorn edible enzyme. Food Ind. 2021, 11, 232–236. [Google Scholar]
- Wang, M.; Yue, F.; Jing, R.; Hou, Y. Study on Manufacture Craft of Hawthorn Ultrafine Powder Bread. Food Sci. Technol. Econ. 2012, 2, 44–46. [Google Scholar]
- Borczak, B.; Sikora, E.; Sikora, M.; Kapusta-Duch, J.; Kutyła-Kupidura, E.M.; Fołta, M. Nutritional Properties of Wholemeal Wheat-Flour Bread with an Addition of Selected Wild Grown Fruits: Nutritional Properties of Wholemeal Wheat-Flour. Starch Stärke 2016, 68, 675–682. [Google Scholar] [CrossRef]
- Gasiński, A.; Kawa-Rygielska, J.; Szumny, A.; Gąsior, J.; Głowacki, A. Assessment of Volatiles and Polyphenol Content, Physicochemical Parameters and Antioxidant Activity in Beers with Dotted Hawthorn (Crataegus punctata). Foods 2020, 9, 775. [Google Scholar] [CrossRef]
- Zhang, W.; Chi, L.; Wu, Y.; Zhang, L.; Xu, C. Quality Comparison of Hawthorn Wines Fermented by Saccharomyces cerevisiae with and without Pulp Contact and Pectase Treatment. J. Chem. 2017, 2017, 6431818. [Google Scholar] [CrossRef]
- Özdemir, G.B.; Özdemir, N.; Ertekin-Filiz, B.; Gökırmaklı, Ç.; Kök-Taş, T.; Budak, N.H. Volatile Aroma Compounds and Bioactive Compounds of Hawthorn Vinegar Produced from Hawthorn Fruit (Crataegus tanacetifolia (Lam.) Pers.). J. Food Biochem. 2022, 46, e13676. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Du, J.; Zhang, L. Textural Characteristics and Sensory Evaluation of Yogurt Fortified with Pectin Extracted from Steeped Hawthorn Wine Pomace. Int. J. Food Eng. 2021, 17, 131–140. [Google Scholar] [CrossRef]
- Lai, K.; How, Y.; Pui, L. Storage Stability of Microencapsulated Lactobacillus rhamnosus GG in Hawthorn Berry Tea with Flaxseed Mucilage. J. Food Process. Preserv. 2020, 44, e14965. [Google Scholar] [CrossRef]
- Ozcelik, F.; Akan, E.; Kinik, O. Use of Cornelian Cherry, Hawthorn, Red Plum, Roseship and Pomegranate Juices in the Production of Water Kefir Beverages. Food Biosci. 2021, 42, 101219. [Google Scholar] [CrossRef]
- Chang, T.; Bi, Y.; Jing, L.; Liu, X.; Fan, M.; Yao, S.; Feng, L.; Zhao, Y. Simultaneous Adsorption of Acid and Flavonoids from Hawthorn Juice onto Resins. J. Food Eng. 2021, 291, 110195. [Google Scholar] [CrossRef]
- Zhou, Q.; Liu, X.; An, X. Study on the Processing Technology and Storage Period Quality of Low Nitrate Hawthorn Rose Sausage. Anhui Nongye Kexue 2020, 11, 194–197. [Google Scholar]
- Li, X.; Yang, W.; Zhou, Y.; Sun, K.; Wang, Z. The Development of Hawthorn Leaf Flavonoid Jam. Processing Technol. 2019, 16, 54–58. [Google Scholar]
- Lu, M.; Tan, H.; Ma, L. Preparation of Hawthorn and Passion Fruit Compound Jam. China Condiment 2022, 2, 93–96. [Google Scholar]
- Chen, J. Study on the production process of orange peel hawthorn soft candy. China Fruit Veg. 2019, 2, 16–19. [Google Scholar]
- Liu, X.; Yang, S.; Ma, J.; Yu, J.; Yan, Q.; Jiang, Z. Efficient Production of Acetylated Xylooligosaccharides from Hawthorn Kernels by a Xylanase from Paecilomyces aerugineus. Ind. Crops Prod. 2020, 158, 112962. [Google Scholar] [CrossRef]
- Golabiazar, R.; Qadir, G.S.; Faqe, Z.A.; Khalid, K.M.; Othman, K.I.; Rasool, N.F.; Saeed, H.F. Green Biosynthesis of CdS NPs and CdS/Fe3O4 NCs by Hawthorn Plant Extract for Photodegradation of Methyl Orange Dye and Antibacterial Applications. J. Clust. Sci. 2021, 3, 1223–1238. [Google Scholar] [CrossRef]
- Qiao, L.; Wang, H.; Shao, J.; Lu, L.; Tian, J.; Liu, X. A Novel Mitigator of Enzymatic Browning—Hawthorn Leaf Extract and Its Application in the Preservation of Fresh-Cut Potatoes. Food Qual. Saf. 2021, 5, fyab015. [Google Scholar] [CrossRef]
- Lis, M.; Szczypka, M.; Suszko-Pawłowska, A.; Sokół-Łętowska, A.; Kucharska, A.; Obmińska-Mrukowicz, B. Hawthorn (Crataegus monogyna) Phenolic Extract Modulates Lymphocyte Subsets and Humoral Immune Response in Mice. Planta Med. 2020, 86, 160–168. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, X.; Ji, Y. Total Flavonoid Extract from Hawthorn (Crataegus pinnatifida) Improves Inflammatory Cytokines-Evoked Epithelial Barrier Deficit. Med. Sci. Monit. 2020, 26, e920170. [Google Scholar] [CrossRef]
- Dong, P.; Pan, L.; Zhang, X.; Zhang, W.; Wang, X.; Jiang, M.; Chen, Y.; Duan, Y.; Wu, H.; Xu, Y.; et al. Hawthorn (Crataegus pinnatifida Bunge) Leave Flavonoids Attenuate Atherosclerosis Development in ApoE Knock-out Mice. J. Ethnopharmacol. 2017, 198, 479–488. [Google Scholar] [CrossRef]
- Rao, H.; Li, P.; Wu, H.; Liu, C.; Peng, W.; Su, W. Simultaneous Determination of Six Compounds in Destructive Distillation Extracts of Hawthorn Seed by GC-MS and Evaluation of Their Antimicrobial Activity. Molecules 2019, 24, 4328. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, H.; Dong, Q.; Hao, X.; Qiao, L. Maslinic Acid Induces Anticancer Effects in Human Neuroblastoma Cells Mediated via Apoptosis Induction and Caspase Activation, Inhibition of Cell Migration and Invasion and Targeting MAPK/ERK Signaling Pathway. AMB Express 2020, 10, 104. [Google Scholar] [CrossRef]
- Zhao, C.; Meng, X.; Li, Y.; Li, S.; Liu, Q.; Tang, G.; Li, H. Fruits for Prevention and Treatment of Cardiovascular Diseases. Nutrients 2017, 9, 598. [Google Scholar] [CrossRef] [PubMed]
- Arslan, R.; Bektas, N. Potential Antithrombotic Effect of Crataegus Species. IJPER 2018, 52, S155–S157. [Google Scholar] [CrossRef]
- Pahlavan, S.; Tousi, M.S.; Ayyari, M.; Alirezalu, A.; Ansari, H.; Saric, T.; Baharvand, H. Effects of Hawthorn (Crataegus pentagyna) Leaf Extract on Electrophysiologic Properties of Cardiomyocytes Derived from Human Cardiac Arrhythmia-specific Induced Pluripotent Stem Cells. FASEB 2018, 32, 1440–1451. [Google Scholar] [CrossRef]
- Yoo, J.-H.; Liu, Y.; Kim, H.-S. Hawthorn Fruit Extract Elevates Expression of Nrf2/HO-1 and Improves Lipid Profiles in Ovariectomized Rats. Nutrients 2016, 8, 283. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Jiang, W.; Xiong, X.; Chen, J.; Xiong, Y.; Li, Y. Ethanol Extract of Chinese Hawthorn (Crataegus pinnatifida) Fruit Reduces Inflammation and Oxidative Stress in Rats with Doxorubicin-Induced Chronic Heart Failure. Med. Sci. Monit. 2020, 6, 667–674. [Google Scholar] [CrossRef]
- Shih, C.; Lin, C.; Lin, Y.; Wu, J. Validation of the Antidiabetic and Hypolipidemic Effects of Hawthorn by Assessment of Gluconeogenesis and Lipogenesis Related Genes and AMP-Activated Protein Kinase Phosphorylation. Evid.-Based Complementary Altern. Med. 2013, 2013, 597067. [Google Scholar] [CrossRef] [PubMed]
- Aierken, A.; Buchholz, T.; Chen, C.; Zhang, X.; Melzig, M.F. Hypoglycemic Effect of Hawthorn in Type II Diabetes Mellitus Rat Model: Hypoglycemic Effect of Hawthorn in Type II Diabetes Mellitus. J. Sci. Food Agric. 2017, 97, 4557–4561. [Google Scholar] [CrossRef]
- Verma, T.; Sinha, M.; Bansal, N.; Yadav, S.R.; Shah, K.; Chauhan, N.S. Plants Used as Antihypertensive. Nat. Prod. Bioprospect. 2021, 11, 155–184. [Google Scholar] [CrossRef]
- Zheng, X.; Li, X.; Chen, M.; Yang, P.; Zhao, X.; Zeng, L.; OuYang, Y.; Yang, Z.; Tian, Z. The Protective Role of Hawthorn Fruit Extract against High Salt-Induced Hypertension in Dahl Salt-Sensitive Rats: Impact on Oxidative Stress and Metabolic Patterns. Food Funct. 2019, 10, 849–858. [Google Scholar] [CrossRef]
- Orhan, I.E. Phytochemical and Pharmacological Activity Profile of Crataegus oxyacantha L. (Hawthorn)—A Cardiotonic Herb. CMC 2019, 25, 4854–4865. [Google Scholar] [CrossRef]
- Al Humayed, S. Protective and Therapeutic Effects of Crataegus Aronia in Non-Alcoholic Fatty Liver Disease. Arch. Physiol. Biochem. 2017, 123, 23–30. [Google Scholar] [CrossRef]
- Wang, Y.; Lv, M.; Wang, T.; Sun, J.; Wang, Y.; Xia, M.; Jiang, Y.; Zhou, X.; Wan, J. Research on Mechanism of Charred Hawthorn on Digestive through Modulating “Brain-Gut” Axis and Gut Flora. J. Ethnopharmacol. 2019, 245, 112166. [Google Scholar] [CrossRef]
- Zeng, L.; Luo, L.; Xue, Q.; He, Q.; Chen, X.; Meng, J.; Wang, S.; Liang, S. LC–MS Based Plasma Metabolomics Study of the Intervention Effect of Different Polar Parts of Hawthorn on Hyperlipidemia Rats. J. Sep. Sci. 2021, 44, 963–972. [Google Scholar] [CrossRef]
- Hu, H.; Luo, X.; Dong, Q.; Mu, A.; Shi, G.; Wang, Q.; Chen, X.; Zhou, H.; Zhang, T.; Pan, L. Ethanol Extract of Zhongtian Hawthorn Lowers Serum Cholesterol in Mice by Inhibiting Transcription of 3-Hydroxy-3-Methylglutaryl-CoA Reductase via Nuclear Factor-Kappa B Signal Pathway. Exp. Biol. Med. 2016, 241, 667–674. [Google Scholar] [CrossRef]
- Holubarsch, C.J.F.; Colucci, W.S.; Eha, J. Benefit-Risk Assessment of Crataegus Extract WS 1442: An Evidence-Based Review. Am. J. Cardiovasc. Drugs 2018, 18, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Xin, C.; Zhao, M.; Wang, J.; Wang, Z. Hawthorn Polyphenols, D-chiro-inositol, and Epigallocatechin Gallate Exert a Synergistic Hypoglycemic Effect. J. Food Biochem. 2021, 7, e13771. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.; Li, Y.; Cao, Q.; Wu, H.; Tang, X.; Gao, X.; Yu, J.; Chen, Z.; Yang, Y. In Vitro and In Vivo Evidence That Quercetin Protects against Diabetes and Its Complications: A Systematic Review of the Literature. Biomed. Pharmacother. 2019, 109, 1085–1099. [Google Scholar] [CrossRef]
- Sagaradze, V.; Babaeva, E.; Ufimov, R.; Trusov, N.; Kalenikova, E. Study of the variability of rutin, vitexin, hyperoside, quercetin in “Crataegifolium cum flore” of hawthorn (Crataegus L.) species from Russian flora. J. Appl. Res. Med. Aromat. Plants 2019, 15, 100217. [Google Scholar]
- Li, T.; Chen, X.; Huang, Z.; Xie, W.; Tong, C.; Bao, R.; Sun, X.; Li, W.; Li, S. Pectin Oligosaccharide from Hawthorn Fruit Ameliorates Hepatic Inflammation via NF-ΚB Inactivation in High-Fat Diet Fed Mice. J. Funct. Foods 2019, 57, 345–350. [Google Scholar] [CrossRef]
- Li, C.; Wang, M. Anti-Inflammatory Effect of the Water Fraction from Hawthorn Fruit on LPS-Stimulated RAW 264.7 Cells. Nutr. Res. Pract. 2011, 5, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Wang, H.; Mu, X.; He, X.; Zhao, F.; Meng, Q. Advances in Research on the Preparation and Biological Activity of Maslinic Acid. MRMC 2021, 21, 79–89. [Google Scholar] [CrossRef]
- Liu, H.; Liu, J.; Lv, Z.; Yang, W.; Zhang, C.; Chen, D.; Jiao, Z. Effect of Dehydration Techniques on Bioactive Compounds in Hawthorn Slices and Their Correlations with Antioxidant Properties. J. Food Sci. Technol. 2019, 56, 2446–2457. [Google Scholar] [CrossRef]
- Liu, S.; Sui, Q.; Zou, J.; Zhao, Y.; Chang, X. Protective Effects of Hawthorn (Crataegus pinnatifida) Polyphenol Extract against UVB-Induced Skin Damage by Modulating the P53 Mitochondrial Pathway In Vitro and In Vivo. J. Food Biochem. 2019, 43, e12708. [Google Scholar] [CrossRef]
- Lou, X.; Guo, X.; Wang, K.; Wu, C.; Jin, Y.; Lin, Y.; Xu, H.; Hanna, M.; Yuan, L. Phenolic Profiles and Antioxidant Activity of Crataegus pinnatifida Fruit Infusion and Decoction and Influence of in Vitro Gastrointestinal Digestion on Their Digestive Recovery. LWT 2021, 135, 110171. [Google Scholar] [CrossRef]
- Niu, Z.; Yan, M.; Zhao, X.; Jin, H.; Gong, Y. Effect of Hawthorn Seed Extract on the Gastrointestinal Function of Rats with Diabetic Gastroparesis. S. Afr. J. Bot. 2020, 130, 448–455. [Google Scholar] [CrossRef]
- Rababa’h, A.M.; Al Yacoub, O.N.; El-Elimat, T.; Rabab’ah, M.; Altarabsheh, S.; Deo, S.; Al-Azayzih, A.; Zayed, A.; Alazzam, S.; Alzoubi, K.H. The Effect of Hawthorn Flower and Leaf Extract (Crataegus spp.) on Cardiac Hemostasis and Oxidative Parameters in Sprague Dawley Rats. Heliyon 2020, 6, e04617. [Google Scholar] [CrossRef]
- Pawlaczyk-Graja, I. Polyphenolic-Polysaccharide Conjugates from Flowers and Fruits of Single-Seeded Hawthorn (Crataegus monogyna Jacq.): Chemical Profiles and Mechanisms of Anticoagulant Activity. Int. J. Biol Macromol. 2018, 116, 869–879. [Google Scholar] [CrossRef]
- Bae, H.J.; Kim, J.; Kim, J.; Goo, N.; Cai, M.; Cho, K.; Jung, S.Y.; Kwon, H.; Kim, D.H.; Jang, D.S.; et al. The Effect of Maslinic Acid on Cognitive Dysfunction Induced by Cholinergic Blockade in Mice. Br. J. Pharmacol. 2020, 177, 3197–3209. [Google Scholar] [CrossRef]
- Lee, J.; Cho, E.; Kwon, H.; Jeon, J.; Jung, C.J.; Moon, M.; Jun, M.; Lee, Y.C.; Kim, D.H.; Jung, J.W. The Fruit of Crataegus pinnatifida Ameliorates Memory Deficits in β-Amyloid Protein-Induced Alzheimer’s Disease Mouse Model. J. Ethnopharmacol. 2019, 243, 112107. [Google Scholar] [CrossRef]
- Lim, D.W.; Han, T.; Jung, J.; Song, Y.; Um, M.Y.; Yoon, M.; Kim, Y.T.; Cho, S.; Kim, I.-H.; Han, D. Chlorogenic Acid from Hawthorn Berry (Crataegus pinnatifida Fruit) Prevents Stress Hormone-Induced Depressive Behavior, through Monoamine Oxidase B-Reactive Oxygen Species Signaling in Hippocampal Astrocytes of Mice. Mol. Nutr. Food Res. 2018, 62, 1800029. [Google Scholar] [CrossRef]
- Zhang, Q.; Xiong, Y.; Li, B.; Deng, G.; Fu, W.-W.; Cao, B.; Zong, S.; Zeng, G. Total Flavonoids of Hawthorn Leaves Promote Motor Function Recovery via Inhibition of Apoptosis after Spinal Cord Injury. Neural Regen. Res. 2021, 2, 350–356. [Google Scholar] [CrossRef]
- Qiao, A.; Wang, Y.; Xiang, L.; Zhang, Z. Novel triterpenoids isolated from hawthorn berries functioned as antioxidant and antiproliferative activities. J. Funct. Foods 2015, 13, 308–313. [Google Scholar] [CrossRef]
- Guo, R.; Lin, B.; Shang, X.; Zhou, L.; Yao, G.; Huang, X. Phenylpropanoids from the fruit of Crataegus pinnatifida exhibit cytotoxicity on hepatic carcinoma cells through apoptosis induction. Fitoterapia 2018, 127, 301–307. [Google Scholar] [CrossRef]
- He, Z.; Kwek, E.; Hao, W.; Zhu, W.; Liu, J.; Ma, K.; Chen, Z. Hawthorn fruit extract reduced trimethylamine-N-oxide (TMAO)-exacerbated atherogenesis in mice via anti-infammation and anti-oxidation. Nutr. Metab. 2021, 18, 6. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Deng, J.; Wen, L.; You, L.; Zhao, Z.; Zhou, L. Release of phenolic compounds and antioxidant capacity of Chinese hawthorn “Crataegus pinnatifida” during in vitro digestion. J. Funct. Foods 2018, 40, 76–85. [Google Scholar] [CrossRef]
- Wu, P.; Li, F.; Zhang, J.; Yang, B.; Ji, Z.; Chen, W. Phytochemical compositions of extract from peel of hawthorn fruit, and its antioxidant capacity, cell growth inhibition, and acetylcholinesterase inhibitory activity. BMC Complementary Altern. Med. 2017, 17, 151. [Google Scholar] [CrossRef]
- Alirezalu, A.; Salehi, P.; Ahmadi, N.; Sonboli, A.; Aceto, S.; Maleki, H.; Ayyari, M. Flavonoids profile and antioxidant activity in flowers and leaves of hawthorn species (Crataegus spp.) from different regions of Iran. Genet. Resour. Crop Evol. 2018, 21, 452–470. [Google Scholar] [CrossRef]
| Species | C. Monogyna Jacq. Var. Monogyna | C. pinnatifida Bunge | NM | C. pinnatifida | |||||
|---|---|---|---|---|---|---|---|---|---|
| Country | Source | Turkey | Wild | China | Wild | China | Wild | China | Wild |
| Protein | 3.03% | 0.7 | 0.5 | 3.14% | |||||
| Water | 68.98% | ND | 73 | 77.48% | |||||
| Fat | ND | 0.2 | 0.6 | 1.3% | |||||
| Pectin | ND | 3–4 | ND | 13 | |||||
| Energy(kJ) | ND | ND | 397 | 364 | |||||
| Dietary fiber | ND | ND | 3.1 | 33% | |||||
| Ca | 0.1 | ND | 52 | 0.06 | |||||
| K | 1.6 | ND | 299 | 1.02 | |||||
| Fe | 6.2 | 2.1 | 0.9 | 0.003 | |||||
| Na | 5.7 | 68 | 5.4 | 0.005 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Chai, X.; Zhao, F.; Hou, G.; Meng, Q. Food Applications and Potential Health Benefits of Hawthorn. Foods 2022, 11, 2861. https://doi.org/10.3390/foods11182861
Zhang J, Chai X, Zhao F, Hou G, Meng Q. Food Applications and Potential Health Benefits of Hawthorn. Foods. 2022; 11(18):2861. https://doi.org/10.3390/foods11182861
Chicago/Turabian StyleZhang, Juan, Xiaoyun Chai, Fenglan Zhao, Guige Hou, and Qingguo Meng. 2022. "Food Applications and Potential Health Benefits of Hawthorn" Foods 11, no. 18: 2861. https://doi.org/10.3390/foods11182861
APA StyleZhang, J., Chai, X., Zhao, F., Hou, G., & Meng, Q. (2022). Food Applications and Potential Health Benefits of Hawthorn. Foods, 11(18), 2861. https://doi.org/10.3390/foods11182861






